Abstract
When saccades consistently overshoot their targets, saccade amplitudes gradually decrease, thereby maintaining accuracy. This adaptive process has been seen as a form of motor learning that copes with changes in physical parameters of the eye and its muscles, brought about by aging or pathology. One would not expect such a motor-repair mechanism to be specific to the visual properties of the target stimulus. We had subjects make saccades to sudden movements of either of two targets—a steadily illuminated circle or a flickering circle—one of which stepped back during each saccade it elicited, simulating the effect of a hypermetric saccade. Saccade gain (saccade amplitude/target amplitude) decreased by 15% for the target that stepped back versus 6% for the target that did not step back. Most of the change in gain between successive blocks of trials of each type occurred on the first saccade of the block, decreasing by 0.12 on the first trial of a step-back block and increasing by 0.1 on the first trial of a no-step-back block. The differential adaptation of the two targets required postsaccadic feedback of both target types, as shown in a separate experiment, in which saccades to only one target received feedback, and the gain did not differ between the two target types. This demonstration that a context defined by a visual stimulus can serve as an effective cue for switching saccade gain between states suggests that saccade adaptation may have a heretofore unsuspected dimension of adaptability.
INTRODUCTION
Because saccades are so brief (on the order of tens of milliseconds), they cannot be adjusted on-line through the use of visual information (feedback), which takes 40–50 ms to reach the superior colliculus (Wurtz and Goldberg 1972). Instead, any inaccuracies in the motor plan must be compensated for after the movement's completion, with corrections being manifested on subsequent saccades. In the laboratory, saccade adaptation can be demonstrated in humans by the double-step paradigm (McLaughlin 1967), in which a target steps and, when the subject initiates a saccade toward it, the target makes a small second step back toward its starting position, resulting in the eye landing beyond the target and provoking a second, corrective saccade. Repetitions of this series of target steps and intrasaccadic target back steps cause a decrease in saccade amplitude so that the initial saccade brings the eye progressively closer to landing on the target. Saccade adaptation has also been observed both in human patients with disease-related weakness of the extraocular muscles (Optican et al. 1985) and in monkeys, in which muscles have been experimentally debilitated (Optican and Robinson 1980; Snow et al. 1985). It is widely held that postsaccadic retinal error (the difference between target and gaze positions) drives the adaptation both in the double-step paradigm (Noto and Robinson 2001; Seeberger et al. 2002; Wallman and Fuchs 1998) and in cases of attenuated muscle strength (Scudder et al. 1998).
Experiments using the double-step paradigm have shown saccade adaptation to be specific to the vector of the adapted saccade: Adaptation of saccades in one direction (left vs. right) does not affect the amplitudes of saccades in the opposite direction (Albano 1996; Deubel et al. 1986; Frens and van Opstal 1994; Moidell and Bedell 1988; Semmlow et al. 1987) and large and small saccades can be adapted somewhat independently (Miller et al. 1981; Semmlow et al. 1987). Thus small saccades in one direction can be made larger whereas large saccades in the same direction can be made smaller, so that the endpoints of these saccades come progressively closer together (Watanabe et al. 2000).
The specificity of saccade adaptation also extends beyond the saccade vector to the saccade type. Adapting saccades that either are reactive responses to novel stimuli, or are voluntary shifts of gaze to existing stimuli, or are guided by memory will lead to incomplete and asymmetric transfer to the other two types (Deubel 1995b; Erkelens and Hulleman 1993; Fujita et al. 2002).
Saccade adaptation can also be specific to a sensorimotor context: whether the eye is deviated up or down at the time of a horizontal saccade or whether the head is tilted to the left or right, potentially providing the subject with proprioceptive information (Alahyane and Pélisson 2004; Shelhamer and Clendaniel 2002a,b; Shelhamer et al. 2004). These results, however, do not challenge the view of saccade adaptation as a motor-repair mechanism. When the eyes are deviated in any direction, the set of tensions on the extraocular muscles is changed and thus the commands sent to the muscles must be changed accordingly to achieve a particular movement. Similarly, because gaze position is the sum of head and eye positions, accurate redirection of gaze necessarily depends on head orientation. In this sense, it would be expected that a motor-domain repair mechanism would be sensitive to head and eye positions.
However, saccade adaptation may also reflect changes beyond those required for a motor-domain saccade-repair mechanism. Amplitude adaptation of voluntary saccades transfers to arm movements (Cotti et al. 2007). There is also some evidence that directional adaptation of reactive saccades transfers to arm movements and vice versa (Bock et al. 2008). These examples of transfer are suggestive of the adjustment of a common sensory map. This point is strengthened by the demonstration that changes in visual judgment of position accompany adaptation (Awater et al. 2005; Bahcall and Kowler 1999; Hernandez et al. 2008). Finally, it has been reported that adaptation of saccades between visually defined objects does not transfer to similar saccades made within an object (Collins et al. 2007), suggesting that visual selection is necessary for adaptation effects to reveal themselves.
The flexibility displayed by the saccade system in adaptation may be a manifestation of a generalized learning mechanism. Here we ask whether saccade adaptation can depend on a purely visual context. We used two experimental paradigms: in the first, we trained subjects to selectively decrease their saccade gain (ratio of saccade amplitude to target displacement) to one of two visually distinct targets by stepping back only one of the targets during the subject's saccade to it. In the second experiment, we adapted saccades to one target and assessed to what extent the gain change transferred to the other target, which was presented infrequently and without any retinal error feedback. Our results indicate that a visual stimulus can serve as an effective contextual cue for saccade adaptation.
METHODS
General
Subjects in a darkened room viewed stimuli at a distance of 57 cm while on a bite board to minimize head movements. Stimuli were generated on a computer running VisionWorks (Vision Research Graphics, Durham, NH) and displayed on a 21-in. monochrome CRT display with a fast phosphor and a vertical refresh rate of 200 Hz (Image Systems, LaFox, IL).
Pupil position was digitized at 240 Hz, using an infrared video eye-tracking system (ISCAN, Woburn, MA), controlled by a computer running SuperScope II (GW Instruments, Somerville, MA). Immediately preceding each experimental session, a 50-point horizontal calibration was carried out by having the subject fixate a 0.3° target 10 times at each of five randomized screen locations and strike a key to acquire a 200-ms average of pupil position. Locations were randomized to ensure that there was always a saccade between measurements. A least-squares fit to these measurements was used for off-line analysis.
