Abstract
The neural control of heart rate is determined primarily by the activity of preganglionic parasympathetic cardiac vagal neurons (CVNs) originating in the nucleus ambiguus (NA) in the brain stem. GABAergic inputs to CVNs play an essential role in determining the activity of these neurons including a robust inhibition during each inspiratory burst. The origin of GABAergic innervation has yet to be determined however. A transgenic mouse line expressing green florescent protein (GFP) in GABAergic cells was used in conjunction with caged glutamate to identify both clusters and individual GABAergic neurons that evoke inhibitory GABAergic synaptic responses in CVNs. Transverse slices were taken with CVNs patch-clamped in the whole cell configuration. Sections containing both the pre-Botzinger complex as well as the calamus scriptorius were divided into ∼90 quadrants, each 200 × 200 μm and were sequentially photostimulated. Inhibitory post synaptic currents (IPSCs) were recorded in CVNs after a 5-ms photostimulation of 50 μM caged glutamate. The four areas that contained GABAergic cells projecting to CVNs were 200 μm medial, 400 μm medial, 200 μm ventral, and 1,200 μm dorsal and 1,000 μm medial to patched CVNs. Once foci of GABAergic cells projecting to CVNs were determined, photostimulation of individual GABAergic neurons was conducted. The results from this study suggest that GABAergic cells located in four specific areas project to CVNs, and that these cells can be individually identified and stimulated using photouncaging to recruit GABAergic neurotransmission to CVNs.
INTRODUCTION
The modulation of heart rate is dominated by the activity of the cardioinhibitory parasympathetic nervous system, originating from cardiac vagal neurons (CVNs) located in the nucleus ambiguus (NA) and the dorsal motor nucleus of the vagus (Loewy and Spyer 1990). The activity of these neurons is dictated by synaptic neurotransmission as these neurons do not possess any inherent pacemaker properties of their own (Mendelowitz 1996).
CVNs receive numerous synaptically released neurotransmitters, including glutamate, ATP, serotonin, glycine, and GABA (Kamendi et al. 2006; Mendelowitz 1999; Neff et al. 1998a,b; Wang et al. 2003). GABAergic neurotransmission is one of the most important and endogenously active inputs to CVNs. GABA plays an essential role in one of the most common cardio-respiratory interactions, the generation of respiratory sinus arrhythmia, in which heart rate increases with each inspiration (Neff et al. 2003). Synaptic release of GABA activates multiple types of postsynaptic GABAergic receptors, including GABAzine-sensitive receptors that regulate phasic currents and GABAzine-insensitive, picrotoxin-sensitive tonic currents (Bouairi et al. 2006). Acetylcholine has been shown to be an important modulator of GABAergic synaptic transmission. Spontaneous GABAergic inhibitory postsynaptic inputs to CVNs are facilitated by activation of nicotinic receptors. Neostigmine, an acetylcholinesterase inhibitor, increases the frequency of GABAergic inhibitory postsynaptic currents (IPSCs) in CVNs and can be blocked by the α4 nicotinic receptor blocker DHβE (Wang et al. 2003). These nicotinic receptors are located on the presynaptic terminal, as application of tetrodotoxin, a sodium channel blocker, does not block the nicotine-mediated increase in IPSCs (Wang et al. 2003). Moreover, presynaptic α4 nicotinic receptors mediate the increase in GABAergic frequency that occurs during inspiratory activity (Neff et al. 2003).
