Abstract
Disease progression of glioblastoma involves a complex interplay between tumor cells and the peri-tumor microenvironment. The propensity of malignant glioma cells to disperse throughout the brain typifies the disease and portends a poor response to surgical resection, radio-therapy, and current chemotherapeutics. The focal adhesion kinases FAK and Pyk2 function as important signaling effectors in glioma through stimulation of pro-migratory and proliferative signaling pathways. In the current study, we examined the importance of Pyk2 and FAK in the pathobiology of malignant glioma in an intracranial xenograft model. We show that mice with xenografts established with glioma cells with specific knockdown of Pyk2 or FAK expression by RNA interference had significantly increased survival compared to control mice. Furthermore, the effect of inhibition of Pyk2 activity in xenografts was compared to the effect of knockdown of Pyk2 expression. Inhibition of Pyk2 activity by stable expression an autonomous FERM domain in glioma cells slowed disease progression in the intracranial xenograft model. In contrast, expression of a variant FERM domain that does not inhibit Pyk2 activity did not alter survival. These results substantiate the disease relevance of both Pyk2 and FAK in glioma and suggest a novel approach to target Pyk2 for therapeutic benefit.
Keywords: Glioma, Focal adhesion kinase, FAK, Pyk2, Xenografts, Invasion
Introduction
Glioblastoma multiforme, the most common and malignant central nervous system cancer, carries an exceptionally poor prognosis with median survival of approximately one year following diagnosis [1, 2]. Current treatment typically includes surgical resection followed by radiation and chemotherapy yet the highly infiltrative behavior of malignant glioma cells negates the effectiveness of this approach. Although tumor invasion of surrounding normal brain is a significant reason for treatment failure, the molecular mechanisms that underlie glioma invasion remain largely undefined. Invasion relies mechanically on cellular interaction with the extracellular matrix (ECM) and alterations in the composition of the ECM have been associated with glioma invasion [3–5]. Moreover, adhesion to ECM strongly stimulates cell survival signaling pathways and plays an important role in modulating the apoptotic response. Therefore, understanding the mechanisms that couple alterations in the tumor microenvironment to activation of intracellular signaling pathways that regulate glioma invasion and proliferation could provide important insights into new therapeutic targets.
The non-receptor tyrosine kinases FAK and Pyk2 integrate signals from integrins and growth factor receptors with signaling pathways that regulate cell migration and proliferation [6]. Knockout studies, in vitro overexpression studies, as well as analysis of transformed cells and primary tumor samples have provided compelling support for an important role for FAK in the control of cell motility in a variety of cell types [7]. Unlike the expression of FAK, the expression of Pyk2 has a more restricted tissue distribution with highest levels in the brain and cells of hematopoietic origin where it has been linked to regulation of cell migration. Thus, the migration of macrophages and lymphocytes from Pyk2 null mice is significantly impaired [8, 9]. As both kinases share a conserved domain structure, possess significant sequence identity, and have been reported to interact with many of the same adapter proteins and downstream effectors, defining the specific contributions of FAK and Pyk2 to cell proliferation and migration has been problematic. Indeed, it has been proposed that FAK and Pyk2 may function in an antagonistic manner [10, 11] as well as in a compensatory or synergistic role [12, 13]. The differences in these conclusions may well reflect the important influence of cell type specific elements. Since gliomas express both FAK and Pyk2, the balance of activity of these two kinases may be particularly important to the temporal regulation of the proliferative or invasive behavior of the malignant cells that is intimately linked to disease progression.
Previous findings from our laboratory and others have established roles for FAK and Pyk2 in the biology of malignant gliomas. Notably, increased expression of FAK increased glioma cell anchorage independent growth in vitro and increased tumor cell proliferation in vivo [14, 15]. Increased Pyk2 activity has been correlated with increased migration/invasiveness of gliomas in vitro [16]. In addition, Pyk2 expression is differentially upregulated in invasive glioma cells relative to cells in their cognate tumor cores in glioblastoma tumor samples [17]. Similarly, immunohistochemical analysis of astrocytoma tissue samples of varying pathological grades indicated significant co-expression of both FAK and Pyk2 with Pyk2 expression occurring much more frequently and with higher expression scores than FAK with increasing tumor grade [18]. The present study was undertaken to further investigate the role of Pyk2 and FAK in glioma proliferation and invasion. We demonstrate that using siRNAs to specifically silence expression of Pyk2 or FAK or modulating endogenous Pyk2 activity significantly extends survival in a glioma xenograft model. Validating and characterizing the relevant pathobiology of FAK and Pyk2 with glioma invasion and proliferation will provide insights into more complex questions centered upon tumor sensitivities to adjuvant therapies.
Materials and methods
Vector construction and lentiviral transduction
Small interfering RNAs (siRNA) targeting either human Pyk2 or human FAK were assembled in the lentiviral plasmid vector pLVTHM (Addgene, Cambridge, MA) [19] that expresses a fluorescent marker protein (GFP or RFP) under transcriptional control of the EF-1α promoter. Oligonucleotide duplexes encoding a human Pyk2 siRNA or a human FAK siRNA were cloned downstream of the H1 promoter in a second transcriptional cassette inserted into the 3′ LTR to knockdown expression of Pyk2 or FAK. An empty pLVTHM construct expressing GFP was used as a control. Constructs expressing the Pyk2 FERM domain or the Pyk2 FERM I308E variant [20] were engineered in the lentiviral plasmid vector pWPXL (Addgene). The wild type Pyk2 FERM domain, encoding residues R39-A367, or the Pyk2 FERM I308E variant were expressed by the EF1-α promoter as part of a bicistronic unit encoding DsRed using the encephalomyocarditis virus 5′ internal ribosome entry site [21]. All sequences were verified by DNA sequencing.
