Abstract
The National Institute on Aging Interventions Testing Program (ITP) was established to evaluate agents that are purported to increase lifespan and delay the appearance of age-related disease in genetically heterogeneous mice. Up to five compounds are added to the study each year and each compound is tested at three test sites (The Jackson Laboratory, TJL; University of Michigan, UM; and University of Texas Health Science Center, San Antonio, UT). Mice in the first cohort were exposed to one of four agents: aspirin, nitroflurbiprofen (NFP), 4-OH-α-phenyl-N-tert-butyl nitrone (4-OH-PBN), or nordihydroguiaretic acid (NDGA). Sample size was sufficient to detect a 10% difference in lifespan in either sex, with 80% power, using data from two of the three sites. Pooling data from all three sites, a log-rank test showed that both NDGA (p = 0.0006) and aspirin (p = 0.01) led to increased lifespan of male mice. Comparison of the proportion of live mice at the age of 90% mortality was used as a surrogate for measurement of maximum lifespan; neither NDGA (p = 0.12) nor aspirin (p = 0.16) had a significant effect in this test. Measures of blood levels of NDGA or aspirin and its salicylic acid metabolite suggest that the observed lack of effects of NDGA or aspirin on lifespan in females could be related to gender differences in drug disposition or metabolism. Further studies are warranted to find whether NDGA or aspirin, over a range of doses, might prove to postpone death and various age-related outcomes reproducibly in mice.
Keywords: longevity, nordihydroguiaretic acid, aspirin, aging, mice
INTRODUCTION
Both dietary restriction (DR) and single-gene dwarfing mutations in rodents demonstrate that mammalian aging can be retarded and mortality postponed (Miller, 2001; Flurkey et al., 2001). Furthermore, dietary additives with the potential for human application exist. Unfortunately, clinical screening studies for anti-aging drugs in humans are impractical because of time, cost, and ethical constraints. Mouse models have a vital place here because mice age 30 times faster than human beings (Flurkey et al, 2007), yet their biology and genetics are remarkably similar to humans (Peters et al., 2007; Petkov et al., 2007; Svenson et al., 2007).
Dietary additives that delay aging would potentially have a major effect on human health, an effect far in excess of preventive measures that affect only individual forms of late-life disease such as cancer or heart disease (Miller, 2002; Olshansky et al.,1990; 2006). Even negative data would be useful if based on strong science. The published literature contains sporadic reports (Schneider & Miller, 1998; Archer & Harrison, 1996; Schneider & Reed, Jr., 1985) of dietary additives purported to have beneficial effects in rodent models, but none that has been replicated and accepted by the scientific community as a reliable and reproducible result. Stimulated by a consensus conference in 1999 (Warner et al., 2000), the National Institute on Aging has developed a program, the NIA Interventions Testing Program (ITP), to evaluate agents that are considered plausible candidates for delaying aging or preventing multiple forms of late-life disease in laboratory mice.
The central features of the ITP protocol and the compounds were detailed by Miller et al. (2007), which also presented an interim analysis of survival data for mice exposed to one of four agents. Briefly, test agents are nominated by scientists whose recommendations are reviewed by an expert committee. Details about the selection process are found at www.nia.nih.gov/ResearchInformation/ScientificResources/InterventionsTestingProgram.htm. Studies were simultaneously replicated at three test sites: The Jackson Laboratory in Bar Harbor, Maine (TJL), the University of Michigan at Ann Arbor (UM), and the University of Texas Health Science Center at San Antonio (UT). The protocol uses genetically heterogeneous mice, the progeny of CB6F1 females bred to C3D2F1 males, with breeders supplied to each site at the same time by The Jackson Laboratory. Each animal in the test population is thus genetically unique, but all the animals are full siblings. None of the four agents in the current report altered mean weights. Therefore, positive effects on lifespan could not result from unintended dietary restriction. The ITP standard protocol enrolls 36 female and 44 male mice in each treatment group, at each of the three test sites, as well as 72 females and 96 males in the untreated control group. The extra males compensate for the larger number of early-life deaths of male mice due to fighting. According to the power calculations, a design of this size provides 80% power to detect a 10% increase (or decrease) in mean lifespan with respect to unmanipulated controls of the same sex, even if data from one of the three test sites are unavailable.
This article presents the full lifespan analysis of survival data for mice exposed to one of four agents. Aspirin is a non-steroidal anti-inflammatory agent that also has anti-thrombotic and anti-oxidant properties (Shi et al., 1999; Vane, 2000; Weissmann, 1991) and is widely used clinically. Nitroflurbiprofen (NFP) is a nitric oxide (NO)-releasing flurbiprofen derivative (Brunelli et al., 2007; Ongini & Bolla, 2006). Like flurbiprofen, NFP is a non-steroidal anti-inflammatory agent showing about an equal inhibitory potential against the cyclooxygenases, COX-1 and COX-2. Its NO-releasing moiety should make the drug safer than ibuprofen in chronic administration. Nordihydroguaiaretic acid (NDGA; also called masoprocol) has both anti-oxidant and anti-inflammatory properties, and it resembles other polyphenols that can activate sirtuins and which have shown life-extension properties in metazoan models (Wood et al., 2004). 4-OH-PBN is the 4-hydroxy derivative and principal metabolite of PBN (α-phenyl-N-tert-butyl nitrone), a nitrone that is well known in analytical chemistry to trap and stabilize free radicals. PBN has potent pharmacologic activities in stroke (Floyd, 1990; Zhao et al., 1994) and cancer (Floyd et al., 2002; Nakae et al., 2003).
We report here that both NDGA (p = 0.0006) and aspirin (p = 0.01) led to a significant increase in survival of male mice. Neither NFP nor 4-OH-PBN altered survival of male mice, and none of the four test agents had a significant effect on survival of female mice.
METHODS
Mouse production, maintenance, and estimation of lifespan
UM-HET3 mice were produced at each of the three test sites. The mothers of the test mice were CByB6F1/J, JAX stock #100009, whose female parents are BALB/cByJ and whose male parents are C57BL/6J. The fathers of the test mice were C3D2F1/J, JAX stock #100004, whose mothers are C3H/HeJ and whose fathers are DBA/2J. The first litter from each breeding cage was discarded, so that all of the experimental animals would be the product of a second or subsequent pregnancy. This decision was made to avoid the possibility that mice born to primiparous dams might receive inferior nutrition or maternal care compared to offspring from subsequent pregnancies. Each test site enrolled approximately equal numbers of weanlings each month over a six month period. Mice were weaned into same-sex cages (3 males or 4 females/cage) at the age of 19 – 21 days. Sires were not separated from pregnant dams during the period of pregnancy to allow subsequent impregnation at post-partum estrous. Mice did not receive ear punches or toe clips or other identifying marks at weaning. Each site used diets based on the NIH-31 standard. For breeding cages, UM used Purina 5008, UT used Teklad 7912, and TJL used Purina 5K52. For weanlings prior to 4 months of age, UM used Purina 5001, UT used Teklad 7912, and TJL used Purina 5LG6.
