Abstract
Conditioned stimulus pathway protein 24 (Csp24) is a β-thymosin-like protein that is homologous to other members of the family of β-thymosin repeat proteins that contain multiple actin binding domains. Actin co-precipitates with Csp24 and co-localizes with it in the cytosol of type-B photoreceptor cell bodies. Several signal transduction pathways have been shown to regulate the phosphorylation of Csp24 and contribute to cellular plasticity. Here, we report the identification of the adapter protein 14-3-3 in lysates of the Hermissenda circumesophageal nervous system and its interaction with Csp24. Immunoprecipitation experiments using an antibody that is broadly reactive with several isoforms of the 14-3-3 family of proteins showed that Csp24 co-precipitates with 14-3-3 protein, and nervous systems stimulated with 5-HT exhibited a significant increase in co-precipitated Csp24 probed with a phosphospecific antibody as compared with controls. These results indicate that posttranslational modifications of Csp24 regulate its interaction with 14-3-3 protein, and suggest that this mechanism may contribute to the control of intrinsic enhanced excitability.
Keywords: actin, β-thymosin repeat proteins, Csp24, 14-3-3 proteins, Hermissenda B-photorecceptors, protein phosphorylation
The 14-3-3 family of acidic proteins consists of seven mammalian isoforms (β, ε, γ, η, θ, ρ, and ζ) that exist primarily as homo- and heterodimers within all eukaryotic cells. A number of functions have been established for the family of 14-3-3 proteins including modulation of the interactions between proteins (for reviews see [2–4,23,25]). Major roles for the 14-3-3 family of proteins include the mediation of the formation of protein complexes involved in signal transduction, regulation of the cell cycle, intracellular trafficking/targeting, cytoskeletal structure and transcription [16–19,24,27]. Because of the interest in the role of 14-3-3 proteins in signal transduction pathways in general, and specifically in its contribution to ERK activation and the regulation of PKC, we have examined the potential interaction between 14-3-3 proteins and the β-thymosin-repeat protein (Csp24) [7] in the nervous system of the marine mollusk Hermissenda.
Posttranslational modification of Csp24 is regulated by one-trial Pavlovian conditioning [7–10]. Here we show that a polyclonal antibody that is broadly reactive with members of the 14-3-3 family of proteins recognizes a 32 kDa band on western blots from lysates of Hermissenda circumesophageal nervous systems. Mass spectrometric analysis of the 32 kDa protein bands from nervous system lysates resolved by 1-D PAGE identified peptides with amino acid sequences homologous to 14-3-3 proteins. Moreover, mass spectrometric analysis of 32 kDa immunoprecipitates also revealed the same amino acid sequence that is homologous to protein 14-3-3. The interaction between Csp24 and 14-3-3 proteins was supported by the results of immunoprecipitation experiments showing that Csp24 co-precipitates with immunoprecipitated protein 14-3-3 and stimulation of circumesophageal nervous systems with 5-HT results in a significant increase in co-precipitated phosphoCsp24.
Samples of isolated circumesophageal nervous systems from adult Hermissenda crassicornis obtained from Sea Life Supply, Sand City, CA were rinsed with PBS and lysed in ice-cold radioimmunoprecipitation (RIPA) assay buffer containing PBS, 1% nonidet NP-40, 0.5% deoxycholate, 0.1% SDS, 0.1 mg/ml AEBSF, 0.6 U/ml aprotinin, and 1 mM sodium orthovanadate. All steps were conducted at 4°C. Lysates were centrifuged at 18000×g for 20 min, and the supernatants were recovered and incubated with rabbit polyclonal anti-14-3-3 (ZYMED) antibody for 1 hr. Protein A/G-agarose (Santa Cruz Biotechnology, Santa Cruz, CA) was then added for overnight incubation with rotation. Immunoprecipitates were collected by centrifugation (18000×g) for 10 min, and the pellets were carefully washed in the RIPA buffer four times. The washed pellets were rinsed an additional two times in RIPA buffer, then suspended in 40 μl sample buffer (0.5 M Tris, 2.3% SDS, 10% glycerol), boiled 3 min, and centrifuged. The immunoprecipitated samples were resolved on non-reducing SDS gels (without β-mercaptoethanol) to facilitate the detection of protein 14-3-3 due to the shift of the antibody heavy and light chains to a higher molecular weight under non-reducing conditions. Co-precipitation experiments involved 14-3-3 immunoprecipitated samples that were transferred to PVDF membranes and probed with anti-Csp24 antibody. For the analysis of the interaction between phosphorylated Csp24 and 14-3-3 protein, nervous systems (N=4) were exposed to 5-HT (10−4M, 5 min), lysed, and 14-3-3 immunoprecipitates were blotted and probed with two different antibodies directed at two distinct Csp24 phosphorylation sites. Control samples were incubated in normal ASW. The immunocomplexes were detected with enhanced chemiluminescent reagent (Amersham) following the manufacturer’s procedures. Normalization of the samples to total Csp24 levels involved stripping the membranes and re-probing with anti-Csp24 antibody. Ratios of phosphoCsp24 to total Csp24 were generated by densitometric scanning of the PVDF membranes (Molecular Dynamics, NJ).