Recombinant lentiviruses were produced by transient transfection of 293T cells with 20 µg of the appropriate lentiviral transfer vector construct, 15 µg of psPAX2 packaging plasmid, and 5 µg of pMD2G-VSVG envelope vector by calcium phosphate precipitation. Recombinant lentivirus containing supernatants were harvested 48 h after transfection. For lentiviral transduction, medium containing recombinant lentiviruses was added to sub-confluent cultures of SF767 cells. Forty-eight hours after infection, cells were harvested and GFP or RFP positive cells were collected by mass sorting on a FACS Vantage flow cytometer (BD Biosciences, San Jose, CA). SF767 control cells transduced with empty vector GFP expressing were designated SF767 WT. SF767 cells with knockdown of Pyk2 were designated SF767 Pyk2i and those with knockdown of FAK were designated SF767 FAKi. SF767 cells stably expressing the wild type Pyk2 FERM domain were designated SF Pyk2 FERM and those expressing the Pyk2 FERM I308E variant were designated as SF PF I308E.
Immunoblotting
Cells were washed in cold PBS and lysed by addition of 1 ml IPB buffer: 137 mM NaCl, 20 mM Tris, pH 7.5, 1% NP-40, and 10% glycerol containing protease inhibitors (100 µM leupeptin, 5 IU/ml aprotinin, 10 µg/ml soybean trypsin inhibitor, 10 mM benzamidine, and 1 mM phenylmethylsulfonyl fluoride) and phosphatase inhibitors (1 mM orthovanadate). Lysates were incubated on ice for 30 min and clarified by clarified by centrifugation at 16,000×g for 10 min at 4°C. Protein content of the lysate was determined using the BCA assay (Pierce, Rockford, IL). For immunoblotting, equal amounts of protein (10–20 µg) were electrophoresed on 8–16% gradient SDS-PAGE gels (Invitrogen, Carlsbad, CA) and transferred to nitrocellulose. Immunoblotting of transferred proteins was performed with the appropriate antibodies for 1 h at room temperature and was visualized by enhanced chemiluminescence (Perkin Elmer Life Sciences, Boston, MA).
Cell cycle analysis
Wild type SF767 cells or stably transduced SF767 cells were seeded into six well plates and cultured in DMEM media containing 0.5% fetal calf serum for 16 h. Cells were washed and cultured in complete media containing 10% serum for 24 h. Cells were removed from the plate with trypsin, washed in PBS, centrifuged, and resuspended in 1 ml PBS. Cells were then fixed by adding cold ethanol dropwise while vortexing followed by incubation on ice for 1 h. Cells were washed in PBS and resuspended in 100 µl RNase A (100 mg/ml) for 5 min at room temperature. The cells were then stained in 400 µl of propidium iodide (50 µg/ml) and analyzed on a Becton Dickinson FACScan (BD Biosciences) flow cytometer. The percentage of cells in S-phase was calculated using the Modfit program (Verity Software House, Inc., Topsham, ME).
Generation of intracranial xenograft tumors
Female athymic nude mice (age 4–5 weeks) were randomized into groups of eight. Power analysis indicated that a sample size of 8 animals for each group will have 80% power to detect a probability of 0.90 that the time till onset of a moribund state in one group is less than the time until onset of a moribund state in another group using a Wicoxon (Mann–Whitney) rank sum test with a 0.05 two sided significance level. Each animal received either SF767 WT control transduced cells, SF767 Pyki, SF767 FAKi, SF767 Pyk2 FERM, or SF767 Pyk2 FERM I308E variant glioma cells delivered by intraparenchymal injection into the right cerebral hemisphere. Animals were first anesthetized with ketamine (10 mg/kg) and xylazine (90 mg/kg) and a 0.75 cm skin incision was made over the cranial midline. A burr hole was made through the skull 3 mm posterior and 3 mm lateral of bregma and afterwards, the mice were placed into the small animal stereotaxic frame. A micromanipulator bearing a 10 µl Hamilton syringe (30 gauge needle) was advanced through the burr hole until and intraparenchymal depth of 3 mm was reached. Tumor cells (7.5 × 105) were delivered in 10 µl of PBS at a rate of 1 µl/min after which the needle was left an additional 10 min before removal. Following injection, the craniotomy was filled with bone wax and the skin closed with 5–0 silk suture. Mice were weighed daily and observed for the onset of neurological symptoms or until moribund. When reaching the study end-point, animals were euthanized and formalin-perfused brains were harvested for tissue analysis.
Histology and immunohistochemistry
Frozen sections of cryostat sectioned xenograft tissue was stained using antibodies to FAK (Upstate, Lake Placid, NY 10 µg/ml) or Pyk2 (Upstate, Lake Placid, NY 10 µg/ml). Briefly, 5 µm frozen sections were fixed in 10% formalin for 10 min. Antigen retrieval was performed using a pressure cooker at 90 degrees for 10 min in 10 mM sodium citrate buffer. Endogenous peroxidases were quenched using 3% hydrogen peroxide in PBS. Slides were blocked for 15 min in Background Sniper (Biocare, Concord CA). Rabbit polyclonal antibodies against FAK and Pyk2 were applied respectively, at a concentration of 10 µg/ml for 1 h. Slides were rinsed 5× in TBST. A probe/polymer-HRP detection system was used by applying the rabbit probe for 15 min followed by the rabbit polymer-HRP for 15 min (Biocare, Concord CA). The slides were developed with DAB and counterstained with hematoxylin. Assessment of tumor proliferation was performed on deparaffinized, microwave epitope-retrieved brain sections by immunohistochemical staining using a primary antibody to Ki67 (Santa Cruz Biotechnology, Santa Cruz, CA). An automatic immunohistostainer (TechMate 500; Dako, Glostrup, Denmark) was used with a standard avidin-bio-tinylated immunoperoxidase technique for visualization of the probe antibody.
Statistics
Survival of mice with xenografts was determined by Kaplan–Meier analysis using software from SAS. Differences between survival curves were compared with the log-rank test. Statistical significance was set at criterion level P < 0.01. Confidence intervals for the difference between means was also calculated and compared at the 95% confidence level.