GABAergic neurons have been found in several brain regions involved in the control of respiration and cardiovascular function (Dehkordi et al. 2007; Ellenberger 1999; Stornetta and Guyenet 1999; Stornetta et al. 2004), including the nucleus tractus solitarius (NTS), which sends both excitatory and inhibitory projections to the NA (Neff et al. 1998b; Wang et al. 2001), and the ventrolateral medulla (Carolina Takakura et al. 2007; Urbanski and Sapru 1988). However, despite the importance of GABAergic neurotransmission to CVNs, the origin of GABAergic pathways to CVNs has not been elucidated. This study was designed to identify the source(s) of GABAergic pathways to CVNs in the brain stem. To accomplish this goal, we used a novel experimental design using a UV uncaging system where a laser is used to focally photo-uncage glutamate in a small, defined area of tissue. The size of this area can be controlled by the objective used as well as the concentration of caged compound, allowing for more rapid spatial and temporal control when compared with other drug administration or stimulation methods. In addition, this method of stimulation is effective only on cell bodies and not fibers of passage that could be stimulated with electrical stimulation. The glutamate is made biologically inert by the addition of a caging group, and on exposure to light of a specific wavelength, the caging group is cleaved from the glutamate. We mapped, using spatially limited photostimulation of caged glutamate, regions in the brain stem that could evoke a GABAergic pathway to CVNs in transgenic mice in which GABAergic neurons are identified with expression of enhanced GFP under the control of the mouse Gad1 (GAD67) gene promoter.
METHODS
Transgenic mice (P1-5) expressing GFP under the control of the Gad1 (GAD67) gene promoter (Jackson Laboratories, Bar Harbor, MA) were exposed to hypothermia to slow heart rate. The heart was exposed by a right thoractomy and the retrograde fluorescent tracer X-rhodamine-5-(and 6)-isothiocyanate (Molecular Probes, Eugene, OR) was injected into the pericardial sac. The fluorescent tracer was absorbed by the terminals of the preganglionic parasympathetic neurons and then retrogradely transported to the cell bodies of CVNs in the NA in the brain stem. After a 24- to 48-h recovery, pups were anesthetized with isoflurane and killed by cervical dislocation, and the hindbrain was rapidly removed and placed in cold physiological saline solution [containing (in mM)140 NaCl, 5 KCl, 2 CaCl2, 5 glucose, and 10 HEPES, bubbled with 100% O2, pH 7.4]. Using a vibrotome, two 500-μm slices, rostral to the calamus scriptorius were taken (which contained labeled CVNs) and were used for electrophysiological experiments. All animal procedures were performed in compliance with the institutional guidelines at George Washington University and are in accordance with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association and the National Institutes of Health publication “Guide for the Care and Use of Laboratory Animals.”
Slices were mounted in a perfusion chamber and submerged in a perfusate of the following composition (in mM): 125 NaCl, 3 KCl, 2 CaCl2, 26 NaHCO3, 5 glucose, and 5 HEPES, constantly bubbled with gas (95% O2-5% CO2) and maintained at pH 7.4. Individual CVNs were identified by the presence of the fluorescent tracer. These identified CVNs were then imaged with differential interference contrast optics, infrared illumination, and infrared-sensitive video-detection cameras (Nikon) to gain better spatial resolution and to visually guide and position the patch pipette onto the surface of the identified neuron. Patch pipettes were mounted onto a pipette holder and amplifier head stage (Axon Instruments; Axopatch 200B), which were connected to micromanipulators (Narashige, Tokyo, Japan). The indifferent electrode was an Ag-AgCl plug submerged in the bath.
Patch pipettes (2.5–4.5 MΩ) were visually guided to the surface of individual CVNs. The pipette was advanced until a seal was obtained over 1 GΩ between the pipette tip and the cell membrane of the identified neuron. The membrane under the pipette tip was then ruptured with a brief suction to obtain whole cell patch-clamp configuration. For spatial experiments, pipettes were filled with potassium gluconate solution of the following composition (in mM): 135 gluconic acid, 10 HEPES, 10 EGTA, 1 CaCl2, and 1 MgCl2, pH 7.4. For quadrant experiments, pipettes were filled with a solution containing (in mM) 150 KCl, 4 MgCl2, 2 EGTA, 2 Na-ATP, and 10 HEPES, pH 7.4. Strychnine (1 μM) was included in the bath to block glycinergic IPSCs to isolate GABAergic events.