Mice were housed in plastic cages with metal tops, using corn-cob bedding, specifically 1/4 inch Bed O’Cobs, produced by The Andersons, PO Box 114, Maumee, Ohio. Mice were given free access to water, acidified (pH 2.5 – 2.7) by addition of hydrochloric acid, using water bottles rather than an automated watering system. Mice were housed in ventilated cages, and were transferred to fresh cages every 14 days, except that at UT mice were transferred to fresh cages every 7 days. Temperature was maintained within the range of 21°C to 23°C.
At the age of 42 days, each cage was assigned to a control or test group by use of a random number table. Each mouse was then briefly anesthetized by isoflurane inhalation administered either by nose cone or by an instrument designed for small animal anesthesia. Measures were taken of weight, body length, and tail length; and an electronic ID chip was implanted by sterile syringe beneath the dorsal skin between the shoulder blades, after which the wound was closed by a drop of superglue (Loctite gel, purchased locally, or Nexaband S/C, purchased from Abbott Laboratories, North Chicago, IL). UM and UT used chips purchased from AVID Microchip ID Systems (Folsom, LA; Catalog AVID3002); TJL used chips purchased from Locus Technology (Manchester, MD; catalog 1D-100A). A portion of the distal tail (1 cm) was taken and frozen for later analysis of DNA polymorphisms, after which the mouse was permitted to awaken from the anesthesia. The duration of anesthesia was approximately 1 to 2 min.
Mice received control diet until the age of 4 months, after which mice in the control group received Purina 5LG6 at all three sites and mice in the NFP, 4-OH-PBN, and aspirin groups received Purina 5LG6 containing these additives. Mice in the NDGA group started receiving 5LG6 supplemented with NDGA at the age of 9 months.
At the age of 7 months, a subset of the mice (100 controls plus 50 of each test group at each site) were evaluated for activity, using a test in which each mouse is housed individually in a standard mouse cage for 50 hrs, while a computer records episodes of ambulation or movement in place. This test was repeated at the age of 18 months, for each live mouse that had been previously tested. Three weeks after the activity test, a blood sample was obtained from each mouse that had been tested for activity; these samples were used for analyses of T cell subsets and hormone levels that will be reported elsewhere.
Details of the methods used for health monitoring were provided in Miller et al. (2007); in brief, each of the three colonies was evaluated four times each year for infectious agents, including pinworm. All such tests were negative throughout the entire study period.
Removal of mice from the longevity population
As described in detail in Miller et al. (2007), mice were removed from the study because of fighting (36 mice), or accidental death, typically during chip implantation or chip failure (26 mice), or because they were used for another experimental purpose, such as testing for blood levels of a test agent (51 mice). For survival analyses, all such mice were treated as alive at the date of their removal from the protocol, and lost to follow-up thereafter. These mice were not included in calculations of median longevity.
Estimation of age at death (lifespan)
Mice were examined at least daily for signs of ill health. Mice were euthanized for humane reasons if so severely moribund that they were considered, by an experienced technician, unlikely to survive for more than an additional 48 hrs. A mouse was considered severely moribund if it exhibited more than one of the following clinical signs: (a) inability to eat or to drink; (b) severe lethargy, as indicated by reluctance to move when gently prodded with a forceps; (c) severe balance or gait disturbance; (d) rapid weight loss over a period of one week or more; or (e) an ulcerated or bleeding tumor. The age at which a moribund mouse was euthanized was taken as the best available estimate of its natural lifespan. Mice found dead were also noted at each daily inspection.
Control and experimental diets
TestDiet, Inc. (Richmond, IN) prepared batches of Purina 5LG6 food containing each of the test substances, as well as control diet batches, at intervals of approximately 4 months, and shipped each batch of food at the same time to each of the three test sites. NDGA was purchased from Cayman Chemicals (Ann Arbor, MI) and was mixed with chow at a concentration of 2500 milligrams of NDGA per kg of food. Nitroflurbiprofen was obtained from NicOx Research Institute (Milan, Italy) and used at a dose of 200 mg NFP per kg of food. Aspirin was obtained from a local supplier and used at a dose of 21 mg per kg of food. 4-OH-PBN was synthesized in the laboratory of Robert Floyd and used at a dose of 350 mg per kg of food. On the assumption that each mouse weighs 30 gm and consumes 5 gm food/day, the estimated daily doses of these agents would be NDGA 417; NFP 33; 4-OH-PBN 53, and aspirin 3.3 mg/kg body weight/day.
Measurement of concentrations of NDGA, acetylsalicylic acid (ASA), and salicylic acid (SA) in plasma
Male mice (9 per condition) and female mice (8 per condition) were treated for four weeks at the UM site with two different doses of aspirin or NDGA administered in the diet. Doses of NDGA were 2.5 g/kg food (low dose, same as for the current study) and 5 g/kg (high dose). Doses of aspirin were 20 mg/kg food (low dose, same as for the current study) and 60 mg/kg food (high dose). Concentrations of aspirin and NDGA in food were verified by HPLC analysis. Animals had free access to food and water 24 hours per day. At the end of the treatment period, blood samples (200 – 300 μl each) were obtained from the tail vein. Samples were taken at approximately 9:00 AM, three hours after the lights come on in the animal facility. Blood samples were centrifuged to pellet the cell and the plasma supernatant was carefully removed. The tubes were shipped on dry ice to the UT site and stored frozen at −80°C until assayed for NDGA, acetylsalicylic acid, and salicylic acid.
Measurement of Nordihydroguiaretic Acid (NDGA)
NDGA was quantified in mouse plasma using HPLC with UV detection. Briefly, 200 μL of calibrators and unknown samples were mixed with 10 μL of 100 μg/mL flurbiprofen (internal standard) and 2 mL of 50:50 acetonitrile:methanol. The samples were vortexed vigorously and then centrifuged at 1500 g for 15 min. Supernatants were transferred to glass test tubes and dried to residue under a gentle stream of nitrogen. The residues were redissolved in 250 μL of mobile phase and then filtered using a microfilterfuge tube. Then, 150 μL of the final samples were injected into the HPLC. The ratios of the peak area of NDGA to that of the internal standard were compared against a linear regression of calibrators at concentrations of 0, 50, 100, 500, and 1000 ng/ml to quantify NDGA in the samples. NDGA concentration in blood or plasma was reported in ng/mL.
The HPLC system consisted of an Alltima C18 column (4.6 × 150 mm, 5 micron), Waters 2487 UV detector, Waters 717 autosampler, Waters 515 HPLC pump. The mobile phase was 35% acetonitrile, 64.9% Milli-Q water, and 0.1% phosphoric acid (pH 2.5). The flow rate of the mobile phase was 1.5 mL/min and the wavelength of absorbance was 214 nm.