Results
Generation of glioma cells with stable knockdown of FAK or Pyk2
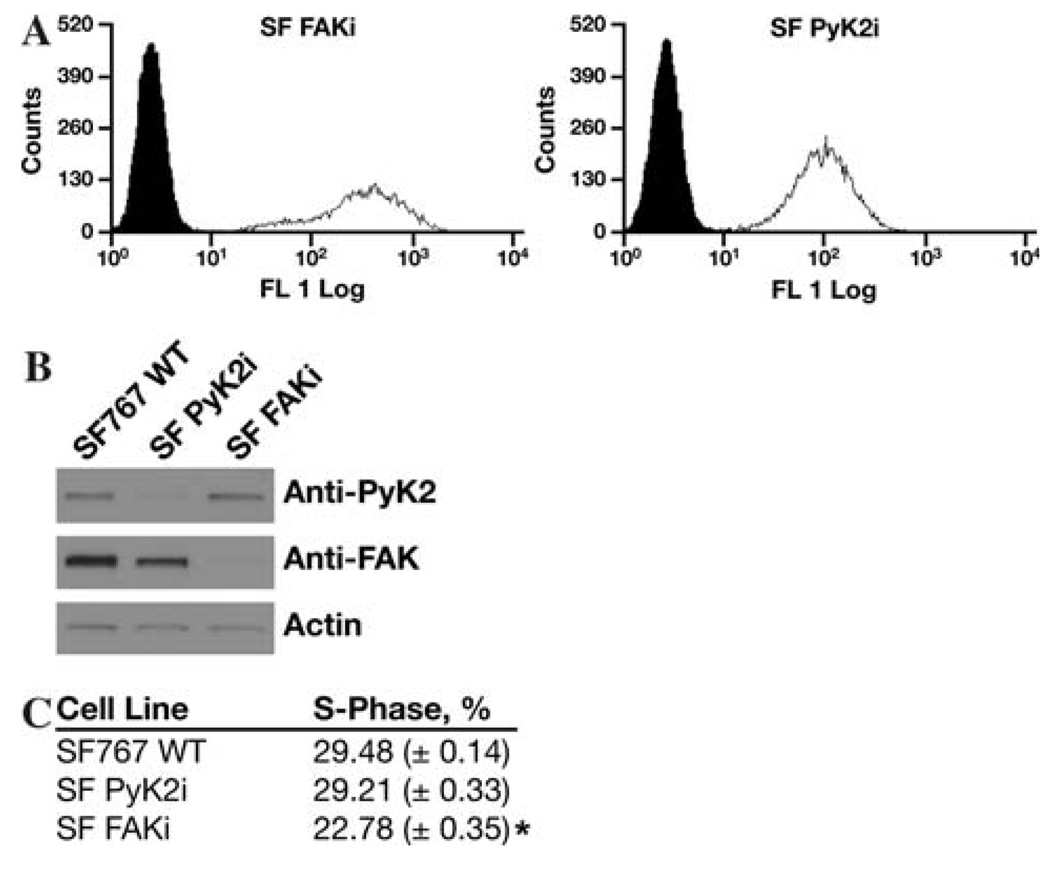
Previously, we demonstrated that Pyk2 and FAK activity exert differential effects on glioma cell phenotype in vitro [16, 22]. Increased expression of Pyk2 stimulated migration of glioma cells on defined ECM substrates and expression of a shRNA targeting Pyk2 inhibited migration in vitro and invasion in an orthotopic brain slice model. Conversely, overexpression of FAK stimulated cell cycle progression but did not alter glioma cell migration. To further characterize the role of each of these focal adhesion kinases in the biology of malignant gliomas, SF767 glioma cells stably expressing a siRNA targeting Pyk2 or SF767 cells stably expressing a siRNA targeting FAK were established by lentiviral transduction and enriched by mass sorting on a flow cytometer. The resulting cell populations were greater than 95% positive (Fig. 1). Immunoblotting of cell lysates demonstrated that knockdown of Pyk2 or FAK was significant and specific in the SF Pyk2i and SF FAKi cell lines, respectively. The effect of silencing Pyk2 or FAK expression on cell proliferation was examined by analysis of cell cycle progression by flow cytometry. Cell cycle analysis of the different cell populations indicated that knockdown of FAK expression decreased the percentage of cells in S-phase relative to control SF767 cells transduced with an empty lentiviral virus. This is consistent with the previously described positive role for FAK in cell cycle progression [23]. On the other hand, knockdown of Pyk2 expression did not alter the percentage of cells in S-phase relative to control cells.
Fig. 1.
