Abstract
The apelinergic system has a widespread expression in the central nervous system (CNS) including the paraventricular nucleus, supraoptic nucleus and median eminence, and isolated cells of the anterior lobe of the pituitary. This pattern of expression in hypothalamic nuclei known to contain corticotrophin-releasing factor (CRF) and vasopressin (AVP) and to co-ordinate endocrine responses to stress has generated interest in a role for apelin in the modulation of stress, perhaps via the regulation of hormone release from the pituitary. In this study, to determine whether apelin has a central role in the regulation of CRF and AVP neurones, we investigated the effect of i.c.v. administration of pGlu-apelin-13 on neuroendocrine function in male mice pre-treated with the CRF receptor antagonist, α-helical CRF9–41, and in mice-lacking functional AVP V1b receptors (V1bR KO). Administration of pGlu-apelin-13 (1 mg/kg i.c.v.) resulted in significant increases in plasma ACTH and corticosterone (CORT), which were significantly reduced by pre-treatment with α-helical CRF9–41, indicating the involvement of a CRF-dependent mechanism. Additionally, pGlu-apelin-13-mediated increases in both plasma ACTH and CORT were significantly attenuated in V1bR KO animals when compared with wild-type controls, indicating a role for the vasopressinergic system in the regulation of the effects of apelin on neuroendocrine function. Together, these data confirm that the in vivo effects of apelin on hypothalamic–pituitary–adrenal neuroendocrine function appear to be mediated through both CRF- and AVP-dependent mechanisms.
Introduction
The apelin peptide, isolated from extracts of bovine stomach (Tatemoto et al. 1998), selectively binds to the G-protein-coupled apelin receptor (APJ; O'Dowd et al. 1993). Apelin, a 36-amino acid peptide, is derived from a 77-amino acid precursor, preproapelin (Tatemoto et al. 1998, Lee et al. 2000). Although initial studies suggested that apelin-36 was the predominant cleavage product in vivo, subsequent studies have suggested that other cleavage products, notably the pyroglutamyl form of apelin-13 (pGlu-apelin-13) and apelin-17, may be more abundant in the rat (De Mota et al. 2004, Reaux-Le Goazigo et al. 2004). These cleavage products have greater affinity for APJ (Medhurst et al. 2003) and exhibit greater biological activity than the parent peptide on extracellular acidification rates, promote APJ internalisation and strongly inhibit forskolin-stimulated cAMP production (Medhurst et al. 2003, El Messari et al. 2004).
The apelinergic system has a widespread expression in the CNS, including the paraventricular nucleus (PVN), preoptic area, periventricular hypothalamus and ventromedial and dorsomedial nuclei (Lee et al. 2000), while high densities of apelin immunoreactive fibres and cell bodies have been detected in the PVN, supraoptic nucleus (SON), the median eminence (Reaux et al. 2002) and in the posterior pituitary (Brailoiu et al. 2002). Outside the CNS, apelin immunoreactivity and mRNA are present in vascular endothelial cells (Kleinz & Davenport 2004) and other peripheral tissues such as heart, mammary gland, lung and adrenal (Habata et al. 1999, O'Carroll et al. 2000). The pattern of expression of the rat receptor implies that APJ has tissue-specific functions in adult animals. High levels of APJ mRNA expression are found in the brain, the pineal gland, the parenchyma of the lung, the heart and in isolated cells of the anterior lobe of the pituitary (O'Carroll et al. 2000). APJ expression is restricted in the CNS and is particularly striking in the medial parvocellular regions of the hypothalamic PVN (pPVN) and scattered magnocellular neurones of the PVN (mPVN) and SON (De Mota et al. 2000, O'Carroll et al. 2000), sites that control hypothalamic–pituitary–adrenal (HPA) axis and hypothalamo-neurohypophysial system activity respectively (Ynag et al. 1977). APJ mRNA and apelin immunoreactivity are expressed within a proportion of vasopressinergic neurones originating from the SON and mPVN and projecting to the posterior pituitary (Reaux et al. 2001, O'Carroll & Lolait 2003), where the two peptides are segregated within distinct subcellular compartments (Reaux-Le Goazigo et al. 2004). Extrahypothalamic structures such as the piriform cortex, the dentate gyrus, the nucleus of the lateral olfactory tract and the dorsal raphe nucleus also express APJ mRNA (De Mota et al. 2000).
The key CNS site integrating neuroendocrine adjustments to stress is the hypothalamic PVN. This nucleus is comprised of two major neurosecretory components – the mPVN and pPVN subdivisions. Neurones originating from the more medially situated pPVN (where vasopressin (AVP) is co-localised in a proportion of corticotrophin-releasing factor (CRF) neurones; Tramu et al. 1983) project to the median eminence where, in response to stress, CRF and AVP are released from nerve terminals into the portal vessels bathing the anterior pituitary. CRF and AVP stimulate ACTH secretion by interacting with the CRF-type 1 (CRFR1) and AVP V1b (V1bR) receptors respectively, on the pituitary corticotrope (Gillies et al. 1982). ACTH in turn potently induces the secretion of glucocorticoids (corticosterone (CORT) in rodents) by the adrenal cortex. Regulation of CORT via ACTH secretion is critical for life and is necessary for the mammalian response to stress.
