Abstract
Aims
Compared with other non-steroid anti-inflammatory drugs (NSAIDs), aspirin is not correlated to hypertension. It has been shown that aspirin has unique vasodilator action in vivo, offering an explanation for the unique blood pressure effect of aspirin. In the present study, we investigate the mechanism whereby salicylates (aspirin and sodium salicylate) dilate blood vessels.
Methods and results
Rat aortic or mesenteric arterial rings were used to test the vascular effect of salicylates and other NSAIDs. RhoA translocation and the phosphorylation of MYPT1, the regulatory subunit of myosin light chain phosphatase, were measured by western blot, as evidenced for RhoA/Rho-kinase activation. Salicylates, but not other NSAIDs, relaxed contraction induced by most tested constrictors except for calyculin A, indicating that RhoA/Rho-kinase-mediated calcium sensitization is involved. The involvement of RhoA/Rho kinase in vasodilation by salicylates was confirmed by measurements of RhoA translocation and MYPT1 phosphorylation. The calculated half maximal inhibitory concentration (IC50) of vasodilation was apparently higher than that of cyclooxygenase inhibition, but comparable to that of proline-rich tyrosine kinase 2 (PYK2) inhibition. Over-expression of PYK2 induced RhoA translocation and MYPT1 phosphorylation, and these effects were markedly inhibited by sodium salicylate treatment. Consistent with the ex vitro vascular effects, sodium salicylate acutely decreased blood pressure in spontaneous hypertensive rats but not in Wistar Kyoto rats.
Conclusion
Salicylates dilate blood vessels through inhibiting PYK2-mediated RhoA/Rho-kinase activation and thus lower blood pressure.
KEYWORDS: G proteins, Contractile function, Smooth muscle, Hypertension
1. Introduction
Non-steroid anti-inflammatory drugs (NSAIDs) are some of the most frequently prescribed drugs worldwide that exert their therapeutic effects by the inhibition of cyclooxygenase (COX).1 Epidemiological studies have demonstrated that use of NSAIDs is associated with an increase in blood pressure.2–4 However, although aspirin is the prototype of NSAIDs, use of aspirin does not increase the risk of hypertension,5,6 but actually decreases blood pressure.7,8 The mechanism whereby aspirin has a unique blood pressure remains elusive. It has been shown that high doses of salicylates, including aspirin and sodium salicylate, dilate blood vessels in vivo, probably through direct effect on vascular smooth muscle.1 Vascular tone determines peripheral resistance and thus blood pressure. Therefore, this vasodilator action of aspirin may contribute to its unique blood pressure effect. However, despite considerable interest in the unique blood pressure effect of aspirin, there are few studies investigating the mechanism whereby aspirin dilates blood vessels.
Vascular smooth muscle contraction is determined by the phosphorylation level of the myosin light chain (MLC), which can be achieved through activating MLC kinase (MLCK) and/or inhibiting MLC phosphatase (MLCP).9 MLCK is activated by increased cytoplasmic [Ca2+]. In contrast, regulation of MLCP activity appears to be Ca2+-independent, and a reduction in MLCP activity allows less intracellular Ca2+ to phosphorylate MLC, thus known as Ca2+ sensitization. A number of studies have established that a small G protein, RhoA, and its downstream effecter Rho kinase are critical in Ca2+ sensitization of vascular smooth muscle.9 Their role in blood pressure regulation has also been demonstrated. An enhanced RhoA/Rho-kinase activation in vascular smooth muscle has been shown in various hypertensive animals and human patients.9–13 More importantly, Rho kinase inhibitors decrease blood pressure in hypertensive animals, but not in normotensive animals,14 which further supports a significant role of RhoA/Rho kinase in the pathophysiology of hypertension.
As aspirin inhibits COX similar to other NSAIDs, its unique blood pressure-lowering effect is likely COX-independent. Notably, in addition to COX, aspirin also inhibits a non-receptor tyrosine kinase, proline-rich tyrosine kinase 2 (PYK2), in vascular smooth muscle cells (VSMCs).15 PYK2 is activated by various physiological stimuli including angiotensin II.16 Gene-targeting studies have shown that PYK2 is essential for RhoA activation by chemokines in macrophages.17 PYK2 activity is increased in VSMCs from spontaneous hypertensive rats (SHR),18 and PYK2 knockdown by antisense oligonucleotides significantly abolishes signalling downstream of the angiotensin II AT1 receptor.19 These studies together indicate that PYK2 may modulate RhoA/Rho-kinase-mediated Ca2+ sensitization in vascular smooth muscle. In the present studies, we tested the hypothesis that aspirin relaxes blood vessels by inhibiting PYK2-mediated RhoA/Rho-kinase activation.
2. Methods
2.1. Animals
All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and approved by the Institutional Animal Care and Use Committees at the Medical College of Georgia. Sprague–Dawley rats (male, 12-week-old) were obtained from Harlan. SHRs (male, 12-week-old) and Wistar Kyoto rats (WKY) (male, 12-week-old) were bought from Taconic Farms.