RNAi mediated knockdown of Pyk2 and FAK expression. SF767 glioma cells were stably transduced with a lentiviral vector expressing a shRNAi targeting Pyk2 or FAK and the DsRed fluorescent marker. (a) FACS histogram of the mass sorted cell populations. Cell number is on the ordinate and log fluorescence intensity on the abscissa. Solid histogram is wild type SF767 cells. (b) Cell lysates of wild-type SF767, SF Pyk2i, or SF FAKi cells immunoblotted with the indicated antibodies. (c) Subconfluent cultures of the indicated cell lines were serum starved for 16 h and the percentage of cells in S-phase was determined by flow cytometry 24 h after addition of serum. * P<0.05 relative to SF767 WT
Silencing Pyk2 or FAK expression extends survival of orthotopic xenograft mice
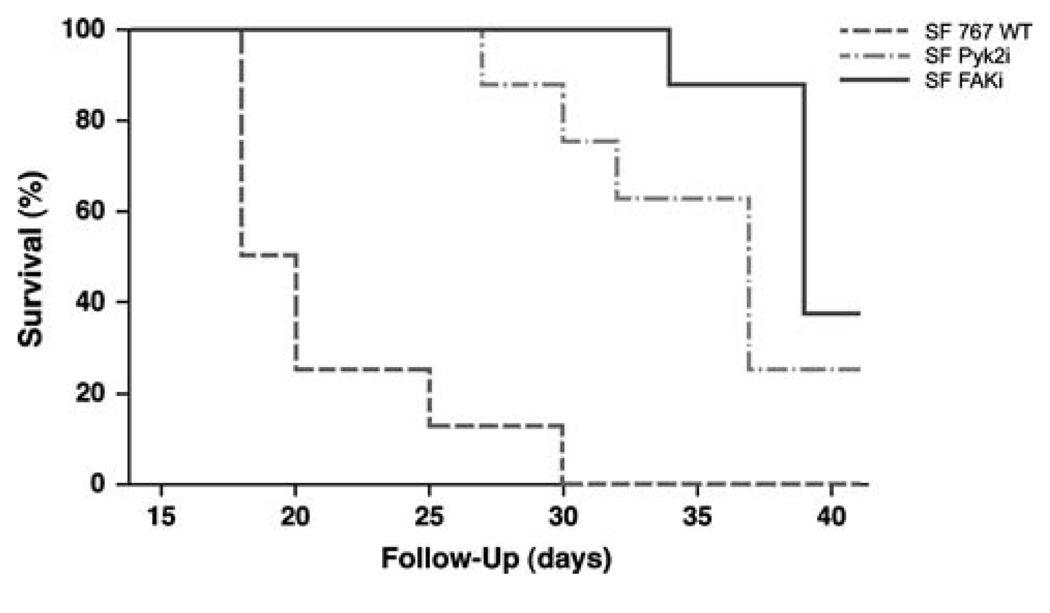
To investigate the effects of Pyk2 or FAK knockdown in a clinically relevant disease model of glioma, SF Pyk2i or SF FAKi cells were used to establish intracranial xenografts in the brains of athymic nude mice. Survival of these mice was compared to that of animals with xenografts established with the control transduced SF767 WT cells. Athymic nude mice grafted with control SF767 WT cells survived a mean of 19 days (95% CI = 18–25 days) (Fig. 2). In contrast, mice with xenografts established with SF Pyk2i cells survived a mean of 37 days (95% CI = 30–41 days, P<0.0001). Similarly, mice with SF FAKi xenografts survived significantly longer than animals with SF767 WT cell xenografts with a mean survival of 39 days (95% CI = 39–41 days, P<0.0001). These results indicate that that stable knockdown of either Pyk2 or FAK results in delayed tumor progression.
Fig. 2.
Survival curves for mice with SF767 wild type, SF Pyk2i, or SF FAKi intracranial tumors. Athymic nude mice were randomly assigned to receive intracranial xenografts of SF767 WT, SF767 Pyki, or SF767 FAKi cells and observed until mice became moribund
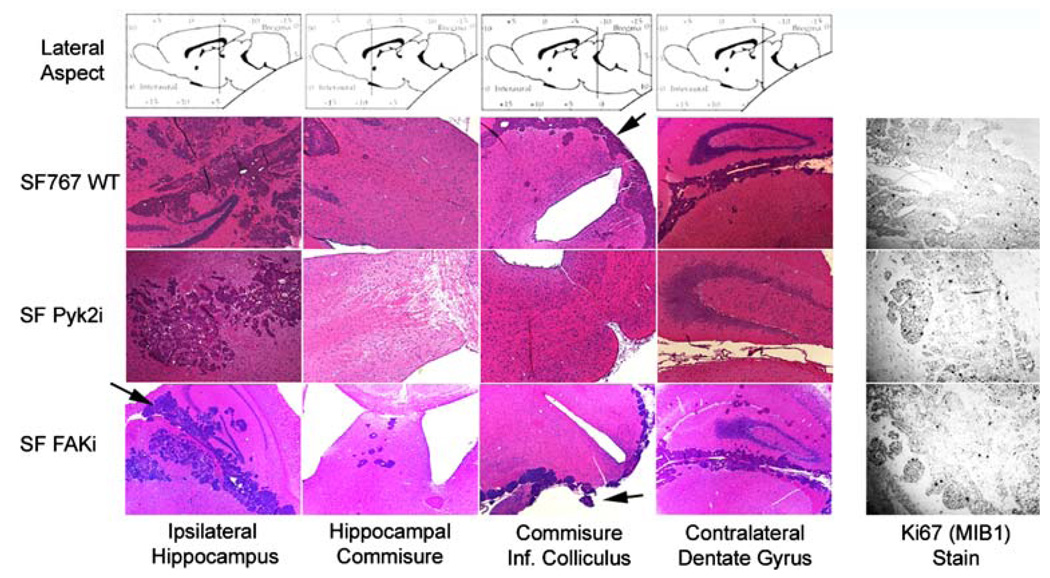
Hematoxylin and eosin staining of tissue sections from throughout the brain demonstrated that the control SF767 WT glioma cells were both locally invasive and dispersed (Fig. 3). Similar staining patterns were observed in brains harvested from the SF FAKi xenografts. In both cases, satellite tumor foci were observed distant from the main tumor mass. In contrast, the SF Pyk2i cells were observed to invade locally, however, distant satellite tumor foci were rarely observed in the xenografts from the SF Pyk2i mice. Dissemination of glioma cells through cerebrospinal fluid was observed in all cases as foci of tumor could be detected in the lateral ventricles and cisterna (Fig. 3, arrows). Inhibition of invasive signaling pathways in malignant glioma cells could potentially result in an associated increase in cellular proliferation. This possibility did not appear to occur in the xenografts as immunostaining of sections from each group with the proliferation marker Ki67 did not reveal any significant differences between the different xenograft tumors (Fig. 3).
Fig. 3.