Glutamate uncaging was performed using a DPSS 355 nm UV laser launch system (Prairie Technologies, Madison WI) to photoexcite caged glutamate (Sigma, St. Louis MO). The laser was focused through an acousto-optic tunable filter (AOTF) that allowed for selection of the desired wavelength, adjustment of laser intensity, acted as a shutter and focused the laser beam on the tissue for the desired amount of time. Positioning of the visible aiming spot for the photouncaging beam allowed for precise control over the area to be uncaged. Multiple uncaging durations were examined in preliminary experiments, including 1, 2, 5, and 10 ms; 5 ms was chosen for this study because we found that this duration of time was sufficient to excite a reproducible burst of activity in GABAergic neurons but not produce excito-toxicity that occasionally occurred with longer durations. Caged glutamate was bath-applied at a concentration of either 10 or 50 μM and uncaged for a duration of 5 ms. Analysis of spontaneous synaptic currents was performed using MiniAnalysis (version 5.6.12, Synaptosoft), and analysis of voltage-gated currents was done with Clampfit (Axon Instruments, Union City, CA, version 8.0). Data were analyzed for the 2 s prior to and 2.5 s after uncaging, and plotted in 500-ms bins. Results are presented as means ± SE. Statistical comparisons were performed using Graphpad Statistical software using one-way ANOVA and Neumann-Keuls post hoc. Statistical significance for all data were set at P < 0.05.
RESULTS
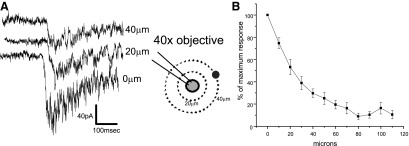
To determine the spatial range of the release of caged glutamate using this uncaging system, currents evoked in GABAergic neurons in response to the uncaging of glutamate were examined with different objectives, concentrations of caged glutamate, and at varying distances from the patched GABAergic neuron. Using a concentration of 50 μM caged glutamate and a ×10 objective, uncaging of glutamate elicited inward currents, typically 100–1,000 pA, that decayed over a distance of ∼100–120 μm. The traces from a typical experiment are shown in Fig. 1A with summary data in B. The inward currents in these GABAergic cells were completely blocked by addition of the N-methyl-d-aspartate (NMDA) receptor antagonist 2-amino-5-phosphonopentanoic acid (AP-5) and the AMPA/kainate receptor antagonist 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX) to the perfusion bath (data not shown). At a distance of 120 μm from the patched GABAergic neuron, uncaging of glutamate evoked responses ∼10% of the peak response obtained when the surface of the neuron was stimulated. This degree of spatial specificity of uncaging was considered appropriate for stimulating regions or clusters of GABAergic neurons.
FIG. 1.

The spatially limited release of glutamate was accomplished by focal photostimulation of 50 μM caged glutamate using a ×10 objective for 5 ms. A, left: the results from a typical experiment. The inward currents evoked by the photorelease of glutamate were typically 100–1,000 pA at the surface of the neuron and decreased with increased distance between the patched cell and the uncaging location. The responses at distances >100 μm were negligible. Middle: illustration shows the experimental paradigm of examining the responses on uncaging glutamate at varying distances from the patched neuron. Data from 9 experiments are summarized in B.
To determine which regions or clusters of GABAergic cells in the brain stem slice project to CVNs, the brain slice was divided into a grid of over 90 sections, each with an area of 200 × 200 μm (Fig. 2). Each foci was stimulated with caged glutamate a minimum of two times for each cell tested, and although it was not possible to test all foci in each CVN, each foci was tested in at least six CVNs. Surprisingly, stimulation of only four regions, of 90–100 potential regions, recruited inhibitory GABAergic neurotransmission to CVNs. These areas were 200 μm directly ventral to the recorded CVNs, 200 μm directly medial, 400 μm directly medial, and 1,200 μm dorsal and 1,000 μm medial to the CVNs in the NA (P < 0.05).
FIG. 2.