In the HPA axis, the presence of apelin and APJ transcripts and immunoreactivity in hypothalamic nuclei known to contain AVP and CRF and to co-ordinate endocrine responses to stress suggests that apelin may play a role in the control of anterior hormone release. Central, i.e. administration (i.c.v.) of apelin increases c-fos expression in the PVN (Kagiyama et al. 2005), and appears to have a role in the regulation of AVP and CRF neurones as it has been found to stimulate both AVP and CRF release from hypothalamic explants in vitro (Taheri et al. 2002), effects consistent with activation of PVN neurones and stimulation of the stress axis. This role was confirmed by increased levels of APJ mRNA resulting from acute and chronic stress and following adrenalectomy (O'Carroll et al. 2003). The involvement of apelin in the regulation of CRF neurones was substantiated by physiological experiments showing apelin-stimulated basal CORT secretion in rats to be partially blocked by pre-treatment with a CRF antagonist, α-helical CRF9–41 (Jaszberenyi et al. 2004); however, the role of additional mediators such as AVP cannot be excluded.
In this study, to determine whether apelin has a central role in the regulation of CRF and AVP neurones, we investigated the effect of i.c.v. administration of pGlu-apelin-13 on neuroendocrine function in male mice pre-treated with the CRF receptor antagonist, α-helical CRF9–41, and in mice lacking functional AVP V1bRs (V1bR KO).
Materials and Methods
Animals
Male adult (8–12 weeks) mice (a mix of the C57BL/6J and 129×1/SvJ strains) were used for in vivo experiments. For V1bR KO experiments, male adult littermates of crosses using mice heterozygous for the AVP V1bR mutation were used. Animals were group-housed (two to four per cage) under controlled light conditions (12 h light:12 h darkness cycle, lights on at 0700 h) and temperature (21±2 °C) with free access to standard laboratory chow and water. Studies were performed between 0900 and 1200 h. All procedures were conducted in accordance with the Animal Scientific Procedures Act (1986) United Kingdom and the appropriate University of Bristol Ethical Review Process.
Surgical procedure
Mice were anaesthetised with isoflurane. Permanent 23-gauge stainless steel guide cannulae (Coopers Needleworks, Birmingham, UK) were stereotactically placed into the lateral ventricle (AP, −0·8; LM, +1·0; DV, −2·5 mm from bregma). Guide cannulae were secured with dental cement and two tether screws (Precision Technology Supplies, East Grinstead, UK). A 29-gauge stainless steel stylet was inserted down the guide cannula to ensure patency. On completion of surgery, animals were single housed in a clean cage, placed on a heatpad (36±1 °C) and administered antibiotics (Baytril, Bayer UK). Animals were allowed to recover for a minimum of 7 days before being used in experiments. To verify correct positioning, methylene blue was subsequently injected into decapitated heads and the brains dissected.
On the day of injection, stylets were removed from the guide cannulae and a 30-gauge stainless steel injection needle (40 mm long, Coopers Needleworks) inserted down the guide cannula, projecting 0·5 mm below the tip. The injection needle was attached to a gas-tight 10 μl Hamilton microsyringe (Fisher Scientific, Loughborough, UK) via 30 cm of flexible portex tubing (0·28 mm internal diameter, Southern Scientific Supplies, Lancing, UK). The animal was returned to the home cage and allowed to move freely during the injection and diffusion process. All substances were injected in a total volume of 2 or 5 μl (depending on solubility) over a period of 30 s, with a further 30 s allowed for complete diffusion. After injection, the injection needle was removed from the guide cannula and the home cage returned to the holding room.
Experiment 1
Mice (n=12–16/group) were administered either vehicle (0·9% saline; 5 μl i.c.v.) or α-helical CRF9–41 (25 μg; 5 μl i.c.v.), followed 30 min later by administration of pGlu-apelin-13 (1 mg/kg; 2 μl i.c.v.) or vehicle (0·9% saline; 2 μl i.c.v.) and killed 30 min later.
Experiment 2
V1bR KO and wild-type (WT) mice (n=9–12/group) were administered pGlu-apelin-13 (1 mg/kg; 2 μl i.c.v.) or vehicle (0·9% saline; 2 μl i.c.v.) and killed 30 min later.
Experiment 3
V1bR KO mice (n=12–16/group) were administered either vehicle (0·9% saline; 5 μl i.c.v.) or α-helical CRF9–41 (25 μg; 5 μl i.c.v.), followed 30 min later by administration of pGlu-apelin-13 (1 mg/kg; 2 μl i.c.v.) or vehicle (0·9% saline; 2 μl i.c.v.) and killed 30 min later.