Stimuli
Target steps were horizontal, with amplitudes of 9–11° and directions (leftward or rightward) selected with equal probability under the constraint that the target remain within a range of ±15°. The first trial began with the target at the center of the screen and subsequent trials always began with the target at the end position of the trial that preceded it.
The saccade target in each trial was a 0.3° filled circle either steadily illuminated at 17 cd/m2 0.3° or flickering (square wave) at a rate of 5 Hz with a temporal contrast of 48.4% (peak: 17 cd/m2; trough: 5.9 cd/m2). Both were presented on a 4.7 cd/m2 background.
Experiment I: stimulus-dependent step-back
Experiment I consisted of a baseline phase, a blocked-presentation phase, and a random-presentation phase (Fig. 1A). In each baseline trial, one of the two target types was stationary for 720–1,220 ms and subsequently stepped to a new position. It then remained illuminated for 450 ms and was briefly extinguished (for 50 ms), providing the subject with an opportunity to blink.
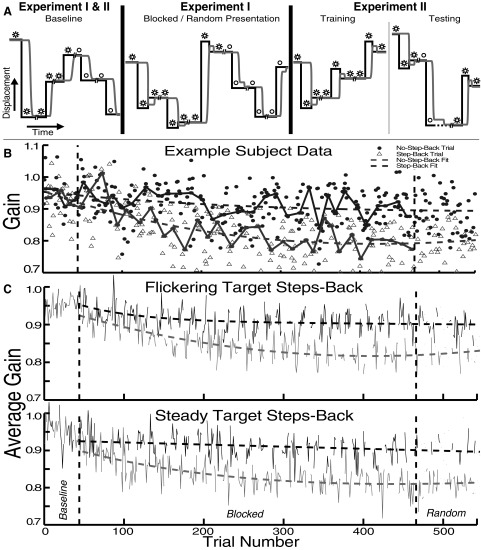
FIG. 1.
Experiment schematics and experiment I data. A, left: in baseline trials, neither target type stepped back during saccades. Middle: in experiment I, one target (stars) stepped back, whereas the other (open circle) did not. Right: experiment II consisted predominantly of step-back trials, plus infrequent trials with the no-step-back stimulus blanked at saccade onset (off-at-saccade [OAS] trials; dashed line) to deprive the subject of feedback. Black trace, target position; gray trace, eye position. Pairs of slashes represent the blink time. B: an example of a single subject's trial-by-trial saccade gain. In this session, the flickering target stepped back on saccades to it. Solid traces connect median gains in each block; dashed traces are double-exponential fits; vertical dashed lines separate experiment phases. C: averages across subjects showing a gradually increasing difference between the gains of saccades to targets that stepped back (light traces) vs. those that did not (dark traces).
To strike a balance between having a sufficient number of switches in target type (to analyze changes in saccade metrics at transitions between trial types) and enough consecutive trials of one type (to establish context), we arranged the trials during the baseline and blocked-presentation phases into blocks each having 3–10 trials of one target type, so that the target type alternated at an average interval of 6.5 trials.
The baseline phase consisted of 7 blocks (43 trials total), 21 trials with one target and 22 with the other. The blocked-presentation phase consisted of blocks of no-step-back trials with one stimulus (like those in the baseline phase) and blocks of step-back trials with the other. In step-back trials, after the target stepped to a new position, when the subject made a saccade to acquire, it the target stepped back toward its initial location by 3° and remained there for 450 ms until the blink time. Initiation of saccades was determined by eye velocity exceeding a threshold adjusted to optimize detection [on average the backstep was triggered 15.5 ± 2.6 ms (mean ± SD) after the start of the saccade, 29.9 ± 4.1 ms from the end of the saccade]. The blocked-presentation phase contained 63 blocks (423 trials total), odd blocks always consisting of nonstep-back trials and even blocks consisting of step-back trials. Approximately every 40 trials, 1-min-long breaks occurred, during which time the subject was instructed to either fixate the target (which remained illuminated) or close his/her eyes (18 breaks total). The random-presentation phase consisted of 40 nonstep-back and 40 step-back trials, pseudorandomly intermixed, which retained the pairing of target and trial type from the blocked-presentation phase. The overall trial ordering was the same for all sessions of experiment I.
Experiment II: transfer between stimulus types
Experiment II consisted of baseline, training, and testing phases (Fig. 1A). The baseline phase consisted of 20 consecutive no-step-back trials with one target type followed by 20 with the other type. Training comprised 150 step-back trials with one of the targets to lower the gain of the saccades. The testing phase consisted of a further 410 trials, a pseudorandom mixture of 370 step-back trials and 40 off-at-saccade (OAS) trials with the other, nonadapted target. In OAS trials, after the target stepped to a new location and the subject made a saccade to it, the target was extinguished for 450 ms. It then reappeared at the same location for 450 ms until the blink time. The testing phase began with an OAS trial and an OAS trial was interspersed after approximately every 10 step-back trials (Fig. 6A). This ordering was the same for all sessions and subjects.
FIG. 6.
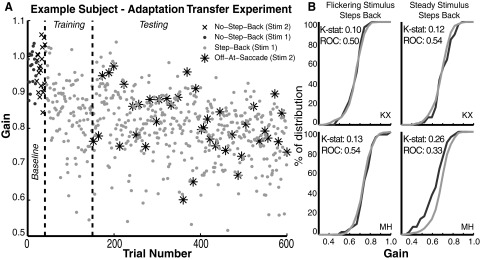
Adaptation transfer results. A: similar gain changes of saccades to both targets when no-step-back target (stars) is extinguished at saccade onset to eliminate feedback (OAS trials), amid predominant presentation of the step-back target with feedback (gray dots). Vertical dashed lines separate baseline, training, and testing phases. B: comparison of cumulative distributions of the gains of saccades to the step-back (light trace) vs. the no-step-back OAS target (dark trace), with Kolmogorov–Smirnov and receiver operating characteristic (ROC) statistics showing nearly complete transfer of adaptation.
Control experiment: conventional adaptation of flickering target
Because there were two unconventional elements to our experimental design (flickering targets and contextual switching), we ran a control experiment on three subjects (KX, MH, and XZ) with the flickering target presented alone throughout. There were 600 trials total: 100 baseline trials (no step-backs), followed by 400 step-back trials, and a further 100 recovery trials (no step-backs). As before, the primary target movement was a randomized mixture of left and rightward steps, 9–11° in amplitude, with 3° intrasaccadic step-backs.