2.2. Cell culture
Primary rat aortic VSMCs were prepared from the thoracic aortas of SD rats as described previously, and maintained in Dulbecco's modified Eagle's medium supplemented with 10% foetal bovine serum (Invitrogen), 100 µg/mL streptomycin, and 100 U/mL penicillin at 37°C under a 95% air/5% CO2 atmosphere. VSMCs with no more than five passages were used in all experiments.
2.3. Constructs
Human PYK2 full-length cDNA was amplified, respectively, from cultured HEK293 by PCR, and inserted into pGEM-T. The inserts were verified by sequencing. To be easily measured, PYK2 was tagged with hemagglutinin (HA) at 5′. Adenoviral vector expressing human PYK2 was generated using Adeno-X™ Expression Systems 2 (Clotech).
2.4. Western blotting
To measure translocation, samples were homogenized as described previously.20 Otherwise, samples were homogenized in radioimmunoprecipitation assay buffer (Upstate). Western blotting was performed using standard techniques with primary antibodies as follows: monoclonal anti-β-actin (Sigma), rabbit anti-PYK2 (Sigma), rabbit anti-PDZ-RhoGEF (Alpha Diagnostics), monoclonal anti-phosphotyrosine (Upstate), monoclonal anti-myc (Roche Applied Sciences), rat anti-HA (Roche Applied Sciences), monoclonal anti-RhoA (BD Biosciences), rabbit anti-phosphoMYPT1 (Santa Cruz), and monoclonal anti-MYPT1 (BD Biosciences).
2.5. Vascular reactivity
Briefly, rats were anaesthetized with pentobarbital sodium, and the thoracic aorta was quickly removed and cleaned in physiological salt solution (PSS) containing (mM): NaCl, 130; NaHCO3, 14.9; KCl, 4.7; KH2PO4, 1.18; MgSO4•7H2O, 1.18; CaCl2•2H2O, 1.56; EDTA, 0.026; glucose, 5.5. The aorta was cut into 2 mm rings, and in some experiments, the endothelium was mechanically removed by gently rubbing the intimal surface with a stainless steel wire. The aortic rings were then mounted in a muscle bath containing PSS at 37°C and bubbled with 95% O2–5% CO2. Isometric force generation was recorded with a Multi Myograph System (Danish Myo Technology A/S, Aarhus, Denmark). A resting tension of 30 mN was imposed on each ring, and the rings were allowed to equilibrate for 1 h.
To test the effect of salicylates on resistance arteries, the mesentery was rapidly excised and placed in an ice-cold PSS. Second-order branches of mesenteric artery (≃2 mm in length with internal diameter ≃150–200 µm) were carefully dissected and mounted as ring preparations on two stainless steel wires. The second-order mesenteric arteries were mounted in an isometric Mulvany–Halpern small-vessel myograph (40 µm diameter; Model 610 M; Danish Myo Technology A/S), and data were acquired by a PowerLab 8/SP data acquisition system (ADInstruments Pty Ltd, Castle Hill, Australia). A resting tension of 3 mN was imposed on the second-order mesenteric arteries, and vessels were equilibrated for 45 min in PSS at 37°C and continuously bubbled with 5% CO2 and 95% O2.
Arterial integrity was assessed first by stimulation of vessels with 80 mM KCl. Endothelium integrity was assessed by measuring the dilatory response to acetylcholine (ACh) (10 µM) in phynelephrine (PE)-contracted vessels (3 µM). The failure of ACh to relax denuded aortic rings was considered proof of endothelium disruption. Some of the experiments were performed in endothelium-denuded arteries to avoid the interference of endothelium-derived vasoactive mediators. In second-order mesenteric arteries, salicylate responses were also evaluated after a 40 min incubation with vehicle or with the NO synthase inhibitor Nω-nitro-l-arginine methyl ester (l-NAME 100 µM) plus indomethacin (10 µM), an inhibitor of prostanoid synthesis.
2.6. Blood pressure
Male SHR and WKY rats (275–325 g, Taconic Farms) were anaesthetized with 50 mg/kg sodium pentobarbital (Nembutal), and atropine sulphate (0.1 mg/kg) was administered to prevent excess airway secretions. Arterial and venous catheters were implanted as described previously.21 Briefly, using aseptic techniques, a laparotomy was performed and a sterile non-occlusive polyvinyl catheter was inserted into the abdominal aorta, distal to the kidneys. Through a left femoral vein incision, a sterile catheter was placed in the vena cava. Both catheters were exteriorized through a subcutaneously implanted stainless steel button. Experiments were performed after 5 days of recovery from surgery.
