Abstract
Aims
Type 2 diabetes mellitus is frequently associated with hypertension, but the underlying mechanisms are not completely understood. We tested the hypothesis that activation of type 1 prostaglandin E2 (PGE2) receptor (EP1) increases skeletal muscle arteriolar tone and blood pressure in mice with type 2 diabetes.
Methods and results
In 12-week-old, male db/db mice (with homozygote mutation in leptin receptor), systolic blood pressure was significantly elevated, compared with control heterozygotes. Isolated, pressurized gracilis muscle arterioles (∼90 µm) of db/db mice exhibited an enhanced pressure- and angiotensin II (0.1–10 nM)-induced tone, which was reduced by the selective EP1 receptor antagonist, AH6809 (10 µM), to the level observed in arterioles of control mice. Exogenous application of PGE2 (10 pM–100 nM) or the selective agonist of the EP1 receptor, 17-phenyl-trinor-PGE2 (10 pM–100 nM), elicited arteriolar constrictions that were significantly enhanced in db/db mice (max: 31 ± 4 and 29 ± 5%), compared with controls (max: 20 ± 2 and 14 ± 3%, respectively). In the aorta of db/db mice, an increased protein expression of EP1, but not EP4, receptor was also detected by western immunoblotting. Moreover, we found that oral administration of the EP1 receptor antagonist, AH6809 (10 mg/kg/day, for 4 days), significantly reduced the systolic blood pressure in db/db, but not in control mice.
Conclusion
Activation of EP1 receptors increases arteriolar tone, which could contribute to the development of hypertension in the db/db mice.
KEYWORDS: Diabetes, Hypertension, Arteriole, Prostanoid, EP receptor
1. Introduction
Type 2 diabetes mellitus is frequently associated with hypertension, but the inter-relationship and the underlying mechanisms are not completely understood, so that effective preventive therapeutic strategies cannot be adopted.1,2 It is known that many forms of hypertension are characterized by an augmented functional constriction of resistance arteries, which may contribute to the increased peripheral resistance and elevation in systemic blood pressure.3–7 Similarly, in type 2 diabetes mellitus, an enhanced myogenic tone and agonist-induced constriction of resistance arteries have been described in humans8 and in animal models of type 2 diabetes.9,10 In this context, we have shown previously that mice with type 2 diabetes (db/db mice with homozygous mutation of leptin receptor) exhibit an enhanced arteriolar tone of skeletal muscle resistance arteries, an alteration, which was found to be associated with elevated blood pressure of these animals.11 A previous study by Lagaud et al. indicated that in db/db mice, basal- and agonist-stimulated NO release is reduced in mesenteric arterioles, similar to our findings showing a diminished contribution of NO to flow-mediated dilations of coronary arterioles of db/db mice.12,13 Interestingly, Lagaud et al.9 also found that arteriolar relaxation was countered by an endogenous production of vasoconstrictor prostanoids. Motivated by this observation, we have recently demonstrated that the enhanced release of constrictor prostaglandins can be specifically attributed to the increased expression of cyclooxygenase-2 (COX-2) in the vascular wall of db/db mice.11 These and other observations14 indicated that COX-2-derived constrictor prostanoids have a pivotal role in increasing arteriolar tone in type 2 diabetic mice. However, little is yet known about the nature of COX-2-derived constrictor prostanoids and how they increase the arteriolar tone and agonist-induced constriction in type 2 diabetes.
Previous studies have found that the induction of COX-2 in vascular smooth muscle cells is associated primarily with an enhanced production of prostaglandin E2 (PGE2).15,16 These findings were further supported by the observations showing that COX-2 is coupled to and compartmentalized with PGE2 synthase.17,18 It known that PGE2 can cause either vasodilatation, via activating EP2/EP4 receptors, or vasoconstriction, via activating EP1/EP3 receptors.19 Thus, either a diminished EP2/EP4 receptor-mediated vasodilatation or an augmented EP1/EP3 receptor signalling may be responsible for an enhanced vascular tone. This latter scenario seems to be supported by a recent study, in which a crucial role for vasoconstrictor EP1 receptor activation has been demonstrated, contributing to the increased angiotensin II (Ang II)-induced vasoconstriction and elevation of systemic blood pressure in spontaneously hypertensive rats.20 In addition, PGE2, via primarily activating EP1 receptors, has been shown to play a key role in the development of hypertensive renal disease.21
These aforementioned studies prompted us to test the specific hypothesis that EP1 receptor activation plays a pivotal role in the augmentation of arteriolar tone and consequently, elevation of systemic blood pressure in type 2 diabetes mellitus. This idea has not yet been tested in subjects with type 2 diabetes. Thus, in the present study, we set out to characterize the contribution of EP1 receptors to the augmented pressure- and agonist (Ang II)-induced constriction of resistance arteries, as well as its impact on systemic blood pressure in a mouse model of type 2 diabetes, db/db mice.