The brain slice was divided into 200 × 200 μm areas with each foci being stimulated with a 5-ms pulse of 50 μM glutamate. Of 90–100 areas tested, only 4 areas were found to elicit an increase in inhibitory events in the patched cardiac vagal neuron (P < 0.05). The results shown are the typical evoked responses in a single GABAergic neuron (top) and the average results in 8–10 neurons (bottom) from each of these localized areas. The bar graphs are plotted in 500-ms bins, with the 1st 4 bars representing the 2 s prior to the photouncaging, and the last 5 bars representing the 0.5 ms associated with the photostimulation, and the 2-s poststimulation, respectively.
Once regions were found that contain GABAergic neurons that project to CVNs, individual GABAergic neurons were identified and studied. To accomplish the goal of stimulating individual GABAergic neurons, the concentration of caged glutamate was reduced to 10 μM and a ×40 objective was utilized. Under these conditions, direct stimulation of GABAergic neurons was much more discreet and spatially limited. The release of caged glutamate activated inward currents in GABAergic cells that decayed to 50% of the peak response at a distance of ∼20 μm from the recorded GABAergic neuron (Fig. 3).
FIG. 3.
The spatially limited release of glutamate on an individual GABAergic neuron was accomplished by 5 ms focal photostimulation of 10 μM caged glutamate using a ×40 objective. As shown in A, the inward inhibitory currents evoked by the photorelease of glutamate were typically 50–150 pA at the surface of the neuron, which decreased with increased distance between the patched cell and the uncaging location. B: the responses at distances >50 μm were negligible. Left: traces are the responses from a typical experiment; middle: illustration depicts the experimental procedure of uncaging glutamate at increasing distances from the patched cell. Right: the average results from 10 neurons.
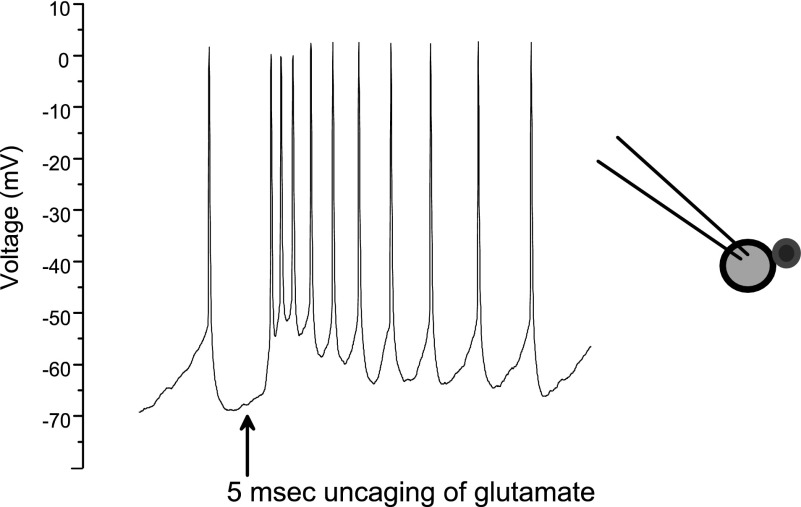
To characterize the firing patterns of GABAergic neurons in response to this localized excitatory stimulus, GABAergic cells were recorded in current-clamp configuration, and the response to a 5-ms uncaging pulse of 10 μM caged glutamate was examined. As shown in Fig. 4, GABAergic neurons' firing rate increased from 4 ± 0.8 to 22.4 ± 5.2 Hz in response to one uncaging pulse of UV light to uncage the glutamate (n = 8).
FIG. 4.
Depicted are the changes in action potential firing on release of caged glutamate (10 μM) using a ×40 objective. Glutamate was uncaged for a duration of 5 ms on the patched GABAergic cell, which fired multiple action potentials in response to the uncaging of caged glutamate. Cells were patched in the current clamp configuration (n = 8). GABAergic cells fired an average of 4 ± 0.8 Hz before stimulation with glutamate and then an average of 22.4 ± 5.2 Hz in the 1 s after the UV uncaging. All cells responded with action potentials to the uncaging within 33.25 ± 1.8 ms. The cells returned to baseline by 600 ms post stimulation. Right: illustration depicts the experimental paradigm.