Subjects
Six experienced subjects (ages 18–35 yr) with normal or corrected vision, performed experiment I (AK, JH, KX, LM, MH, and XZ) and two of these performed experiment II (KX, MH). Subjects performed each experiment twice, once with each target stepping back. Three of the six subjects (AK, JH, and MH) performed experiment I with the flickering target stepping back during the first of two sessions and the other three (KX, LM, and XZ) performed the experiment with the steady target stepping back first. One subject (KX) performed experiment II with the flickering target stepping back during the first of two sessions, whereas the other (MH) performed experiment II with the steady target stepping back first. There was always ≥24 h between experimental sessions. Subjects were instructed to follow the target and were told about the break periods and the blink time. Written consent was obtained from all subjects and the experimental protocol was approved by the Institutional Review Board of the City College of New York.
Analysis
All analyses were performed using MATLAB (The MathWorks, Natick, MA). During off-line analysis, saccades were detected automatically using a velocity threshold of 10°/s and a minimum latency criterion of 100 ms to exclude anticipatory movements. The start and end of each saccade were confirmed and, if necessary, corrected by the experimenter. Approximately 2% of all saccades were excluded from further analysis.
RESULTS
General
In general, our results show that when one target type (flickering or steady) steps back during saccades and the other does not, saccades to the step-back target develop consistently smaller gain than those to the no-step-back target. Furthermore, if one target type steps back and after 150 trials the other target type is presented sporadically and extinguished during a saccade, no difference in gain is observed between the two targets after adaptation.
Experiment I: stimulus-dependent step-back
The results of experiment I indicate that subjects are able to selectively reduce the gain of saccades made to one of two visually distinct targets. Figure 1B shows the gain on each trial for one subject in a session in which the flickering target stepped back (SB) and the steady target did not (NSB). With the onset of the blocked-presentation phase (vertical dashed lines), the gain of saccades to the flickering target decreased at a greater rate than those made to the steady target. The gain of this subject's saccades to the two targets was significantly different in both the blocked-presentation and random-presentation phases (mean difference in gain: blocked-presentation phase, 0.08 ± 0.1 SD; random-presentation phase, 0.09 ± 0.1; both P < 0.01, two-tailed t-test). The gain of saccades to the SB target continued to decrease gradually over the course of the session, despite a small increase during the random-presentation phase; meanwhile, the gain of saccades to the NSB target showed a much slower rate of decline.
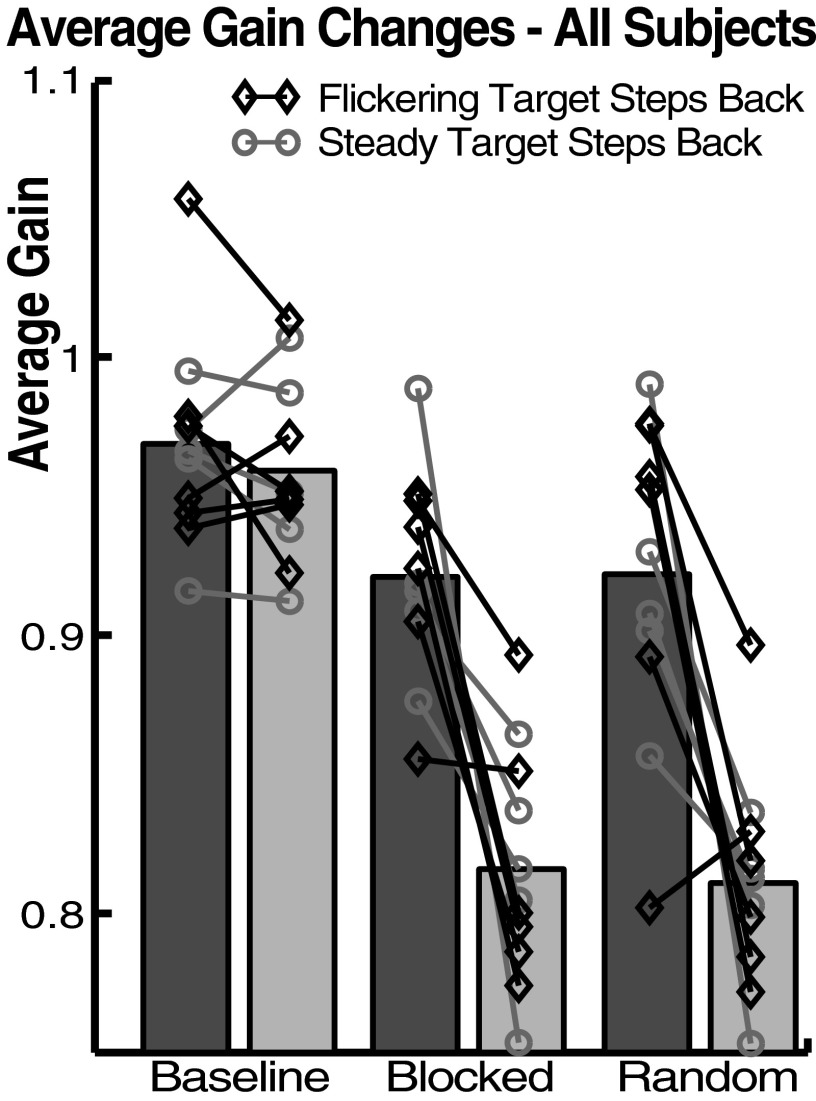
Averaging each trial across subjects (after an additive normalization of each session's data set to equalize mean baseline gain), we found lower gain for saccades to the target that stepped back, whether it was the flickering target (Fig. 1C, top) or the steady target (Fig. 1C, bottom). Specifically, gain was reduced to 85% of baseline for the SB target and 94% for the NSB target (ratio of the average of the final 20 trials in the random-presentation phase to the average baseline trials, across subjects). Thus the context specificity of the adaptation was not absolute. Considering each subject in each target-type condition, the gain was significantly lower to the SB target in 11 of 12 experimental sessions in both the blocked-presentation and random-presentation phases. On average (across subjects), saccade gain was 0.92 ± 0.04 for the NSB and 0.82 ± 0.04 for the SB target during the second half of the blocked-presentation phase (trials 212–466) and was 0.92 ± 0.06 for the NSB target and 0.81 ± 0.04 for the SB target during the random-presentation phase (Fig. 2). A mixed-effects ANOVA [four factors: trial type, session phase, experiment type (which stimulus stepped back), and subject] showed that the gain of saccades to the SB target was significantly smaller than that of those to the NSB target (F = 61.62, P < 0.01). This analysis also showed that the saccades to the NSB target were significantly smaller during blocked- and random-presentation phases than they were during the baseline phase (P < 0.01, Tukey–Kramer post hoc test). Finally, the same ANOVA revealed no significant effect of which stimulus was the SB and which was the NSB target on saccade gain (F = 0.56, P = 0.45).