Bands were excised and digested in-gel with trypsin. Protein digests were spotted on a MALDI target plate, with matrix, dried and the analysis performed in reflector mode on an ABI/SCIEN 4700 proteomic analyzer TOF/TOF mass spectrometer. Detected monoisotopic peptide masses were analyzed using the systems software interfaced with a database search engine to identify the proteins by peptide mass fingerprinting. Selected peptide precursor ions were subjected to high-energy collision induced dissociation to generate fragment ions (MS/MS fragmentation data, MS/MS sequence tags) that were analyzed to generate amino acid sequences of the peptides. In some cases, digests were also analyzed by liquid chromatography MS/MS using a hybrid triple quadrupole linear ion trap MS (ABI 4000 Q TRAP) following LC reverse phase chromatography (LC packings nanoflow LC system).
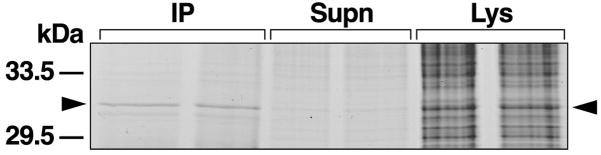
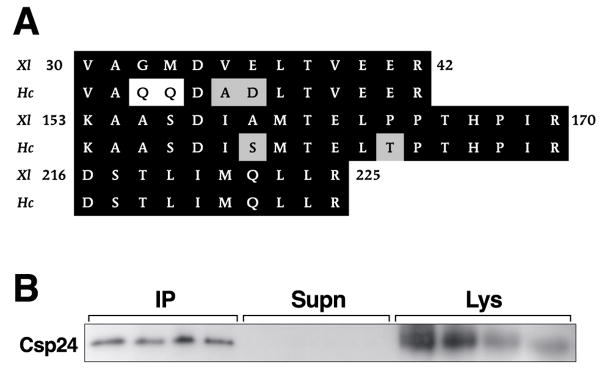
The circumesophageal nervous systems from three animals were isolated, lysed, and prepared for immunoprecipitation with anti 14-3-3 antibody. Protein bands from the lysate samples were resolved with 1-D PAGE and stained with Coomassie blue. Protein bands in the expected apparent molecular weight range of 14-3-3, 32 kDa, were excised from the gel followed by in-gel digestion with trypsin. Protein digests were analyzed using MALDI-TOF MS. In addition, MS/MS fragmentation data was generated to identify amino acid sequences of peptides. In some cases confirmation of the MALDI-TOF analysis was obtained by electrospray LC/MS/MS analysis of protein digests. A representative example of two replicates showing 1-D gel resolution of protein bands from lysate samples, supernatants, and immunoprecipitates from a non-reducing gel is shown in Figure 1. The effectiveness of the immunoprecipitation procedure is indicated by an absence of the 32 kDa protein bands in the supernatant samples and the identification of the bands in the immunoprecipitates (arrow). The arrow next to the lysate samples indicates putative 14-3-3 protein band that was analyzed by MS. The MS analysis of the 32 kDa band from the lysates resulted in the identification of three peptides with an amino acid sequence homologous to 14-3-3 proteins from Xenopus (see Fig. 2A). The bands from the immunoprecipitates with an apparent molecular weight of 32 kDa were excised from the gel and subjected to the same MS analysis as the lysate samples. The results of the MS analysis of the immunoprecipitates generated the same three peptides with an amino acid sequence homologous to 14-3-3 proteins (Fig. 2A). There appears to be a high degree of conservation between Xenopus 14-3-3 protein and Hermissenda 14-3-3 protein based upon the sequence alignment of the three peptides shown in Figure 2A. An examination of sequence alignments of evolutionary diverse 14-3-3 proteins suggests a high degree of conservation [4].