3. Results
3.1. Salicylates have a vasodilator action in vitro
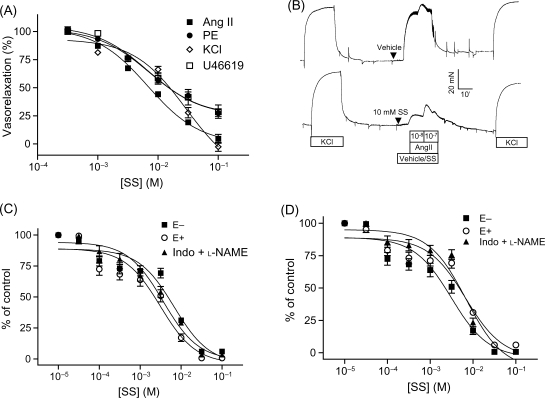
To test whether salicylates relax vascular smooth muscle contraction in vitro, endothelium-denuded rat aortic rings were contracted by various constrictors and then treated with aspirin or sodium salicylate. Results (Supplementary material online, Figure S1) revealed that both sodium salicylate (Figure 1A) and aspirin (Supplementary material online, Figure S1) concentration-dependently relaxed contraction induced by angiotensin II, KCl, PE, and U-46619 (a thromboxane mimic). The restoration of aortic contraction by washing out sodium salicylate (Figure 1B) excluded the possibility that salicylates relaxed vasoconstriction by reducing the viability of VSMCs.
Figure 1.
Salicylates relax smooth muscle contraction in vitro. (A) Endothelium-denuded rat aortic rings were pre-contracted by KCl (120 mM), U-46619 (1 µM), or PE (1 µM), and then treated with various concentrations of sodium salicylate. Results are expressed as percentage of pre-contraction. To test the effect of sodium salicylate on contraction by angiotensin II, endothelium-denuded rat aortic rings were pre-treated with various concentrations of sodium salicylate and then contracted by angiotensin II (100 nM). Results are expressed as percentage of vehicle control. n ≥ 5/group. (B) Endothelium-denuded rat aortic rings were contracted by angiotensin II in the presence of sodium salicylate or vehicle, and then all the chemicals were washed out, following by contraction by KCl (120 mM). A representative recording is presented. n = 5. Mesenteric artery preparations were pre-contracted by PE (1 µM, C) or U-46619 (1 µM, D), and sodium salicylate was added in a cumulative manner. E−, endothelium-denuded; E+, endothelium intact; Indo+l-NAME, indomethacin (10 µM) plus l-NAME (100 µM). n = 4/group.
As the mechanism of vascular tone regulation may vary in different blood vessel beds, and the peripheral resistance and blood pressure are mainly determined by the contractibility of resistance arteries, we examined the effect of sodium salicylate on mesenteric artery tone. Consistent with aortic results, sodium salicylate concentration-dependently relaxed mesenteric arterial contraction induced by PE and U-46619 (Figure 1C and D). Endothelium plays a critical role in the regulation of vascular tone. To confirm that the effect of sodium salicylate on mesenteric arterial contraction is independent of endothelium, before treatment with sodium salicylate, we either removed the endothelium of mesenteric arteries or blocked the endothelial function of mesenteric arteries with l-NAME (NO synthase inhibitor) plus indomethacin (an inhibitor of prostanoid synthesis). Figure 1C and D showed that endothelium removal or treatment with l-NAME plus indomethacin did not change the effect of sodium salicylate on mesenteric arterial contraction induced by PE and U-46619.
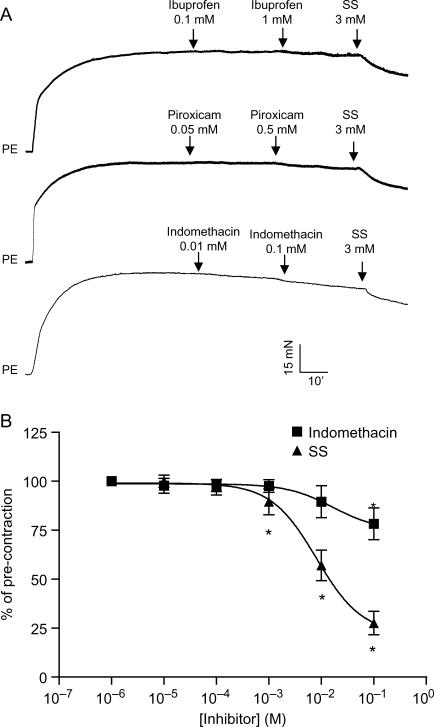
Notably, the calculated IC50s to relax aortic contraction (Table 1) were apparently higher than those reported to inhibit COX,22 indicating that salicylates may COX-independently relax blood vessels. To verify that the vasodilator action of salicylates is COX-independent, we tested the vascular effect of other NSAIDs, including indomethacin, piroxicam, and ibuprofen. None of these compounds relaxed aortic rings, which had been pre-contracted by PE at the highest concentration reported in patient plasma23 (Figure 2A). Moreover, indomethacin, the COX inhibitor with an IC50 lower than those of salicylates,22 only slightly relaxed PE-contracted aortic rings at high concentrations, whereas the same concentration of sodium salicylate markedly relaxed PE-contracted aortic rings (Figure 2B).
Table 1.