FIG. 2.
Average gain changes during experiment I. Bars represent averages across subjects without normalization. Dark bars: no-step-back trials; light bars: step-back trials. Symbols and lines represent average gains of individual subjects. Data from blocked-presentation phase are from the second half of that phase (trials 212–466).
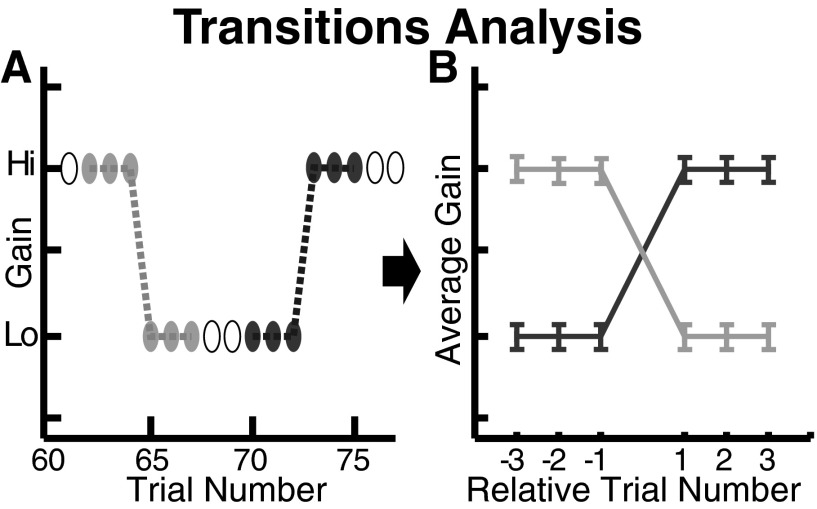
To quantify the purely contextual aspect of the adaptation, independent of the gain changes within each block of trials of one type, we examined the transitions from one stimulus type to the other. For the blocked-presentation phase of each session, we extracted the last three trials of a block of one stimulus type and the first three trials in the next block, making a vector of length 6 of the saccadic gains (Fig. 3). Because each block had a minimum of three trials, this kept the sample sizes constant for statistical purposes. This procedure resulted in one set of 32 vectors for the transitions from step-back to no-step-back and another for the transitions from no-step-back to step-back. For the random-presentation phase, in which the stimulus types were randomly interleaved, we extracted the last trial of one stimulus type and the first trial of the following stimulus type, to avoid having different numbers of points extracted from different transitions (20 transitions of each type). We plotted each subject's average transition vector from SB to NSB and NSB to SB separately for blocked-presentation and random-presentation phases and for data from sessions in which the flickering target stepped back versus those in which the steady target stepped back (Fig. 4). Across sessions, during the blocked-presentation phase, we found that when the target type switched, the gain of the first saccade was 0.08 ± 0.024 (mean ± SE) higher to the NSB target and 0.08 ± 0.022 lower to the SB target. During the random-presentation phase, the gain of the first saccade was 0.1 ± 0.03 higher to the NSB target and 0.12 ± 0.029 lower to the SB target. To test the significance of these gain changes, both within and across subjects, we compared the gain on the trials before a switch to the gain on the trials after a switch with a two-factor ANOVA (four levels in one factor: last step-back trial, first no-step-back trial, last no-step-back trial, first step-back trial, with subjects as a second factor). Using a Tukey–Kramer post hoc test, we found that the last step-back trial was significantly different from the first no-step-back trial and that the last no-step-back trial was significantly different from the first step-back trial for each subject (all P < 0.01) and across subjects (P < 0.01), combining the blocked- and random-presentation phases.
FIG. 3.
Transition analysis. Schematic of method for examining changes in gain at transitions from one target type to the other in the blocked-presentation phase. A: the gain values for 3 trials before and after each transition were extracted; light and dark ovals indicate transitions from no-step-back to step-back and step-back to no-step-back blocks, respectively; unfilled ovals indicate trials not included in transition analysis. In blocks of fewer than 6 trials, some trials contributed to both traces. B: idealized traces resulting from averaging across transitions shown in A.
FIG. 4.
Average gain changes around transitions. Transitions from step-back to no-step-back (dark) and vice versa (light). In all but one case the gain changes at the 2 transitions differed significantly, with no difference between cases in which the target that stepped back was flickering (1st and 3rd columns), vs. steady (2nd and 4th columns) (*P < 0.01 by one-tailed Tukey–Kramer post hoc test). Note: as indicated in the top left, each ordinate has the same span (0.2) but has a different central value.
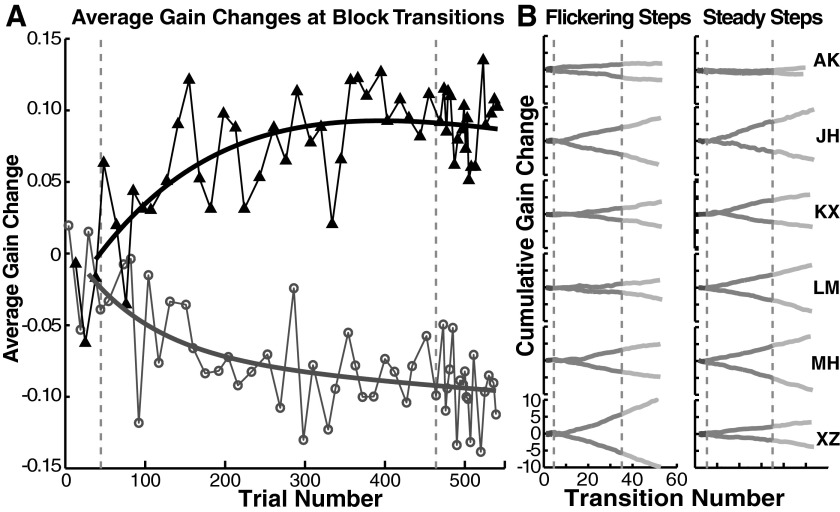
Gain change on the first trial of each block increased progressively across the adaptation session (Fig. 5A). To show individual variations we plotted the cumulative sums of these gain changes over the course of each session (Fig. 5B). In other words, each time that the stimulus switched, we computed the change in gain, summing these up according to the transition type to obtain the cumulative record (step-back to no-step-back or vice versa). Some subjects seemed to learn the context almost immediately, indicated by a difference in slope of the cumulative sums that began after just a few blocks (Fig. 5B: JH, XZ). Other subjects required more substantial exposure to the differential adaptation (Fig. 5B: KX, both columns; LM, left column; MH, left column). In some of the traces the slopes start to increase a bit, late in the session, as though the gains to the SB and NSB targets were continuing to diverge (Fig. 5B: LM, XZ). This is also reflected in the larger transition sizes for some subjects we observed in the random-presentation phase versus the blocked-presentation phase of the experiment (Fig. 4: MH, XZ).