Fig. 1.
14-3-3 protein is found in the Hermissenda circumesophageal nervous system. Scan of Coomassie blue-stained 1- D gel of protein bands from lysate (Lys) samples, supernatant (Supn) and anti-14-3-3 immunoprecipitates (IP). Three circumesophageal nervous systems were included in each of the two replications. Immunoprecipitation with anti-14-3-3 antibody dramatically reduced the 32 kDa protein levels in the supernatant samples. The arrowhead on the right identifies the bands in the lysates and immunoprecipitates excised from the gel for mass spectrometric analysis.
Fig. 2.
Hermissenda peptide sequences are homologous to 14-3-3 proteins. (A) Alignment of the three peptides identified from mass spectrometry with corresponding amino acid sequences for Xenopus protein 14-3-3. Accession number AAH45025. Identical amino acids are shaded in black and similar amino acids are shaded in gray. The same peptides were identified from the lysate samples and immunoprecipitate samples. (B) Csp24 co-precipitates with 14-3-3 protein. Co-precipitation of Csp24 and 14-3-3 protein is shown by the western blots generated from a non reducing gel of anti-14-3-3 immunoprecipitates probed with anti-Csp24 antibody. The blots of the lysate samples indicate relative levels of Csp24. Three nervous systems were used in each of the four replications.
To determine whether 14-3-3 interacts with Csp24, lysates were immunoprecipitated with anti-14-3-3 and the resulting western blots were probed with an antibody raised against Csp24. A representative example of immunoblots of lysates, supernatants, and immunoprecipitates probed with anti-Csp24 from four replicates is shown in Figure 2B. The immuno procedures with anti-14-3-3 successfully precipitated most of the Csp24 from the lysates as shown by an absence of Csp24 in the supernatant sample. In contrast, Csp24 was present in all of the immunoprecipitate lanes (see Fig. 2B). The finding that Csp24 from the Hermissenda circumesophageal nervous system co-precipitates with 14-3-3 protein suggests that the two proteins interact.
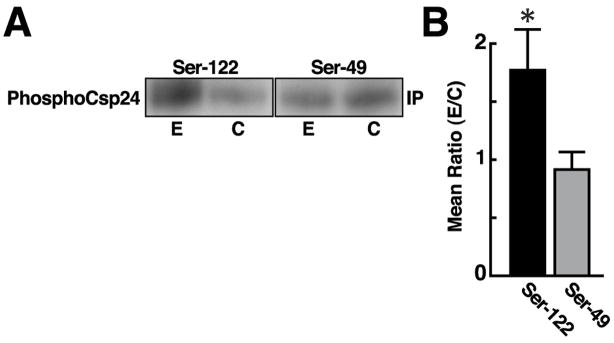
Two phosphorylation sites on Csp24 have been recently identified using electrospray mass spectrometry. One-trial in vitro conditioning regulates the phosphorylation of Ser122, but not Ser49 of Csp24 [11]. The potential regulation of the interaction between 14-3-3 protein and Csp24 by phosphorylation was assessed by the analysis of 5-HT-dependent phosphorylation of Csp24 co-precipitates generated by immunoprecipitated 14-3-3 protein. Isolated circumesophageal nervous systems (N=4) received a 5 min incubation with 10−4M 5-HT and control nervous systems (N=4) were incubated with ASW. The nervous systems were lysed and immunoprecipitation experiments were conducted with anti 14-3-3 antibody. The immmunoprecipitates from experimental and control groups were blotted and probed with phosphospecific antibodies directed to Ser122 or Ser49 as previously described [11]. Following densitometric scanning the membranes were stripped and reprobed with anti-Csp24 antibody and scanned to provide assessment of total Csp24 levels. The densitometric scans of the immunoprecipitates from the experimental groups (N=6) and ASW controls (N=6) probed with the two phosphospecific antibodies were normalized to the respective total Csp24 levels. The statistical analysis of the ratios of 5-HT treatment relative to total Csp24 and ASW controls relative to total Csp24 for the densitometric scans of phosphospecific antibodies directed at the two phosphorylation sites were significantly different (t10=2.07; P < .05). The 5-HT treatment resulted in an increase in co-precipitated phosphoCsp24 at Ser122 (X=1.76 ± .35), but not phosphoCsp24 at Ser49 (X=.91 ± .15) (see Fig. 3). Examples of blots from the experimental and control procedures for the phosphospecific antibodies directed at Ser49 or Ser122 are shown in Fig. 3A. The group summary data shown in Fig. 3B depicts the mean ratios of the experimental and the control procedures generated from densitometric scans of the membranes probed with the phosphospecific antibodies. These results suggest that the interaction between Csp24 and 14-3-3 proteins is influenced by the phosphorylation of Csp24 at a specific serine residue, the same site which is regulated by in vitro conditioning [11].
