Abstract
Purpose
Accelerated partial breast irradiation (PBI) is a new treatment paradigm for early-stage breast cancer. Although PBI may lead to higher local recurrence rates, it may be cost-effective because of better tolerability and lower cost. We aimed to determine the incremental cost-effectiveness of PBI compared to whole breast radiation therapy (WBRT) for estrogen-receptor positive postmenopausal women treated for early-stage breast cancer.
Methods and Materials
We developed a Markov model to describe health states in the 15 years following radiotherapy (RT) for early-stage breast cancer. External beam (EB) and MammoSite (MS) PBI were considered and assumed to be equally effective but carried different costs. Patients received tamoxifen but not chemotherapy. Utilities, recurrence risks, and costs were adapted from the literature; the baseline utility for no disease after RT was set at 0.92. Probabilistic sensitivity analyses were performed to model uncertainty in the PBI hazard ratio (HR), recurrence pattern, and patient utilities. Costs (in 2004 $US) and QALYs were discounted at 3%/year.
Results
The incremental cost-effectiveness ratio for WBRT compared to EB-PBI was $630,000/QALY; WBRT strongly dominated MS-PBI. One-way sensitivity analysis found the results were sensitive to PBI HR, recurrence pattern, baseline recurrence risk, and NED-PBI utility values. Probabilistic sensitivity showed that EB-PBI was the most cost-effective technique over a wide range of assumptions and societal willingness-to-pay values.
Conclusions
EB-PBI was the most cost-effective strategy for postmenopausal women with early-stage breast cancer. Unless the quality-of-life after MS-PBI proves to be superior, it is unlikely to be cost-effective.
Keywords: Breast cancer, cost-effectiveness analysis, partial breast irradiation, whole breast radiotherapy
Introduction
Accelerated partial breast irradiation (PBI) is a relatively new radiotherapy technique that treats only part of the breast over a dramatically shorter time than traditional whole breast radiotherapy (WBRT). A typical course of PBI involves twice daily treatments over 4–5 days, versus 5–7 weeks of daily treatment incurred with WBRT. Therefore, advantages of PBI include improved convenience and morbidity, and preliminary evidence suggests similar control rates.1 Currently, three modalities of PBI are commonly used – external beam (EB-PBI), interstitial brachytherapy (IB-PBI), and MammoSite brachytherapy (MS-PBI) – with vastly different costs. Interstitial brachytherapy delivers radiation through surgically-placed catheters, while MammoSite brachytherapy delivers radiation via a small sphere placed into the surgical cavity. The two brachytherapy techniques are nearly twice as expensive as EB-PBI.2 Thus far no difference in local control rates has been shown among these three.
Prior cost-effectiveness analyses of conventional breast or chest wall radiotherapy have generally shown that its incremental cost-effectiveness ratio is below the societal willingness-to-pay threshold of $50,000 when used for adjuvant treatment of ductal carcinoma in situ,3 early-stage breast cancer,4 and high-risk post-mastectomy breast cancer.5 The cost-effectiveness of PBI has not yet been examined, but given the prevalence of breast cancer, the results from such an analysis may have broad implications.
We have previously published a decision analysis comparing PBI with whole breast radiotherapy for early-stage breast cancer.6 Our baseline analysis revealed that even with the assumption of equivalent quality-of-life following PBI and conventional breast radiotherapy (which may in fact underestimate the quality-of-life benefit afforded by PBI), PBI is only a marginally inferior treatment strategy. Given the near equivalence in outcomes of the two approaches, the significantly lower cost of external beam PBI raises the possibility for EB-PBI to be cost-effective compared with whole breast radiotherapy. We therefore performed a cost-effectiveness analysis comparing EB-PBI and MS-PBI to whole breast radiotherapy for adjuvant treatment of early-stage breast cancer.