FIG. 5.
Development of gain changes at transitions. A: average gain changes at stimulus transitions. Dark trace/triangles: gain change on switching to a no-step-back trial; light trace/open circles: gain change on switching to a step-back trial. Curves are double-exponential fits. B: cumulative sums for each session show individual differences in the rate of acquisition of fast gain changes at transitions. Vertical dashed lines separate baseline, blocked, and random phases.
We found it curious that, on average, most of the gain change between successive blocks occurred abruptly at the first trial of each block. Because these gain changes were larger than would be expected for any one trial under conventional adaptation, it appears that the gain values reached for each target are stored with little decrement between blocks. To evaluate whether there was any gain change beyond the first trial of each block, we tested how quickly the gain of the first, third, and last trials in a block deviated from baseline by using an ANOVA with Tukey–Kramer post hoc tests as a metric (two factors: first, third, and last trials as the first factor; block number as the second factor; data pooled across sessions). The gain of the last trial of a step-back block deviated significantly from baseline after only 4 blocks and the third trial deviated from baseline after 8 blocks, whereas the first trial deviated only after 11 blocks. We take this as an indication that the adaptation progressed within each block and that it took some time within a session for the fast switching of gain on the first trial of a block to be manifested.
If the reaction time of saccades to one or both of the stimuli was longer during the differential adaptation, it might reflect a cognitive strategy. Furthermore, when two perceptual tasks are interleaved, the reaction time of the first response to the new task is frequently longer than that to the subsequent ones—the switch cost (Altmann 2007; Barton et al. 2006). In experiment I, reaction times were much shorter than one might expect for cognitively driven saccades (Deubel 1995) and did not significantly differ between adaptation and baseline phases [median = 168 ± 29 (SD) ms for random-presentation phase vs. 164 ± 28 ms for baseline phase; Tukey–Kramer post hoc test, P = 0.6]. Furthermore, the latency of the first saccade of a new block was, on average, only 5.5 ± 2 ms longer than that of the next saccade, a nonsignificant change (P = 0.31, t-test).
We did observe a significant difference in the distribution of latencies of saccades made to the steady (165 ± 33 ms) versus the flickering (179 ± 40 ms) stimulus (F = 458.43, P < 0.01). However, because the choice of stimulus used as the step-back target had no statistically significant effect on the experimental outcome, this difference in latency is unlikely to have influenced our main result.
Experiment II: transfer between stimulus types
Experiment I used stimulus-dependent step-backs, which concurrently guided subjects to decrease the amplitude of their saccades to the SB target and to keep the amplitude of their saccades to the other target unchanged. To determine the necessity of this differential feedback, we measured the transfer of adaptation from the SB to the other target by sporadically presenting the other target and extinguishing it at saccade onset (OAS) to remove postsaccadic retinal error (Fig. 1A, right). One subject's results are plotted in Fig. 6A, in which gains of saccades to the OAS stimulus were indistinguishable from those to the SB stimulus; that is, in the absence of differential feedback, complete transfer occurred. To compare the gains of saccades to the two targets, cumulative distributions of OAS trials and step-back trials (after trial 140) were calculated for each subject under conditions in which either the flickering or steady target stepped back. These distributions were compared individually using the Kolmogorov–Smirnov test statistic (Fig. 6B). In none of the four sessions were the gains of saccades to the OAS target greater than those to the SB target. A receiver operating characteristic (ROC) analysis (Fig. 6B) confirmed this result. The ROC analysis in this case provides an estimate of the fraction of trials that one would be able to classify, on the basis of a single saccade, as having contained the SB or OAS target. Further, we examined the gains using a two-factor ANOVA with step-back trials versus OAS trials as one factor and subjects as the second factor. In Tukey–Kramer post hoc tests, the step-back saccades were not statistically distinguishable from the OAS saccades for each subject (all P > 0.5).
There was no noticeable difference in the degree of adaptation, depending on whether the flickering or the steady target served as the SB stimulus; these were also indistinguishable from the control experiment. A single-factor ANOVA, comparing gains of saccades (across sessions) from the final 40 trials in each session (including only step-back trials from experiment I and both step-back and OAS trials from experiment II), showed them to be statistically indistinguishable [experiment I: flickering: 0.81 ± 0.078 (median gain ± SD), steady: 0.81 ± 0.08, P = 0.32; experiment II: flickering: 0.79 ± 0.067, steady: 0.81 ± 0.062; Control: 0.8 ± 0.06, F = 0.92, P = 0.44].
To compare the rates of adaptation during step-back trials for flickering and steady targets, we split the first 120 step-back trials into six blocks, separately for each session type (flickering vs. steady target stepping back) of experiments I and II and the control experiment of conventional adaptation with the flickering target (5 groups). We again pooled across subjects after additively normalizing to the mean baseline gains, so that the variances reflected differences only in the course of adaptation and not individuals’ baseline variances. We found that as gains gradually decreased there were no significant differences between these five groups after 20, 40, 60, 80, 100, or 120 trials (two-factor ANOVA; first factor: experiment type; second factor: number of preceding step-back trials; F = 156.95, P < 0.01), leading us to conclude that the rate of adaptation in these experiments was approximately the same, despite the fact that during experiment I the interspersed NSB trials might have decreased the rate of adaptation for the SB target.
DISCUSSION
Our results show that the same target step can elicit saccades of different gain, depending on the visual attributes of the target, as a consequence of a learned contextual association (Fig. 2). Furthermore, the results demonstrate that the context-dependent gain difference develops gradually (Figs. 1C and 5A), much as in conventional saccade adaptation experiments, and most of the gain difference is evident on the first saccade after a switch of target type (Figs. 4 and 5A), implying that only a small part of the gain difference is due to the progressive gain changes within each block of trials of one target type. Finally, the context dependence of gain is not absolute in that the gain of saccades to the target that does not step back is also reduced (Figs. 1 and 2).