Fig. 3.
The interaction between 14-3-3 proteins and Csp24 is regulated by phosphorylation. (A) Representative examples of blots from a non-reducing gel of anti-14-3-3 immunoprecipitates probed with phosphospecific antibodies directed at Ser49 or Ser122 of Csp24 for experimental (N=6) and the control procedure (N=6). The membranes were stripped following densitometric analysis and reprobed for total Csp24 for each respective group. (B) Group data showing mean ± SE ratios of densitometric scans for phosphoSer49 co-precipitates and phosphoSer122 co-precipitates from experimental and control groups (N=6 respectively) * P < .05
In this study we demonstrate for the first time that 14-3-3 proteins are present in the circumesophageal nervous system of Hermissenda, and that the β-thymosin repeat protein Csp24 co-precipitates with 14-3-3 proteins. In addition, we provide evidence that the interaction between Csp24 and 14-3-3 protein is regulated by posttranslational modifications. The phosphorylation of Csp24 is regulated by both Pavlovian conditioning and several signal transduction pathways [7–10,26]. Specifically, protein kinase C and ERK have been shown to contribute to the induction and expression of cellular and synaptic plasticity associated with Pavlovian conditioning of Hermissenda [5,6,14]. Interestingly, 14-3-3 proteins have been implicated in PKC regulation [22], and they interact with Raf-1 kinase, resulting in an increase in Raf-1 activity and the activation of ERK [1,13]. Taken collectively, the interaction of specific 14-3-3 isoforms with Raf-1 in the mitogen-activated protein kinase cascade is well-documented in many systems. Therefore the potential role of 14-3-3 activation of Raf-1 and its subsequent activation of MEK1 and ERK is suggested in Hermissenda, since conditioning is dependent upon the activation of ERK and the expression of the cytoskeletal-related protein Csp24 [6,9]. Indeed, previous studies have suggested that 14-3-3 proteins have a role in synaptic plasticity and learning and memory. Mutations of alleles of 14-3-3ζ result in deficits of olfactory learning in Drosophila [12]. In the mouse cerebellum, LTP of the granule cell-Purkinje cell synapses involves binding of 14-3-3 protein to phosphorylated Ser413 of the active-zone protein RIM1α [21]. We previously reported that Csp24 co-precipitated with actin and co-localizes with actin in the cytosol of type B-photoreceptors [8]. Downstream of ERK are several protein kinases that control cytoskeletal architecture [20]. One substrate of an ERK-activated protein kinase is 14-3-3 protein. The activity of p160 ROCK phosphorylates Lim kinase which results in cofilin phosphorylation and the stabilization of F-actin [15]. Cofilin in its inactive phosphorylated conformation is bound to 14-3-3 proteins and its dephosphorylation by protein phosphatases is inhibited. Following stimulation, small Hsps are phosphorylated and compete with phosphorylated cofilin for 14-3-3 protein binding. This results in the dephosphorylation of cofilin and its binding to the barbed ends of actin, which blocks actin polymerization and results in actin remodeling. The interaction of Csp24 and 14-3-3 protein may be important for the posttranslational modifications of proteins contributing to actin-filament dynamics underlying conditioning-dependent structural remodeling.