Measurement of Aspirin (ASA) and Salicylic Acid (SA)
ASA and SA were quantified in mouse plasma using HPLC with UV detection. The HPLC system and settings were the same as that described for measurement of NDGA except that the mobile phase was 8% methanol, 6% acetonitrile, 3.3 mM phosphate in Milli-Q water (pH 2.5). Briefly, 200 μL of calibrators and unknown samples were mixed with 700 μL of EDTA solution, 10 μL of 500 μg/mL homovanillic acid (internal standard), 500 μL of 1 M HCl, and 2 mL of water-saturated ethyl acetate. The samples were vortexed vigorously, shaken for 15 min, and then centrifuged at 1500 g for 15 min. Next, 1.7 mL of the supernatants were transferred to glass test tubes and dried to residue under a gentle stream of nitrogen. The residues were redissolved in 200 μL of mobile phase and then filtered using a microfilterfuge tube. Then, 150 μL of the final samples were injected into the HPLC apparatus. The ratios of the peak area of ASA and SA to those of the internal standard were compared against a linear regression of calibrators at concentrations of 0, 250, 500, 1000, and 2000 ng/mL to quantify ASA and SA in the samples. ASA and SA concentrations in blood or plasma were reported in ng/mL.
Statistical methods
Each mouse originally entered into the study was, at the time of analysis, considered to be in one of two categories: either dead (from natural causes), or censored. Mice were considered to be censored at the age at which they were no longer subjected to the mortality risks typical of unmanipulated mice. In some cases, this was because the mouse was removed because of fighting; in other cases, mice died as the result of an accident (for example, death when anesthetized for implantation of a radio-emitting chip). In still other cases, mice were considered censored on the day in which they received an experimental treatment (such as blood sampling or injection with an inflammatory agent) to which the control mice were not exposed. Kaplan-Meier analysis and log-rank comparisons among groups consider censored mice to be lost from follow-up on the day at which they were removed from the longevity protocol. There were no mice remaining alive at the time of the analyses reported here.
Unless stated otherwise, all significance tests about survival effects are based upon the two-tailed log-rank test at p < 0.05, stratified by test site, with censored mice included up until their date of removal from the longevity population. Other statistical tests are described in the text; all p-values were two-tailed.
RESULTS
Survival analysis: NDGA and aspirin increased longevity in male (but not female) UM-HET3 mice
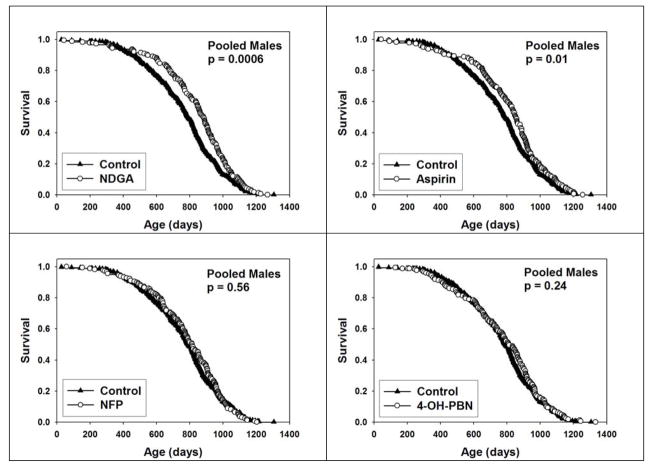
Figure 1 shows survival, as a function of age, in male mice treated with each of the four test agents, pooling data across all three test sites and using a site-stratified log-rank test. Both NDGA (p = 0.0006) and aspirin (p = 0.01) led to a significant increase in survival, but neither NFP nor 4-OH-PBN altered survival of male mice. None of the four test agents had a significant effect on survival of female mice (see Supplementary Figure 1). The hypothesis that these agents altered survival to exceptional old age, a surrogate for maximal lifespan, was evaluated using the method of Wang and Allison (Wang et al., 2004), in each instance by calculation of the proportion of mice still alive at the age when the pooled population, including controls and mice in the treatment group, had reached 90% mortality. This calculation was stratified by site, so that the proportion of live mice in control and treatment groups was evaluated separately at each site, with the pooled counts then combined for the Fisher Exact test statistic. For NDGA, 8.6% of control mice (i.e. 30 of 350) were alive at this time point, compared to 13.1% of treated mice (22 of 168); this difference, at p = 0.12, does not meet our significance criterion. A parallel calculation for aspirin treatment found 8.9% of control males and 13.1% of aspirin-treated males alive at the 90% mortality point, again not significant at p = 0.16. Median survival for control mice was 786 days, compared to 881 days (12% increase) for NDGA and 849 days (8% increase) for aspirin.
Figure 1.
Survival plots for male mice treated with NDGA (upper left), aspirin (upper right), NFP (lower left) or 4-OH-PBN (lower right.) Each symbol represents an individual mouse dying at the age indicated. Log-rank test was used to calculate p-values for differences between treated and control mice.
Sub-group analyses: site-to-site differences in weights and survival curves
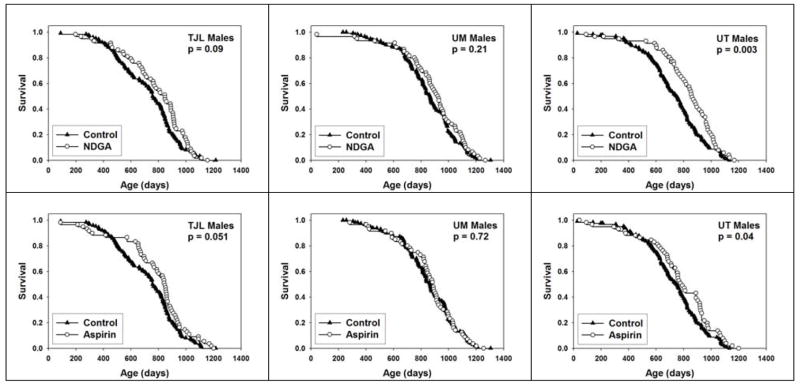
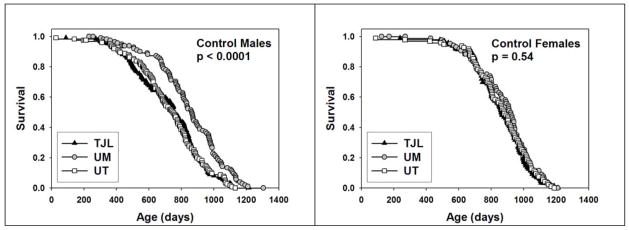
To see if the effects of NDGA or aspirin differed depending on the site at which the mice were housed, we evaluated life tables separately for TJL, UM, and UT. Figure 2 presents survival curves for each of these two interventions, presented by site, for males alone. For NDGA, a significant effect was noted for mice at UT (p = 0.003), and we noted a trend (p = 0.09) at TJL, but there was no significant effect at UM. Aspirin led to a significant improvement at UT (p = 0.04), and a similar trend at TJL (p = 0.051), but not at UM. Although these site-specific analyses have much lower statistical power than the primary analysis (pooling across sites), the results suggest that the effect of the agents may depend on site-specific factors. Indeed, there was a significant difference (p = 0.0001) in survival of male mice among the three test sites (Figure 3a), with UM males living longer than males at either of the other two sites. In contrast, there was no difference in survival of female mice among sites (Figure 3b). We have previously noted site-specific differences in the weight trajectory of control mice (Miller et al., 2007), with mice at UM lighter in weight than mice at either of the other two sites; these differences among sites in body weight were, however, seen in both male and female animals. We have seen equivalent differences in site-specific survival patterns of male mice, and weight trajectories of both male and female mice, in independent cohorts of control UM-HET3 mice involved in subsequent lifespan studies initiated in 2005 (data not shown), and we can thus conclude that these site-specific variations reflect replicable disparities in one or more aspects of husbandry, diet, or other non-genetic variables among the three test sites.