Peptides
pGlu-apelin-13 was purchased from Bachem (St Helens, UK). α-helical CRF9–41 was purchased from Phoenix Pharmaceuticals (Burlingame, CA, USA). All peptides were made up fresh in 0·9% sterile physiological saline (pH 5) on the day of the experiment and kept on ice until use.
Hormone analysis
All experiments were performed at least twice and hormone concentrations in samples were measured in duplicate. Mice were killed by decapitation and trunk blood was collected into chilled heparinised tubes. Plasma was obtained by centrifugation and stored at −20 °C until assayed for ACTH and CORT. Total plasma ACTH concentration was quantified using a two-site ELISA kit (IDS, Tyne & Wear, UK) with a sensitivity of 0·46 pg/ml and intra-assay variation of <10% (supplier's product data (http://www.idsltd.com/Downloads/)). Plasma concentrations of CORT were measured using an enzyme immunoassay (EIA) kit (IDS). The sensitivity is 0·55 ng/ml with an intra-assay variation of <10% (product data at: http://www.idsltd.com/Downloads/AC-14PL.pdf). For both ACTH ELISA and CORT EIA, absorbance readings were taken at 450 nm using a Versamax plate reader (Molecular Devices Corporation, Sunnyvale, CA, USA).
Statistical analysis
Hormone concentrations are expressed as a percentage of baseline of saline-treated control animals. All results are expressed as mean±s.e.m. Where appropriate, plasma hormone concentrations were analysed by one-way ANOVA followed by Dunnett's post hoc using GraphPad Prism (version 4.0b) software (GraphPad Software, San Diego, CA, USA). P<0·05 was considered statistically significant. Effects of genotype and treatment were determined by two-way ANOVA with repeated measures and subsequent post hoc tests as appropriate.
Results
Experiment 1: effect of α-helical CRF9–41 on pGlu-apelin-13 stimulated plasma ACTH and CORT
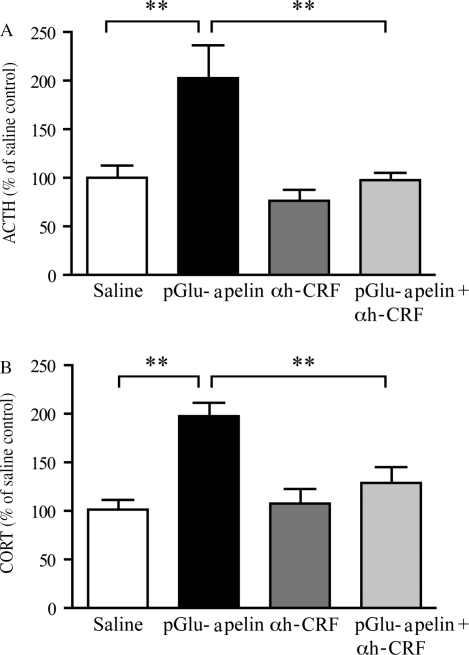
To investigate whether apelin has a central role in regulating CRF neurons, the effect of i.c.v. administration of pGlu-apelin-13 on neuroendocrine function in mice pre-treated with the CRF receptor antagonist, α-helical CRF9–41, was determined. Absolute levels of ACTH secreted after administration of saline were 50±24 pg/ml. Administration of pGlu-apelin-13 (1 mg/kg i.c.v.) resulted in a statistically significant increase in ACTH (202·2±29·3% of saline control; see Fig. 1A). This effect was blocked by pre-treatment with α-helical CRF9–41 (25 μg; 5 μl i.c.v.), which reduced the ACTH response to 97·4±8·3% of saline-treated animals. Administration of α-helical CRF9–41 alone had no significant effect on plasma ACTH (77±12·0%), when compared with saline controls.
Figure 1.
The effect of α-helical CRF9–41 on pGlu-apelin-13 stimulated plasma ACTH and CORT. Pre-treatment with α-helical CRF9–41 (αh-CRF) blocked the pGlu-apelin-13-induced increase in (A) ACTH and (B) CORT concentrations in male mice. Animals received either α-helical CRF9–41 (25 μg; 5 μl i.c.v.) or vehicle (0·9% saline; 5 μl i.c.v.), followed 30 min later by administration of pGlu-apelin-13 (1 mg/kg; 2 μl i.c.v.) or vehicle (0·9% saline; 2 μl i.c.v). Data are expressed as mean±s.e.m. **P<0·01, one-way ANOVA followed by Dunnett's post hoc test.