Materials and Methods
Decision model
We designed a Markov model to simulate the clinical history of 55 year-old women with estrogen-receptor (ER) positive, stage I breast cancer (pT1N0 by the tumor-node-metastasis system7) after lumpectomy. Markov simulation allows hypothetical cohorts of women to transition between different health states in fixed increments of time.8
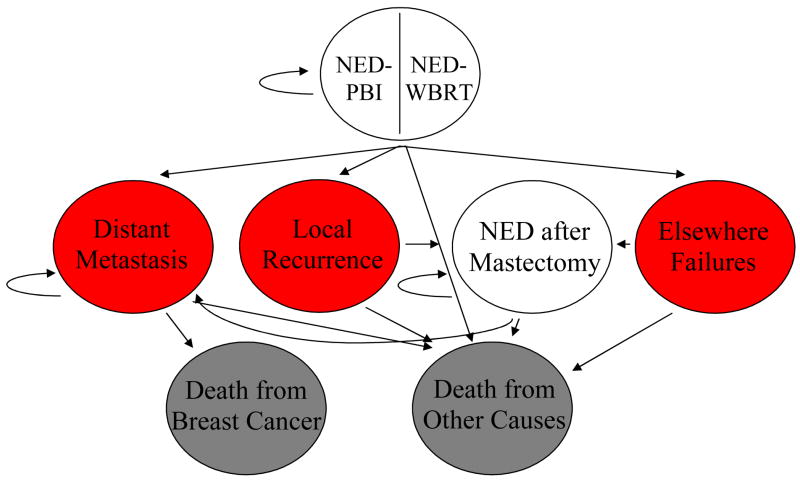
Patients begin in the model in the well state (no evidence of disease, NED), having received treatment with either WBRT or PBI. These initial states are titled NED-PBI or NED-WBRT (Figure 1). From these states, a patient can transition in the model to the disease states, including local recurrence (LR), defined as recurrence in the same quadrant of the breast, elsewhere failures (EF), defined as recurrence in a different quadrant of the breast, well after salvage mastectomy (NED-MTX), and distant metastasis (DM). Possible terminal states into which a woman could transition are death from breast cancer and death from other causes.
Figure 1.
Markov model. NED = no evidence of disease. The utility of the NED health states may vary according to modality of radiotherapy (WBRT vs. PBI) and to time since RT (0–5 years versus 6–15 years).
A 15-year time horizon was used in the model, due to evidence that improved local control manifests a survival benefit over this time period.9 The model was created and analyzed using Data TreeAge Pro (Williamstown, MA).
Model assumptions and data
Assumptions and data sources for the decision model are described elsewhere and are shown in Table 1.6 Costs for PBI regimens were taken from a societal-perspective economic analysis published by Suh et al.2
Table 1.
Probabilities, hazard ratios, and utilities used in this study. Years refers to the years in which the probabilities apply. Tamoxifen hazard ratios are relative to no hormonal therapy. PBI hazard ratios for local recurrence (LR) and elsewhere failure (EF) are relative to WBRT. Costs are expressed in US2004$.
| Event | Years | Baseline Value | Range Studied | Reference |
|---|---|---|---|---|
|
| ||||
| Probabilities | ||||
|
| ||||
| NED ⇒ IBTR | 0–5 | 6.7% (5-year) | 2%–20% | 9 |
| 6–10 | 3.3% (5-year) | 2–5% | ||
| 11–15 | 0 | -- | ||
|
| ||||
| IBTR ⇒ Metastasis | 0–15 | 20% (10-year) | 10–40% | 20 |
|
| ||||
| NED ⇒ Metastasis | 0–10 | 11% (10-year) | 5–20% | 21 |
| 11–15 | 6.7% (5-year) | -- | ||
|
| ||||
| Metastasis ⇒ Death | 0–15 | 32.8% (1-year) | -- | 22 |
|
| ||||
| % failures that are local | 0–5 | 93% | 50–93% | 16 |
| 6–10 | 62% | 50–75% | ||
| 11–15 | N/A | -- | ||
|
| ||||
| Hazard ratios | ||||
|
| ||||
| Tamoxifen Distant HR | 0–5 | 0.64 | -- | 9 |
| 6–10 | 0.69 | -- | ||
| 11–15 | 1 | -- | ||
|
| ||||
| Tamoxifen IBTR HR | 0–5 | 0.47 | -- | 9 |
| 6–10 | 0.69 | -- | ||
| 11–15 | 1 | -- | ||
|
| ||||
| PBI LR HR | 0–5 | 1 | 0.75–2.0 | -- |
| 6–10 | 1 | 0.75–2.0 | ||
| 11–15 | 1 | -- | ||
|
| ||||
| PBI EF HR | 0–5 | 3 | 2–4 | -- |
| 6–10 | 1 | 1–3 | ||
| 11–15 | 1 | -- | ||
|
| ||||
| Utilities | ||||
|
| ||||
| NED-WBRT | 0–15 | 0.92 | -- | 10 |
|
| ||||
| NED-PBI | 0–5 | 0.92 | 0.92–0.96 | -- |
| 6–15 | 0.92 | 0.88–0.96 | ||
|
| ||||
| Well after salvage mastectomy | 0–15 | 0.82 | -- | 10 |
|
| ||||
| Distant metastasis | 0–15 | 0.62 | -- | 5 |
|
| ||||
| Disutility of tamoxifen | 0–10 | 0.05 | 0–0.05 | 12 |
|
| ||||
| Costs | ||||
|
| ||||
| PBI-External Beam | 0 | $7,900 | -- | 2 |
|
| ||||
| PBI-MammoSite | 0 | $18,800 | -- | 2 |
|
| ||||
| WBRT | 0 | $11,190 | -- | 2 |
|
| ||||
| Treatment of local recurrence | Total | $20,879 | -- | 23 |
|
| ||||
| Treatment of metastases | Total | $13,627 | -- | 23 |
Utilities