Figure 2.
Survival plots for male mice treated with either NDGA (top panels) or aspirin (bottom panels), compared to control mice, at each of the three test sites. Log-rank test was used to calculate p-values for differences between treated and control mice.
Figure 3.
Survival plots for control (untreated) males (left panel) and females (right panel) showing the comparison among the three test sites. Log-rank test was used to calculate p-values for differences among the three test sites.
Assessment of the effects of gender on plasma NDGA, Aspirin (ASA) and Salicylic acid (SA)
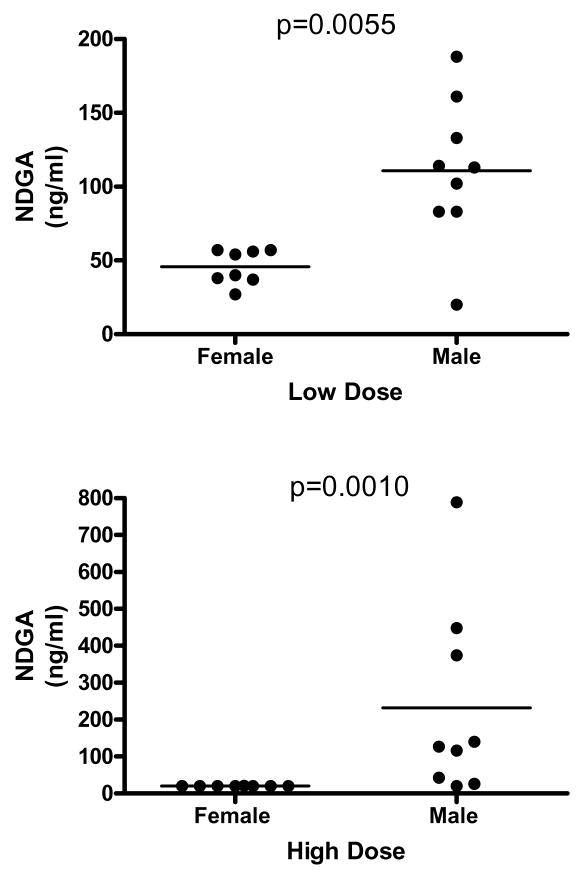
To see whether gender differences in the effects of NDGA and aspirin on survival resulted from gender differences in drug disposition, we assessed drug levels in male and female mice given free access for four weeks to diets that contained either one of two different doses of aspirin or one of two different doses of NDGA. The results for NDGA are shown in Figure 4. Median plasma NDGA levels were significantly (Mann-Whitney U=8.0; p=0.0055) higher in males as compared to female mice fed the low dose diet (2.5 g/kg food), the dose of NDGA used in the survival study reported here. Additionally, males fed the high dose diet (5 g/kg food) had a significantly (Mann-Whitney U=4.5; p=0.001) higher median concentration of plasma NDGA than females in that treatment group.
Figure 4.
NDGA levels in plasma of young UM-HET3 mice after four weeks of consumption of food containing NDGA at either 2.5 gm per kg food (“low dose”, upper panel) or 5 gm per kg food (“high dose”, lower panel). Each symbol represents an individual mouse, and median levels are shown by horizontal lines. Statistical significance was evaluated by the Mann-Whitney test.
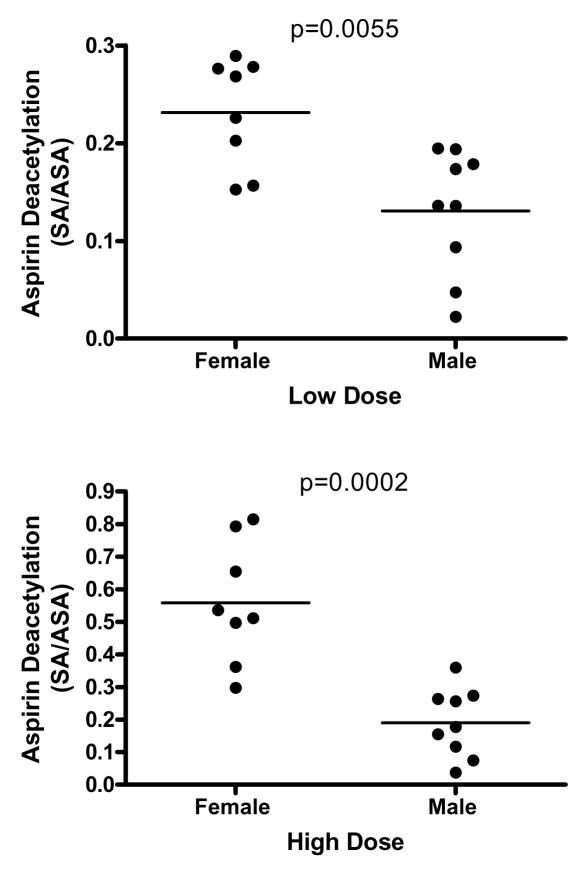
Results of the aspirin feeding study are shown in Figure 5 and Supplementary Figure 2. There was a wide range of plasma ASA and SA values in both male and female mice, which may reflect the genetic heterogeneity in this population, or may reflect differences in the time between when each mouse last ate and when blood was taken for analysis. There were no significant differences in ASA or SA levels between males and females at either tested dose as shown in Supplementary Figure 2. However, as shown in Figure 5, males had a significantly lower ratio of SA/ASA at each dose (High dose, Mann-Whitney U=1.0, p=0.0002; Low dose, Mann Whitney U=8.000, p=0.0055), consistent with the idea that conversion of ASA to its less active metabolite SA may occur more rapidly in females than in males.