The effect of pGlu-apelin-13 on plasma CORT is shown in Fig. 1B. Absolute levels of CORT secreted after administration of saline were 73±17 ng/ml. pGlu-apelin-13 administration (1 mg/kg i.c.v.) resulted in a significant increase in CORT (189·5±15·3%) compared with saline-injected controls. This increase in plasma CORT was significantly reduced by pre-treatment with α-helical CRF9–41 (25 μg; 5 μl i.c.v.) to 129±16% of saline-treated control. Pre-treatment with α-helical CRF9–41 (25 μg; 5 μl i.c.v.) alone had no significant effect on plasma CORT (107·4±15·4%) when compared with saline controls.
Experiment 2: effect of pGlu-apelin-13 on plasma ACTH and CORT in WT and V1bR KO mice
The potential role of apelin in regulating AVP neurones was investigated by determining the effect of i.c.v. administration of pGlu-apelin-13 on neuroendocrine function in V1bR KO mice (Fig. 2). There was no significant effect of genotype on the plasma ACTH response to administration of saline. Absolute levels of ACTH secreted after administration of saline were WT 50±24 versus KO 59±16 pg/ml plasma; P>0·05, two-way ANOVA. After administration of pGlu-apelin-13 (1 mg/kg i.c.v.), a significant increase in plasma ACTH was seen in WT (231·9±24·4%) but not in V1bR KO (119·5±16·6%) mice compared with WT control (Fig. 2A).
Figure 2.
Effect of pGlu-apelin-13 on plasma ACTH and CORT in wild-type (white bars) and V1bR KO mice (black bars). (A) Administration of pGlu-apelin-13 (1 mg/kg; 2 μl i.c.v.) resulted in a significant increase in plasma ACTH in wild-type but not in V1bR KO mice. (B) Administration of pGlu-apelin-13 (1 mg/kg; 2μl i.c.v.) resulted in a significant increase in plasma CORT in both wild-type and V1bR KO mice; however, the CORT response to pGlu-apelin-13 was significantly reduced in V1bR KO compared to wild-type mice. Data are expressed as mean±s.e.m. +++P<0·001, **P<0·01 two-way ANOVA using Bonferroni post hoc test.
The effect of pGlu-apelin-13 administration (1 mg/kg i.c.v.) on plasma CORT in WT and V1bR KO mice is shown in Fig. 2B. As for ACTH, there was no significant effect of genotype on plasma CORT response to the administration of saline, WT 73±17 ng/ml plasma versus KO 109±24 ng/ml plasma; P>0·05, two-way ANOVA. Administration of pGlu-apelin-13 (1 mg/kg i.c.v.) resulted in a significant increase in plasma CORT in both WT and V1bR KO mice; however, this CORT response to pGlu-apelin-13 was significantly reduced in V1bR KO compared with WT mice (WT 554·3±41·1% versus V1bR KO 402·3±28·9%; P<0·01).
Experiment 3: effect of α-helical CRF9–41 on pGlu-apelin-13 stimulated plasma CORT in V1bR KO mice
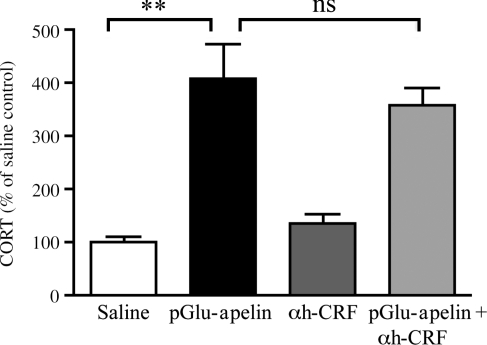
To investigate whether the CORT response seen after the administration of pGlu-apelin-13 to V1bR KO mice is due to an involvement of CRF-mediated actions, the effect of i.c.v. administration of pGlu-apelin-13 on the CORT response of V1bR KO mice pre-treated with α-helical CRF9–41 was determined. The CORT response to pGlu-apelin-13 alone was similar to that seen in experiment 2 with an increase to 408·8±63·3% of baseline control. This increase in CORT secretion was unaffected by pre-treatment with α-helical CRF9–41 (359·0±30·7%; P<0·001; Fig. 3).
Figure 3.
Effect of α-helical CRF9–41 on pGlu-apelin-13 stimulated plasma CORT in V1bR KO mice. Administration of pGlu-apelin-13 (1 mg/kg; 2 μl i.c.v.) resulted in a significant increase in plasma CORT in V1bR KO mice that was not blocked by pre-treatment with α-helical CRF9–41 (αh-CRF) (25 μg; 5 μl i.c.v.). Data are expressed as mean±s.e.m. **P<0·01 one-way ANOVA followed by Dunnett's post hoc test; ns, not significant.
Discussion
The present study demonstrates that the in vivo effects of apelin on HPA axis neuroendocrine function are mediated through both CRF- and AVP-dependent mechanisms. pGlu-apelin-13 activation of the HPA axis, as evidenced by an increase in plasma ACTH and CORT in control animals, was significantly attenuated both in mice pre-treated with the CRF receptor antagonist, α-helical CRF9–41, and in mice lacking functional AVP V1b receptors.