IC50 values of salicylates' vasodilator action
| Aspirin (95% CI, mM) | Sodium salicylate (95% CI, mM) | |
|---|---|---|
| Phenylephrine | 2.98–17.84 | 3.72–13.66 |
| KCl | 5.88–45.70 | 4.91–53.33 |
| Angiotensin II | 2.84–16.96 | 3.73–12.20 |
| U-46619 | ND | 3.00–15.08 |
| Calyculin A | ND | 15.72–53.99 |
Endothelium-denuded rat aortic rings were pre-contracted by the indicated vasoconstrictors (except for angiotensin II, in which aortic rings were contracted by angiotensin II in the presence of SS) and then various concentrations of salicylates were added. IC50 values were calculated by non-linear curve fit using the sigmoidal dose–response equation. Results are presented as 95% confidence interval (CI). n ≥ 4.
Figure 2.
Salicylates relax smooth muscle contraction independently of cyclooxygenase inhibition. (A) Endothelium-denuded rat aortic rings were pre-contracted by phynelephrine and then treated with indicated concentration of non-steroid anti-inflammatory drugs. A representative result from two independent experiments was presented. SS, sodium salicylate. (B) Concentration-dependent response of phynelephrine-contracted endothelium-denuded rat aortic rings to indomethacin (n = 3) and sodium salicylate (n = 5). *P < 0.05 vs. pre-contraction; Student's t-test with Bonferroni post hoc test.
3.2. Salicylates relax vasoconstriction by inhibiting RhoA/Rho–kinase pathway
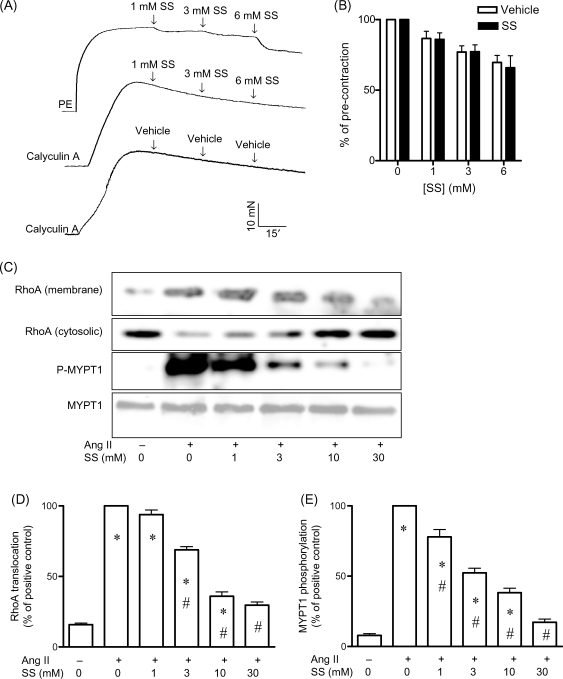
Calyculin A is a compound that causes contraction by inhibiting MLCP.14 Interestingly, aortic contraction by calyculin A appeared to be resistant to salicylates. In the range of concentration reported in patient plasma (<6 mM),23 sodium salicylate did not relax calyculin A-induced contraction, whereas the same concentration of sodium salicylate markedly relaxed PE-induced contraction (Figure 3A and B). Moreover, compared with contraction by other constrictors, the calculated IC50 was significantly higher (Table 1). RhoA/Rho–kinase pathway plays a critical role in MLCP regulation.9 We therefore examined whether salicylates inhibited RhoA/Rho kinase in aortic rings. Figure 3C–E demonstrated that angiotensin II markedly induced RhoA translocation and MYPT1 phosphorylation and that these observations were inhibited by sodium salicylate with an IC50 [95% confidence interval (CI): 3.8–13.1 and 1.6–43 mM, RhoA translocation and MYPT1 phosphorylation, respectively] comparable to that of its vasodilator action. Moreover, pre-treatment of aortic rings with the Rho-kinase inhibitor Y-27632 markedly decreased the vasodilator action of sodium salicylate (see Supplementary material online, Figure S2), further supporting that salicylates exert vasodilator action by inhibiting RhoA/Rho kinase.
Figure 3.
Salicylates relax aortic contraction through inhibiting RhoA/Rho kinase. (A) and (B) Endothelium-denuded rat aortic rings were pre-contracted by calyculin A (300 nM), and various concentrations of sodium salicylate were added. Meanwhile, at least one aortic ring was pre-contracted by phynelephrine (1 µM) and treated with the same concentrations of sodium salicylate. A representative recording (A) and the summary (B) of three independent experiments are presented. (C–E) Endothelium-denuded rat aortic rings were treated with angiotensin II (100 nM) for 3 min in the presence of the indicated concentrations of sodium salicylate, and RhoA translocation and MYPT1 phosphorylation were then analysed. A representative gel image (C) and the summary (D, RhoA translocation; E, MYPT1 phosphorylation) of three independent experiments are shown. m, membrane; c, cytosolic. *P < 0.05 vs. negative control; #P < 0.05 vs. positive control; Student's t-test with Bonferroni post hoc test.