Acknowledgments
This research was supported by Grant MH 40860 to T.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aitken A, Howell S, Jones D, Madrazo J, Patel Y. 14-3-3 α and δ are the phosphorylated forms of Raf-activating 14-3-3 β and ζ In vivo stoichiometric phosphorylation in brain at a Ser-Pro-Glu-Lys motif. J Biol Chem. 1995;270:5706–5709. doi: 10.1074/jbc.270.11.5706. [DOI] [PubMed] [Google Scholar]
- 2.Aitken A, Baxter H, Dubbis T, Clokie S, Mackie S, Mitchell K, Peden A, Zemlickova E. Specificity of 14-3-3 isoform dimmer interactions and phosphorylation. Biochem Soc Trans. 2002;30:351–360. doi: 10.1042/bst0300351. [DOI] [PubMed] [Google Scholar]
- 3.Aitken A. 14-3-3 proteins: A historic overview. Seminars in Cancer Biol. 2006;16:163–172. doi: 10.1016/j.semcancer.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Bridges D, Moorhead GBG. 14-3-3 proteins: a number of functions for a numbered protein. Sci STKE. 2005:re10. doi: 10.1126/stke.2962005re10. [DOI] [PubMed] [Google Scholar]
- 5.Crow T, Forrester J, Williams M, Waxham MN, Neary JT. Down-regulation of protein kinase C blocks 5-HT-induced enhancement in Hermissenda B photoreceptors. Neurosci Letts. 1991;121:107–110. doi: 10.1016/0304-3940(91)90660-l. [DOI] [PubMed] [Google Scholar]
- 6.Crow T, Xue-Bian JJ, Siddiqi V, Kang Y, Neary JT. Phosphorylation of mitogen-activated protein kinase by one-trial and multi-trial classical conditioning. J Neurosci. 1998;18:3480–3487. doi: 10.1523/JNEUROSCI.18-09-03480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crow T, Xue-Bian JJ. Identification of a 24 kDa phosphoprotein associated with an intermediate stage of memory in Hermissenda. J Neurosci. 2000;20:RC74. doi: 10.1523/JNEUROSCI.20-10-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crow T, Xue-Bian JJ. One-trial in vitro conditioning regulates a cytoskeletal-related protein (CSP24) in the conditioned stimulus pathway of Hermissenda. J Neurosci. 2002;22:10514–10518. doi: 10.1523/JNEUROSCI.22-24-10514.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crow T, Redell JB, Tian LM, Xue-Bian J, Dash PK. Inhibition of conditioned stimulus pathway phosphoprotein 24 expression blocks the development of intermediate-term memory in Hermissenda. J Neurosci. 2003;23:3415–3422. doi: 10.1523/JNEUROSCI.23-08-03415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crow T, Xue-Bian JJ, Dash PK, Tian LM. Rho/ROCK and Cdk5 effects on phosphorylation of a β-thymosin repeat protein in Hermissenda. Biochem Biophys Commun. 2004;323:395–401. doi: 10.1016/j.bbrc.2004.08.103. [DOI] [PubMed] [Google Scholar]
- 11.Crow T, Xue-Bian JJ. One-trial in vitro conditioning of Hermissenda regulates phosphorylation of Ser122 of Csp24. Ann NY Acad Sci. 2007 doi: 10.1196/annals.1415.012. in press. [DOI] [PubMed] [Google Scholar]
- 12.Efthimios M, Skoulakis G, Davis RL. Olfactory learning deficits in mutants for leonardo, a Drosophila gene encoding a 14-3-3 protein. Neuron. 1996;17:931–944. doi: 10.1016/s0896-6273(00)80224-x. [DOI] [PubMed] [Google Scholar]
- 13.Fanti WJ, Muslin AJ, Kikuchi A, Martin JA, MacNicol AM, Gross RW, Williams LT. Activation of Raf-1 by 14-3-3 proteins. Nature. 1994;371:612–614. doi: 10.1038/371612a0. [DOI] [PubMed] [Google Scholar]