For the well state following treatment with either whole or partial breast radiotherapy we used the same utility value of 0.92 (on a scale of 0–1, 0=being dead and 1=optimal health), elicited by Hayman et al. from women treated for early-stage breast cancer (using standard gamble technique).10 There are no published data on utilities for the health states during and immediately after either type of radiotherapy. Others have suggested that PBI has health-related quality of life benefits in several domains.11 We used an equivalent utility value for the time period following either intervention, and tested this value in sensitivity analyses. Because there may be a later incidence of fibrosis and skin changes associated with shorter course, high dose per fraction therapy, we included a later stage post-PBI utility (for years 6–15 following radiotherapy) which we adjusted separately from the early stage period of the first 5 years in sensitivity analyses. Utilities for health states after salvage mastectomy and distant metastases were extracted from published literature.5 The effect of tamoxifen on utility has been estimated in terms of a disutility of 0.05,12 which we used in our model with the assumption of an additive relationship among utilities (and we tested this inclusion in sensitivity analyses).
Sensitivity analyses
Sensitivity analyses allow the modeler to adjust the assumptions of the model and measure the effect of these adjustments on the model results. We performed these analyses over a wide range of assumptions for all parameters that are listed with a range in Table 1. We performed a series of two-way sensitivity analyses to determine the optimal treatment outcome when the PBI hazard ratio (HR) and either the 5-year breast recurrence rate or pattern was varied. A two-way sensitivity analysis was also performed to study the influence of varying early and late PBI utilities. This latter analysis represents the potential for an initial quality-of-life benefit from PBI, followed by a quality-of-life decrement from late fibrosis. These parameters were chosen for analysis because they are the key clinical features of PBI that may be significantly different than conventional radiotherapy. Each sensitivity analysis was performed twice, once with the societal willingness-to-pay (WTP) assumed to be $50,000/QALY, and once with the WTP assumed to be $150,000/QALY, a value much higher than commonly used.
Probabilistic sensitivity analyses
Probabilistic sensitivity analysis is a technique in which unknown parameters are assigned a probability distribution according to prior data, and Monte Carlo simulations are performed in which the unknown parameter(s) are drawn from those distributions. If the resulting cost-effectiveness of the more effective technology (i.e. WBRT) is less than the societal WTP, then it is considered cost-effective. This result is graphed on an acceptability curve, which reports the percentage of trials in which a strategy is cost-effective at a series of societal willingness-to-pay values.
We performed 3 probabilistic sensitivity analyses. The first solely drew from a distribution of PBI hazard ratios (i.e., hazard for LR with respect to EB-PBI) with an expected value of 1.25, modeled by a gamma distribution with an alpha of 25 and a lambda of 20. The second analysis drew on both the distribution of PBI HR and a distribution of recurrence patterns, in which the expected value of the fraction of IBTRs that were local was 79%; this distribution was chosen to emulate the published data on failure patterns.13–16 That distribution was created such that there was a 5% chance that 50–60% of the IBTRs were local, a 17% chance that 60–70% of the IBTRs were local, a 27% chance that 70–80% of the IBTRs were local, a 37% chance that 80–90% of the IBTRs were local, and a 15% chance that 90–100% of the IBTRs were local. Finally, the third probabilistic sensitivity analysis included the prior two distributions, plus normal distributions on the NED-PBI utilities for the first five years and the last ten years. The mean value for the early utility was 0.93, and the mean value of the late utility was 0.92, with a standard deviation of 0.01 for both.