2. Methods
2.1. Animals and experimental procedures
In the experiments, a well-characterized mouse model of the human type 2 diabetes was used (db/db mouse with homozygote mutation in leptin receptor).11–13 A 12-week-old, male db/db (C57BL/KsJ-db−/db−) and heterozygous (C57BL/KsJ-db+/db−) mice were fed standard chow and had free access to water. All protocols were approved by the Institutional Animal Care and Use Committee. The investigation conforms with the ‘Guide for the Care and Use of Laboratory Animals' published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg). Under anaesthesia, the gracilis muscle was excised and placed in ice-cold and oxygenated Krebs solution. Euthanasia was then performed by additional intraperitoneal injection of pentobarbital sodium (150 mg/kg), and the aorta of mice was excised.
2.2. Isolation of skeletal (gracilis) muscle arteriole
Microsurgery instruments and an operating microscope were used for the isolation of a gracilis muscle arteriole (∼0.5 µm in length) running intramuscularly. The arteriole was isolated and transferred into an organ chamber containing two glass micropipettes filled with Krebs solution composed of (in mmol/L): 110 NaCl, 5.0 KCl, 2.5 CaCl2, 1.0 MgSO4, 1.0 KH2PO4, 5.0 glucose, and 24.0 NaHCO3 equilibrated with a gas mixture of 10% O2 and 5% CO2, balanced with nitrogen, at pH 7.4. Vessels were cannulated on both ends, and micropipettes were connected with silicone tubing to a pressure servo control system (Living Systems Instrumentation, VT, USA). Temperature was set at 37°C by a temperature controller. Changes in arteriolar diameter were continuously measured with a videomicroscope system (Nikon Eclipse 80i, CCD camera, frame grabber, National Instruments, PCI 1405).
2.3. Experimental protocols
After a 1 h incubation period, spontaneous basal arteriolar tone developed in response to 80 mmHg intraluminal pressure, without the use of any constrictor agent. Then, changes in the diameter of arterioles were measured in response to step increases in intraluminal pressure from 20 to 120 mmHg. Arterioles were incubated with the EP1 receptor antagonist, AH6809 (10 µM for 30 min), and pressure-induced diameter changes were reassessed. To obtain the passive arteriolar characteristics, pressure-induced arteriolar responses were measured in the presence of Ca2+-free Krebs solution. Normalized arteriolar diameter (in Ca2+-containing Krebs solution) was expressed as a percentage of corresponding passive diameters (in Ca2+-free Krebs solution).
In separate experiments, at an intraluminal pressure of 80 mmHg, cumulative concentrations of PGE2 (10 pM–100 nM), the selective EP1 receptor agonist, 17-phenyl-trinor-PGE2 (10 pM–100 nM), or Ang II (0.1 nM–10 nM) were applied to the organ chamber, and diameter changes of arterioles were continuously recorded. These agonist-induced responses were observed after incubation with AH6809 (10 µM for 30 min).
In one set of experiments, PGE2-induced arteriolar responses were investigated in the simultaneous presence of AH6809 and the TP receptor antagonist, SQ29548 (1 µM for 30 min), in both groups of vessels. In addition, PGE2-induced arteriolar responses were investigated in the presence of L-161,982 (1 µM for 30 min), the selective antagonist of EP4 receptors, or in the presence of the NO synthesis inhibitor, l-NAME (200 µM for 20 min), in both groups of vessels.
2.4. Measurement of blood pressure and administration of AH6809
Blood pressure was measured in 12-week-old conscious mice using an automated tail cuff manometer system.11 After 3 days of measurements, eight control and eight db/db mice were randomized to two groups. Groups of control (n = 4) and db/db (n = 4) mice were given a selective EP1 receptor antagonist, AH6809 (10 mg/kg/day), by daily oral gavage. Administration and dosage of AH6809 was based on previous studies, in which in vivo administration of AH6809 was performed in mice22 and in which another EP1 receptor antagonist, SC51322, was used in a similar experimental design.20 Other groups of control (n = 4) and db/db (n = 4) mice were given vehicle. AH6809 or vehicle administration was continued for 4 days, and blood pressure measurements were continued for two consecutive days after the treatments were terminated.
2.5. Western immunoblotting
Aorta was dissected from control and db/db mice, cleared of connective tissue, and briefly rinsed in ice-cold, oxygenated Krebs solution. After the addition of 200 µL of Laemmli sample buffer (Sigma Inc.), tissues were homogenized. Immunoblot analysis was carried out as described earlier.11 The polyclonal antibodies used for the detection of EP1 and EP4 receptors were obtained from Cayman Chemicals. Anti-β-actin IgG obtained from Abcam was used as loading control. Signals were revealed with chemiluminescence and visualized autoradiographically. Optical density of bands was quantified and normalized for β-actin by using NIH Image software.
