Abstract
There is a strong clinical relationship between stress and stress-related disorders and the incidence of alcohol abuse and alcoholism, and this relationship appears to be partly genetic in origin. There are marked strain differences in ethanol (EtOH)-related behaviors and reactivity to stress, but little investigation of the interaction between the two. The present study assessed the effects of chronic exposure to swim stress on EtOH-related behavior in three common inbred strains of mice, C57BL/6J, DBA/2J and BALB/cByJ. After establishing baseline (10%) EtOH self-administration in a two-bottle free choice test, mice were exposed to daily swim stress for 14 consecutive days and EtOH consumption measured as a percent of baseline both during stress and for 10 days afterwards. A separate experiment examined the effects of 14 days of swim stress on sensitivity to the sedative/hypnotic effects of an acute injection of 4 g/kg EtOH. Results showed that stress produced a marked and prolonged decrease in EtOH consumption in DBA/2J and BALB/cByJ, but not C57BL/6J mice. By contrast, stress increased sensitivity to the sedative/hypnotic effects of EtOH across all 3 strains. These findings demonstrate that chronic swim stress produces reductions in EtOH self-administration in a strain-dependent manner, and that these effects may be restricted to low-consuming strains. Present data also indicate a dissociation between effects of this stressor on EtOH self-administration and sensitivity to EtOH’s sedative/hypnotic effects. In conclusion, strain differences, that are likely in large part genetic in nature, modify the effects of this stressor on EtOH’s effects in a behavior-specific manner.
Keywords: mouse, strain, ethanol, sedation, gene, drinking, swim stress, preference, two-bottle choice
There is a strong epidemiological link between stress-related psychiatric disorders and alcoholism. Individuals suffering from depression or anxiety disorders, conditions often associated with stress, are more likely to abuse alcohol and become alcoholic, and tend to respond less well to therapeutic interventions [8, 9, 29, 54]. Moreover, a history of adverse life events positively correlates with increased rates of alcoholism; although, interestingly, a proportion of individuals become abstinent following stress [19, 35, 46, 51]. These clinic data lend support to the influential hypothesis that stress represent a significant risk factor for alcoholism [12, 28].
Recent evidence demonstrates that individuals at risk for alcoholism exhibit abnormal responses to stress. Subjects that are family history positive (FHP) for alcoholism exhibit lesser sensitivity to certain behavioral effects of EtOH than family history negatives (FHN), due to increased acute functional tolerance to the drug’s effects [42, 52]. Furthermore, some studies find that FHP’s have exaggerated autonomic and hypothalamic-pituitary-adrenal (HPA)-axis activation in response to stress or EtOH challenge, as compared to FHN’s [13, 17, 18, 22, 24, 31, 39, 43, 58, 63]. Taken together these data suggest that individual differences in stress reactivity are associated with differences in sensitivity to EtOH’s behavioral effects and vulnerability to alcoholism. Such individual differences are likely affected by multiple factors, including genetics.
In this context, there is a major genetic component to the risk for both stress-related disorders [27] and alcoholism [23], while genetically distinct strains of rats and mice differ in their responsivity either to stress and/or to EtOH’s behavioral effects. For example, inbred mouse strains differ from one another in the locomotor-stimulant, sedative/hypnotic, hypothermic, ataxic, and withdrawal-related effects of EtOH [14]. Inbred strains also differ in their propensity to consume EtOH, with large differences evident between the (preferring) C57BL/6J and (avoiding) DBA/2J strains in the two-bottle choice test [2, 50]. Moreover, a number of studies have found marked differences in stress-related behaviors and stress-responsivity between inbred strains. Of note, on some, but not all, assays for anxiety and depression-related behaviors and stress-responsivity, the C57BL/6J strain scores low relative to certain other strains such as BALB/cByJ [3, 15, 36, 56]. We recently reported that repeated exposure (14-days) to forced swim stress in the C57BL/6J inbred strain increased sensitivity to the sedative/hypnotic and hypothermic effects of EtOH [5]. However, the same stress regime did not alter voluntary EtOH self-administration in a two-bottle free choice test.