Discounting
Both costs and QALYs were discounted at an annual rate of 3%.
Results
Model validity
The external validity of our model was assessed by comparing the results from our model with those of another, external prediction tool. Our model’s 10-year overall survival and breast cancer mortality were compared with the predicted results from Adjuvant!Online. For a 55 year-old woman with lymph-node negative, ER-positive breast cancer, our model predicted a 10-year overall survival of 86.3% and breast cancer mortality of 5.5%, while Adjuvant!Online estimated overall survival of 85.1% and breast cancer mortality of 5.4%. This comparison suggests that our model is comparable to Adjuvant!Online for predicting clinical outcomes.
Base case incremental cost-effectiveness
The incremental cost-effectiveness ratio (ICER) for whole breast radiotherapy was $630,000/QALY when compared with external beam-PBI. However, because whole breast radiotherapy is more effective and less expensive than MammoSite-PBI, whole breast radiotherapy strongly dominated MS-PBI. Since utility values may vary from person-to-person, we repeated the analysis using life-years as the effectiveness measure. As would be expected, the ICER rose to $1.6 million/LY.
Sensitivity analyses
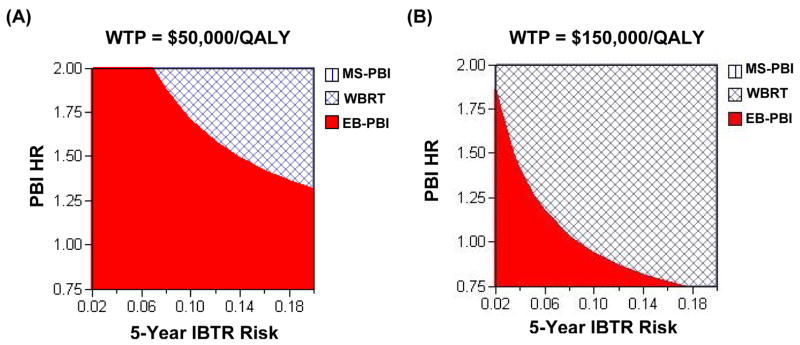
As the risk of ipsilateral breast tumor recurrence (HR for PBI) decreased and PBI became more effective, external beam-PBI became an increasingly cost-effective strategy specifically because of the reduced risk of recurrence. Figure 2 displays the results of this two-way sensitivity analysis. At a societal willingness-to-pay (WTP) of $50,000/QALY (Figure 2a), PBI was cost-effective over the vast majority of combinations of PBI hazard ratios for recurrence. When the WTP was increased to $150,000/QALY (Figure 2b), whole breast radiotherapy was more likely to be the preferred treatment, because its increased cost was overcome by its reduced breast tumor recurrence risk.
Figure 2.
Two-way sensitivity analysis of early (i.e. 5-year) IBTR risk and PBI hazard ratio (PBI HR). The X-axis represents the 5-year breast recurrence rate, and the Y-axis is the PBI HR. The hatched area represents conditions (i.e. a 5-year IBTR risk and a given PBI HR) under which WBRT is the cost-effective treatment (i.e. incremental cost-effectiveness ratio < $50,000/QALY). The remaining solid area reflects conditions under which PBI is the cost-effective strategy. As the IBTR risk increases and PBI becomes less effective, WBRT is more likely to be the cost-effective strategy. Panel (A) assumes the societal willingness-to-pay is $50,000/QALY. Panel (B) assumes the societal willingness-to-pay is $150,000/QALY.
Furthermore, when there was an increase in the fraction of ipsilateral breast tumor recurrences that were local, EB-PBI was more likely to be the cost-effective treatment compared with whole breast radiotherapy (Figure 3). In fact, the incremental cost-effectiveness ratio of WBRT was above $50,000/QALY (Figure 3a) but below $150,000/QALY (Figure 3b) over a wide range of PBI hazard ratios and recurrence patterns.
Figure 3.