2.6. Statistics
Data are expressed as means ± SEM. Statistical analyses were performed by two-way analysis of variance for repeated measures (ANOVA) followed by the Tukey post hoc test. P < 0.05 was considered statistically significant.
3. Results
3.1. Basic characteristics of db/db mice
Previously, we have found that at 12 weeks of age, body weight, serum glucose, and serum insulin of male, db/db mice were significantly elevated, compared with age-matched control heterozygous animals.11 These alterations in the db/db mice resemble to characteristics of human type 2 diabetes. In this study, we have found that systolic blood pressure was significantly elevated in db/db compared with control mice (control: 136 ± 4 mmHg vs. db/db: 155 ± 5 mmHg, P < 0.05), whereas heart rates were similar in the two groups of animals (control: 612 ± 18, db/db 579 ± 24 1/min, P > 0.05).
3.2. Role of EP1 receptor in enhanced arteriolar tone in db/db mice
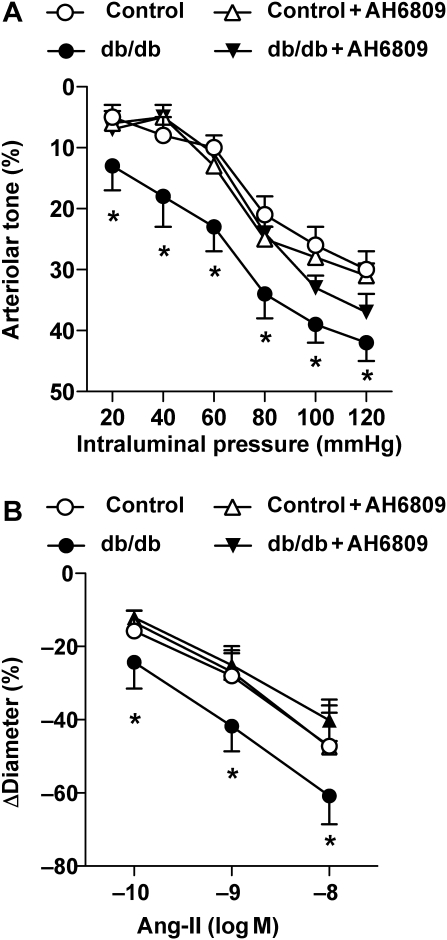
First, the potential contribution of EP1 receptor activation to the intraluminal pressure- and agonist (Ang-II)-induced arteriolar tone was investigated. Stepwise increases in intraluminal pressure from 20 to 120 mmHg elicited significantly greater constrictions in arterioles from db/db mice compared with control vessels at each pressure step (Figure 1A), a finding that corresponds with our previous observations.11 Ang II-induced constrictions were also augmented in skeletal muscle arterioles of db/db mice (Figure 1B), similar to the observation in the aorta of the obese Zucker rat.23
Figure 1.
Effect of the EP1 receptor antagonist AH6809 on intraluminal pressure- [from 20 to 120 mmHg (A)] and angiotensin II [Ang II, 0.1–10 nM (B)]-induced constrictions of arterioles isolated from control (n = 11) and db/db mice (n = 11). Data are mean ± SEM. Asterisks indicate significant difference (P < 0.05).
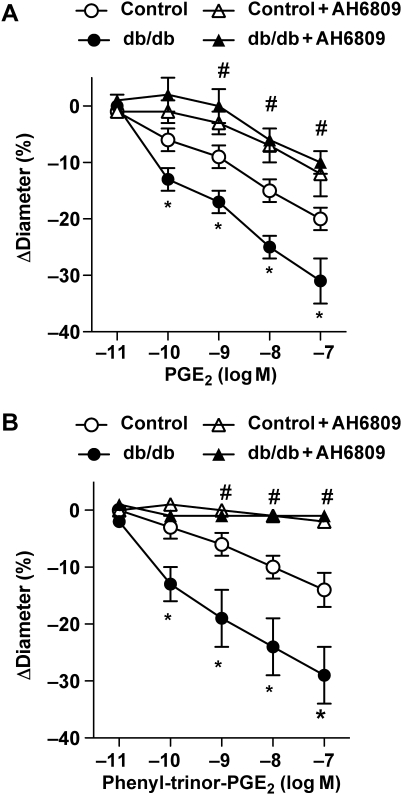
Incubation with the selective EP1 receptor antagonist, AH6809, did not affect pressure- and Ang II-induced responses in arterioles of control mice, but it reduced pressure- and Ang II-induced tone in arterioles of db/db mice, back to the control level (Figure 1). Next, arteriolar responses were obtained to exogenously administered PGE2 (10 pM–100 nM) or to the selective EP1 receptor agonist 17-phenyl-trinor-PGE2 (10 pM–100 nM) in the absence and presence of the EP1 receptor antagonist, AH6809. PGE2, in a dose-dependent manner, elicited constrictions in both group of vessels; however, the magnitude of constrictions was significantly enhanced in arterioles of db/db mice (Figure 2A). 17-phenyl-trinor-PGE2 elicited constriction of arterioles, which was also significantly greater in arterioles of db/db mice (Figure 2B). The EP1 receptor antagonist, AH6809, significantly reduced constrictions to PGE2 and diminished arteriolar responses to 17-phenyl-trinor-PGE2 in both groups of mice (Figure 2). Of note, higher concentrations of PGE2 (10 and 100 nM) elicited significant constrictions even in the presence of AH6809 (Figure 2A). We have found that the remaining constrictions in the presence of the EP1 receptor antagonist were completely abolished by additional administration of the TP receptor antagonist, SQ29548, in both groups of vessels (control: to 1 ± 4% and db/db: to −3 ± 2%).