Our study of contextual saccade adaptation follows an earlier, unsuccessful attempt to differentially adapt saccade gain (Deubel 1995a). That study differed from our own in that: 1) the ordering of the presentation of the two stimuli was random throughout; 2) the subject foveated a fixation target that changed into one of the two stimuli at the time of the initial target step; 3) the stimuli were differentiated by color and shape (the targets were a red circle and a green cross). What might account for the difference in the results between these two studies? First, trial structure may be important. If postsaccadic error acts most strongly to change the gain of the immediately following saccade, random target presentation may slow contextual learning since half of the trials would be followed by the other target type. On the other hand, long blocks of each stimulus would allow the subject to ignore the target type and be guided by whether the target stepped back; that is, the target step-back during blocked presentation could itself act as a context, since each trial predicts with high probability that the following trial will be of the same type. For these reasons, we used short blocks of irregular length. Second, in experiment I, the subject could have fixated the saccade target for as long as 1.2 s prior to the target step. Perhaps a long fixation period provides the subject with time to assign the context-specific gain appropriate to the presented target, whereas in Deubel's paradigm the target step may have initiated the programming of a saccade before the context had been decoded. Finally, it is conceivable that shape and color are simply not effective contextual cues for saccade adaptation, as opposed to the random trial structure in Deubel's experiment having masked learning with these features. Thus the difference between our results and those of Deubel suggests that there are limits to the generality of the associations that saccade adaptation can learn.
One might question whether the adaptation shown in this study is the same as conventional adaptation or whether it is a manifestation of a conscious targeting strategy, in which the subject explicitly recognizes the contextual association and voluntarily produces hypometric saccades or “aims” his or her saccades to a position short of the target. We are disinclined to accept this explanation, largely because the adaptation develops gradually over hundreds of trials (Fig. 1C). Even after the first 100 adaptation trials the gain continued to fall in 10 of the 12 experimental sessions (in which the slopes of linear regressions of gain against trial numbers 101–546 were negative and significantly nonzero; P < 0.05). If a subject was consciously directing saccades to a particular unmarked point on the screen, one might expect that the variability would diminish with practice, but the gain would not change progressively over hundreds of trials. Furthermore, the finding that there was some transfer between the gains to the two target types is more suggestive of incompletely separated contextual adaptation than of cognitive strategy. Indeed, we suspect that the target specificity we have observed reflects the acquisition of two contexts, one for each target. In experiment II, in which one target type was always extinguished on saccade initiation, the gain of saccades to that target decreased as much as those to the other target, which stepped back. Therefore it is likely that in experiment I, subjects were actively maintaining an elevated saccade gain for the NSB target while decreasing gain for the SB target, as a result of their differing postsaccadic consequences.
Despite our contention that it is not highly cognitive, the contextual adaptation we observed clearly uses a more elaborate error signal than simply accumulating retinal error. Although many experiments support the importance of simple retinal error (e.g., Noto and Robinson 2001; Seeberger et al. 2002; Wallman and Fuchs 1998), these do not exclude an error signal incorporating a prediction of the retinal error expected, given the saccade executed. Indeed, there is some evidence that predicted retinal error does have a role to play in saccade adaptation (Bahcall and Kowler 2000) and predicting the sensory consequences of action is seen, more widely, as an important teaching signal in motor adaptation (Wolpert and Ghahramani 2000). If the functional utility of motor adaptation is to improve accuracy, then it is plausible that other, higher-level predictions could also be taken into account.
A theoretical framework for multiple predictions (or “forward models”) has been widely used in the study of arm movement adaptation and has been supported by many empirical data (Kluzik et al. 2008; Wagner and Smith 2008; Wolpert et al. 1998). In motor learning theories of this type, an inverse model is used to compute the motor commands necessary to achieve a desired trajectory and a forward model predicts the sensory consequences as the movement occurs. In keeping with the known involvement of the cerebellum in motor learning, it has been suggested that there are multiple, overlapping internal models localized in the cerebellum (Kluzik et al. 2008; Wolpert et al. 1998). Multiple models could facilitate efficient context-dependent motor learning and execution, if the cerebellum used a sensory context signal to flexibly select the model (or sets of models) appropriate for motor execution and/or the models to be modified during motor learning (Wolpert et al. 1998). Although one might argue that this type of contextual flexibility is essential only for motor systems that have to deal with varying loads, adaptation of the vestibuloocular reflex, which is known to be mediated by the cerebellum, can be specific to head-tilt and head-rotation frequency, velocity, and acceleration (Boyden et al. 2004, 2006). Moreover, the separate adaptabilities of saccades to sudden target steps compared with voluntary saccades to stationary targets (Alahyane et al. 2007; Deubel 1995b; Erkelens and Hulleman 1993; Fujita et al. 2002; Gaveau et al. 2005) may reflect separate cerebellar loci (Alahyane et al. 2008), consistent with at least partially separate internal models for each movement type. In experiment I, we speculate that the lack of consistent trial-to-trial retinal error—in contrast to conventional saccade adaptation experiments—led to a persistent dissonance between predictions and observations of that error. This continued dissonance may have coaxed the oculomotor system into searching for another signal that might be predictive of retinal error, exploiting the consistent relationship between stimulus type and target movement. If multiple context-dependent internal models exist, it would not be surprising if some subset of models were shared, with the effect that adapting one movement would transfer somewhat to the other, as we have found.
Contextual learning might be a general feature of motor adaptation. In the directional adaptation of arm movements, the presence of distinct proprioceptive contexts permits two tasks to alternate without interference (Krakauer et al. 2006). In the case of saccade adaptation as well, distinct proprioceptive contexts prevent increased-gain adaptation from interfering with the progressive course of decreased-gain adaptation (Aboukhalil et al. 2004; Shelhamer and Clendaniel 2002a,b). In addition, there is an indirect suggestion of a contextual effect on saccade adaptation in that decreased gain can be retained for long intervals (up to 5 days) following the initial step-back adaptation session, despite the subject's presumably having made hundreds of thousands of saccades in between (Alahyane and Pélisson 2005). Because, in the laboratory, having subjects make saccades without step-backs following adaptation restores “normal” saccade gain in a few hundred trials, the prolonged retention observed may result from the contextual difference between daily life and the laboratory setting—the sights, smells, and sounds experienced during the experimental session(s), as well as the taste of the bite board and the seated posture of the subject. Alternatively, the retention may be due to the low frequency in daily life of reflexive saccades (driven by abrupt stimulus steps) compared with voluntary saccades and the partial independence of the adaptation of these two types mentioned earlier.