Histological analysis of tumor sections from xenografts. Following sacrifice, intact brains from mice harboring xenografts established with the indicated cell lines were removed, sectioned and processed for histochemical analysis with hematoxylin and eosin (H&E) or processed for immunohistochemical analysis with Ki67. Sections were obtained from SF767 WT at 20 days post-implantation, SF Pyk2i at 36 days post-implantation, and SF FAKi at 39 days post-implantation. Arrows indicate tumor foci in the lateral ventricles and cisterna
Pyk2 inhibition extends survival of orthotopic xenograft mice
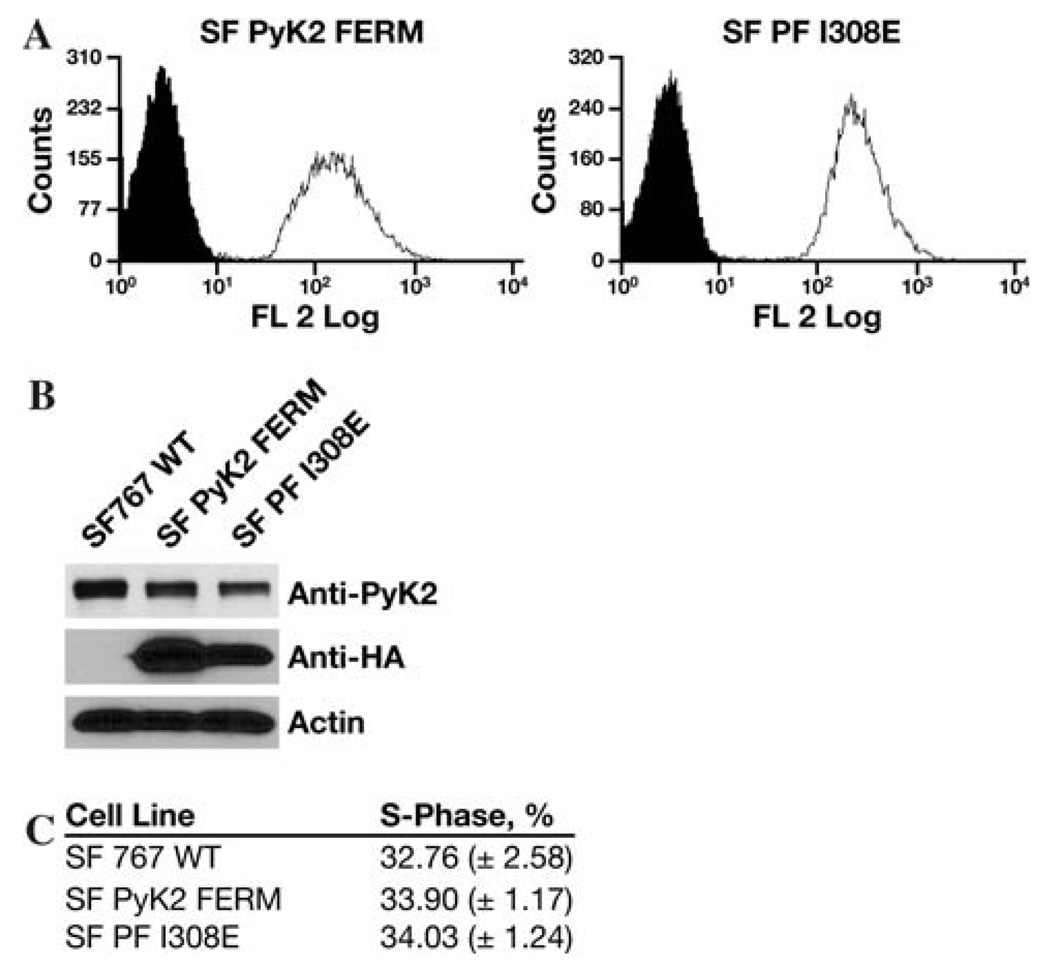
RNA interference is a robust method for evaluating the phenotypic contribution of a signaling effector in tumor models. However, total removal of a protein from signaling pathway may not necessarily be congruent with direct inhibition of the same protein. Thus, in addition to their activity as kinases, both FAK and Pyk2 function as protein scaffolds for the formation of multi-protein signaling complexes. We previously demonstrated that expression of the Pyk2 FERM domain as an autonomous fragment functioned as a Pyk2 inhibitor [20]. Expression of the wild type Pyk2 FERM domain significantly inhibited Pyk2 phosphorylation but did not alter FAK phosphorylation. Moreover, expression of the Pyk2 FERM domain potently inhibited glioma cell migration while expression of a Pyk2 FERM domain containing an I308E substitution did not inhibit Pyk2 phosphorylation or glioma cell migration. To investigate the effect of inhibition of Pyk2 activity on tumor progression, individual SF767 cell lines stably expressing either the wild type Pyk2 FERM domain (SF Pyk2 FERM) or stably expressing the Pyk2 FERM I308E variant (SF PF I308E) were established by lentiviral transduction. The resulting cells lines were enriched by mass sorting on a flow cytometer and expression of the FERM domains verified by immunoblotting (Fig. 4). The expression of either the wild type Pyk2 FERM domain or the Pyk2 FERM I308E variant did not alter the expression of endogenous Pyk2 on the percentage of cells in S-phase relative to control SF767 cells (Fig. 4).
Fig. 4.