The aim of the present study was to compare the effects of stress on sensitivity to acute EtOH intoxication and on voluntary EtOH consumption. C57BL/6J, DBA/2J and BALB/cByJ mice were exposed to chronic swim stress [5] and twenty-four hours later tested for the sedative/hypnotic effects of an acute dose of EtOH (4 g/kg) previously shown to increase sensitivity to this measure in C57BL/6J [5]. In a separate cohort, EtOH self-administration was measured in a two-bottle free choice paradigm prior to, during chronic swim stress, and after the cessation of stress.
Subjects were male C57BL/6J, DBA/2J and BALB/cByJ mice obtained from The Jackson Laboratory (Bar Harbor, ME). For each experiment, all three strains were delivered in a single shipment and were housed side-by-side in groups of 2–4 (except for the 2-bottle choice experiment) in a temperature- and humidity-controlled vivarium under a 12 h light/dark cycle (lights on 0600 h). Mice were 8–12 weeks old at the start of testing. The number of mice used in each experiment is shown in the corresponding Figures legends. All experimental procedures were approved by the National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee, and followed the National Institute of Health guidelines outlined in ‘Using Animals in Intramural Research.’
Voluntary EtOH consumption was measured using a 2-bottle choice paradigm as previously described [6]. EtOH-naïve mice were individually-housed in ‘Space Saver’ cages (Model 1145T, Tecniplast, Buguggiate, Italy) with lids fitted for 2 fluid bottles (Model 1145T482SUDB--Polysulfone cage-top to accommodate 2 water bottles (top flow design; Tecniplast, Buguggiate, Italy)). Two bottles, 1 containing 10% v/v EtOH in water and the other containing tap water, were available throughout the experiment. After a 14-day pre-stress baseline period, mice were exposed to daily swim stress for 14 days followed by a 10-day post-stress period. Mice were stressed by being placed in a transparent Plexiglas cylinder (20 cm diameter) filled halfway with water (24 ±1°C) for 10 min, as previously described [5, 25]. We have previously shown that this stress regime produces a progressive, significant increase in immobility (a putative measure of depression-related behavior) across days, and significant activation of the hypothalamic-pituitary-adrenal axis in C57BL/6J mice [5]. Every 2 days the EtOH and water were changed and the left/right position of the bottles was switched daily to control for any side bias. Food was available ad libitum. EtOH and water consumption were measured every 24 hr correcting for evaporation and spillage (i.e., the average loss of fluid measured from bottles in an empty chamber was subtracted from the amounts ‘consumed’) and body weight was measured every 48 hr. These data were used to calculate EtOH consumption and percent EtOH preference (EtOH consumption/total fluid consumption × 100). The effect of stress and strain on EtOH consumption and EtOH preference was analyzed both during stress and the 10 days after the cessation of stress using multifactorial analysis of variance (ANOVA) and Newman-Keuls post-hoc tests where appropriate.
EtOH-induced sedation/hypnosis was assessed as previously described [6]. EtOH-naïve mice were given 0 or 14 consecutive days of stress as above and tested 24 hr later for sleep time responses to 4 g/kg EtOH (200 proof, prepared in 0.9% v/v saline to produce a 20% v/v ethanol solution). Immediately after injection, mice were placed in the supine position in Plexiglas ‘V’shaped chambers. The time from EtOH injection to recovery of the righting reflex (turning onto all 4 paws twice in 30 sec after initial self-righting) was defined as sleep time. The effect of stress and strain on sleep time duration was analyzed using multifactoral ANOVA and Newman-Keuls post-hoc tests where appropriate.