3.3. Salicylates target PYK2 for their vasodilator action
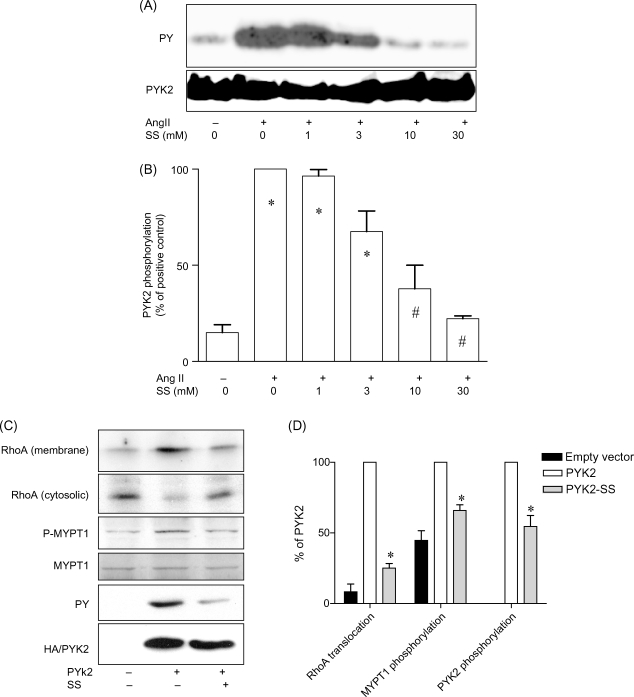
To examine how salicylates inhibit RhoA/Rho-kinase activation, we over-expressed constitutively active RhoA in VSMCs. Supplementary material online, Figure S3 showed that over-expression of constitutively active RhoA markedly induced MYPT1 phosphorylation, but this effect was not affected by 10 mM sodium salicylate, suggesting that salicylates target upstream of RhoA. PYK2 is essential for RhoA activation in macrophages by chemokines,17 and it can be inhibited by salicylates in VSMCs.15 Therefore, we tested whether PYK2 inhibition by salicylates contributes to their vasodilator action. Figure 4A and B shows that sodium salicylate concentration-dependently inhibited PYK2 with the IC50 (95% CI: 0.4–34 mM) comparable to that of its vasodilator action. Treatment with a non-specific tyrosine kinase inhibitor, genistein, markedly reduced the vasodilator action of sodium salicylate on contraction by angiotensin II (see Supplementary material online, Figure S4), further supporting that sodium salicylate targets PYK2 for its vasodilator action. To confirm that PYK2 mediates RhoA/Rho-kinase activation in VSMCs, human PYK2 over-expression adenoviral vector was generated. Treatment with this vector markedly increased RhoA translocation and MYPT1 phosphorylation (Figure 4C and D), and 10 mM sodium salicylate markedly inhibited these effects of the human PYK2 adenoviral vector, paralleled by markedly inhibiting tyrosine phosphorylation of over-expressed PYK2 (Figure 4C and D).
Figure 4.
Salicylates inhibit RhoA/Rho kinase by targeting PYK2. (A and B) Rat primary aortic vascular smooth muscle cells were treated with angiotensin II for 3 min in the presence of the indicated concentration of sodium salicylate. The PYK2 tyrosine phosphorylation level was then measured by immunoprecipitation following western blotting analysis. A representative image (A) and the summary (B) of three independent experiments are presented. *P < 0.05 vs. negative control; #P < 0.05 vs. positive control; Student's t-test with Bonferroni post hoc test. (C) and (D) Rat primary aortic vascular smooth muscle cells had been infected with the adenoviral vector that expresses human full-length PYK2 or control vector for 24 h, and then treated with sodium salicylate (10 mM) or vehicle for 30 min. Tyrosine phosphorylation of over-expressed PYK2, RhoA translocation, and MYPT1 phosphorylation were measured. A representative image (C) and the summary (D) of three independent experiments with similar results are presented. −, empty vector.
3.4. Salicylates have acute blood pressure-lowering effect in spontaneous hypertensive rats
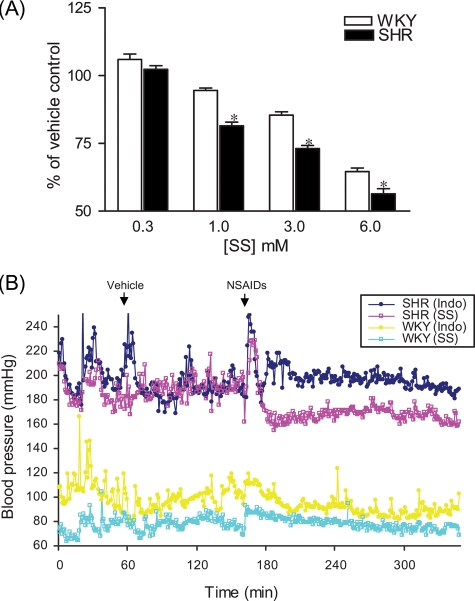
PYK2 activity and RhoA/Rho-kinase activation have been shown to be increased in SHR.18 Figure 5A and Supplementary material online, Figure S5 showed that aortic rings from SHR were more sensitive to sodium salicylate than those from WKY. Consistent with the increased vasodilator action in SHR, sodium salicylate decreased blood pressure in SHR in minutes. A fall of 18.2 ± 3.1 mmHg was observed 2 h after administration of 100 mg/kg of sodium salicylate, whereas the same dose of sodium salicylate did not significantly alter blood pressure in WKY (Figure 5B). In contrast, 20 mg/kg of indomethacin decreased blood pressure in neither SHR nor WKY (Figure 6A).