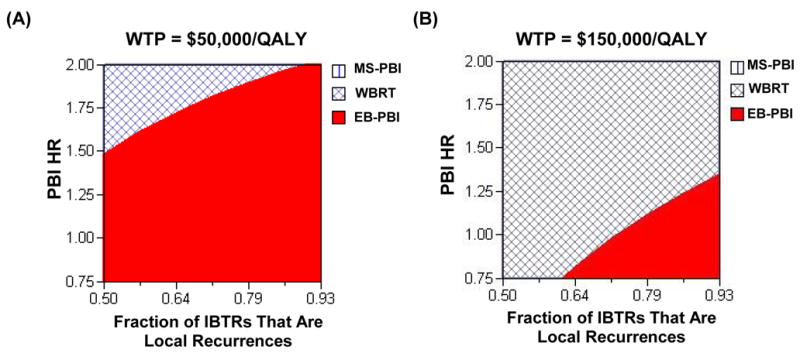
Two-way sensitivity analysis of fraction of early IBTRs (i.e. years 0–5) that are local recurrences and PBI hazard ratio (PBI HR). The X-axis represents the fraction of breast recurrences that are local recurrences instead of elsewhere failures, and the Y-axis is the PBI HR. The hatched area represents conditions (i.e. a given fraction of IBTRs that are LR and a given PBI HR) under which WBRT is the cost-effective treatment. The remaining solid area reflects conditions under which PBI is the optimal strategy. As the fraction of IBTRs that are local increases and PBI becomes more effective, PBI is more likely to be the cost-effective strategy. Panel (A) assumes the societal willingness-to-pay is $50,000/QALY. Panel (B) assumes the societal willingness-to-pay is $150,000/QALY.
Our previous analysis showed that this model is very sensitive to the early and late utilities for partial breast irradiation. This sensitivity is seen in Figure 4, which shows that if the NED utilities are equivalent in the first five years, even if the late NED-PBI utility is less than the late NED-WBRT utility, PBI could be the preferred treatment strategy; that is, PBI may still be cost-effective even if there is unexpected late toxicity.
Figure 4.
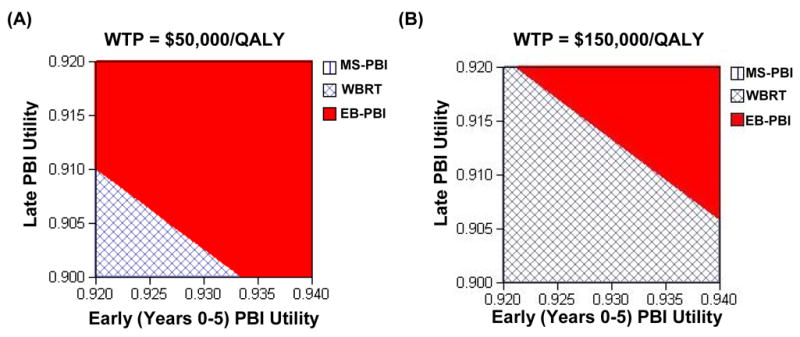
Two-way sensitivity analysis of the NED-PBI utility values in the first 5 years after RT (X-axis) and in years 6–15 (Y-axis). The hatched area represents a combination of utility values for which WBRT is the cost-effective treatment, while the remaining solid area represents the combination of utility values for which PBI is the cost-effective therapy. Panel (A) assumes the societal willingness-to-pay is $50,000/QALY. Panel (B) assumes the societal willingness-to-pay is $150,000/QALY.
MammoSite-PBI was not cost-effective at either WTP threshold, owing to its equivalent efficacy to EB-PBI but with a significantly higher cost.