Baseline levels of EtOH self-administration differed significantly across strains. There was a significant effect of strain for EtOH consumption (F2,23=56.30, P<.01), due to lesser consumption in BALB/cByJ (1.33 ±0.09 g/kg/day) and DBA/2J (1.34 ±0.19 g/kg/day) than C57BL/6J (5.59 ±0.52 g/kg/day). There was a significant effect of strain for EtOH preference (F2,23=77.31, P<.01), due to EtOH avoidance in BALB/cByJ (12.00 ±0.88 %) and DBA/2J (7.24 ±1.11 %), and EtOH preference in C57BL/6J (67.40 ±6.21 %). Because of these marked strain differences in baseline consumption and preference, the effect of stress was analyzed as a percent change from pre-stress baseline (e.g., (during stress consumption/pre-stress consumption)*100)
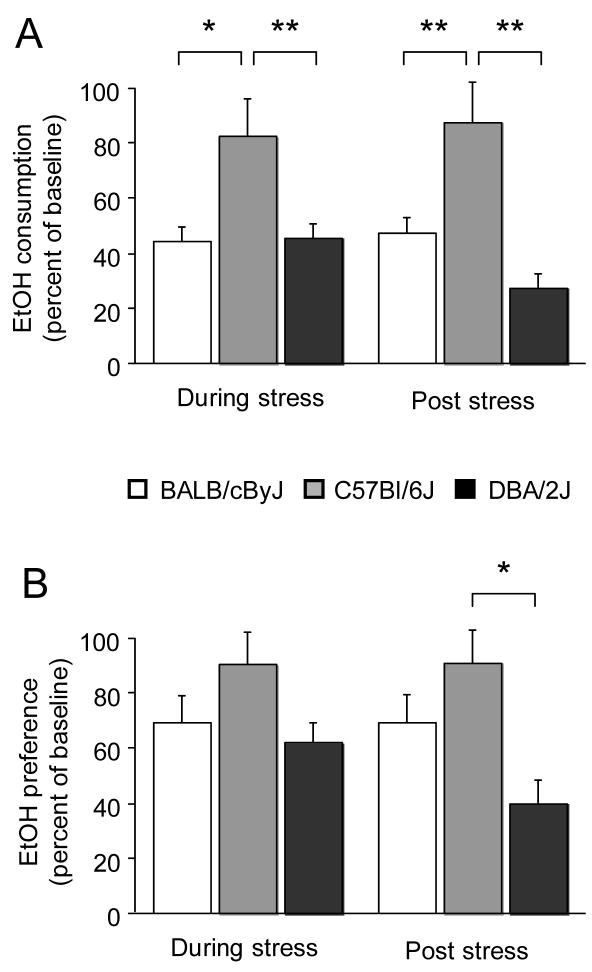
There was a significant effect of strain for (baseline normalized) EtOH consumption during stress (F2,23=6.09, P<.01) and post-stress (F2,23=9.29, P<.01). There was also a significant effect of strain for the percent change in (baseline normalized) EtOH preference post-stress (F2,23=5.12, P<.05) but not during stress. Post-hoc analysis showed significantly less (baseline-normalized) EtOH consumption in DBA/2J and BALB/cByJ than C57BL/6J both during stress and 10 days after stress (Figure 1A). Strains did not differ in EtOH preference during stress while, post-stress, DBA/2J showed significantly less (baseline-normalized) EtOH preference than C57BL/6J (Figure 1B).
Figure 1.
Effects of stress on voluntary EtOH self-administration in C57BL/6J, DBA/2J and BALB/cByJ mice. (A) DBA/2J and BALB/cByJ showed significantly less (baseline-normalized) EtOH consumption than C57BL/6J both during stress and 10 days after stress. (B) There were no strain differences in EtOH preference during stress, while DBA/2J showed significantly less (baseline-normalized) EtOH preference than C57BL/6J post-stress. n=7–10/strain. Data in Figures 1–2 are means ± SEM. **P<.01, *P<.05.
In the experiment assessing the sedative/hypnotic effects of stress, there was a significant effect of stress (F1,58=31.28, P<.01) and of strain (F2,58=3.98, P<.05) but no stress × strain interaction for sleep time. Post-hoc analysis of the effect of stress, collapsed across strain, showed that stress significantly increased sleep time (P<.01) (Figure 2). Post-hoc analysis of the effects of strain, collapsed across stress condition, showed that no strain significantly differed from another (Figure 2).
Figure 2.
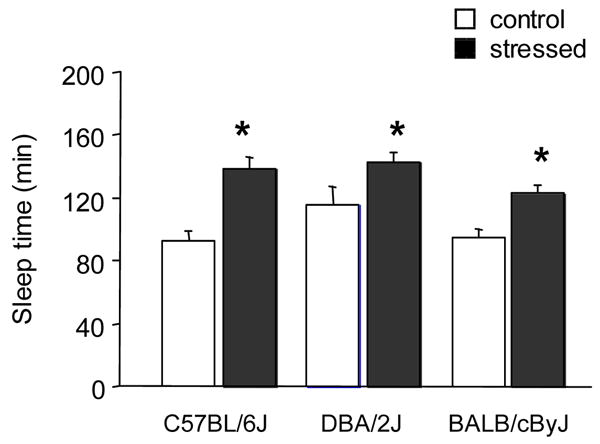
Effects of stress on sensitivity to the sedative/hypnotic effects of EtOH in C57BL/6J, DBA/2J and BALB/cByJ mice. Exposure to 14 days of swim stress produced a significant increase in sleep time responses to injection with 4.0 g/kg EtOH injection, irrespective of strain. n=9–12/strain/stress condition. *P<.05 vs. no stress control.