Figure 5.
Ratio of SA to ASA in plasma of young UM-HET3 mice after four weeks of consumption of food containing ASA at either 20 gm per kg food (“low dose”, upper panel) or 60 gm per kg food (“high dose”, lower panel). Each symbol represents an individual mouse, and median levels are shown by horizontal lines. Statistical significance was evaluated by the Mann-Whitney test.
DISCUSSION
Our results showed that NDGA and aspirin each increase survival in male UM-HET3 mice, but not in females. The body weight data (Miller et al., 2007) suggest that these beneficial effects are not mediated by caloric restriction. Previous studies of agents reported to extend rodent lifespan when added in food (see Schneider & Miller, 1998; Schneider & Reed, Jr., 1985) for reviews), have been criticized frequently for small sample sizes, use of short-lived control groups, or the lack of information about a colony’s infectious status; and none, as far as we can determine, has ever been replicated either at the original test site or by a second laboratory group. Nearly all prior studies have employed isogenic animal groups, raising the possibility that positive results might reflect strain-specific idiosyncrasies that would not be reproducible in other genetic stocks. Our own results, using genetically heterogeneous mice, three specific pathogen-free vivaria, and a relatively long-lived set of control mice, may provide a better foundation for future replication.
We were only able to test each compound at a single dose, because of cost considerations, and the dose selected was in each case based on limited previous experience with that agent in rodents. It is possible, therefore, that different results, including a larger positive effect on survival, might have been seen at doses different from those used in this study. In planned Phase II testing of NDGA and aspirin, the survival studies will be repeated with multiple doses of each agent.
Our interim report (Miller et al., 2007) found a positive effect of NDGA on male survival during the first half of the lifespan, but there are precedents in which an intervention, such as exercise (Holloszy, 1993), has been shown to increase median lifespan or some other index of mortality risks in young and middle-aged adults, without any effect on maximum lifespan or an equivalent index of survival among the oldest test subjects. Therefore, we assessed the effect of NDGA and aspirin on the proportion of mice that live past the age at which 90% of the population has died using a quantile test (Wang et al., 2004). Although the proportion of mice alive at 90% mortality was greater in the NDGA and aspirin groups as compared to the control group, the effects were not statistically significant (p = 0.12 and p = 0.16, respectively). Therefore, we are unable to conclude at this stage that either treatment had an effect on maximum lifespan at the dose used. If NDGA and/or aspirin do indeed modulate the rate of aging or the risks of specific lethal illnesses, it is entirely possible that the optimal benefit, including perhaps a change in probability of survival to the 90th percentile, might be seen at doses higher or lower than those used in this initial study.
Although our experimental design did not include sufficient animals to allow us to detect small intervention effects at each of the three test sites, we note from Fig 2 that the effects of aspirin and of NDGA were strongest at the two sites where control males had the shortest lifespan (see Fig 3). Significant differences among the three sites in survival of control males have also been seen in a separate cohort of control mice being used for comparison to other ITP interventions initiated in 2005 (“Cohort 2” mice; data not shown), with UM males again showing lowest mortality risks in the first half of the lifespan. The site-specific differences seem unlikely to reflect systematic effects on mouse health, in that there were no differences among sites in survival of control females in either Cohort 1 (Fig 3) or Cohort 2 (not shown). Median lifespan for females at all three ITP sites is similar to that found in previous lifespan studies of UM-HET3 mice (all conducted at UM; see Miller et al., 2007, for citations). Median lifespan for ITP males at UM is slightly higher than in any of the three previous studies, but median lifespan for UT and TJL males is somewhat lower than in the historical controls conducted at UM. We suspect that these differences in control male life tables reflect differences among the sites in the specific dietary formulations administered to the breeders used to produce UM-HET3 test mice at each site, or to the formulations to which the test mice were exposed between weaning and transfer to the drug-containing test diets at age 4 months (aspirin, NFP, 4-OH-PBN, and control groups) or at 9 months (NDGA groups). The diets used for breeders and weanlings (prior to drug exposure) differ in fat content (4.5% to 6.5%), supplemental levels of thiamine and other heat-sensitive vitamins, protein content (18% – 24%) and source of protein (e.g. fish, beet, or pork meal). Control mice, and drug-exposed mice, at the UM site were routinely and significantly smaller than those at the other two sites throughout adult life, for both males and females, and these differences could well reflect lasting influences of differences in dietary regimen on breeders, weanlings, or both. It is also possible that other site-specific factors, such as minor differences in water quality, noise level, ventilation details, extraneous odors, cage-changing frequency, etc., might contribute to site-specific differences in survival of male (but not of female) mice. Starting with Cohort 4 (born in 2007), the three ITP sites have adopted a uniform protocol for diet composition at all stages of the test process, including diets for breeder mice and for test mice prior to drug administration. Replicate studies, at the ITP test sites and at other facilities, will be needed to determine whether the present findings on aspirin and NDGA are robust and reproducible in multiple colonies.
The observation that NDGA or aspirin increased survival in males, but not females, could be accounted for by gender differences in steady-state levels and/or in metabolizing the drugs. We conducted a four-week study in which male and female mice were fed diets containing two different doses of each compound; the original dose used in the survival studies reported here and a higher dose. The results for NDGA revealed that males in each dosage group had higher median plasma concentrations of NDGA than females; this difference was two-fold at the original dose used in the survival studies. Thus, it is plausible that NDGA may have extended the lifespan of male mice and not females because of differences between the sexes in peak or average NDGA serum concentrations. More definitive conclusions await Phase II studies in which the effects of a higher dose of NDGA on survival in male and female mice will be studied. Similarly, the results of feeding aspirin to male and female mice provided evidence for gender differences in aspirin metabolism. Thus, there was a 2- to 3-fold higher ratio of salicylic acid metabolite to acetylsalicylic acid in female mice fed either dose of aspirin, indicating that a greater proportion of the ingested aspirin is converted to salicylic acid in females as compared to males. Aspirin’s principal mechanism of action is to inhibit the activity of COX-1 and 2. ASA is 100-fold more potent than SA in inhibiting COX-1 activity and 2-fold more potent than SA in inhibiting COX-2 (Mitchell et al., 1994). Thus, a higher ratio of SA to ASA in females would be consistent with a reduced therapeutic effect of aspirin in females. This result is consistent with that of human studies of the effects of aspirin on the risk of myocardial infarction that indicate females are less responsive to aspirin (Yerman et al., 2007). This may explain why aspirin increased survival in male mice and not female mice. More comprehensive pharmacokinetic studies and feeding a higher dose of aspirin, in addition to the original dose, in planned Phase II studies, will allow for more definitive conclusions.