Figure 2.
Effects of exogenously administered PGE2 [10 pM–100 nM (A)] and 17-phenyl-trinor-PGE2 [10 pM–100 nM (B)] in skeletal muscle arterioles isolated from control (n = 7) and db/db mice (n = 7), in the absence and presence of the EP1 receptor antagonist, AH6809. Data are mean ± SEM. *Indicates significant difference between control and db/db mice; #Indicates significant difference before and after the treatment with AH6809 in both groups (P < 0.05).
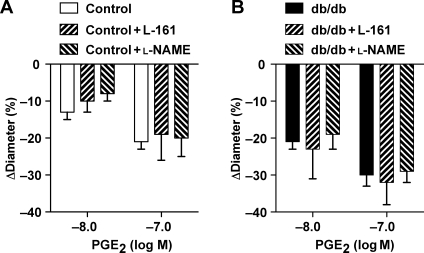
We have also found that PGE2-induced arteriolar tone was not significantly affected by the presence of the selective EP4 receptor antagonist, L-161,982, or by the presence of an NO synthesis inhibitor, l-NAME, either in control or db/db mice (Figure 3).
Figure 3.
Effects of exogenously administered PGE2 (10 pM–100 nM) in skeletal muscle arterioles isolated from control [n = 6 (A)] and db/db mice [n = 6 (B)], in the absence and presence of the EP4 receptor antagonist, L-161,982, or the NO synthesis inhibitor, l-NAME. Data are mean ± SEM.
3.3. Increased protein expression of EP1 receptor in db/db mice
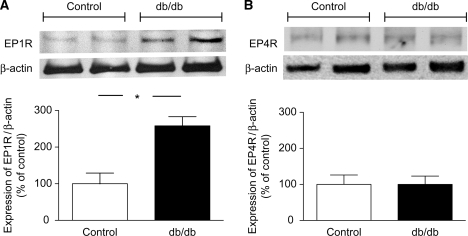
Functional experiments indicated a contribution of EP1 receptors in the augmented constriction of skeletal muscle arterioles of db/db mice. To reveal changes in vascular expression of EP1 receptors, western immunoblot analysis was performed in the aorta of mice. We have found that the protein expression of the EP1 receptor was significantly increased in the aorta of db/db mice (Figure 4A), when compared with control animals, whereas protein expression of the EP4 receptor was similar in the two groups (Figure 4B).
Figure 4.
Western blot analysis of the expression of EP1 and EP4 receptors (EP1R and EP4R) in the aorta of control (n = 4) and db/db (n = 4) mice. Anti-β-actin was used to normalize for loading variations. Bar graphs represent the summary of normalized densitometric ratios (n = 4, for each group). Asterisk indicates significant difference (P < 0.05).
3.4. EP1 receptor activation and elevated blood pressure in db/db mice
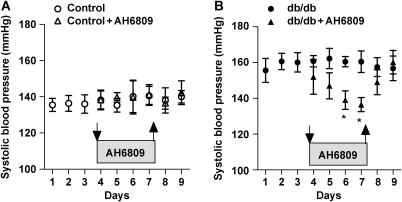
To provide in vivo evidence for an enhanced EP1 receptor activation in db/db mice, the effects of an EP1-selective antagonist on systemic blood pressure were assessed. Systolic blood pressure was monitored in conscious animals by the tail cuff method. After 2 days of treatment with the EP1 receptor antagonist, AH6809 (10 mg/kg/day), significantly reduced the systolic blood pressure of db/db mice, but did not affect the blood pressure of control animals. Upon discontinuing AH6809 administration, systolic blood pressure returned back to the initial, elevated level in db/db mice (Figure 5A and B).
Figure 5.