Previous studies have found inconsistent effects of various stress procedures on EtOH self-administration in rats and mice [1, 10, 32, 33, 41, 47, 49, 55, 59–62]. Similarly, work examining the effects of stress on sensitivity to the behavioral effects of acute EtOH challenge has not produced a clear consensus [7, 16, 20, 21, 26, 34, 45, 48]. Supporting the notion that strain differences may be one factor contributing to this variability, the main finding of the present study was that repeated exposure to swim stress produced alterations in EtOH-related behaviors that were to some extent dependent upon the inbred mouse studied.
Present data replicate the recent finding that swim stress failed to alter two-bottle EtOH consumption in C57BL/6J mice [5]. By contrast, DBA/2J and BALB/cByJ mice exposed to the same stress showed a marked reduction in EtOH self-administration as compared to C57BL/6J. Strikingly, the effect of stress in DBA/2J and BALB/cByJ was evident during exposure to stress as well as during a ten day period following the cessation of stress, demonstrating that the mechanisms underlying these effects were long-lasting. While reduced EtOH consumption as a result of stress was accompanied by a decrease in EtOH preference in DBA/2J and BALB/cByJ, the effects on stress were less clear cut and not significantly different between strains with the exception of the post-stress decrease in DBA/2J. This indicates that swim stress partially reduced water intake as well as EtOH intake, as previously reported in mice (129 × C57BL/6J mixed genetic background mice) [53].
The observation that stress had a robust affect on EtOH self-administration in DBA/2J and BALB/cByJ mice was particularly striking given the low (relative to C57BL/6J) pre-stress baseline levels of self-administration in these strains. Low baseline EtOH drinking in DBA/2J is consistent with numerous previous studies [2, 50], and while there are fewer studies of EtOH consumption in BALB/cByJ mice, preference scores of <15% for a 10% v/v EtOH concentration is comparable to data recently reported by Blizard and colleagues in this strain (albeit in adolescent mice) [4]. These data discount the possibility that the previously reported lack of stress-induced changes in C57BL/6J was due to a floor effect [5]. Rather, they show that stress can produce reductions in EtOH self-administration in mice even under conditions of low baseline intake. Taken together, one interpretation of these data is that the relatively strong propensity to self-administer EtOH in C57BL/6J renders this strain resilient to stress-induced reductions in EtOH consumption, while already low-consuming strains are highly sensitive to these effects.
In the context of the aforementioned link between stress and increased rates of alcoholism, the observation that swim stress produced a decrease rather than an increase in EtOH self-administration in DBA/2J and BALB/cByJ would seem counterintuitive. Although it is pertinent to note that stress is associated with abstinence in a certain percentage of individuals [19, 35, 46, 51], the most parsimonious conclusion to draw from the present data is that either the stress paradigm and/or the measure of EtOH self-administration employed do not provide a good model for stress-related increases in risk for alcoholism. Further studies will be needed to identify such a model in mice. This might entail the use of an alterative stressor (e.g., restraint, footshock) that has less of a physical component than swimming given evidence that physical exercise can itself affect EtOH-related behaviors in C57BL/6J mice [38]) and strain differences in swimming [37]. Another potentially fruitful approach would be to test stress effects on EtOH drinking after enforced abstinence, as successfully employed in rats [30].
Present data fail to support the hypothesis that strain differences in stress-reactivity would predict sensitivity to stress-induced changes in EtOH behaviors. Thus, although the relatively stress-insensitive C57BL/6J strain [3, 36, 56] did not respond to stress while ‘stress-sensitive’ BALB/cByJ did, DBA/2J mice also responded, even though this strain is typically similar to C57BL/6J on measures of anxiety-like behavior and stress-reactivity [3, 36, 56]. While we did not conduct a strain comparison of glucocorticoid response profiles to our stress paradigm, these data suggest that the relationship between strain differences in stress reactivity and two-bottle EtOH self-administration is likely to be complex and modulated by multiple factors.