In the HPA axis, localisation of apelin and APJ mRNA and protein to hypothalamic nuclei known to contain AVP and CRF, and to modulate HPA endocrine responses to stress, has generated interest in a role for apelin in the regulation of HPA axis activity. Apelin may possibly act by controlling hypothalamic neuropeptide synthesis or release in parvocellular AVP and/or CRF neurones, to directly or indirectly alter ACTH secretion. However, apelin may also have a direct effect on HPA axis activity at the pituitary corticotroph level, as the peptide and receptor are both co-localised with ACTH in corticotrophs suggesting autocrine and/or paracrine interactions between apelin and ACTH. Our previous work suggested aspects of the apelinergic system present in the hypothalamus to be involved in the modulation of stress responses, as restraint stress was shown to be associated with a significant increase in APJ mRNA expression in the pPVN (O'Carroll et al. 2003). Removal of endogenous glucocorticoids by adrenalectomy resulted in an increased expression of APJ mRNA in the pPVN, implying negative regulation of APJ mRNA expression by glucocorticoids (O'Carroll et al. 2003). After repeated restraint stress, there was a sustained increase in expression of APJ mRNA in the pPVN, similar to that observed for AVP mRNA (De Goeij et al. 1992), suggesting that apelin acting through APJ facilitates parvocellular function under conditions of repeated stress. While CRF is the major activator of pituitary corticotroph ACTH secretion in acute stress, AVP appears to be important in maintaining the HPA axis response to a number of repeated and chronic stressors (Harbuz & Lightman 1992, Aguilera 1994), and, although both pituitary CRFR1 receptors and AVP V1b receptors are activated and undergo regulatory variations during stress, only the changes in V1b receptor levels parallel the changes in pituitary ACTH responsiveness (Rabadan-Diehl et al. 1995, Aguilera & Rabadan-Diehl 2000). Recent studies, however, in V1bR KO receptor-deficient mice, have substantiated the role of AVP in the HPA axis response to some acute stressors (Lolait et al. 2007a,b, Stewart et al. 2008).
Previous studies involving central administration of pGlu-apelin-13 have focussed on the activity of this peptide in the regulation of a broad range of homeostatic behaviours in the rat, notably food (Sunter et al. 2003) and water intake (Reaux et al. 2001,Taheri et al. 2002), or cardiovascular effects (Kagiyama et al. 2005). Few studies have investigated the role of central apelin and APJ in neuroendocrine activation as reflected by ACTH and CORT secretion (Taheri et al. 2002, Jaszberenyi et al. 2004). Central administration of pGlu-apelin-13 has been shown to activate PVN neurones and to stimulate the HPA axis as indicated by increased c-fos expression in the PVN (Kagiyama et al. 2005), and increased secretion of both ACTH and CORT into the plasma (Taheri et al. 2002). Additionally, apelin appears to have a central role in the regulation of AVP and CRF neurones, as apelin stimulated release of both of these peptides from hypothalamic explants in vitro (Taheri et al. 2002). Recently, in an in vitro perfusion system of anterior pituitaries, apelin-17 significantly increased basal ACTH secretion (Reaux-Le Goazigo et al. 2007).
This study represents the first in vivo investigation of the mechanism of action of pGlu-apelin-13 on the secretion of ACTH and CORT in the mouse. We investigated whether pGlu-apelin-13 may exert its actions via stimulation of the release of CRF from neurones of the pPVN, which acts on the anterior pituitary to stimulate the secretion of ACTH into the periphery, resulting in the secretion of CORT from the adrenal glands. We show that pre-treatment of mice with the CRF receptor antagonist, α-helical CRF9–41, attenuated the pGlu-apelin-13-induced elevations in plasma ACTH and CORT observed in control mice to levels seen in saline controls. This result is in agreement with a study performed on rats where apelin-evoked CORT secretion was diminished by pre-treatment with α-helical CRF9–41 (Jaszberenyi et al. 2004). Our data clearly indicate that apelin may exert its effects through CRF-dependent mechanisms; however, the role of additional mediators such as AVP cannot be excluded. The relative contributions of AVP and CRF in activation of the HPA axis depend on the nature of the stimulus, but it is well established that both peptides are required for a full ACTH response to many acute stressors (Kjaer et al. 1992, Suda et al. 1992).
In the neurohypophysial system, the physiological effects of apelin appear to be mediated by AVP. We and others have found apelin and APJ to be expressed within a proportion of magnocellular PVN (mPVN) and SON vasopressinergic neurones (Reaux et al. 2001, O'Carroll & Lolait 2003), where the two peptides are segregated within separate subcellular compartments (Reaux-Le Goazigo et al. 2004), suggesting an interaction between the apelinergic and vasopressinergic systems in the regulation of body fluid homeostasis. Recent studies report a cross-regulation of endogenous levels of apelin and AVP (Reaux-Le Goazigo et al. 2004), and that apelin acts to regulate AVP actions through modulation of AVP neurone activity and AVP release (Reaux-Le Goazigo et al. 2004, Tobin et al. 2008). Interestingly, there is unequivocal evidence that the neurohypophysial and HPA axes functionally overlap and that AVP produced in the mPVN contributes to the regulation of HPA axis activity (secretion of ACTH; reviewed in Engelmann et al. 2004). Moreover, some stressors that activate the HPA axis appear to preferentially activate mPVN more than pPVN AVP neurones e.g. forced swim test (Engelmann et al. 2004).