Effect of EP1 receptor antagonist AH6809 (10 mg/kg/day by daily gavage) or vehicle on systolic blood pressure in control (n = 8) and db/db (n = 8) mice. Downward arrows indicate the start and upward arrows indicate the end of the treatments. Data are mean ± SEM. Asterisks indicate significant difference (P < 0.05).
4. Discussion
This study demonstrates that, in addition to the pathological factors described earlier,24 endogenous EP1 receptor activation in resistance arteries may also contribute to the development of high blood pressure in a well-established model of human type 2 diabetes (db/db mice). This conclusion is supported by the findings that the augmented pressure- and Ang II-induced arteriolar tone, as well as the elevated systolic blood pressure of db/db mice are normalized by the EP1-selective antagonist, AH6809.
Recently, we have reported that mice with type 2 diabetes (db/db mice) develop high blood pressure and increased peripheral vascular resistance.11 We have found that these haemodynamic changes were associated with an enhanced basal tone of skeletal muscle resistance arteries.11 Along with recent studies, proposing a pivotal role for low-grade vascular inflammation in type 2 diabetes mellitus,25,26 we have shown that COX-2-derived constrictor prostanoids contribute to this enhanced constriction in resistance-sized vessels.11 It was thought that the predominant effects of endogenous prostanoids are posed to reduce blood pressure, consistent with observations showing that inhibition of prostaglandin synthesis by non-steroidal anti-inflammatory drugs or selective COX-2 inhibitors may increase blood pressure in humans.27 In contrast, clinical studies also demonstrated that COX-2 inhibition does not increase blood pressure,27,28 whereas selective inhibition of COX-1 has been implicated in the reduction of blood pressure.29 In light of this existing controversy and due to the unwanted actions of the systemic inhibition of prostanoid synthesis, other studies focused on particular prostanoid receptors that can be targeted to reduce vascular resistance and systemic blood pressure. In this context, type 1 PGE2 (EP1) receptors have been demonstrated to play a potential role in the development of hypertension.20,21
Based on the aforementioned, we hypothesized that EP1 receptor activation may be responsible for the enhanced arteriolar tone and consequently elevated systemic blood pressure in db/db mice. Supporting our assumption, in the present study, we have found that both pressure- and Ang II-induced constrictions were augmented in skeletal muscle arterioles of db/db mice, responses that were normalized by the selective EP1 receptor antagonist, AH6809 (Figure 1). These findings raised the possibility that in arterioles of db/db mice, endogenous PGE2, via primarily activating EP1 receptors, enhances pressure- and Ang II-induced arteriolar tone. It is known that PGE2 can activate four different types of G-protein-coupled receptors, which may result in either vasodilation (via activating EP2 and EP4 receptors) or vasoconstriction (via activating EP1 and EP3 receptors).19,30 Exogenously administered PGE2 has been shown to reduce31 or enhance the basal tone of resistance-sized arteries.32 In this study, we have found that exogenous application of PGE2 or the selective EP1 receptor agonist, 17-phenyl-trinor-PGE2, elicited substantial constrictions in isolated arterioles, responses that were significantly greater in arterioles of db/db mice and were inhibited by EP1 receptor antagonist, AH6809, in vessels of both groups of animals (Figure 2). These findings indicated that exogenous PGE2 causes primarily vasoconstriction in mouse skeletal muscle arterioles and that increased responsiveness of EP1 receptors is mainly responsible for the augmented pressure and Ang II-induced constriction in arterioles of db/db mice. To exclude the possible contribution of a diminished EP4 receptor-mediated dilator responses,19 PGE2-induced arteriolar responses were also evaluated in the presence of selective antagonist of EP4 receptor, L-161,982, both in control and db/db mice. We have found that L-161,982 did not exaggerate PGE2-induced arteriolar constrictions in either control or db/db mice (Figure 3), suggesting no or only a minimal role for the involvement of EP4 receptors in this process. Furthermore, it is possible that a reduced NO bioavailability, often present in diabetes, may also exaggerate prostanoid-induced vascular tone.33 However, we have found that NO synthesis inhibitor, l-NAME, did not significantly affect arteriolar tone and constrictions to PGE2 in control and db/db mice (Figure 3), suggesting only a limited contribution of NO in mediating PGE2-induced arteriolar responses. It should be noted that PGE2-mediated vasoconstriction was not entirely inhibited by the EP1 receptor antagonist, but was completely diminished by additional application of the TP receptor blocker, SQ29548, both in control and diabetic mice. Thus, it is likely that PGE2, especially at higher concentrations (10−8 and 10−7 M), may co-activate TP receptors in the arteriolar wall. This observation might explain why TP receptor blockade by SQ29548 reduced the enhanced arteriolar tone in db/db mice9,11,14 and also PGE2-induced constriction in mesenteric arterioles,32 as reported previously. Thus, it is possible that the augmented constriction of arterioles of db/db mice can be attributed to both EP1 and TP receptors in the microvascular wall. Notwithstanding, our present data support a pivotal role for EP1 receptors, since we detected an increased vascular expression of EP1 receptors in db//db mice, when compared with controls (Figure 4). However, it should be noted that an increased expression of EP1 receptors does not necessarily cause a greater response, as EP1 receptor signal transduction/second messenger pathways could be also enhanced, and receptors may not be present in the plasma membrane, but rather in an intracellular location. Thus, one cannot exclude the possibility that EP1 receptor-initiated signalling mechanisms are augmented in microvessels of type 2 diabetic mice, an idea yet to be elucidated.