Stable expression of wild-type or I308E Pyk2 FERM domain. SF767 cells were stably transduced with a lentiviral vector expressing the wild-type Pyk2 FERM domain or the I308E FERM domain variant. (a) FACS histogram of the mass sorted cell populations. Indicated cells are in the open histogram and wild type SF767 cells in the filled histogram. (b) Cell lysates of SF767 WT, SF Pyk2 FERM, or SF PF I308E cells were immunoblotted with the indicated antibodies. (c) Subconfluent cultures of the indicated cell lines were serum starved for 16 h and the percentage of cells in S-phase was determined by flow cytometry 24 h after addition of serum
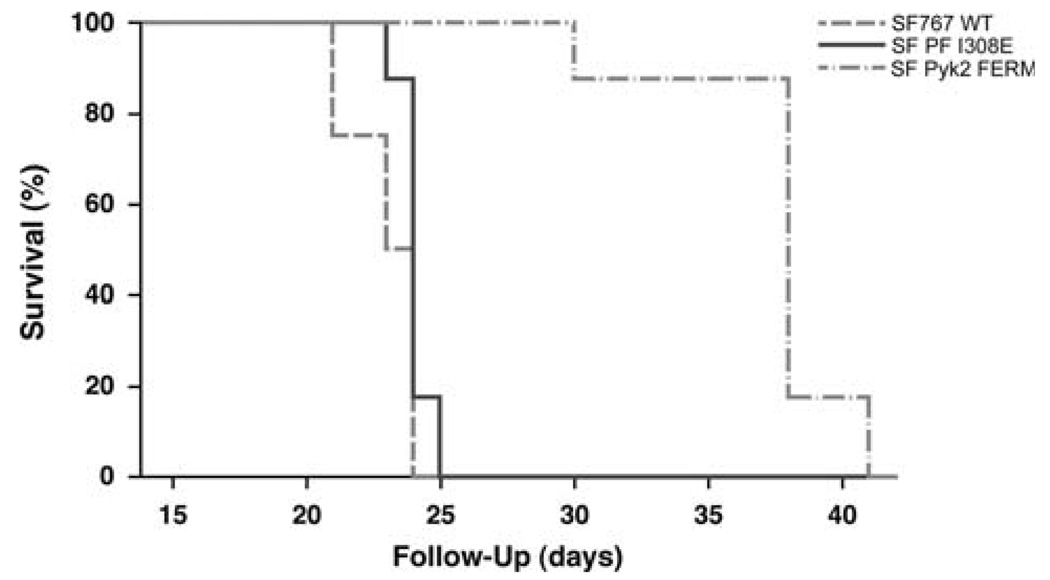
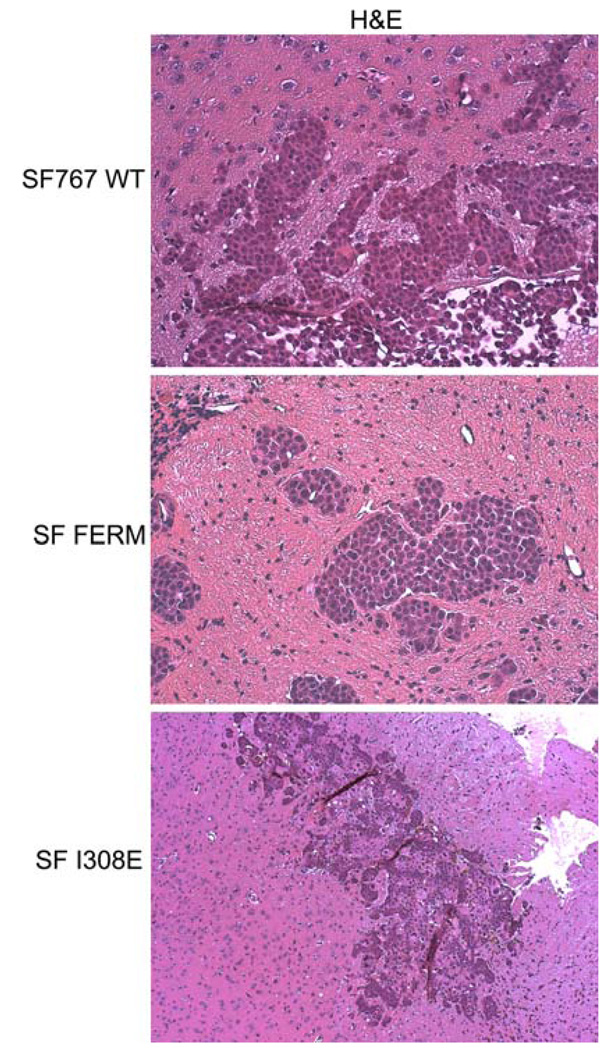
To examine the effect of inhibition of Pyk2 activity on tumor progression in vivo, intracranial xenografts were established with SF767 WT cells, SF Pyk2 FERM cells, or SF PF I308E cells (Fig. 5). Mice with xenografts established with control SF767 WT cells survived a mean of 20 days (95% CI = 17–23 days). Mice bearing SF PF I308E xenografts survived a mean of 21 days (95% CI = 19–25 days) which was not significantly different than animals with SF767 WT xenografts. Mice with SF Pyk2 FERM xenografts survived significantly longer than mice with either SF767 WT or SF PF I308E xenografts with a mean survival of 34 days (95% CI = 28–41 days; P<0.001). Histological analysis of tissue sections showed that there was significant local invasion in the SF767 WT and SF PF I308E tumors (Fig. 6). In contrast, the tumors that arise from the SF Pyk2 FERM cells showed much less dispersion and tended to grow as a more discrete mass. These data indicate that similar to knockdown of Pyk2 expression, inhibition of Pyk2 favorably altered tumor progression in this disease model consistent with the results obtained with knockdown of Pyk2 expression.
Fig. 5.
Effect of inhibition of Pyk2 on survival. Athymic nude mice were randomly assigned to receive intracranial xenografts of wild-type SF767 cells, SF767 cells expressing the Pyk2 FERM domain, or SF767 cells expressing the I308E FERM mutant and observed until mice became moribund. Survival curves show a significant survival benefit for the mice with SF PF I308E xenografts
Fig. 6.
SF767 cell distribution within xenograft tumors. Histological staining (H&E) of tissue sections from endpoint animals illustrates tumor morphology of the indicated intracranial xenografts
Discussion
In this study, we present data that the focal adhesion kinases Pyk2 and FAK play important roles in the pathobiology of malignant gliomas. Notably, silencing the expression of Pyk2 or FAK utilizing RNA interference significantly increased survival in an intracranial xenograft model. Furthermore, specific inhibition of Pyk2 activity significantly increased survival and reduced tumor invasion. Together, these data support the utility of Pyk2 and FAK as therapeutic targets to inhibit glioma tumor progression.