Increased sensitivity to the sedative/hypnotic effects of EtOH is one factor associated with lesser EtOH drinking in mice [11, 40, 44, 57]. Confirming our recent finding [5], chronic swim stress significantly potentiated sleep time responses to 4 g/kg EtOH measured twenty-four hours after the final stressor in C57BL/6J mice. Here we show that these effects generalize to the DBA/2J and BALB/cByJ strains, and thereby provide further support for the finding that exposure to chronic swim stress potentiates this measure of sensitivity to the acute intoxicating effects of a relatively high dose of EtOH. However, present findings do not support a simple inverse relationship between the effects of this stressor on increased EtOH sensitivity and decreased EtOH drinking, with stress increasing sensitivity to sedation/hypnosis in all strains, but decreasing EtOH drinking in DBA/2J and BALB/cByJ but not C57BL/6J. Additional work will be necessary to more fully elucidate the nature of the interrelationship between stress and various EtOH-related behaviors.
In summary, the present study found that exposure to chronic swim stress produced a reduction in voluntary EtOH self-administration that was maintained for at least ten days after stress. These effects occurred in the DBA/2J and BALB/cByJ, but not C57BL/6J, inbred mouse strains. The same stress regimen produced a significant potentiation of the sedative/hypnotic effects of an acute dose of 4 g/kg EtOH in all three strains tested. These data demonstrate that strain differences, that are likely in large part genetic in nature, modify the effects of this stressor on EtOH’s effects in a behavior-specific manner.
Acknowledgments
We would like to thank Kathryn M. Hefner for technical assistance. Research supported by the Intramural Research Program of the National Institute of Alcohol Abuse and Alcoholism (Z01-AA000411).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acosta GB, Otero Losada ME, Rubio MC. Area-dependent changes in GABAergic function after acute and chronic cold stress. Neurosci Lett. 1993;154:175–178. doi: 10.1016/0304-3940(93)90200-5. [DOI] [PubMed] [Google Scholar]
- 2.Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- 3.Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- 4.Blizard DA, Vandenbergh DJ, Jefferson AL, Chatlos CD, Vogler GP, McClearn GE. Effects of periadolescent ethanol exposure on alcohol preference in two BALB substrains. Alcohol. 2004;34:177–185. doi: 10.1016/j.alcohol.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Boyce-Rustay JM, Cameron HA, Holmes A. Chronic swim stress alters sensitivity to acute behavioral effects of ethanol in mice. Physiol Behav. 2007;91:77–86. doi: 10.1016/j.physbeh.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 6.Boyce-Rustay JM, Holmes A. Ethanol-related behaviors in NMDA receptor NR2A KO mice. Psychopharmacology (Berl) 2006;187:455–466. doi: 10.1007/s00213-006-0448-6. [DOI] [PubMed] [Google Scholar]
- 7.Brown PL, Hurley C, Repucci N, Drugan RC. Behavioral analysis of stress controllability effects in a new swim stress paradigm. Pharmacol Biochem Behav. 2001;68:263–272. doi: 10.1016/s0091-3057(00)00460-3. [DOI] [PubMed] [Google Scholar]
- 8.Burns L, Teesson M. Alcohol use disorders comorbid with anxiety, depression and drug use disorders. Findings from the Australian National Survey of Mental Health and Well Being. Drug Alcohol Depend. 2002;68:299–307. doi: 10.1016/s0376-8716(02)00220-x. [DOI] [PubMed] [Google Scholar]
- 9.Burns L, Teesson M, O’Neill K. The impact of comorbid anxiety and depression on alcohol treatment outcomes. Addiction. 2005;100:787–796. doi: 10.1111/j.1360-0443.2005.001069.x. [DOI] [PubMed] [Google Scholar]