Figure 5.
Spontaneous hypertensive rats are more sensitive to sodium salicylate. (A) Endothelium-denuded aortic rings from spontaneous hypertensive and Wistar Kyoto rats were contracted by angiotensin II (100 nM) in the presence of the indicated concentrations of sodium salicylate. Results were expressed as percentage of the vehicle control. n = 4. *P < 0.05. One-way analysis of variance. (B) After recovery from surgery of arterial and venous catheter implantation and 30 min of baseline recording, rats were administrated intravenoulsy with respective vehicle. After 1 h, rats were administrated intravenoulsy with 100 mg/kg of sodium salicylate or 20 mg/kg of indomethacin. A representative recording for each group (n ≥ 3) is presented. Blood pressure spikes following administration of drugs are injection artefacts.
Figure 6.
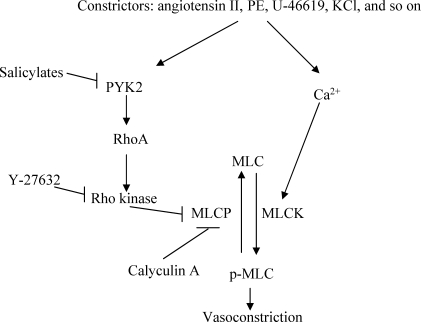
A proposed scheme depicting the main players involved in the present study. Ca2+, calcium; MLCK, myosin light chain kinase; MLCP, myosin light chain phosphatase; PYK2, proline-rich tyrosine kinase 2; MLC, myosin light chain; p-MLC, phosphor-myosin light chain.
4. Discussion
One important finding in the present study is that salicylates relax vasoconstriction independently of COX, offering a potential explanation for the unique blood pressure effect of aspirin. In contrast to other NSAIDs, which are associated with the risk of hypertension or an increase in blood pressure,2–4 aspirin does not increase the risk of hypertension,5,6 but decreases blood pressure.7,8 COX, the common target of NSAIDs, may play a critical role in blood pressure regulation through multiple mechanisms including modulating vascular tone.24 However, the blood pressure effect of aspirin is likely to be COX-independent, since this effect is unique. Consistent with the unique blood pressure effect, our results reveal that salicylates, including aspirin and sodium salicylate, have a unique vasodilator action. This vasodilator action of salicylates appeared to be independent of COX, since other tested COX inhibitors did not have a similar vasodilator action. Moreover, aspirin and sodium salicylate have different IC50s in inhibiting COX,22 but they have comparable IC50s in vasodilation, which are apparently higher than those reported to inhibit COX, further strongly supporting that the vasodilator action of salicylates is COX-independent. These results are consistent with a recent epidemiological study, in which low frequency of aspirin use increased the risk of hypertension, but high frequency of aspirin use did not further increase the risk of hypertension; in contrast, other NSAIDs continuously increased the risk of hypertension according to the frequency of use.4 The COX-independent pharmacological effects of salicylates have come to be identified. For example, a recent study demonstrates that salicylates inhibit angiogenesis through an unclear COX-independent mechanism.25 Our data thus not only support the concept that aspirin may exert pharmacological effects through COX-independent mechanisms, but also indicate the potential clinical importance of these COX-independent pharmacological effects.
Our present data also demonstrate that salicylates relax vasoconstriction through inhibition of the RhoA/Rho–kinase pathway. RhoA/Rho kinase is a critical component of vascular tone regulation through phosphorylation and inhibition of MLCP.9 In the present study, we show that contraction by calyculin A is resistant to salicylates. Since calyculin A contracts vascular smooth muscle through inhibiting MLCP, thus independently of RhoA/Rho kinase,14 this result strongly indicates that salicylates may relax vasoconstriction through inhibiting RhoA/Rho-kinase activation. The effects of salicylates on RhoA/Rho-kinase activation are then confirmed by analysing RhoA translocation and MYPT1 phosphorylation induced by angiotensin II. RhoA/Rho-kinase activation is increased in hypertensive animal models and patients,9,11 and inhibition of Rho kinase lowers blood pressure in hypertensive but not in normotensive animals.14 Consistently, our results demonstrate that sodium salicylate lowers blood pressure in SHR but not in WKY, further supporting that inhibiting RhoA/Rho kinase is the major mechanism for the unique blood pressure effect of salicylates.