To identify the individual GABAergic cells that project to CVNs, the regions where an inhibitory response was seen using the ×10 objective with 50 μM caged glutamate were studied using instead, the ×40 objective with 10 μM caged glutamate.
As shown from a representative experiment in Fig. 5, individual GABAergic neurons could be stimulated to evoke postsynaptic GABAergic neurotransmission to CVNs. Stimulation of an individual GABAergic neuron for 5 ms with 10 μM caged glutamate elicited an increase in the frequency of GABAergic IPSCs in the CVNs (P < 0.05, Fig. 5, A and B), and this pathway was subsequently blocked by bath application of 25 μM GABAzine, a GABAA antagonist (C).
FIG. 5.
Stimulation of an individual GABAergic cell projecting to a patched cardiac vagal neuron (CVN) was accomplished by the spatially limited release of glutamate (10 μM) onto the GABAergic cell using a ×40 objective (n = 17; A. and B. Top: the results from a typical experiment. Glutamate uncaged on the cell body of a GABAergic neuron evokes an increase in inhibitory events in a CVN from 2.8 ± 0.2 Hz prestimulation to 4.3 ± 0.3 Hz (P < 0.05). C: the application of GABAzine to the bath solution blocked the increase in inhibitory events. Right: illustration depicts the experimental design.
DISCUSSION
In this study, we sought to identify the GABAergic neurons that project to CVNs. The results from this study indicate there are a few select foci of GABAergic neurons, both in the immediate vicinity of the NA and in the area of the nucleus tractus solitarius that contain GABAergic neurons projecting to CVNs. The areas, more specifically, are directly medial (200 and 400 μm, respectively), directly ventral (200 μm), and dorsal and medial (1,200 × 1,000 μm) to the CVNs in the NA. On stimulation of these selected areas by the uncaging of glutamate, IPSCs were evoked in CVNs. Similarly, but using a more powerful objective for more focal stimulation, individual GABAergic neurons projecting to cardiac vagal neurons can be identified in several sites which when stimulated evoke an inhibitory response in CVNs.
Mapping synaptic connections and neural circuitry with stimulation of caged glutamate has been used in other networks and provides several advantages over the more traditional “puffer” pipette experimental setup. For instance, photo-uncaging of caged glutamate onto the CA3 region and dentate gyrus of the hippocampus elicits an inward current in neurons located in the CA1 hippocampal region (Callaway and Katz 1993). Similarly, Lam et al. used photo-uncaging to study the interconnections of the thalamic reticular nucleus and isolated a GABAergic synaptic input to reticular cells (Lam et al. 2006). The use of photouncaging allows the stimulation of a larger grid of tissue in a shorter amount of time than normally would be accomplished.
Previous studies from this lab, and others, have indicated CVNs receive GABAergic innervation (Batten 1995; Wang et al. 2001, 2003), and these GABAergic neurons are found throughout the brain stem, especially in regions essential in respiration and cardiovascular function, including the pre-Botzinger and Botzinger complexes. GABAergic cells in these regions are able to fire numerous action potentials when excited with either glutamate or a depolarizing pulse, corresponding with the typical bursting pattern of postsynaptic GABAergic IPSCs recorded in CVNs when GABAergic cells projecting to them are stimulated.
Acetylcholine plays a major modulatory role in CVNs both pre- and postsynaptically, and cholinergic receptors have been found to modulate GABAergic neurotransmission to CVNs. CVN GABAergic activity is endogenously facilitated by activation of nicotinic receptors (Wang et al. 2003), and blockade of nicotinic β2 receptors abolishes the respiratory-related increase of GABA neurotransmission to CVNs (Neff et al. 2003). GABAergic neurons in the RVM and RVLM have been shown to regulate numerous autonomic functions including cardiorespiratory regulation and function (Dehkordi et al. 2007). When glutamate is micro-injected into the RVLM, an increase in heart rate occurs (Takayama and Miura 1991; Vayssettes-Courchay et al. 1992), and this increase in heart rate can be attenuated with blockade of GABAergic neurotransmission in the NA, indicating a reciprocal role for these two neurotransmitter systems (Nakamura et al. 2008).