Probabilistic sensitivity analyses
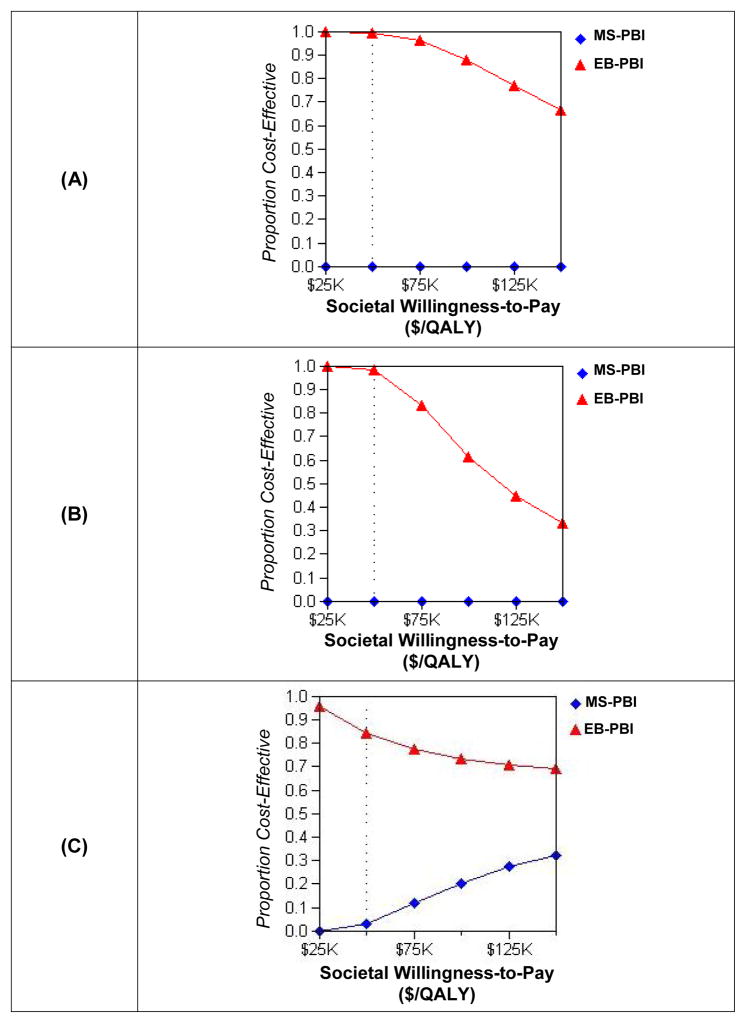
Three probabilistic sensitivity analyses were performed, revealing that external beam PBI is cost-effective over a wide range of willingness-to-pay thresholds and model parameters, whereas MammoSite PBI was rarely cost-effective over those same assumptions. The results are displayed in Figure 5. When only the PBI hazard ratio was varied (normal distribution around 1.25), external beam PBI was cost-effective for the vast majority of the trials (Figure 5a). In contrast, MS-PBI was not cost-effective at any of the WTP thresholds.
Figure 5.
Acceptability curves. These curves represent the proportion of trials drawn on a hypothetical distribution of unknown parameters that result in an incremental cost-effectiveness ratio for PBI less than the societal willingness-to-pay. Panel (A): Only unknown parameter is the PBI hazard ratio (HR). Panel (B): Unknown parameters are PBI HR (mean 1.25) and fraction of IBTRs that are local (mean 0.75). Panel (C): Unknown parameters are PBI HR, fraction of IBTRs that are local, and NED-PBI utilities for both the first five years (mean 0.93) and the following ten years (mean 0.92).
When both the PBI HR and the fraction of recurrences that were local were varied, EB-PBI was still cost-effective for the majority of the trials through a WTP of $100,000/QALY, beyond which WBRT was more often cost-effective. MS-PBI was still not cost-effective at any of the WTP thresholds.
Finally, when PBI HR, fraction of IBTRs that were local, and NED-PBI utilities were varied, EB-PBI was again the cost-effective strategy for the vast majority of the trials. Of note, under these conditions, MS-PBI was cost-effective compared to WBRT in some trials, due to the small chance that the well-state utilities after PBI were significantly higher than after whole breast RT, mitigating its increased cost.
Discussion
The growing interest in accelerated partial breast irradiation has largely been driven by considerations of cost and patient convenience.1 Although the convenience benefit of receiving 1 week (most deliver treatment over 5 days) of radiotherapy instead of 6 weeks are self-evident, there have been no assessments of the potential cost-effectiveness. Our analysis has shown that external beam PBI is cost-effective compared with whole breast radiotherapy over a wide range of reasonable assumptions. In contrast, MammoSite-based treatment is not cost-effective over those same parameters, both in deterministic and probabilistic sensitivity analyses over a wide range of WTP thresholds.
This study has several limitations. First, any cost-effectiveness analysis is based on key assumptions which may dramatically affect its results. In this analysis, we based our clinical parameters (i.e. PBI efficacy and patterns of recurrence) on single-arm phase I and II data. The NSABP is currently performing a large phase III randomized trial comparing WBRT and PBI, but the results will not be available for 5–10 years. This trial allows physician to deliver PBI using external beam, MammoSite, or interstitial brachytherapy techniques. Our analysis is therefore based on the best existing data, though limited in scope and external validity. In addition, some outcomes of PBI are not well established, such as late-stage morbidity after PBI, so our analysis included assumptions about these occurrences and parameters. In such cases we attempted to bias our results toward the null hypothesis (i.e., towards whole breast radiation therapy), minimizing differences between the modalities. One must also consider that utility values and quality-of-life measures vary from individual to individual, and thus the QALY is a subjective measure of efficacy, although QALYs are the standard metric used in cost-effectiveness analyses. Finally, model-based cost-effectiveness analyses such as the present study are inherently retrospective, and as described above, they depend on published data and deterministic and probabilistic sensitivity analyses to generalize the results. Prospective cost-effectiveness trials are based on actual patient data and more accurately reflect real-world conditions, but the time and expense associated with their completion will likely preclude such a study of this question.