- 14.Farley J, Auerbach S. Protein kinase C activation induces conductance changes in Hermissenda photoreceptors like those seen in associative learning. Nature. 1986;319:220–223. doi: 10.1038/319220a0. [DOI] [PubMed] [Google Scholar]
- 15.Gaestel M. MAPKAP kinases – MKs – two’s company, three’s a crowd. Nature Rev. 2006;7:120–130. doi: 10.1038/nrm1834. [DOI] [PubMed] [Google Scholar]
- 16.Gu YM, Jin YH, Choi JK, Back KY, Yeo CY, Lee K-Y. Protein kinase A phosphorylates and regulates dimerization of 14-3-3ζ. FEBS Letts. 2006;580:305–310. doi: 10.1016/j.febslet.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Hashiguchi M, Sobue K, Paudel HK. l4-3-3ζ is an effector of tau protein phosphorylation. J Biol Chem. 2000;275:25247–25254. doi: 10.1074/jbc.M003738200. [DOI] [PubMed] [Google Scholar]
- 18.Matto-Yelin M, Aitken A, Ravid S. 14-3-3 inhibits the Dictyostelium myosin II heavy-chain specific protein kinase C activity by a direct interaction: identification of the 14-3-3 binding domain. Mol Biol Cell. 1997;10:1889–1899. doi: 10.1091/mbc.8.10.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen TA, Takemoto LJ, Takemoto DJ. Inhibition of gap junction activity through the release of the C1B domain of protein kinase Cγ (PKCγ) from 14-3-3. J Biol Chem. 2004;279:52714–52715. doi: 10.1074/jbc.M403040200. [DOI] [PubMed] [Google Scholar]
- 20.Raftopoulou M, Hall A. Cell migration: Rho GTPase leads the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Simsek-Duran F, Linden DJ, Lonert G. Adapter protein 14-3-3 is required for a presynaptic form of LTP in the cerebellum. Nat Neurosci. 2004;7:1246–1298. doi: 10.1038/nn1348. [DOI] [PubMed] [Google Scholar]
- 22.Toker A, Ellis CA, Sellers LA, Aitken A. Protein kinase C inhibitor proteins, purification from sheep brain and sequence similarity to lipocortins and 14-3-3 protein. Europ J Biochem. 1990;191:421–429. doi: 10.1111/j.1432-1033.1990.tb19138.x. [DOI] [PubMed] [Google Scholar]
- 23.Tzivion G, Avruch J. 14-3-3 proteins: active cofactors in cellular regulation of serine/threonine phosphorylation. J Biol Chem. 2002;277:3061–3064. doi: 10.1074/jbc.R100059200. [DOI] [PubMed] [Google Scholar]
- 24.Woodcock JM, Murphy J, Stomaski FC, Berndt MC, Lopez AF. The dimeric versus monomeric status of 14-3-3ζ is controlled by phosphorylation of Ser58 at the dimmer interface. J Biol Chem. 2003;278:36323–36327. doi: 10.1074/jbc.M304689200. [DOI] [PubMed] [Google Scholar]
- 25.Yaffe MB. How do 14-3-3 proteins work? – Gatekeeper phosphorylation and the molecular anvil hypothesis. FEBS Letts. 2002;513:53–57. doi: 10.1016/s0014-5793(01)03288-4. [DOI] [PubMed] [Google Scholar]
- 26.Yamoah EN, Levic S, Redell JB, Crow T. Inhibition of conditioned stimulus pathway phosphoprotein 24 expression blocks the reduction in A-type transient K+ current produced by one-trial in vitro conditioning of Hermissenda. J Neurosci. 2005;25:4793–4800. doi: 10.1523/JNEUROSCI.5256-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Wang H, Liu D, Liddington R, Fu H. Raf-1 kinase and exoenzyme S interact with 14-3-3ζ through a common site involving lysine 49. J Biol Chem. 1997;272:13717–13724. doi: 10.1074/jbc.272.21.13717. [DOI] [PubMed] [Google Scholar]