PYK2 is a non-receptor tyrosine kinase regulated by various extracellular signals that activate G protein coupled receptors (GPCR) and/or elevated cytoplasmic Ca2+ such as angiotensin II.16 Knockdown of PYK2 by antisense oligonucleotides abolishes various downstream signalling induced by angiotensin II in VSMCs.19 The involvement of tyrosine phosphorylation in RhoA/Rho-kinase activation has also been demostrated.26 Studies on PYK2-deficient macrophages have revealed an essential role of PYK2 in RhoA activation induced by chemokines.17 In cultured VSMCs, PYK2 activation by angiotensin II can be inhibited by salicylates.15 In the present study, we extend this report to reveal that salicylates inhibit angiotensin II-induced PYK2 activation with an IC50 comparable to that of its vasodilator action, indicating that salicylates may relax vasoconstriction by targeting PYK2. This is further confirmed by results showing that over-expression of PYK2 is sufficient to induce RhoA translocation and MYPT1 phosphorylation in VSMCs; these effects are markedly reduced by sodium salicylate. Various studies have demonstrated a role of tyrosine phosphorylation in vascular tone regulation, but the mechanism remains elusive.27,28 Our data therefore offer a mechanistic insight into how tyrosine phosphorylation is involved in vascular tone regulation.
SHR is one of the most frequently used animal models for human essential hypertension. It has been shown that both baseline and agonist-induced PYK2 activities are increased in SHR.18 Consistently, our results show that sodium salicylate has a more potent vasodilator action on the aorta from SHR and lowers blood pressure in SHR but not in WKY rats. These results not only support the concept that salicylates exert unique blood pressure effects by targeting PYK2, but also highlight the role of PYK2 in hypertension. Most recently, consistent with our results, a study using PYK2-deficient mice revealed an essential role of PYK2 in angiotensin II-induced hypertension.29 Our results are also consistent with epidemiological studies, demonstrating that the different blood pressure effects of aspirin may be more evident in hypertensive patients.4
However, to confirm that salicylates lower blood pressure through targeting PYK2, specific PYK2 inhibitors or PYK2-deficient mice are required in the future studies. Moreover, PYK2 can not directly regulate RhoA activity. The underlying molecular mechanism for how PYK2 mediates RhoA/Rho-kinase activation remains to be defined in the future.
5. Conclusions
The present studies indicate that salicylates, including aspirin and sodium salicylate, relax vasoconstriction through inhibiting PYK2-mediated RhoA/Rho-kinase activation, offering a mechanistic insight into the unique blood pressure effect of salicylates.
Supplementary material
Supplementary Material is available at Cardiovascular Research Online.
Funding
This work was supported by National Institutes of Health grants HL-71138 and HL-74167 (R.C.W.).
Acknowledgements
We thank Wedegaertner (Department of Microbiology and Immunology, Thomas Jefferson University) for kindly providing us Myc-tagged human PDZ-RhoGEF expression vector.
Conflict of interest: none declared.
References
- 1.Roberts LJ, Morrow JD. Analgesic-antipyretic and antiinflammatory agents and drugs employed in the treatment of gout. In: Hardman JG, Limbird LE, Gilman AG, editors. Goodman & Gilman's The Pharmarcological Basis of Therapeutics. Columbus, Ohio, USA: The McGraw-Hill Companies; 2001. pp. 687–731. [Google Scholar]
- 2.Pope JE, Anderson JJ, Felson DT. A meta-analysis of the effects of nonsteroidal anti-inflammatory drugs on blood pressure. Arch Intern Med. 1993;153:477–484. [PubMed] [Google Scholar]
- 3.Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Intern Med. 1994;121:289–300. doi: 10.7326/0003-4819-121-4-199408150-00011. [DOI] [PubMed] [Google Scholar]
- 4.Wilson SL, Poulter NR. The effect of non-steroidal anti-inflammatory drugs and other commonly used non-narcotic analgesics on blood pressure level in adults. J Hypertens. 2006;24:1457–1469. doi: 10.1097/01.hjh.0000239278.82196.a5. [DOI] [PubMed] [Google Scholar]
- 5.Chan AT, Manson JE, Albert CM, Chae CU, Rexrode KM, Curhan GC, et al. Nonsteroidal antiinflammatory drugs, acetaminophen, and the risk of cardiovascular events. Circulation. 2006;113:1578–1587. doi: 10.1161/CIRCULATIONAHA.105.595793. [DOI] [PubMed] [Google Scholar]
- 6.Curhan GC, Willett WC, Rosner B, Stampfer MJ. Frequency of analgesic use and risk of hypertension in younger women. Arch Intern Med. 2002;162:2204–2208. doi: 10.1001/archinte.162.19.2204. [DOI] [PubMed] [Google Scholar]
- 7.Hermida RC, Ayala DE, Calvo C, Lopez JE. Aspirin administered at bedtime, but not on awakening, has an effect on ambulatory blood pressure in hypertensive patients. J Am Coll Cardiol. 2005;46:975–983. doi: 10.1016/j.jacc.2004.08.071. [DOI] [PubMed] [Google Scholar]