In addition to the RVM, the pre-Botzinger complex, ventral to the NA in the rostro-ventral lateral medulla, is the proposed site of respiratory rhythmogenesis (Smith et al. 1991). This region also contains GABAergic neurons (Ellenberger 1999). Because CVNs receive a robust burst of GABAergic neurotransmission during inspiratory activity, it seems probable that GABAergic neurons in the pre-Botzinger complex that project to CVNs may constitute this cardiorespiratory interaction and are likely contained within the ventral foci of GABAergic neurons.
CVNs in the NA receive inputs from a multitude of areas in the brain stem, one of which is the NTS. The NTS is thought to play an important role in cardiovascular regulation as well as respiration, and recent work has shown electrical stimulation of the NTS evokes a monosynaptic GABAergic pathway to CVNs (Wang et al. 2001) as well as an excitatory monosynaptic glutamatergic pathway (Neff et al. 1998b). Likewise stimulation of the NTS causes an increase in the baseline respiratory frequency in rats (Braccialli et al. 2008). The NTS is the site that receives cardiovascular and respiratory sensory information from baroreceptors located in the aortic arch and carotid sinus (Ciriello 1983; Kumada et al. 1990; Mendelowitz 1998; Mendelowitz et al. 1992; Sapru 1996; Wallach and Loewy 1980). In this study, we identified an area in the vicinity of the NTS that, when stimulated with caged glutamate, evoked inhibitory postsynaptic GABAergic currents in CVNs. The GABAergic neurons originating near the NTS that project to CVNs are most likely involved in the baroreflex control of parasympathetic cardiac activity, the phasic pattern of parasympathetic cardioinhibitory activity dependent on phasic pulsatile arterial pressure, and/or the respiratory modulation of CVNs.
An increase in IPSCs was also noted in two regions medial to the NA, 200, and 400 μm, respectively. Although these are not necessarily thought of as major respiratory centers, neurons within this region do project to respiratory nuclei. When pseudo-rabies virus was injected into the C4–C6 region of the feline spinal cord, labeling appeared within 1 day in the lateral reticular formation (LRt), an area roughly medial to the NA (Lois et al. 2008). Similarly, injection of both fluorogold and rhodamine into the NA and the NTS was retrogradely transported into the magnocellular reticular nucleus and adjacent lateral paragigantocellular nucleus (LPGi), areas approximately medial to the NA (Babic et al. 2008).
In summary, this study identifies four major origins of GABAergic neurotransmission to CVNs. It is likely that the GABAergic neurons immediately ventral and potentially medial to these CVNs in the NA play a role in cardiorespiratory network interactions, whereas the foci of GABAergic neurons in the dorsomedial medulla are involved in the baroreflex and/or chemoreflex control of heart rate.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-49965, HL-72006, and HL-59895 to D. Mendelowitz and American Heart Association predoctoral fellowship to J. G. Frank.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Babic et al. 2008.Babic T, de Oliveira CV, Ciriello J. Collateral axonal projections from rostral ventromedial medullary nitric oxide synthase containing neurons to brainstem autonomic sites. Brain Res 1211: 44–56, 2008. [DOI] [PubMed] [Google Scholar]