The potential role of EP1 receptor activation on cardiovascular homeostasis is complex and is still open to debate. PGE2, via activating EP receptors, may affect several blood pressure regulatory mechanisms, among which the regulation of extracellular fluid volume in the kidney and the central nervous system is important.34 The aforementioned study by Guan et al.20 has demonstrated that EP1 receptor activation in vivo contributes to blood pressure elevation in spontaneously hypertensive rats and also in rats given a chronic Ang II infusion. Therefore, in a similar experimental setting, in our study, we have investigated the effect of EP1 receptor inhibition on systemic blood pressure in control and db/db mice. We have found that, after 2 days of treatment, EP1 receptor inhibition elicited a significant reduction in systolic blood pressure of db/db mice, whereas it had no effect on control animals (Figure 5). These data suggested that interfering with EP1 receptor in vivo results in a reduction of systemic blood pressure in db/db mice, but not in control animals. Although evidence supports that acute, in vivo administration of PGE2 causes a reduction in systemic blood pressure,35,36 chronic effects of PGE2 on systemic blood pressure are inconsistent and variable and seem to depend on experimental conditions, such as mode of administration.35,37,38 For instance, Stock et al.39 have found that in male mice, the absence of EP1 receptor is associated with significant reduction in blood pressure, suggesting a crucial role for EP1 receptors in elevating systemic blood pressure, in the long term. Correspondingly, our data showing that significant blood pressure fall in db/db mice developed only after 2 days of treatment with the EP1 receptor antagonist suggest that EP1 receptors are involved in the chronic regulation of systemic blood pressure, yet an indirect effect of EP1 receptor inhibition cannot be entirely excluded. Also, we should emphasize that due to the limitation of our blood pressure monitoring method (tail cuff), a careful interpretation of these results is still needed. Further studies have yet to be performed to demonstrate the long-term haemodynamic effects of EP1 receptor inhibition in type 2 diabetes mellitus.
In conclusion, the present study showed that up-regulation of EP1 receptors, in part, contributes to the augmented pressure- and Ang II-induced arteriolar tone in db/db mice. We propose that targeting of EP1 receptors may provide novel therapeutic modalities for the treatment of type 2 diabetes-associated microvascular vasomotor dysfunction and hypertension.
Funding
This study was supported by grants from the NIH PO1-HL-43023, HL-46813, American Heart Association, 0735540T, and by the Hungarian Ministry of Health: 449/2006, 454/2006 grants.
Conflict of interest: Z.P. holds a Bolyai Fellowship of the Hungarian Academy of Sciences.
References
- 1.Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37:1053–1059. doi: 10.1161/01.hyp.37.4.1053. [DOI] [PubMed] [Google Scholar]
- 2.Kirpichnikov D, Sowers JR. Diabetes mellitus and diabetes-associated vascular disease. Trends Endocrinol Metab. 2001;12:225–230. doi: 10.1016/s1043-2760(01)00391-5. [DOI] [PubMed] [Google Scholar]
- 3.Heistad DD, Lopez JA, Baumbach GL. Hemodynamic determinants of vascular changes in hypertension and atherosclerosis. Hypertension. 1991;17:III7–11. doi: 10.1161/01.hyp.17.4_suppl.iii7. [DOI] [PubMed] [Google Scholar]
- 4.Mulvany MJ. Structure and function of small arteries in hypertension. J Hypertens Suppl. 1990;8:S225–S232. [PubMed] [Google Scholar]
- 5.Schmid-Schonbein GW, Zweifach BW, DeLano FA, Chen PC. Microvascular tone in a skeletal muscle of spontaneously hypertensive rats. Hypertension. 1987;9:164–171. doi: 10.1161/01.hyp.9.2.164. [DOI] [PubMed] [Google Scholar]
- 6.Bohlen HG. Arteriolar closure mediated by hyperresponsiveness to norepinephrine in hypertensive rats. Am J Physiol. 1979;236:H157–H164. doi: 10.1152/ajpheart.1979.236.1.H157. [DOI] [PubMed] [Google Scholar]