The present results are consistent with previous reports that NDGA increases lifespan in both insects and mammals. Thus, NDGA has been reported to increase lifespan in fruit flies, mosquitoes, and rats (Buu-Hoi & Ratsimamang, 1959; Miquel et al., 1982; Richie et al., 1986). Several properties of NDGA may contribute to its beneficial effects on lifespan. For example, NDGA may act to prevent deleterious effects of aging on the brain. Thus, it has been reported to extend the survival of a mouse model of amyotrophic lateral sclerosis, the G93A SOD1 mutant mouse (West et al., 2004). Expression of 5-lipoxygenase is elevated with age and has been proposed to play a role in the pathobiology of aging-associated neurodegenerative diseases (Manev et al., 2000; Qu et al., 2001). Indeed, NDGA has been reported to be effective in preventing neuronal death and cognitive deficits resulting from forebrain ischemia-reperfusion injury (Shishido et al., 2001). Moreover, it has been reported to enhance glucose clearance and insulin sensitivity in a rat diabetes model (Reed et al., 1999). In the same study, it was reported to drastically reduce serum triglycerides (Reed et al., 1999), which may relate in part to NDGA’s reported ability to block fatty acid synthesis in adipocytes through inhibition of fatty acid synthase (FAS) and to inhibit lipoprotein lipase (Li et al., 2005; Park and Pariza, 2001). In numerous studies, NDGA has been reported to have anti-cancer activity through its action as a 5-lipoxygenase inhibitor (e.g. McDonald et al, 2001; Nony et al., 2005; Hoferova et al, 2004). It has also been reported to suppress growth of breast cancer cells through inhibition of the function of two receptor tyrosine kinases (RTKs), the insulin-like growth factor receptor (IGF-1R) and the c-erbB2/HER2/neu (HER2/neu) receptor (Youngren et al., 2005).
The effect of aspirin on lifespan in mice in the present study is consistent with reports of numerous epidemiological studies showing that aspirin use reduces the risk of mortality from a variety of diseases in humans including colon cancer, prostate cancer, and cardiovascular disease (e.g. Jacobs et al., 2005; Chan et al., 2008; Johnson et al., 2002; Lim et al., 2007). Recently, aspirin was reported to activate the NF-kappa B signaling pathway and induce apoptosis in two in vivo models of human colorectal cancer (Stark et al., 2007). Aspirin also normalized blood pressure in a rat model of hypertension (Tuttle et al., 1988). Furthermore, it reportedly improved learning of a spatial memory task in old rats (Smith at al., 2002). Interestingly aspirin use was reported to be associated with increased survival in extreme old age in humans. Aspirin use was associated with increased survival in a five-year follow-up study of subjects in the Finnish Centenarian Study (Aguero-Torres et al., 2001). The authors concluded that the increased survival was probably not attributable simply to the anti-atherogenic effects of aspirin, because the aspirin group had significantly more cardiovascular disease than the non-aspirin users.
Both NDGA and aspirin have anti-oxidative effects and anti-inflammatory properties in addition to the other properties already discussed (Harper et al., 1999; Shi et al., 1999). NDGA, for example, is a potent anti-oxidant and has anti-inflammatory activity by inhibiting leukotriene synthesis (Harper et al., 1999; West et al., 2004). Aspirin potently inhibits oxidative damage, most likely through scavenging the hydroxyl radical and has anti-inflammatory actions by inhibiting prostaglandin synthesis (Shi et al., 1999; Vane, 2000). It is noteworthy that the compounds studied in Cohort I have either anti-inflammatory properties (nitroflurbiprophen), antioxidant properties (4OH-PBN), or both (NDGA and aspirin). Of the four compounds tested, only those with both anti-inflammatory action and antioxidant activity extended lifespan in males. Thus, the combination of antioxidant activity with anti-inflammatory effects may be important in the positive results seen from these two interventions in our initial Phase I study.
The effects of NDGA and aspirin on our measure of extreme longevity, i.e. proportion surviving at the start of the final survival decile, did not reach statistical significance (p = 0.12 and p = 0.16, respectively). It will be interesting to see if greater effects are seen in follow-up studies of these agents at higher or lower doses. Analysis of the effects of NDGA and aspirin on other indices of age-dependent change, including data on terminal pathology, incidence of non-lethal lesions (e.g. cataracts), and the pace of functional changes (e.g. loss of immune response and muscle strength) will all be needed to reach an inference as to whether the effects of NDGA and aspirin on survival reflect a general effect on aging and age-sensitive traits, or instead merely a deceleration of specific forms of lethal disease. Currently, we are planning follow-up (“Phase II”) studies that will include evaluations of the effects of additional doses of aspirin and NDGA on multiple markers of health in middle-aged mice, including analyses of CNS function, metabolic rate, insulin and IGF-1 sensitivity, IGF-I sensitivity, hematopoietic stem cells, DNA strand breaks, body composition, bone mineral density, measures of oxidative stress, T cell subsets, collagen cross-linking, lens turbidity, and early signs of neoplastic and degenerative illnesses. Evaluations of possible modes of action of NDGA and aspirin, including measurements of hormonal, oxidative, and metabolic indices could help to test specific hypotheses about NDGA- and aspirin-sensitive pathways relevant to the aging process and pathogenesis of late-life illnesses. Studies of other compounds with structures or pharmacologic profiles similar to NDGA and aspirin also could be informative. Our data also provide a rationale for evaluation of NDGA and aspirin effects on mortality risks in other varieties of mice and potentially for parallel studies using short-lived primates. Finally, the effects of NDGA and aspirin on long-lived mice, for example mice on calorically restricted diets, or dwarf mutants, could help to determine whether the pathways by which NDGA and aspirin affect survival may overlap with those involved in other models of lifespan extension in mice.
Supplementary Material
Table 1.
Median Survival Values (in days) for Male and Female Control Mice
| Site | Sex | Median ± standard error (N) |
|---|---|---|
| TJL | F | 858 ± 7 (93) |
| UM | F | 909 ± 4 (86) |
| UT | F | 876 ± 4 (96) |
| TJL | M | 781 ± 7 (125) |
| UM | M | 876 ± 8 (106) |
| UT | M | 739 ± 7 (119) |
Acknowledgments
This work was supported by NIA grants AG022303 (RAM), AG025707 and AG022308 (DEH), and AG022307 and AG13319 (RS), and the Department of Veterans Affairs (RM and RS). We wish to thank Vivian Diaz, Elizabeth Fernandez, Greg Friesenhahn, Melissa Han, Patricia Harrison, Roni Kobrosly, Bill Kohler, Pam Krason, Jessica Sewald, and Maggie Lauderdale for technical support. We thank Scott Pletcher and Andrzej Galecki for assistance in power analysis and study design.