- 8.Hermida RC, Ayala DE, Calvo C, Lopez JE, Mojon A, Rodriguez M, et al. Differing administration time-dependent effects of aspirin on blood pressure in dipper and non-dipper hypertensives. Hypertension. 2005;46:1060–1068. doi: 10.1161/01.HYP.0000172623.36098.4e. [DOI] [PubMed] [Google Scholar]
- 9.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 10.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, et al. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res. 2007;100:923–929. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 11.Hirooka Y, Shimokawa H, Takeshita A. Rho-kinase, a potential therapeutic target for the treatment of hypertension. Drug News Perspect. 2004;17:523–527. doi: 10.1358/dnp.2004.17.8.863696. [DOI] [PubMed] [Google Scholar]
- 12.Ito K, Hirooka Y, Kimura Y, Sagara Y, Sunagawa K. Ovariectomy augments hypertension through rho-kinase activation in the brain stem in female spontaneously hypertensive rats. Hypertension. 2006;48:651–657. doi: 10.1161/01.HYP.0000238125.21656.9e. [DOI] [PubMed] [Google Scholar]
- 13.Kanda T, Wakino S, Homma K, Yoshioka K, Tatematsu S, Hasegawa K, et al. Rho-kinase as a molecular target for insulin resistance and hypertension. FASEB J. 2006;20:169–171. doi: 10.1096/fj.05-4197fje. [DOI] [PubMed] [Google Scholar]
- 14.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Brecher P. Salicylate inhibits phosphorylation of the nonreceptor tyrosine kinases, proline-rich tyrosine kinase 2 and c-Src. Hypertension. 2001;37:148–153. doi: 10.1161/01.hyp.37.1.148. [DOI] [PubMed] [Google Scholar]
- 16.Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12:123–133. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 17.Okigaki M, Davis C, Falasca M, Harroch S, Felsenfeld DP, Sheetz MP, et al. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc Natl Acad Sci USA. 2003;100:10740–10745. doi: 10.1073/pnas.1834348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rocic P, Griffin TM, McRae CN, Lucchesi PA. Altered PYK2 phosphorylation by ANG II in hypertensive vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2002;282:H457–H465. doi: 10.1152/ajpheart.00546.2001. [DOI] [PubMed] [Google Scholar]
- 19.Rocic P, Lucchesi PA. Down-regulation by antisense oligonucleotides establishes a role for the proline-rich tyrosine kinase PYK2 in angiotensin II-induced signaling in vascular smooth muscle. J Biol Chem. 2001;276:21902–21906. doi: 10.1074/jbc.M101684200. [DOI] [PubMed] [Google Scholar]
- 20.Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol. 2004;287:H1495–H1500. doi: 10.1152/ajpheart.01006.2003. [DOI] [PubMed] [Google Scholar]
- 21.da Silva AA, Kuo JJ, Tallam LS, Hall JE. Role of endothelin-1 in blood pressure regulation in a rat model of visceral obesity and hypertension. Hypertension. 2004;43:383–387. doi: 10.1161/01.HYP.0000111139.94378.74. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci USA. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McEvoy GK. AHFS Drug Information. Bethesda: American Society of Health-System Pharmacist; 2007. [Google Scholar]
- 24.Welch WJ, Patel K, Modlinger P, Mendonca M, Kawada N, Dennehy K, et al. Roles of vasoconstrictor prostaglandins, COX-1 and -2, and AT1, AT2, and TP receptors in a rat model of early 2K,1C hypertension. Am J Physiol Heart Circ Physiol. 2007;293:H2644–H2649. doi: 10.1152/ajpheart.00748.2007. [DOI] [PubMed] [Google Scholar]
- 25.Borthwick GM, Johnson AS, Partington M, Burn J, Wilson R, Arthur HM. Therapeutic levels of aspirin and salicylate directly inhibit a model of angiogenesis through a Cox-independent mechanism. FASEB J. 2006;20:2009–2016. doi: 10.1096/fj.06-5987com. [DOI] [PubMed] [Google Scholar]
- 26.Seok YM, Baek I, Kim YH, Jeong YS, Lee IJ, Shin DH, et al. Isoflavone attenuates vascular contraction through inhibition of the RhoA/Rho–kinase signaling pathway. J Pharmacol Exp Ther. 2008;326:991–998. doi: 10.1124/jpet.108.138529. [DOI] [PubMed] [Google Scholar]
- 27.Adegunloye BJ, Su X, Camper EV, Moreland RS. Sensitivity of rabbit aorta and mesenteric artery to norepinephrine: role of tyrosine kinases. Eur J Pharmacol. 2003;476:201–209. doi: 10.1016/s0014-2999(03)02183-6. [DOI] [PubMed] [Google Scholar]
- 28.Fang LH, Kwon SC, Zhang YH, Ahn HY. Tyrosine kinase participates in vasoconstriction through a Ca(2+)- and myosin light chain phosphorylation-independent pathway. FEBS Lett. 2002;512:282–286. doi: 10.1016/s0014-5793(02)02235-4. [DOI] [PubMed] [Google Scholar]
- 29.Matsui A, Okigaki M, Katsume A, Urao N, Yamada H, Matsubara H. Calcium-dependent tyrosine kinase PYK2 plays crucial role in angiotensin II—but not norepinephrine—mediated activation of Rac/superoxide pathway that differentially regulates blood pressure and vasoconstriction unmasked by analysis of PYK2-knock-out mice. Circulation. 2008;118:S.316. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.