- 7.Koller A. Signaling pathways of mechanotransduction in arteriolar endothelium and smooth muscle cells in hypertension. Microcirculation. 2002;9:277–294. doi: 10.1038/sj.mn.7800142. [DOI] [PubMed] [Google Scholar]
- 8.Jaap AJ, Shore AC, Tooke JE. The influence of hypertension on microvascular blood flow and resistance to flow in the skin of patients with type 2 (non-insulin-dependent) diabetes. Diabet Med. 1994;11:883–887. doi: 10.1111/j.1464-5491.1994.tb00373.x. [DOI] [PubMed] [Google Scholar]
- 9.Lagaud GJ, Masih-Khan E, Kai S, van Breemen C, Dube GP. Influence of type II diabetes on arterial tone and endothelial function in murine mesenteric resistance arteries. J Vasc Res. 2001;38:578–589. doi: 10.1159/000051094. [DOI] [PubMed] [Google Scholar]
- 10.Naik JS, Xiang L, Hester RL. Enhanced role for RhoA-associated kinase in adrenergic-mediated vasoconstriction in gracilis arteries from obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R154–R161. doi: 10.1152/ajpregu.00245.2005. [DOI] [PubMed] [Google Scholar]
- 11.Bagi Z, Erdei N, Toth A, Li W, Hintze TH, Koller A, et al. Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2-derived constrictor prostaglandins. Arterioscler Thromb Vasc Biol. 2005;25:1610–1616. doi: 10.1161/01.ATV.0000172688.26838.9f. [DOI] [PubMed] [Google Scholar]
- 12.Bagi Z, Koller A, Kaley G. Superoxide–NO interaction decreases flow- and agonist-induced dilations of coronary arterioles in Type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2003;285:H1404–H1410. doi: 10.1152/ajpheart.00235.2003. [DOI] [PubMed] [Google Scholar]
- 13.Bagi Z, Koller A, Kaley G. PPARgamma activation, by reducing oxidative stress, increases NO bioavailability in coronary arterioles of mice with Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2004;286:H742–H748. doi: 10.1152/ajpheart.00718.2003. [DOI] [PubMed] [Google Scholar]
- 14.Guo Z, Su W, Allen S, Pang H, Daugherty A, Smart E, et al. COX-2 up-regulation and vascular smooth muscle contractile hyperreactivity in spontaneous diabetic db/db mice. Cardiovasc Res. 2005;67:723–735. doi: 10.1016/j.cardiores.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Bishop-Bailey D, Pepper JR, Larkin SW, Mitchell JA. Differential induction of cyclooxygenase-2 in human arterial and venous smooth muscle: role of endogenous prostanoids. Arterioscler Thromb Vasc Biol. 1998;18:1655–1661. doi: 10.1161/01.atv.18.10.1655. [DOI] [PubMed] [Google Scholar]
- 16.Bishop-Bailey D, Larkin SW, Warner TD, Chen G, Mitchell JA. Characterization of the induction of nitric oxide synthase and cyclo-oxygenase in rat aorta in organ culture. Br J Pharmacol. 1997;121:125–133. doi: 10.1038/sj.bjp.0701100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campean V, Theilig F, Paliege A, Breyer M, Bachmann S. Key enzymes for renal prostaglandin synthesis: site-specific expression in rodent kidney (rat, mouse) Am J Physiol Renal Physiol. 2003;285:F19–F32. doi: 10.1152/ajprenal.00443.2002. [DOI] [PubMed] [Google Scholar]
- 18.Schneider A, Zhang Y, Zhang M, Lu WJ, Rao R, Fan X, et al. Membrane-associated PGE synthase-1 (mPGES-1) is coexpressed with both COX-1 and COX-2 in the kidney. Kidney Int. 2004;65:1205–1213. doi: 10.1111/j.1523-1755.2004.00493.x. [DOI] [PubMed] [Google Scholar]
- 19.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 20.Guan Y, Zhang Y, Wu J, Qi Z, Yang G, Dou D, et al. Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J Clin Invest. 2007;117:2496–2505. doi: 10.1172/JCI29838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suganami T, Mori K, Tanaka I, Mukoyama M, Sugawara A, Makino H, et al. Role of prostaglandin E receptor EP1 subtype in the development of renal injury in genetically hypertensive rats. Hypertension. 2003;42:1183–1190. doi: 10.1161/01.HYP.0000101689.64849.97. [DOI] [PubMed] [Google Scholar]