REFERENCE LIST
- Agüero-Torres H, Viitanen M, Fratiglioni L, Louhija J. The effect of low-dose daily aspirin intake on survival in the Finnish centenarians cohort. J Am Geriatr Soc. 2001;49:1578–1580. doi: 10.1046/j.1532-5415.2001.4911264.x. [DOI] [PubMed] [Google Scholar]
- Archer JR, Harrison DE. L-deprenyl treatment in aged mice slightly increases life spans, and greatly reduces fecundity by aged males. J Gerontol A Biol Sci Med Sci. 1996;51:B448–53. doi: 10.1093/gerona/51a.6.b448. [DOI] [PubMed] [Google Scholar]
- Brunelli S, Sciorati C, D’Antona G, Innocenzi A, Covarello D, Galvez BG, Perrotta C, Monopoli A, Sanvito F, Bottinelli R, Ongini E, Cossu G, Clementi E. Nitric oxide release combined with nonsteroidal antiinflammatory activity prevents muscular dystrophy pathology and enhances stem cell therapy. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(1):264–9. doi: 10.1073/pnas.0608277104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buu-Hoi NP, Ratsimamanga AR. Retarding action of nordihydroguiaiaretic acid on aging in the rat. [French] Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales. 1959;153:1180–2. [PubMed] [Google Scholar]
- Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Wu K, Fuchs CS. Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology. 2008;134:21–28. doi: 10.1053/j.gastro.2007.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd RA. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J. 1990;4:2587–2597. [PubMed] [Google Scholar]
- Floyd RA, Kotake Y, Hensley K, Nakae D, Konishi Y. Reactive oxygen species in choline deficiency induced carcinogenesis and nitrone inhibition. Mol Cell Biochem. 2002;234–235:195–203. [PubMed] [Google Scholar]
- Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flurkey K, Brandvain Y, Klebanov S, Austad SN, Miller RA, Yuan R, Harrison DE. PohnB6F1: a cross of wild and domestic mice that is a new model of extended female reproductive life span. J Gerontol A Biol Sci Med Sci. 2007;62:1187–98. doi: 10.1093/gerona/62.11.1187. [DOI] [PubMed] [Google Scholar]
- Harper A, Kerr DJ, Gescher A, Chipman JK. Antioxidant effects of isoflavonoids and lignans, and protection against DNA oxidation. Free Radic Res. 1999;31:149–160. doi: 10.1080/10715769900301661. [DOI] [PubMed] [Google Scholar]
- Hoferova Z, Soucek K, Hofmanova J, Hofer M, Chramostova K, Fedorocko P, Kozubik A. In vitro proliferation of fibrosarcoma cells depends on intact functions of lipoxygenases and cytochrome P-450-monooxygenase. Cancer Invest. 2004;22:234–247. doi: 10.1081/cnv-120030212. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Exercise increases average longevity of female rats despite increased food intake and no growth retardation. J Gerontol Biol Sci. 1993;48:B97–B100. doi: 10.1093/geronj/48.3.b97. [DOI] [PubMed] [Google Scholar]
- Jacobs EJ, Rodriguez C, Mondul AM, Connell CJ, Henley SJ, Calle EE, Thun MJ. A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence. J Natl Cancer Inst. 2005;97:975–980. doi: 10.1093/jnci/dji173. [DOI] [PubMed] [Google Scholar]
- Johnson TW, Anderson KE, Lazovich D, Folsom AR. Association of aspirin and nonsteroidal anti-inflammatory drug use with breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:1586–1591. [PubMed] [Google Scholar]
- Li BH, Ma XF, Wang Y, Tian WX. Structure-activity relationship of polyphenols that inhibit Fatty Acid synthase. J Biochem (Tokyo) 2005;138:679–685. doi: 10.1093/jb/mvi171. [DOI] [PubMed] [Google Scholar]
- Lim SS, Gaziano TA, Gakidou E, Reddy KS, Farzadfar F, Lozano R, Rodgers A. Prevention of cardiovascular disease in high-risk individuals in low-income and middle-income countries: health effects and costs. Lancet. 2007;370:2054–2062. doi: 10.1016/S0140-6736(07)61699-7. [DOI] [PubMed] [Google Scholar]
- McDonald RW, Bunjobpon W, Liu T, Fessler S, Pardo OE, Freer IK, Glaser M, Seckl MJ, Robins DJ. Synthesis and anticancer activity of nordihydroguaiaretic acid (NDGA) and analogues. Anticancer Drug Des. 2001;16:261–270. [PubMed] [Google Scholar]
- Manev H, Uz T, Sugaya K, Qu T. Putative role of neuronal 5-lipoxygenase in an aging brain. FASEB J. 2000;14:1464–9. doi: 10.1096/fj.14.10.1464. [DOI] [PubMed] [Google Scholar]
- Miller RA. Genetics of increased longevity and retarded aging in mice. In: Masoro EJ, Austad SN, editors. Handbook of the Biology of Aging. Academic Press; San Diego, CA: 2001. pp. 369–395. [Google Scholar]
- Miller RA. Extending life: scientific prospects and political obstacles. Milbank Quarterly. 2002;80:155–174. doi: 10.1111/1468-0009.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Strong R. An aging interventions testing program: study design and interim report. Aging Cell. 2007;6:565–575. doi: 10.1111/j.1474-9726.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- Miquel J, Fleming J, Economos AC. Antioxidants, metabolic rate and aging in Drosophila. Archives of Gerontology & Geriatrics. 1982;1(2):159–65. doi: 10.1016/0167-4943(82)90016-4. [DOI] [PubMed] [Google Scholar]
- Nakae D, Kishida H, Enami T, Konishi Y, Hensley KL, Floyd RA, Kotake Y. Effects of phenyl N-tert-butyl nitrone and its derivatives on the early phase of hepatocarcinogenesis in rats fed a choline-deficient, L-amino acid-defined diet. Cancer Sci. 2003;94:26–31. doi: 10.1111/j.1349-7006.2003.tb01347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nony PA, Kennett SB, Glasgow WC, Olden K, Roberts JD. 15SLipoxygenase- 2 mediates arachidonic acid-stimulated adhesion of human breast carcinoma cells through the activation of TAK1, MKK6, and p38 MAPK. J Bio l Chem. 2005;280:31413–31419. doi: 10.1074/jbc.M500418200. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Carnes BA, Cassel C. In search of Methuselah: estimating the upper limits to human longevity. Science. 1990;250:634–640. doi: 10.1126/science.2237414. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Perry D, Miller RA, Butler RN. In pursuit of the longevity dividend. The Scientist. 2006;20:28–35. [Google Scholar]