In order to determine the role of AVP in the mechanism of action of pGlu-apelin-13 in activation of the HPA axis, pGlu-apelin-13 was administered to both WT and V1bR KO mice. These mice have impaired HPA responses to a number of stress models (Lolait et al. 2007a,b, Stewart et al. 2008), indicating a key role for V1bR in HPA axis activation. However, we have shown no difference in basal or CRF-stimulated ACTH release from anterior pituitary tissue from V1bR KO or WT mice, suggesting that there is no difference in pituitary sensitivity to CRF between genotypes (Lolait et al. 2007a). Additionally, in the same study, we have shown basal levels of AVP, oxytocin and CRF gene expression in the hypothalamic PVN, and pro-opiomelanocortin (the precursor for ACTH) gene expression in the anterior pituitary to be similar in V1bR KO and WT mice implying no evidence of compensatory gene expression to the genetic change. In this present study, administration of pGlu-apelin-13 resulted in a significant increase in plasma ACTH in WT mice, an effect that was abolished in V1bR KO mice, indicating a significant role for the V1bR in the ACTH response to apelin administration. The absence of an ACTH response in these mutant mice suggests that the stimulatory effect of apelin on CRF release cannot by itself manifest a full ACTH response to apelin administration. Interestingly, V1bR KO mice had a markedly diminished, although not absent, CORT response to pGlu-apelin-13 administration. Dissociation between ACTH and CORT responses to stimulation in V1bR KO animals has been observed previously, albeit in different experimental models (acute lipopolysaccharide challenge (Lolait et al. 2007b), acute and chronic forced swimming and acute and chronic restraint stress (Stewart et al. 2008)). It is possible that ACTH levels in V1bR KO mice, however low, are still high enough to induce a normal CORT response to stress, or that the residual adrenal activity may be mediated by ACTH-independent CORT modulators. Pituitary-independent factors that may modulate adrenal CORT secretion include sympathetic activity (Bornstein & Chrousos 1999, Droste et al. 2007) and cytokines such as interleukin-6 (Bornstein & Chrousos 1999). Levels of CORT secretion in V1bR KO mice did not change when animals were pre-treated with α-helical CRF9–41, indicating that the remaining levels of CORT cannot be attributed to the action of CRF on CRF pituitary receptors.
In conclusion, our data suggest that apelin, mediating its effects through activation of paraventricular CRF and AVP neurones, requires both CRF and AVP for a full ACTH and CORT response and that neither can compensate for the loss of the other in maintaining a normal HPA axis response to apelin. We cannot exclude the possibility, however, that apelin injected i.c.v. in these studies reaches the anterior pituitary and/or other sites that may influence HPA axis activity to exert a direct effect. Our results emphasise an interaction between apelin and paraventricular AVP and CRF and suggest that the integration of neurobehavioural responses to stress is more complicated than previously envisioned.
Declaration of interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by the Biotechnology and Biological Sciences Research Council (grant number BB/D00196X/1) and The Wellcome Trust (grant numbers 076321 and 074690).