- 22.Oliva P, Berrino L, de Novellis V, Palazzo E, Marabese I, Siniscalco D, et al. Role of periaqueductal grey prostaglandin receptors in formalin-induced hyperalgesia. Eur J Pharmacol. 2006;530:40–47. doi: 10.1016/j.ejphar.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqui AH, Hussain T. Enhanced AT1 receptor-mediated vasocontractile response to ANG II in endothelium-denuded aorta of obese Zucker rats. Am J Physiol Heart Circ Physiol. 2007;292:H1722–H1727. doi: 10.1152/ajpheart.00612.2006. [DOI] [PubMed] [Google Scholar]
- 24.Stas SN, El-Atat FA, Sowers JR. Pathogenesis of hypertension in diabetes. Rev Endocr Metab Disord. 2004;5:221–225. doi: 10.1023/B:REMD.0000032410.75638.da. [DOI] [PubMed] [Google Scholar]
- 25.Hsueh WA, Quinones MJ. Role of endothelial dysfunction in insulin resistance. Am J Cardiol. 2003;92:10J–17J. doi: 10.1016/s0002-9149(03)00611-8. [DOI] [PubMed] [Google Scholar]
- 26.Hartge MM, Unger T, Kintscher U. The endothelium and vascular inflammation in diabetes. Diab Vasc Dis Res. 2007;4:84–88. doi: 10.3132/dvdr.2007.025. [DOI] [PubMed] [Google Scholar]
- 27.Sowers JR, White WB, Pitt B, Whelton A, Simon LS, Winer N, et al. The effects of cyclooxygenase-2 inhibitors and nonsteroidal anti-inflammatory therapy on 24 h blood pressure in patients with hypertension, osteoarthritis, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:161–168. doi: 10.1001/archinte.165.2.161. [DOI] [PubMed] [Google Scholar]
- 28.White WB, Kent J, Taylor A, Verburg KM, Lefkowith JB, Whelton A. Effects of celecoxib on ambulatory blood pressure in hypertensive patients on ACE inhibitors. Hypertension. 2002;39:929–934. doi: 10.1161/01.hyp.0000014323.99765.16. [DOI] [PubMed] [Google Scholar]
- 29.Qi Z, Hao CM, Langenbach RI, Breyer RM, Redha R, Morrow JD, et al. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J Clin Invest. 2002;110:61–69. doi: 10.1172/JCI14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 31.Messina EJ, Kaley G. Microcirculatory responses to prostacyclin and PGE2 in the rat cremaster muscle. Adv Prostaglandin Thromboxane Res. 1980;7:719–722. [PubMed] [Google Scholar]
- 32.Bolla M, You D, Loufrani L, Levy BI, Levy-Toledano S, Habib A, et al. Cyclooxygenase involvement in thromboxane-dependent contraction in rat mesenteric resistance arteries. Hypertension. 2004;43:1264–1269. doi: 10.1161/01.HYP.0000127438.39744.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sametz W, Prasthofer S, Wintersteiger R, Juan H. Vascular effects of isoprostanes after endothelial damage. Prostaglandins Leukot Essent Fatty Acids. 1999;61:369–372. doi: 10.1054/plef.1999.0113. [DOI] [PubMed] [Google Scholar]
- 34.Breyer MD, Breyer RM. G protein-coupled prostanoid receptors and the kidney. Annu Rev Physiol. 2001;63:579–605. doi: 10.1146/annurev.physiol.63.1.579. [DOI] [PubMed] [Google Scholar]
- 35.Audoly LP, Tilley SL, Goulet J, Key M, Nguyen M, Stock JL, et al. Identification of specific EP receptors responsible for the hemodynamic effects of PGE2. Am J Physiol. 1999;277:H924–H930. doi: 10.1152/ajpheart.1999.277.3.H924. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Guan Y, Schneider A, Brandon S, Breyer RM, Breyer MD. Characterization of murine vasopressor and vasodepressor prostaglandin E(2) receptors. Hypertension. 2000;35:1129–1134. doi: 10.1161/01.hyp.35.5.1129. [DOI] [PubMed] [Google Scholar]
- 37.Armstrong JM, Boura AL, Hamberg M, Samuelsson B. A comparison of the vasodepressor effects of the cyclic effects of the cyclic endoperoxides PGG, and PGH2 with those of PGD2 and PGE2 in hypertensive and normotensive rats. Eur J Pharmacol. 1976;39:251–258. doi: 10.1016/0014-2999(76)90133-3. [DOI] [PubMed] [Google Scholar]
- 38.Gerber JG, Nies AS. The hemodynamic effects of prostaglandins in the rat. Evidence for important species variation in renovascular responses. Circ Res. 1979;44:406–410. doi: 10.1161/01.res.44.3.406. [DOI] [PubMed] [Google Scholar]
- 39.Stock JL, Shinjo K, Burkhardt J, Roach M, Taniguchi K, Ishikawa T, et al. The prostaglandin E2 EP1 receptor mediates pain perception and regulates blood pressure. J Clin Invest. 2001;107:325–331. doi: 10.1172/JCI6749. [DOI] [PMC free article] [PubMed] [Google Scholar]