Nevertheless, sensitivity analyses vastly improve the validity of cost-effectiveness analyses by testing disease models over a wide range of assumptions and values. We tested our model over a spectrum of plausible assumptions, including the underlying recurrence rate, pattern of recurrences, PBI efficacy, and utility values, and consistently found external beam PBI to be cost-effective compared with whole breast radiotherapy across a wide range of societal willingness-to-pay levels. We conducted further probabilistic sensitivity analyses in which we specifically biased our model against PBI by assuming its mean HR for local recurrences was 1.25 and allowing a relatively high rate of elsewhere failures, but we found our results to be robust. Finally, we also assumed that salvage lumpectomy is equally unlikely after PBI and WBRT, although repeat breast-conserving therapy may be more feasible after PBI,17 and we still found that external beam PBI was the preferred treatment over a broad spectrum of WTP values. In sum, we feel confident in our results despite the assumptions inherent in modeling disease outcomes using limited available data.
Although the final results of NSABP B-39 will be invaluable in comparing the local recurrence rates and patterns of failure after PBI and WBRT, a definitive cost-effectiveness analysis will require more data than that provided by the trial. Additional research must be performed to evaluate the health states following PBI and WBRT, including the possibility of late fibrosis and telangiecstasias. Quality-of-life during and following EB-PBI and MS-PBI need to be compared to determine if the higher cost of MammoSite brachytherapy is an improvement over its external beam counterpart.
In addition, hypofractionated schemes for whole breast radiotherapy may have similar efficacy to treatment delivered with standard fractions at a lower total cost, and this “Canadian fractionation” regimen may also be cost-effective in comparison to standard whole breast radiation treatment.
Our results have broad implications, both within the United States and beyond. Breast cancer is a common disease, afflicting roughly 200,000 American women in 2007.18 Our analysis studied post-menopausal women with breast cancer who account for the majority of new diagnoses and deaths from the disease.19 Given this high prevalence, resource-conscious health care systems may want to consider cost-effectiveness when deciding on appropriate adjuvant therapies for early-stage breast cancer.
In a cost-conscious environment, our results suggest that external beam PBI should be given preference over whole breast radiotherapy to the appropriate patient. Unless the costs associated with MammoSite are significantly lowered, or quality-of-life after its use is superior to its alternatives, MammoSite brachytherapy is not likely to be a cost-effective technique. As local control rates continue to improve with more effective radiation delivery and systemic therapy, quality-of-life concerns become increasingly important, and additional investigation is crucial to characterize the long-term sequelae of both conventional whole breast and accelerated partial breast radiotherapy.
Acknowledgments
Research Support: Supported in part by a post-doctoral fellowship from the Agency for Healthcare Research and Quality (D.J.S.), a grant from the National Institutes of Health (1K07 CA118629 to R.S.P.) and American Society Clinical Oncology (Career Development Award to R.S.P.).
Footnotes
Statement of originality: This work is completely original and was presented at the Annual Meeting of the American Society for Clinical Oncology, June 2008.