- Ongini E, Bolla M. Nitric-oxide based nonsteroidal anti-inflammatory agents. Drug Discovery Today: Therapeutic Strategies. 2006;3:395–400. [Google Scholar]
- Park Y, Pariza MW. Lipoxygenase inhibitors inhibit heparin-releasable lipoprotein lipase activity in 3T3-L1 adipocytes and enhance body fat reduction in mice by conjugated linoleic acid. Biochim Biophys Acta. 2001;1534:27–33. doi: 10.1016/s1388-1981(01)00171-8. [DOI] [PubMed] [Google Scholar]
- Peters LL, Robledo RF, Bult CJ, Churchill GA, Paigen BJ, Svenson KL. The mouse as a model for human biology: a resource guide for complex trait analysis. Nat Rev Genet. 2007;8:58–69. doi: 10.1038/nrg2025. [DOI] [PubMed] [Google Scholar]
- Petkov PM, Graber JH, Churchill GA, DiPetrillo K, King BL, Paigen K. Evidence of a large-scale functional organization of Mammalian chromosomes. PLoS Biol. 2007;5:e127. doi: 10.1371/journal.pbio.0050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu T, Manev R, Manev H. 5-Lipoxygenase (5-LOX) promoter polymorphism in patients with early-onset and late-onset Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2001;13:304–30. doi: 10.1176/jnp.13.2.304. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Brignetti D, Luo J, Khandwala A, Reaven GM. Effect of masoprocol on carbohydrate and lipid metabolism in a rat model of Type II diabetes. Diabetologia. 1999;42:102–106. doi: 10.1007/s001250051121. [DOI] [PubMed] [Google Scholar]
- Richie JP, Jr, Mills BJ, Lang CA. Dietary nordihydroguaiaretic acid increases the life span of the mosquito. Proceedings of the Society for Experimental Biology & Medicine. 1986;183(1):81–5. doi: 10.3181/00379727-183-42389. [DOI] [PubMed] [Google Scholar]
- Shishido Y, Furushiro M, Hashimoto S, Yokokura T. Effect of nordihydroguaiaretic acid on behavioral impairment and neuronal cell death after forebrain ischemia. Pharmacol Biochem Behav. 2001;69:469–474. doi: 10.1016/s0091-3057(01)00572-x. [DOI] [PubMed] [Google Scholar]
- Schneider EL, Miller RA. Anti-Aging Interventions. In: Tallis R, Fillit H, Brockelhurst JC, editors. Brockelhurst’s Textbook of Geriatric Medicine. New York: Churchill Livingstone; 1998. pp. 193–199. [Google Scholar]
- Schneider EL, Reed JD., Jr Life extension. New Engl J Med. 1985;312:1159–1168. doi: 10.1056/NEJM198505023121805. [DOI] [PubMed] [Google Scholar]
- Shi X, Min D, Zigang D, Fei C, Jiangping Y, Suwei W, Stephen S, Leonard Vince C, Val V. Antioxidant properties of aspirin: Characterization of the ability of aspirin to inhibit silica-induced lipid peroxidation, DNA damage, NF-+¦B activation, and TNF-+¦ production. Molecular and Cellular Biochemistry. 1999;199:93–102. doi: 10.1023/a:1006934612368. [DOI] [PubMed] [Google Scholar]
- Smith JW, Al-Khamees O, Costall B, Naylor RJ, Smythe JW. Chronic aspirin ingestion improves spatial learning in adult and aged rats. Pharmacol Biochem Behav. 2002;71:233–238. doi: 10.1016/s0091-3057(01)00675-x. [DOI] [PubMed] [Google Scholar]
- Stark LA, Reid K, Sansom OJ, Din FV, Guichard S, Mayer I, Jodrell DI, Clarke AR, Dunlop MG. Aspirin activates the NF-kappaB signalling pathway and induces apoptosis in intestinal neoplasia in two in vivo models of human colorectal cancer. Carcinogenesis. 2007;28:968–976. doi: 10.1093/carcin/bgl220. [DOI] [PubMed] [Google Scholar]
- Svenson KL, Von SR, Magnani PA, Suetin HR, Paigen B, Naggert JK, Li R, Churchill GA, Peters LL. Multiple trait measurements in 43 inbred mouse strains capture the phenotypic diversity characteristic of human populations. J Appl Physiol. 2007;102:2369–2378. doi: 10.1152/japplphysiol.01077.2006. [DOI] [PubMed] [Google Scholar]
- Tuttle RS, Yager J, Northrup N. Age and the antihypertensive effect of aspirin in rats. Br J Pharmacol. 1988;94:755–758. doi: 10.1111/j.1476-5381.1988.tb11585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane SJ. Aspirin and other anti-inflammatory drugs. Thorax. 2000;55:3S–9. doi: 10.1136/thorax.55.suppl_2.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Li Q, Redden DT, Weindruch R, Allison DB. Statistical methods for testing effects on “maximum lifespan”. Mech Ageing Dev. 2004;125:629–632. doi: 10.1016/j.mad.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Warner HR, Ingram D, Miller RA, Nadon NL, Richardson AG. Program for testing biological interventions to promote healthy aging. Mechanisms of Ageing & Development. 2000;115:199–207. doi: 10.1016/s0047-6374(00)00118-4. [DOI] [PubMed] [Google Scholar]
- Weissmann G. Aspirin. Scientific American. 1991;264:84–90. doi: 10.1038/scientificamerican0191-84. [DOI] [PubMed] [Google Scholar]
- West M, Mhatre M, Ceballos A, Floyd RA, Grammas P, Gabbita SP, Hamdheydari L, Mai T, Mou S, Pye QN, Stewart C, West S, Williamson KS, Zemlan F, Hensley K. The arachidonic acid 5-lipoxygenase inhibitor nordihydroguaiaretic acid inhibits tumor necrosis factor alpha activation of microglia and extends survival of G93A-SOD1 transgenic mice. Journal of Neurochemistry. 2004;91(1):133–43. doi: 10.1111/j.1471-4159.2004.02700.x. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans.[erratum appears in Nature. 2004 Sep 2;431(7004):107] Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Yerman T, Gan WQ, Sin DD. The influence of gender on the effects of aspirin in preventing myocardial infarction. BMC Med. 2007;5:29–35. doi: 10.1186/1741-7015-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngren JF, Gable K, Penaranda C, Maddux BA, Zavodovskaya M, Lobo M, Campbell M, Kerner J, Goldfine ID. Nordihydroguaiaretic acid (NDGA) inhibits the IGF-1 and c-erbB2/HER2/neu receptors and suppresses growth in breast cancer cells. Breast Cancer Res Treat. 2005;94:37–46. doi: 10.1007/s10549-005-6939-z. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Pahlmark K, Smith ML, Siesjo BK. Delayed treatment with the spin trap alpha-phenyl-N-tert-butyl nitrone (PBN) reduces infarct size following transient middle cerebral artery occlusion in rats. Acta Physiol Scand. 1994;152:349–350. doi: 10.1111/j.1748-1716.1994.tb09816.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.