- 10.Chester JA, Blose AM, Zweifel M, Froehlich JC. Effects of stress on alcohol consumption in rats selectively bred for high or low alcohol drinking. Alcohol Clin Exp Res. 2004;28:385–393. doi: 10.1097/01.alc.0000117830.54371.7a. [DOI] [PubMed] [Google Scholar]
- 11.Church AC, Fuller JL, Dann L. Alcohol intake in selected lines of mice: importance of sex and genotype. J Comp Physiol Psychol. 1979;93:242–246. doi: 10.1037/h0077563. [DOI] [PubMed] [Google Scholar]
- 12.Conger JJ. Alcoholism: theory, problem and challenge. II. Reinforcement theory and the dynamics of alcoholism. Q J Stud Alcohol. 1956;17:296–305. [PubMed] [Google Scholar]
- 13.Conrod PJ, Pihl RO, Vassileva J. Differential sensitivity to alcohol reinforcement in groups of men at risk for distinct alcoholism subtypes. Alcohol Clin Exp Res. 1998;22:585–597. doi: 10.1111/j.1530-0277.1998.tb04297.x. [DOI] [PubMed] [Google Scholar]
- 14.Crabbe JC, Metten P, Cameron AJ, Wahlsten D. An analysis of the genetics of alcohol intoxication in inbred mice. Neurosci Biobehav Rev. 2005;28:785–802. doi: 10.1016/j.neubiorev.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham CL, Bischof LL. Stress and ethanol-induced hypothermia. Physiol Behav. 1987;40:377–382. doi: 10.1016/0031-9384(87)90064-3. [DOI] [PubMed] [Google Scholar]
- 17.Dai X, Thavundayil J, Gianoulakis C. Differences in the responses of the pituitary beta-endorphin and cardiovascular system to ethanol and stress as a function of family history. Alcohol Clin Exp Res. 2002;26:1171–1180. doi: 10.1097/01.ALC.0000024129.32956.38. [DOI] [PubMed] [Google Scholar]
- 18.Dai X, Thavundayil J, Gianoulakis C. Response of the hypothalamic-pituitary-adrenal axis to stress in the absence and presence of ethanol in subjects at high and low risk of alcoholism. Neuropsychopharmacology. 2002;27:442–452. doi: 10.1016/S0893-133X(02)00308-1. [DOI] [PubMed] [Google Scholar]
- 19.Dawson DA, Grant BF, Ruan WJ. The association between stress and drinking: modifying effects of gender and vulnerability. Alcohol Alcohol. 2005;40:453–460. doi: 10.1093/alcalc/agh176. [DOI] [PubMed] [Google Scholar]
- 20.Drugan RC, Coyle TS, Healy DJ, Chen S. Stress controllability influences the ataxic properties of both ethanol and midazolam in the rat. Behav Neurosci. 1996;110:360–367. doi: 10.1037//0735-7044.110.2.360. [DOI] [PubMed] [Google Scholar]
- 21.Drugan RC, Scher DM, Sarabanchong V, Guglielmi A, Meng I, Chang J, Bloom K, Sylvia S, Holmes P. Controllability and duration of stress alter central nervous system depressant-induced sleep time in rats. Behav Neurosci. 1992;106:682–689. doi: 10.1037//0735-7044.106.4.682. [DOI] [PubMed] [Google Scholar]
- 22.Finn PR, Zeitouni NC, Pihl RO. Effects of alcohol on psychophysiological hyperreactivity to nonaversive and aversive stimuli in men at high risk for alcoholism. J Abnorm Psychol. 1990;99:79–85. doi: 10.1037//0021-843x.99.1.79. [DOI] [PubMed] [Google Scholar]
- 23.Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Avila CA, Oncken C, Van Kirk J, Wand G, Kranzler HR. Adrenocorticotropin and cortisol responses to a naloxone challenge and risk of alcoholism. Biol Psychiatry. 2002;51:652–658. doi: 10.1016/s0006-3223(01)01334-8. [DOI] [PubMed] [Google Scholar]
- 25.Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones BC, Connell JM, Erwin VG. Isolate housing alters ethanol sensitivity in long-sleep and short-sleep mice. Pharmacol Biochem Behav. 1990;35:469–472. doi: 10.1016/0091-3057(90)90187-m. [DOI] [PubMed] [Google Scholar]
- 27.Kendler KS. Twin studies of psychiatric illness: an update. Arch Gen Psychiatry. 2001;58:1005–1014. doi: 10.1001/archpsyc.58.11.1005. [DOI] [PubMed] [Google Scholar]
- 28.Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- 29.Kranzler HR, Del Boca FK, Rounsaville BJ. Comorbid psychiatric diagnosis predicts three-year outcomes in alcoholics: a posttreatment natural history study. J Stud Alcohol. 1996;57:619–626. doi: 10.15288/jsa.1996.57.619. [DOI] [PubMed] [Google Scholar]