Conflict of Interest Statement: No author has any conflict of interest with this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Sanders ME, Scroggins T, Ampil FL, et al. Accelerated partial breast irradiation in early-stage breast cancer. J Clin Oncol. 2007;25:996–1002. doi: 10.1200/JCO.2006.09.7436. [DOI] [PubMed] [Google Scholar]
- 2.Suh WW, Pierce LJ, Vicini FA, et al. A cost comparison analysis of partial versus whole-breast irradiation after breast-conserving surgery for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2005;62:790–796. doi: 10.1016/j.ijrobp.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 3.Suh WW, Hillner BE, Pierce LJ, et al. Cost-effectiveness of radiation therapy following conservative surgery for ductal carcinoma in situ of the breast. Int J Radiat Oncol Biol Phys. 2005;61:1054–1061. doi: 10.1016/j.ijrobp.2004.07.713. [DOI] [PubMed] [Google Scholar]
- 4.Hayman JA, Hillner BE, Harris JR, et al. Cost-effectiveness of routine radiation therapy following conservative surgery for early-stage breast cancer. J Clin Oncol. 1998;16:1022–1029. doi: 10.1200/JCO.1998.16.3.1022. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Glick HA, Hayman JA, et al. Decision-analytic model and cost-effectiveness evaluation of postmastectomy radiation therapy in high-risk premenopausal breast cancer patients. J Clin Oncol. 2002;20:2713–2725. doi: 10.1200/JCO.2002.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Sher DJ, Wittenberg E, Taghian AG, et al. Partial Breast Irradiation Versus Whole Breast Radiotherapy for Early-Stage Breast Cancer: A Decision Analysis. Int J Radiat Oncol Biol Phys. 2007 doi: 10.1016/j.ijrobp.2007.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singletary SE, Allred C, Ashley P, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20:3628–3636. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–338. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 9.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 10.Hayman JA, Fairclough DL, Harris JR, et al. Patient preferences concerning the trade-off between the risks and benefits of routine radiation therapy after conservative surgery for early-stage breast cancer. J Clin Oncol. 1997;15:1252–1260. doi: 10.1200/JCO.1997.15.3.1252. [DOI] [PubMed] [Google Scholar]
- 11.Flynn C, Wallace M, Balasubramaniam M, et al. An assessment of quality of life for patients undergoing radiotherapy with whole breast irradiation compared to accelerated partial breast irradiation; American Society for Therapeutic Radiology and Oncology Annual Meeting; Philadelphia, PA. 2006. [Google Scholar]
- 12.Bordeleau L, Rakovitch E, Naimark DM, et al. A comparison of four treatment strategies for ductal carcinoma in situ using decision analysis. Cancer. 2001;92:23–29. doi: 10.1002/1097-0142(20010701)92:1<23::aid-cncr1287>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 13.Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol. 2001;12:997–1003. doi: 10.1023/a:1011136326943. [DOI] [PubMed] [Google Scholar]
- 14.Liljegren G, Holmberg L, Bergh J, et al. 10-Year results after sector resection with or without postoperative radiotherapy for stage I breast cancer: a randomized trial. J Clin Oncol. 1999;17:2326–2333. doi: 10.1200/JCO.1999.17.8.2326. [DOI] [PubMed] [Google Scholar]
- 15.Smith TE, Lee D, Turner BC, et al. True recurrence vs. new primary ipsilateral breast tumor relapse: an analysis of clinical and pathologic differences and their implications in natural history, prognoses, and therapeutic management. Int J Radiat Oncol Biol Phys. 2000;48:1281–1289. doi: 10.1016/s0360-3016(00)01378-x. [DOI] [PubMed] [Google Scholar]
- 16.Krauss DJ, Kestin LL, Mitchell C, et al. Changes in temporal patterns of local failure after breast-conserving therapy and their prognostic implications. Int J Radiat Oncol Biol Phys. 2004;60:731–740. doi: 10.1016/j.ijrobp.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Kuerer HM, Arthur DW, Haffty BG. Repeat breast-conserving surgery for in-breast local breast carcinoma recurrence: the potential role of partial breast irradiation. Cancer. 2004;100:2269–2280. doi: 10.1002/cncr.20257. [DOI] [PubMed] [Google Scholar]
- 18.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy EP, Burns RB, Freund KM, et al. Mammography use, breast cancer stage at diagnosis, and survival among older women. J Am Geriatr Soc. 2000;48:1226–1233. doi: 10.1111/j.1532-5415.2000.tb02595.x. [DOI] [PubMed] [Google Scholar]
- 20.Abner AL, Recht A, Eberlein T, et al. Prognosis following salvage mastectomy for recurrence in the breast after conservative surgery and radiation therapy for early-stage breast cancer. J Clin Oncol. 1993;11:44–48. doi: 10.1200/JCO.1993.11.1.44. [DOI] [PubMed] [Google Scholar]
- 21.Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996;88:1529–1542. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 22.Surveillance, Epidemiology, End-Results. SEER Cancer Statistics Review 1973–1998. National Cancer Institute Section 4-Breast. 1998:1973–1998. [Google Scholar]
- 23.Stokes MEDTELM, et al. Value Health. 2008. Ten-Year Survival and Cost Following Breast Cancer Recurrence: Estimate from SEER-Medicare Data. Published online:8. [DOI] [PubMed] [Google Scholar]