- Batten 1995.Batten TF Immunolocalization of putative neurotransmitters innervating autonomic regulating neurons (correction of neurons) of cat ventral medulla. Brain Res Bull 37: 487–506, 1995. [DOI] [PubMed] [Google Scholar]
- Braccialli et al. 2008.Braccialli AL, Bonagamba LG, Machado BH. Glutamatergic and purinergic mechanisms on respiratory modulation in the caudal NTS of awake rats. Respir Physiol Neurobiol 161: 246–252, 2008. [DOI] [PubMed] [Google Scholar]
- Bouairi et al. 2006.Bouairi E, Kamendi H, Wang X, Gorini C, Mendelowitz D. Multiple types of GABAA receptors mediate inhibition in brain stem parasympathetic cardiac neurons in the nucleus ambiguus. J Neurophysiol 96: 3266–3272, 2006. [DOI] [PubMed] [Google Scholar]
- Callaway and Katz 1993.Callaway EM, Katz LC. Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc Natl Acad Sci USA 90: 7661–7665, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carolina Takakura et al. 2007.Carolina Takakura A, Santos Moreira T, Menani JV, Ribeiro Campos R, Colombari E. Commissural nucleus of the solitary tract is important for cardiovascular responses to caudal pressor area activation. Brain Res 1161: 32–37, 2007. [DOI] [PubMed] [Google Scholar]
- Ciriello 1983.Ciriello J Brain stem projections of aortic baroreceptor afferent fibers in the rat. Neurosci Lett 36: 37–42, 1983. [DOI] [PubMed] [Google Scholar]
- Dehkordi et al. 2007.Dehkordi O, Millis RM, Dennis GC, Jazini E, Williams C, Hussain D, Jayam-Trouth A. Expression of alpha-7 and alpha-4 nicotinic acetylcholine receptors by GABAergic neurons of rostral ventral medulla and caudal pons. Brain Res 1185: 95–102, 2007. [DOI] [PubMed] [Google Scholar]
- Ellenberger 1999.Ellenberger HH Distribution of bulbospinal gamma-aminobutyric acid-synthesizing neurons of the ventral respiratory group of the rat. J Comp Neurol 411: 130–144, 1999. [DOI] [PubMed] [Google Scholar]
- Kamendi et al. 2006.Kamendi H, Stephens C, Dergacheva O, Wang X, Huang ZG, Bouairi E, Gorini C, McIntosh JM, Mendelowitz D. Prenatal nicotine exposure alters the nicotinic receptor subtypes that modulate excitation of parasympathetic cardiac neurons in the nucleus ambiguus from primarily alpha3beta2 and/or alpha6betaX to alpha3beta4. Neuropharmacology 51: 60–66, 2006. [DOI] [PubMed] [Google Scholar]
- Kumada et al. 1990.Kumada M, Terui N, Kuwaki T. Arterial baroreceptor reflex: its central and peripheral neural mechanisms. Prog Neurobiol 35: 331–361, 1990. [DOI] [PubMed] [Google Scholar]
- Lam et al. 2006.Lam YW, Nelson CS, Sherman SM. Mapping of the functional interconnections between thalamic reticular neurons using photostimulation. J Neurophysiol 96: 2593–2600, 2006. [DOI] [PubMed] [Google Scholar]
- Loewy and Spyer 1990.Loewy AD, Spyer KM. Central Regulation of Autonomic Functions. New York: Oxford, 1990.