- 30.Le A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol Ther. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 31.Levenson RW, Oyama ON, Meek PS. Greater reinforcement from alcohol for those at risk: parental risk, personality risk, and sex. J Abnorm Psychol. 1987;96:242–253. doi: 10.1037//0021-843x.96.3.242. [DOI] [PubMed] [Google Scholar]
- 32.Little HJ, O’Callaghan MJ, Butterworth AR, Wilson J, Cole J, Watson WP. Low alcohol preference among the “high alcohol preference” C57 strain of mice; preference increased by saline injections. Psychopharmacology (Berl) 1999;147:182–189. doi: 10.1007/s002130051159. [DOI] [PubMed] [Google Scholar]
- 33.Lynch WJ, Kushner MG, Rawleigh JM, Fiszdon J, Carroll ME. The effects of restraint stress on voluntary ethanol consumption in rats. Exp Clin Psychopharmacol. 1999;7:318–323. doi: 10.1037//1064-1297.7.4.318. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto K, Ojima K, Watanabe H. Central corticotropin-releasing factor and benzodiazepine receptor systems are involved in the social isolation stress-induced decrease in ethanol sleep in mice. Brain Res. 1997;753:318–321. doi: 10.1016/s0006-8993(97)00080-2. [DOI] [PubMed] [Google Scholar]
- 35.Mertens JR, Moos RH, Brennan PL. Alcohol consumption, life context, and coping predict mortality among late-middle-aged drinkers and former drinkers. Alcohol Clin Exp Res. 1996;20:313–319. doi: 10.1111/j.1530-0277.1996.tb01645.x. [DOI] [PubMed] [Google Scholar]
- 36.Millstein RA, Holmes A Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2006 doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Mollenauer S, Bryson R, Speck C, Chamberlin JR. Effects of exercise on ethanol-induced hypothermia and loss of righting response in C57BL/6J mice. Pharmacol Biochem Behav. 1992;43:285–290. doi: 10.1016/0091-3057(92)90669-7. [DOI] [PubMed] [Google Scholar]
- 39.Munro CA, Oswald LM, Weerts EM, McCaul ME, Wand GS. Hormone responses to social stress in abstinent alcohol-dependent subjects and social drinkers with no history of alcohol dependence. Alcohol Clin Exp Res. 2005;29:1133–1138. doi: 10.1097/01.alc.0000172459.71517.05. [DOI] [PubMed] [Google Scholar]
- 40.Naassila M, Ledent C, Daoust M. Low ethanol sensitivity and increased ethanol consumption in mice lacking adenosine A2A receptors. J Neurosci. 2002;22:10487–10493. doi: 10.1523/JNEUROSCI.22-23-10487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nanni G, Scheggi S, Leggio B, Grappi S, Masi F, Rauggi R, De Montis MG. Acquisition of an appetitive behavior prevents development of stress-induced neurochemical modifications in rat nucleus accumbens. J Neurosci Res. 2003;73:573–580. doi: 10.1002/jnr.10685. [DOI] [PubMed] [Google Scholar]
- 42.Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- 43.Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81:339–358. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Palmer AA, Sharpe AL, Burkhart-Kasch S, McKinnon CS, Coste SC, Stenzel-Poore MP, Phillips TJ. Corticotropin-releasing factor overexpression decreases ethanol drinking and increases sensitivity to the sedative effects of ethanol. Psychopharmacology (Berl) 2004;176:386–397. doi: 10.1007/s00213-004-1896-5. [DOI] [PubMed] [Google Scholar]
- 45.Peris J, Cunningham CL. Handling-induced enhancement of alcohol’s acute physiological effects. Life Sci. 1986;38:273–279. doi: 10.1016/0024-3205(86)90313-9. [DOI] [PubMed] [Google Scholar]