In previous studies, we have found that the focal adhesion kinases FAK and Pyk2 appear to exert differential effects on the phenotype of glioma cells. In vitro studies with glioma cell lines indicated that migratory potential correlated positively with endogenous Pyk2 activity [22]. Moreover, overexpression of Pyk2 stimulated glioma cell migration while knockdown of Pyk2 expression significantly inhibited glioma cell migration [16]. Notably, increased expression of FAK in Pyk2 knockdown cells was unable to rescue their migration deficit. Studies examining the role of FAK in these assays produced some interesting results [16]. Specifically, overexpression of FAK did not stimulate glioma cell migration but rather inhibited glioma cell migration. Inhibition of migration was accompanied by a stimulation of cell cycle progression. Surprisingly, knockdown of FAK expression in SF767 glioma cells did not significantly inhibit migration relative to control cells. This led to the proposal that the dynamic modulation of FAK and Pyk2 activity may regulate the temporal changes in invasion and proliferation that are attendant to glioma tumor progression.
The current study was undertaken to compare these findings to those obtained in a mouse xenograft model where glioma cells are capable of interacting with authentic brain ECM components and soluble mediators that cannot be duplicated in vitro. Using a mouse model of intracranial xenografts established with control or knockdown glioma cells, data was obtained that largely supported the results of in vitro studies. Tumors established with control transduced SF767 glioma cells developed quickly and were invasive producing satellite tumors at sites distant from the site of implantation. In contrast, knockdown of FAK or Pyk2 expression significantly delayed tumor growth and increased survival. Tumors with knockdown of Pyk2 expression showed some invasion at the tumor margin but satellite tumors largely were not observed consistent with previous data indicating that Pyk2 activity stimulates glioma cell migration. Tumors generated with the FAK knockdown cells showed invasion both at the tumor margin and the development of satellite tumors although the numbers were less than that present in the control tumors. The infiltrative nature of the SF767 glioma cell xenografts was distinct from the minimally invasive, well-demarcated tumors typically observed in xenografts established with the U87 glioma cell line. However, the lack of a well-defined, discrete tumor mass in the SF767 xenografts does present difficulty in assigning causation for outcomes. Although histological differences were apparent between FAKi (dispersive) and Pyk2i (non-dispersive) tumors, immunological staining of each tumor for the proliferation marker Ki67 did not indicate a significant difference between control SF767 cells or the knockdown variants. The nearly identical survival advantage conferred by Pyk2 or FAK knockdown thus cannot be explained solely by differences in proliferation rate or invasiveness. We cannot exclude the possibility that the knockdown of either kinase had an initial inhibitory effect on proliferation in the context of this model, but after some time the cells regained the ability to proliferate to a degree similar to that of wild type cells. The results from these studies suggest that additional factors along with proliferation and invasiveness are likely contributing to the ultimate demise from these xenograft tumors.
Interestingly, recent in vitro studies with small molecule inhibitors of FAK kinase activity have provided mixed results. Slack-Davis et al. reported that treatment of cultured PC3 prostate adenocarcinoma cells or REF52 nontransformed fibroblast cells with an small molecule inhibitor of FAK did not block cell growth or induce apoptosis [24]. In contrast, Shi and colleagues demonstrated that treatment of human glioma cell lines in culture with a different FAK inhibitor induced a decrease in cellular proliferation and an increase in apoptosis [25]. These differences could be attributed to the different cell types used in the respective studies as well as to differences in the specificity of each of the inhibitors. In the current study, although the FAK knockdown cells had a small reduction of cell cycle progression in vitro, tumors in the FAK knockdown xenografts did not show evidence of markedly reduced proliferation suggesting that the increased survival of these animals was not due directly to decreased tumor burden. Together, the results indicate that inhibiting the activities of both FAK and Pyk2 might serve as a beneficial therapeutic approach to inhibit glioblastoma progression.
The propensity of malignant glioma cells to invade the surrounding normal brain makes effective treatment nearly impossible. As the invasion of Pyk2 knockdown cells was reduced compared to control cells, we investigated whether direct inhibition of Pyk2 would reduce glioma cell invasion. To inhibit Pyk2, cells were stably transduced to express the Pyk2 FERM domain as an autonomous fragment. Expression of the Pyk2 FERM domain inhibits Pyk2 phosphorylation and potently inhibits glioma cell migration in vitro [20]. This effect is specific for Pyk2 as expression of the Pyk2 FERM domain does not inhibit FAK [20]. Inhibition of Pyk2 activity in xenografts by stable expression of an autonomous Pyk2 FERM domain reduced tumor invasion and significantly increased survival. However, survival of mice engrafted with SF767 cells stably expressing the Pyk2 FERM I308E variant, which does not inhibit Pyk2 phosphorylation or glioma cell migration, was not different than that of control mice with SF767 WT xenografts and the tumors exhibited invasion patterns similar to tumors from wild type SF767 tumors. Although the mechanism for the inhibition of Pyk2 activity by expression of the Pyk2 FERM domain remains to be determined, it has recently been demonstrated that an autonomously expressed Pyk2 FERM domain was capable of forming a Ca2+ dependent heterodimer with full length Pyk2 [26]. The authors proposed that the formation of this heterodimer, in turn, blocked Pyk2 homodimer formation and the resultant transphosphorylation and activation of Pyk2 [27]. Alternatively, exogenous expression of the FERM domain may competitively inhibit protein interactions that regulate Pyk2 activity. Therefore, reducing Pyk2 activity delays tumor progression and suggests that the Pyk2 FERM domain may represent a novel therapeutic target as protein–protein interaction sites are increasingly being considered as potential drug targets [28].