References
- Aguilera G. Regulation of pituitary ACTH secretion during chronic stress. Frontiers in Neuroendocrinology. 1994;15:321–350. doi: 10.1006/frne.1994.1013. [DOI] [PubMed] [Google Scholar]
- Aguilera G, Rabadan-Diehl C. Vasopressinergic regulation of the hypothalamic–pituitary–adrenal axis: implications for stress adaptation. Regulatory Peptides. 2000;96:23–29. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Chrousos GP. Adrenocorticotropin (ACTH)- and non-ACTH-mediated regulation of the adrenal cortex: neural and immune inputs. Journal of Clinical Endocrinology and Metabolism. 1999;84:1729–1736. doi: 10.1210/jcem.84.5.5631. [DOI] [PubMed] [Google Scholar]
- Brailoiu GC, Dun S, Yang J, Ohsawa M, Chang JK, Dun NJ. Apelin-immunoreactivity in the rat hypothalamus and pituitary. Neuroscience Letters. 2002;327:193–197. doi: 10.1016/s0304-3940(02)00411-1. [DOI] [PubMed] [Google Scholar]
- Droste SK, Chandramohan Y, Hill LE, Linthorst ACE, Reul JMHM. Voluntary exercise impacts on the rat hypothalamic–pituitary–adrenocortical axis mainly at the adrenal level. Neuroendocrinology. 2007;86:26–27. doi: 10.1159/000104770. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R, Wotjak CT. The hypothalamic–neurohypophysial system regulates the hypothalamic–pituitary–adrenal axis under stress: an old concept revisited. Frontiers in Neuroendocrinology. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Gillies GE, Linton EA, Lowry PJ. Corticotropin-releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982;299:355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- De Goeij DCE, Jezova D, Tilders FJ. Repeated stress enhances vasopressin synthesis in corticotropin releasing factor neurons in the paraventricular nucleus. Brain Research. 1992;577:165–168. doi: 10.1016/0006-8993(92)90552-k. [DOI] [PubMed] [Google Scholar]
- Habata YH, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S, Kitada C, Nishizawa N, Murosaki S, Kurokawa T, et al. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochimica et Biophysica Acta. 1999;1452:25–35. doi: 10.1016/s0167-4889(99)00114-7. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Lightman SL. Stress and the hypothalamo-pituitary–adrenal axis: acute, chronic and immunological activation. Journal of Endocrinology. 1992;134:327–339. doi: 10.1677/joe.0.1340327. [DOI] [PubMed] [Google Scholar]
- Jaszberenyi M, Bujdoso E, Telegdy G. Behavioral, neuroendocrine and thermoregulatory actions of apelin-13. Neuroscience. 2004;129:811–816. doi: 10.1016/j.neuroscience.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Kagiyama S, Fukuhara M, Matsumura K, Lin Y, Fujii K, Iida M. Central and peripheral cardiovascular actions of apelin in conscious rats. Regulatory Peptides. 2005;125:55–59. doi: 10.1016/j.regpep.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Kjaer A, Knigge U, Bach FW, Warberg J. Histamine- and stress-induced secretion of ACTH and beta-endorphin: involvement of corticotropin-releasing hormone and vasopressin. Neuroendocrinology. 1992;56:419–428. doi: 10.1159/000126258. [DOI] [PubMed] [Google Scholar]
- Kleinz MJ, Davenport AP. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regulatory Peptides. 2004;118:119–125. doi: 10.1016/j.regpep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, Osmond DH, George SR, O'Dowd BF. Characterisation of apelin, the ligand for the APJ receptor. Journal of Neurochemistry. 2000;74:34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- Lolait SJ, Stewart LQ, Jessop DS, Young WSIII, O'Carroll A-M. The hypothalamic–pituitary–adrenal axis response to stress in mice lacking functional vasopressin V1b receptors. Endocrinology. 2007a;148:849–856. doi: 10.1210/en.2006-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolait SJ, Stewart LQ, Roper JA, Harrison G, Jessop DS, Young WSIII, O'Carroll A-M. Attenuated stress response to acute lipopolysaccharide and ethanol administration in vasopressin V1b receptor knockout mice. Journal of Neuroendocrinology. 2007b;19:543–551. doi: 10.1111/j.1365-2826.2007.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhurst AD, Jennings CA, Robbins MJ, Davis RP, Ellis C, Winborn KY, Lawrie KW, Hervieu G, Riley G, Bolaky JE, et al. Pharmacological and immunohistochemical characterisation of the APJ receptor and its endogenous ligand apelin. Journal of Neurochemistry. 2003;84:1162–1172. doi: 10.1046/j.1471-4159.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- El Messari S, Iturrioz X, Fassot C, De Mota N, Roesch D, Llorens-Cortes C. Functional dissociation of apelin receptor signaling and endocytosis: implications for the effects of apelin on arterial blood pressure. Journal of Neurochemistry. 2004;90:1290–1301. doi: 10.1111/j.1471-4159.2004.02591.x. [DOI] [PubMed] [Google Scholar]
- De Mota N, Lenkei Z, Llorens-Cortes C. Cloning, pharmacological characterisation and brain distribution of the rat apelin receptor. Neuroendocrinology. 2000;72:400–407. doi: 10.1159/000054609. [DOI] [PubMed] [Google Scholar]