- 46.Pohorecky LA. Stress and alcohol interaction: an update of human research. Alcohol Clin Exp Res. 1991;15:438–459. doi: 10.1111/j.1530-0277.1991.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 47.Racz I, Bilkei-Gorzo A, Toth ZE, Michel K, Palkovits M, Zimmer A. A critical role for the cannabinoid CB1 receptors in alcohol dependence and stress-stimulated ethanol drinking. J Neurosci. 2003;23:2453–2458. doi: 10.1523/JNEUROSCI.23-06-02453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts AJ, Lessov CN, Phillips TJ. Critical role for glucocorticoid receptors in stress- and ethanol-induced locomotor sensitization. J Pharmacol Exp Ther. 1995;275:790–797. [PubMed] [Google Scholar]
- 49.Rockman GE, Hall A, Glavin GB. Effects of restraint stress on voluntary ethanol intake and ulcer proliferation in rats. Pharmacol Biochem Behav. 1986;25:1083–1087. doi: 10.1016/0091-3057(86)90089-4. [DOI] [PubMed] [Google Scholar]
- 50.Rodgers DA, McClearn G. Mouse strain differences in preference for various concentrations of alcohol. Q J Stud Alcohol. 1962;23:26–33. [PubMed] [Google Scholar]
- 51.Romelsjo A, Lazarus NB, Kaplan GA, Cohen RD. The relationship between stressful life situations and changes in alcohol consumption in a general population sample. Br J Addict. 1991;86:157–169. doi: 10.1111/j.1360-0443.1991.tb01766.x. [DOI] [PubMed] [Google Scholar]
- 52.Schuckit MA, Smith TL, Kalmijn J. The search for genes contributing to the low level of response to alcohol: patterns of findings across studies. Alcohol Clin Exp Res. 2004;28:1449–1458. doi: 10.1097/01.alc.0000141637.01925.f6. [DOI] [PubMed] [Google Scholar]
- 53.Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgansberger W, Wurst W, Holsboer F, Spanagel R. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2002;296:931–933. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- 54.Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Sunanda Rao BS, Raju TR. Restraint stress-induced alterations in the levels of biogenic amines, amino acids, and AChE activity in the hippocampus. Neurochem Res. 2000;25:1547–1552. doi: 10.1023/a:1026606201069. [DOI] [PubMed] [Google Scholar]
- 56.Tannenbaum B, Anisman H. Impact of chronic intermittent challenges in stressor-susceptible and resilient strains of mice. Biol Psychiatry. 2003;53:292–303. doi: 10.1016/s0006-3223(02)01487-7. [DOI] [PubMed] [Google Scholar]
- 57.Thiele TE, Willis B, Stadler J, Reynolds JG, Bernstein IL, McKnight GS. High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. J Neurosci. 2000;20:RC75. doi: 10.1523/JNEUROSCI.20-10-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uhart M, Oswald L, McCaul ME, Chong R, Wand GS. Hormonal Responses to Psychological Stress and Family History of Alcoholism. Neuropsychopharmacology. 2006;31:2255–2263. doi: 10.1038/sj.npp.1301063. [DOI] [PubMed] [Google Scholar]
- 59.van Erp AM, Miczek KA. Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol Behav. 2001;73:301–311. doi: 10.1016/s0031-9384(01)00458-9. [DOI] [PubMed] [Google Scholar]
- 60.van Erp AM, Tachi N, Miczek KA. Short or continuous social stress: suppression of continuously available ethanol intake in subordinate rats. Behav Pharmacol. 2001;12:335–342. doi: 10.1097/00008877-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Vengeliene V, Siegmund S, Singer MV, Sinclair JD, Li TK, Spanagel R. A comparative study on alcohol-preferring rat lines: effects of deprivation and stress phases on voluntary alcohol intake. Alcohol Clin Exp Res. 2003;27:1048–1054. doi: 10.1097/01.ALC.0000075829.81211.0C. [DOI] [PubMed] [Google Scholar]
- 62.Vengeliene V, Vollmayr B, Henn FA, Spanagel R. Voluntary alcohol intake in two rat lines selectively bred for learned helpless and non-helpless behavior. Psychopharmacology (Berl) 2005;178:125–132. doi: 10.1007/s00213-004-2013-5. [DOI] [PubMed] [Google Scholar]
- 63.Zimmermann U, Spring K, Kunz-Ebrecht SR, Uhr M, Wittchen HU, Holsboer F. Effect of ethanol on hypothalamic-pituitary-adrenal system response to psychosocial stress in sons of alcohol-dependent fathers. Neuropsychopharmacology. 2004;29:1156–1165. doi: 10.1038/sj.npp.1300395. [DOI] [PubMed] [Google Scholar]