Why might it be useful to customize saccadic parameters based on a sensory context? Whatever error signals are used to guide saccade adaptation, the oculomotor system would benefit from being able to distinguish saccades that were inaccurate because the target had moved during the saccade from those that were inaccurate because of a miscalibration of the saccadic system. A recent model of saccade adaptation posited that large errors are attributed to the target having moved, whereas small errors are attributed to errors in the saccadic system (Chen-Harris et al. 2008). We conjecture that, from an ethological perspective, it would be more efficient for the oculomotor system to make use of the stimulus context to distinguish between situations in which the targets behave predictably, in which case errors could be attributed to the motoric performance, and those in which saccades are used to track targets moving irregularly, in which case the errors could be attributed to the target motion. In both situations, saccade adaptation might be used, but it would be useful to separate the parameters of the first case, in which saccade adaptation tunes up the motor system for saccades to stationary objects, from the second case, in which saccade adaptation is used to compensate for statistical regularities in the target movement. From this viewpoint, it might not be surprising to find the aforementioned incomplete and asymmetric transfer of adaptation between internally and externally motivated saccades.
In conclusion, our results extend the notion that saccades can have two gains for the same target vector under different sensorimotor contexts, by showing that purely visual attributes of target stimuli can define the context. It remains to be determined whether this ability serves a particular oculomotor function or is a manifestation of saccade adaptation, like other forms of learning, being sensitive to any informative context.
GRANTS
This work was supported by National Institutes of Health Division of Research Resources Grant RR-03060 and Wellcome Trust Grant GR065025M to M. R. Harwood.
Acknowledgments
We thank L. Madelain, J. A. Edelman, and J. B. Levitt for helpful discussions during preparation of this manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Aboukhalil et al. 2004.Aboukhalil A, Shelhamer M, Clendaniel R. Acquisition of context-specific adaptation is enhanced with rest intervals between changes in context state, suggesting a new form of motor consolidation. Neurosci Lett 369: 162–167, 2004. [DOI] [PubMed] [Google Scholar]
- Alahyane et al. 2008.Alahyane N, Fonteille V, Urquizar C, Salemme R, Nighoghossian N, Pélisson D, Tilikete C. Separate neural substrates in the human cerebellum for sensory-motor adaptation of reactive and of scanning voluntary saccades. Cerebellum 7: 595–601, 2008. [DOI] [PubMed] [Google Scholar]
- Alahyane and Pélisson 2004.Alahyane N, Pélisson D. Eye position specificity of saccadic adaptation. Invest Ophthalmol Vis Sci 45: 123–130, 2004. [DOI] [PubMed] [Google Scholar]
- Alahyane and Pélisson 2005.Alahyane N, Pélisson D. Long-lasting modifications of saccadic eye movements following adaptation induced in the double-step target paradigm. Learn Mem 12: 433–443, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahyane et al. 2007.Alahyane N, Salemme R, Urquizar C, Cotti J, Guillaume A, Vercher JL, Pélisson D. Oculomotor plasticity: are mechanisms of adaptation for reactive and voluntary saccades separate? Brain Res 1135: 107–121, 2007. [DOI] [PubMed] [Google Scholar]
- Albano 1996.Albano JE Adaptive changes in saccade amplitude: oculocentric or orbitocentric mapping? Vision Res 36: 2087–2098, 1996. [DOI] [PubMed] [Google Scholar]
- Altmann 2007.Altmann EM Comparing switch costs: alternating runs and explicit cuing. J Exp Psychol Learn Mem Cogn 33: 475–483, 2007. [DOI] [PubMed] [Google Scholar]
- Awater et al. 2005.Awater H, Burr D, Lappe M, Morrone MC, Goldberg ME. Effect of saccadic adaptation on localization of visual targets. J Neurophysiol 93: 3605–3614, 2005. [DOI] [PubMed] [Google Scholar]
- Bahcall and Kowler 1999.Bahcall DO, Kowler E. Illusory shifts of visual direction accompany adaptation of saccadic eye movements. Nature 400: 864–866, 1999. [DOI] [PubMed] [Google Scholar]
- Barton et al. 2006.Barton JJ, Greenzang C, Hefter R, Edelman J, Manoach DS. Switching, plasticity, and prediction in a saccadic task-switch paradigm. Exp Brain Res 168: 76–87, 2006. [DOI] [PubMed] [Google Scholar]
- Bock et al. 2008.Bock O, Schmitz G, Grigorova V. Transfer of adaptation between ocular saccades and arm movements. Hum Mov Sci 27: 383–395, 2008. [DOI] [PubMed] [Google Scholar]
- Boyden et al. 2006.Boyden ES, Katoh A, Pyle JL, Chatila TA, Tslen RW, Raymond JR. Selective engagement of plasticity mechanisms for motor memory storage. Neuron 51: 823–834, 2006. [DOI] [PubMed] [Google Scholar]
- Boyden et al. 2004.Boyden ES, Katoh A, Raymond JR. Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Annu Rev Neurosci 27: 581–609, 2004. [DOI] [PubMed] [Google Scholar]
- Chen-Harris et al. 2008.Chen-Harris H, Joiner WM, Ethier V, Zee DS, Shadmehr R. Adaptive control of saccades via internal feedback. J Neurosci 28: 2804–2813, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins et al. 2007.Collins T, Vergilino-Perez D, Beauvillain C, Doré-Mazars K. Saccadic adaptation depends on object selection: evidence from between- and within-object saccadic eye movements. Brain Res 1152: 95–105, 2007. [DOI] [PubMed] [Google Scholar]
- Cotti et al. 2007.Cotti J, Guillaume A, Alahyane N, Pélisson D, Vercher J-L. Adaptation of voluntary saccades, but not of reactive saccades, transfers to hand pointing movements. J Neurophysiol 98: 602–612, 2007. [DOI] [PubMed] [Google Scholar]
- Deubel 1995a.Deubel H Is saccadic adaptation context-specific? In: Eye Movement Research: Mechanism, Processes and Applications, edited by Findlay JM, Kentridge RW, Walker R. Amsterdam: Elsevier Science, 1995a.