- De Mota N, Reaux-Le Goazigo A, El Messari S, Chartrel N, Roesch D, Dujardin C, Kordon C, Vaudry H, Moos F, Llorens-Cortes C. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. PNAS. 2004;101:10464–10469. doi: 10.1073/pnas.0403518101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Carroll A-M, Lolait SJ. Regulation of rat APJ receptor messenger ribonucleic acid expression in magnocellular neurones of the paraventricular and supraoptic nuclei by osmotic stimuli. Journal of Neuroendocrinology. 2003;15:661–666. doi: 10.1046/j.1365-2826.2003.01044.x. [DOI] [PubMed] [Google Scholar]
- O'Carroll A-M, Selby TL, Palkovits M, Lolait SJ. Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochimica et Biophysica Acta. 2000;1492:72–80. doi: 10.1016/s0167-4781(00)00072-5. [DOI] [PubMed] [Google Scholar]
- O'Carroll A-M, Don AL, Lolait SJ. APJ receptor mRNA expression in the rat hypothalamic paraventricular nucleus: regulation by stress and glucocorticoids. Journal of Neuroendocrinology. 2003;15:1095–1110. doi: 10.1046/j.1365-2826.2003.01102.x. [DOI] [PubMed] [Google Scholar]
- O'Dowd BF, Heiber M, Chan A, Heng HHQ, Tsui L-C, Kennedy JL, Shi X, Petronis A, George SR, Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–360. doi: 10.1016/0378-1119(93)90495-o. [DOI] [PubMed] [Google Scholar]
- Rabadan-Diehl C, Lolait SJ, Aguilera G. Regulation of pituitary vasopressin V1b receptor mRNA during stress in the rat. Journal of Neuroendocrinology. 1995;7:903–910. doi: 10.1111/j.1365-2826.1995.tb00734.x. [DOI] [PubMed] [Google Scholar]
- Reaux A, De Mota N, Skultetyova I, Lenkei Z, El Messari S, Gallatz K, Corvol P, Palkovits M, Llorens-Cortes C. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. Journal of Neurochemistry. 2001;77:1085–1096. doi: 10.1046/j.1471-4159.2001.00320.x. [DOI] [PubMed] [Google Scholar]
- Reaux A, Gallatz K, Palkovits M, Llorens-Cortes C. Distribution of apelin-synthesising neurons in the adult rat brain. Neuroscience. 2002;113:653–662. doi: 10.1016/s0306-4522(02)00192-6. [DOI] [PubMed] [Google Scholar]
- Reaux-Le Goazigo A, Morinville A, Burlet A, Llorens-Cortes C, Beaudet A. Dehydration-induced cross-regulation of apelin and vasopressin immunoreactivity levels in magnocellular hypothalamic neurons. Endocrinology. 2004;145:4392–4400. doi: 10.1210/en.2004-0384. [DOI] [PubMed] [Google Scholar]
- Reaux-Le Goazigo A, Alvear-Perez R, Zizzari P, Epelbaum J, Bluet-Pajot M-T, Llorens-Cortes C. Cellular localization of apelin and its receptor in the anterior pituitary: evidence for a direct stimulatory action of apelin on ACTH release. American Journal of Physiology. Endocrinology and Metabolism. 2007;292:E7–E15. doi: 10.1152/ajpendo.00521.2005. [DOI] [PubMed] [Google Scholar]
- Stewart LQ, Roper JA, Young WSIII, O'Carroll A-M, Lolait SJ. Pituitary–adrenal response to acute and repeated mild restraint, forced swim and change in environment stress in arginine vasopressin receptor 1b knockout mice. Journal of Neuroendocrinology. 2008;20:597–605. doi: 10.1111/j.1365-2826.2008.01704.x. [DOI] [PubMed] [Google Scholar]
- Suda T, Nakano Y, Tozawa F, Sumitomo T, Sato Y, Yamada M, Demura H. The role of corticotropin-releasing factor and vasopressin in hypoglycemia-induced proopiomelanocortin gene expression in the rat anterior pituitary gland. Brain Research. 1992;579:303–308. doi: 10.1016/0006-8993(92)90065-h. [DOI] [PubMed] [Google Scholar]
- Sunter D, Hewson AK, Dickson SL. Intracerebroventricular injection of apelin-13 reduces food intake in the rat. Neuroscience Letters. 2003;353:1–4. doi: 10.1016/s0304-3940(03)00351-3. [DOI] [PubMed] [Google Scholar]
- Taheri S, Murphy K, Cohen M, Sujkovic E, Kennedy A, Dhillo W, Dakin C, Sajedi A, Ghatei M, Bloom S. The effects of centrally administered apelin-13 on food intake, water intake and pituitary hormone release in rats. Biochemical and Biophysical Research Communications. 2002;291:1208–1212. doi: 10.1006/bbrc.2002.6575. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochemical and Biophysical Research Communications. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- Tobin VA, Bull PM, Arunachalam S, O'Carroll A-M, Ueta Y, Ludwig M. The effects of apelin on the electrical activity of hypothalamic magnocellular vasopressin and oxytocin neurons and somatodendritic peptide release. Endocrinology. 2008;149:6136–6145. doi: 10.1210/en.2008-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramu G, Croix C, Pillez A. Ability of the CRF immunoreactive neurons of the paraventricular nucleus to produce a vasopressin-like material. Neuroendocrinology. 1983;37:467–469. doi: 10.1159/000123595. [DOI] [PubMed] [Google Scholar]
- Ynag H, Wang L, Ju G. Evidence for hypothalamic paraventricular nucleus as an integrative center of neuroimmunomodulation. Neuroimmunomodulation. 1977;4:120–127. doi: 10.1159/000097330. [DOI] [PubMed] [Google Scholar]