- Lois et al. 2008.Lois JH, Rice CD, Yates BJ. Neural circuits controlling diaphragm function in the cat revealed by transneuronal tracing. J Appl Physiol 106: 138–152, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelowitz 1996.Mendelowitz D Firing properties of identified parasympathetic cardiac neurons in nucleus ambiguus. Am J Physiol Heart Circ Physiol 271: H2609–2614, 1996. [DOI] [PubMed] [Google Scholar]
- Mendelowitz 1998.Mendelowitz D Nicotine excites cardiac vagal neurons via three sites of action. Clin Exp Pharmacol Physiol 25: 453–456, 1998. [DOI] [PubMed] [Google Scholar]
- Mendelowitz 1999.Mendelowitz D Advances in parasympathetic control of heart rate and cardiac function. News Physiol Sci 14: 155–161, 1999. [DOI] [PubMed] [Google Scholar]
- Mendelowitz et al. 1992.Mendelowitz D, Yang M, Andresen MC, Kunze DL. Localization and retention in vitro of fluorescently labeled aortic baroreceptor terminals on neurons from the nucleus tractus solitarius. Brain Res 581: 339–343, 1992. [DOI] [PubMed] [Google Scholar]
- Nakamura et al. 2008.Nakamura T, Kawabe K, Sapru HN. Cold pressor test in the rat: medullary and spinal pathways and neurotransmitters. Am J Physiol Heart Circ Physiol 295: H1780–1787, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff et al. 1998a.Neff RA, Humphrey J, Mihalevich M, Mendelowitz D. Nicotine enhances presynaptic and postsynaptic glutamatergic neurotransmission to activate cardiac parasympathetic neurons. Circ Res 83: 1241–1247, 1998a. [DOI] [PubMed] [Google Scholar]
- Neff et al. 1998b.Neff RA, Mihalevich M, Mendelowitz D. Stimulation of NTS activates NMDA and non-NMDA receptors in rat cardiac vagal neurons in the nucleus ambiguus. Brain Res 792: 277–282, 1998b. [DOI] [PubMed] [Google Scholar]
- Neff et al. 2003.Neff RA, Wang J, Baxi S, Evans C, Mendelowitz D. Respiratory sinus arrhythmia: endogenous activation of nicotinic receptors mediates respiratory modulation of brainstem cardioinhibitory parasympathetic neurons. Circ Res 93: 565–572, 2003. [DOI] [PubMed] [Google Scholar]
- Sapru 1996.Sapru HN Carotid chemoreflex. neural pathways and transmitters. Adv Exp Med Biol 410: 357–364, 1996. [PubMed] [Google Scholar]
- Smith et al. 1991.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brain stem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta and Guyenet 1999.Stornetta RL, Guyenet PG. Distribution of glutamic acid decarboxylase mRNA-containing neurons in rat medulla projecting to thoracic spinal cord in relation to monoaminergic brainstem neurons. J Comp Neurol 407: 367–380, 1999. [PubMed] [Google Scholar]
- Stornetta et al. 2004.Stornetta RL, McQuiston TJ, Guyenet PG. GABAergic and glycinergic presympathetic neurons of rat medulla oblongata identified by retrograde transport of pseudorabies virus and in situ hybridization. J Comp Neurol 479: 257–270, 2004. [DOI] [PubMed] [Google Scholar]
- Takayama and Miura 1991.Takayama K, Miura M. Glutamate-immunoreactive neurons of the central amygdaloid nucleus projecting to the subretrofacial nucleus of SHR and WKY rats: a double-labeling study. Neurosci Lett 134: 62–66, 1991. [DOI] [PubMed] [Google Scholar]
- Urbanski and Sapru 1988.Urbanski RW, Sapru HN. Putative neurotransmitters involved in medullary cardiovascular regulation. J Auton Nerv Syst 25: 181–193, 1988. [DOI] [PubMed] [Google Scholar]
- Vayssettes-Courchay et al. 1992.Vayssettes-Courchay C, Bouysset F, Verbeuren TJ, Schmitt H, Laubie M. Cardiovascular effects of microinjections of quipazine into nuclei of the medulla oblongata in anaesthetized cats: comparison with L-glutamate. Eur J Pharmacol 211: 243–250, 1992. [DOI] [PubMed] [Google Scholar]
- Wallach and Loewy 1980.Wallach JH, Loewy AD. Projections of the aortic nerve to the nucleus tractus solitarius in the rabbit. Brain Res 188: 247–251, 1980. [DOI] [PubMed] [Google Scholar]
- Wang et al. 2001.Wang J, Irnaten M, Mendelowitz D. Characteristics of spontaneous and evoked GABAergic synaptic currents in cardiac vagal neurons in rats. Brain Res 889: 78–83, 2001. [DOI] [PubMed] [Google Scholar]
- Wang et al. 2003.Wang J, Wang X, Irnaten M, Venkatesan P, Evans C, Baxi S, Mendelowitz D. Endogenous acetylcholine and nicotine activation enhances GABAergic and glycinergic inputs to cardiac vagal neurons. J Neurophysiol 89: 2473–2481, 2003. [DOI] [PubMed] [Google Scholar]