- Deubel 1995b.Deubel H Separate adaptive mechanisms for the control of reactive and volitional saccadic eye movements. Vision Res 35: 3529–3540, 1995b. [DOI] [PubMed] [Google Scholar]
- Deubel et al. 1986.Deubel H, Wolf W, Hauske G. Adaptive gain control of saccadic eye movements. Hum Neurobiol 5: 245–253, 1986. [PubMed] [Google Scholar]
- Erkelens and Hulleman 1993.Erkelens CJ, Hulleman J. Selective adaptation of internally triggered saccades made to visual targets. Exp Brain Res 93: 157–164, 1993. [DOI] [PubMed] [Google Scholar]
- Frens and van Opstal 1994.Frens M, van Opstal A. Transfer of short-term adaptation in human saccadic eye movements. Exp Brain Res 100: 293–306, 1994. [DOI] [PubMed] [Google Scholar]
- Fujita et al. 2002.Fujita M, Amagai A, Minakawa F, Aoki M. Selective and delay adaptation of human saccades. Cogn Brain Res 13: 41–52, 2002. [DOI] [PubMed] [Google Scholar]
- Gaveau et al. 2005.Gaveau V, Alahyane N, Salemme R, Desmurget M. Self-generated saccades do not modify the gain of adapted reactive saccades. Exp Brain Res 162: 526–531, 2005. [DOI] [PubMed] [Google Scholar]
- Hernandez et al. 2008.Hernandez TD, Levitan CA, Banks MS, Schor CM. How does saccade adaptation affect visual perception? J Vis 8: 1–16, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp and Fuchs 2004.Hopp JJ, Fuchs AF. The characteristics and neural substrate of saccadic eye movement plasticity. Prog Neurobiol 72: 27–53, 2004. [DOI] [PubMed] [Google Scholar]
- Kluzik et al. 2008.Kluzik J, Diedrichsen J, Shadmehr R, Bastian A. Reach adaptation: what determines whether we learn an internal model of the tool or adapt the model of our arm? J Neurophysiol 100: 1455–1464, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer et al. 2006.Krakauer JW, Mazzoni P, Ghazizadeh A, Roshni R, Shadmehr R. Generalization of motor learning depends on the history of prior action. PLoS Biol 4: 1798–1808, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin 1967.McLaughlin S Parametric adjustment in saccadic eye movements. Percept Psychophys 2: 359–362, 1967. [Google Scholar]
- Miller et al. 1981.Miller J, Anstis T, Templeton W. Saccadic plasticity: parametric adaptive control by retinal feedback. Exp Psychol 7: 356–366, 1981. [DOI] [PubMed] [Google Scholar]
- Moidell and Bedell 1988.Moidell B, Bedell H. Changes in oculocentric visual direction induced by the recalibration of saccades. Vision Res 28: 329–336, 1988. [DOI] [PubMed] [Google Scholar]
- Noto and Robinson 2001.Noto CT, Robinson FR. Visual error is the stimulus for saccade gain adaptation. Cogn Brain Res 12: 301–305, 2001. [DOI] [PubMed] [Google Scholar]
- Optican and Robinson 1980.Optican L, Robinson D. Cerebellar-dependent adaptive control of primate saccadic system. J Neurophysiol 54: 940–958, 1980. [DOI] [PubMed] [Google Scholar]
- Optican et al. 1985.Optican LM, Zee DS, Chu FC. Adaptive response to ocular muscle weakness in human pursuit and saccadic eye movements. J Neurophysiol 54: 110–122, 1985. [DOI] [PubMed] [Google Scholar]
- Scudder et al. 1998.Scudder CA, Batourina EY, Tunder GS. Comparison of two methods of producing adaptation of saccade size and implications for the site of plasticity. J Neurophysiol 79: 704–715, 1998. [DOI] [PubMed] [Google Scholar]
- Seeberger et al. 2002.Seeberger T, Noto CT, Robinson FR. Non-visual information does not drive saccade gain adaptation in monkeys. Brain Res 956: 374–379, 2002. [DOI] [PubMed] [Google Scholar]
- Semmlow et al. 1987.Semmlow J, Gautheir G, Vercher J-L. Short term adaptive modification of saccade amplitude. In: Eye Movements: From Physiology to Cognition, edited by O'Regan JK, Levy-Schoen A. Amsterdam: Elsevier Science, 1987.
- Shelhamer and Clendaniel 2002a.Shelhamer M, Clendaniel RA. Context-specific adaptation of saccade gain. Exp Brain Res 146: 441–450, 2002a. [DOI] [PubMed] [Google Scholar]
- Shelhamer and Clendaniel 2002b.Shelhamer M, Clendaniel RA. Sensory, motor, and combined contexts for context-specific adaptation of saccade gain in humans. Neurosci Lett 332: 200–204, 2002b. [DOI] [PubMed] [Google Scholar]
- Snow et al. 1985.Snow R, Hore J, Vilis T. Adaptation of saccadic and vestibulo-ocular systems after extraocular muscle tenectomy. Invest Ophthalmol Vis Sci 26: 924–931, 1985. [PubMed] [Google Scholar]
- Wagner and Smith 2008.Wagner MJ, Smith MA. Shared internal models for feedforward and feedback control. J Neurosci 28: 10663–10673, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallman and Fuchs 1998.Wallman J, Fuchs AF. Saccadic gain modification: visual error drives motor adaptation. J Neurophysiol 80: 2405–2416, 1998. [DOI] [PubMed] [Google Scholar]
- Watanabe et al. 2000.Watanabe S, Noto CT, Fuchs AF. Flexibility of saccade adaptation in the monkey: different gain states for saccades in the same direction. Exp Brain Res 130: 169–176, 2000. [DOI] [PubMed] [Google Scholar]
- Wolpert and Ghahramani 2000.Wolpert DM, Ghahramani Z. Computational principles of movement neuroscience. Nat Neurosci 3: 1212–1217, 2000. [DOI] [PubMed] [Google Scholar]
- Wolpert et al. 1998.Wolpert DM, Miall C, Kawato M. Internal models in the cerebellum. Trends Cog Sci 2: 338–347, 1998. [DOI] [PubMed] [Google Scholar]
- Wurtz and Goldberg 1972.Wurtz RH, Goldberg ME. Activity of superior colliculus in behaving monkey. III. Cells discharging before eye movements. J Neurophysiol 35: 575–586, 1972. [DOI] [PubMed] [Google Scholar]