A central tenet of the “go or grow” hypothesis is that cell proliferation and cell invasion are temporally exclusive events in diffusively infiltrative gliomas [29]. While there is significant debate as to whether glioma tumor cells will defer proliferation for invasion [30], this hypothesis suggests that one behavior will have a direct influence on the other since cells cannot invade and proliferate at the same time. Thus, altering the balance of activity between FAK and Pyk2 might be expected to influence the expression of the proliferative or invasive phenotypes. Silencing Pyk2 expression or inhibiting Pyk2 activity could potentially have resulted in increased cellular proliferation by leaving FAK activity unopposed. Similarly, silencing FAK expression could have increased invasion by leaving Pyk2 activity unopposed. Interestingly, neither of these potentially unfavorable outcomes was observed in the xenografts consistent with the described plasticity of the proliferative and invasive phenotypes [31].
In summary, we propose that the focal adhesion kinases FAK and Pyk2 play important roles in the biology of malignant gliomas. Reduction in FAK or Pyk2 activity significantly altered tumor progression and increased survival in a glioma xenograft model. Examining the effect of inhibition of FAK or Pyk2 in serially passaged primary glioblastoma xenografts shown to maintain the morphologic and molecular characteristics of the corresponding patient tumor [32] is necessary to fully support the results of the current study in glioblastoma cells and in vivo disease models more reflective of those found in patient tumors. Preliminary results in ongoing studies with several invasive primary glioblastoma cell lines, silencing Pyk2 expression significantly prolonged survival in the xenograft model consistent with the current results (unpublished observations). The current results further suggest that inhibition of FAK and Pyk2 may have utility to increase responsiveness when used in combination with current therapeutic agents. Inhibition of both kinases may ultimately provide the best approach to improve the clinical outcome for glioblastoma.
Acknowledgments
This work was supported by grants from the National Institutes of Health (CA 103956 and CA 108961 to J.C.L.).
Contributor Information
Christopher A. Lipinski, Mayo Clinic Arizona, 13400 East Shea Blvd, 85259 Scottsdale, AZ, USA
Nhan L. Tran, Translational Genomics Research Institute, Phoenix, AZ, USA
Carole Viso, Mayo Clinic Arizona, 13400 East Shea Blvd, 85259 Scottsdale, AZ, USA.
Jean Kloss, Mayo Clinic Arizona, 13400 East Shea Blvd, 85259 Scottsdale, AZ, USA.
Zhongbo Yang, Mayo Clinic Arizona, 13400 East Shea Blvd, 85259 Scottsdale, AZ, USA.
Michael E. Berens, Translational Genomics Research Institute, Phoenix, AZ, USA
Joseph C. Loftus, Email: loftus.joseph@mayo.edu, Mayo Clinic Arizona, 13400 East Shea Blvd, 85259 Scottsdale, AZ, USA.
References
- 1.Stupp R, Gander M, Leyvraz S, Newlands E. Current and future developments in the use of temozolomide for the treatment of brain tumours. Lancet Oncol. 2001;2:552–560. doi: 10.1016/S1470-2045(01)00489-2. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Gladson CL. The extracellular matrix of glioma: modulation of cell function. J Neuropathol Exp Neurol. 1999;58:1029–1040. doi: 10.1097/00005072-199910000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Nutt CL, Matthews RT, Hockfield S. Glial tumor invasion: a role for the upregulation and cleavage of BEHAB/Brevican. Neuroscientist. 2001;7:113–122. doi: 10.1177/107385840100700206. [DOI] [PubMed] [Google Scholar]
- 5.Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36:1046–1069. doi: 10.1016/j.biocel.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci. 2001;114:3583–3590. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- 7.Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev. 2004;14:92–101. doi: 10.1016/j.gde.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat Immunol. 2000;1:31–36. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- 9.Okigaki M, Davis C, Falasca M, Harroch S, Felsenfeld DP, Sheetz MP, et al. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc Natl Acad Sci USA. 2003;100:10740–10745. doi: 10.1073/pnas.1834348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du QS, Ren XR, Xie Y, Wang Q, Mei L, Xiong WC. Inhibition of PYK2-induced actin cytoskeleton reorganization, PYK2 autophosphorylation and focal adhesion targeting by FAK. J Cell Sci. 2001;114:2977–2987. doi: 10.1242/jcs.114.16.2977. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J, Zheng C, Guan J. Pyk2 and FAK differentially regulate progression of the cell cycle. J Cell Sci. 2000;113:3063–3072. doi: 10.1242/jcs.113.17.3063. [DOI] [PubMed] [Google Scholar]
- 12.Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK(−) cell migration. EMBO J. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueki K, Mimura T, Nakamoto T, Sasaki T, Aizawa S, Hirai H, et al. Integrin-mediated signal transduction in cells lacking focal adhesion kinase p125FAK. FEBS Lett. 1998;432:197–201. doi: 10.1016/s0014-5793(98)00862-x. [DOI] [PubMed] [Google Scholar]
- 14.Wang D, Grammer JR, Cobbs CS, Stewart JE, Jr, Liu Z, Rhoden R, et al. p125 focal adhesion kinase promotes malignant astrocytoma cell proliferation in vivo. J Cell Sci. 2000;113:4221–4230. doi: 10.1242/jcs.113.23.4221. [DOI] [PubMed] [Google Scholar]
- 15.Hecker TP, Ding Q, Rege TA, Hanks SK, Gladson CL. Overexpression of FAK promotes Ras activity through the formation of a FAK/p120RasGAP complex in malignant astrocytoma cells. Oncogene. 2004;23:3962–3971. doi: 10.1038/sj.onc.1207541. [DOI] [PubMed] [Google Scholar]
- 16.Lipinski CA, Tran NL, Menashi E, Rohl C, Kloss J, Bay RC, et al. The tyrosine kinase pyk2 promotes migration and invasion of glioma cells. Neoplasia. 2005;7:435–445. doi: 10.1593/neo.04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoelzinger DB, Mariani L, Weis J, Woyke T, Berens TJ, McDonough WS, et al. Gene expression profile of glioblastoma multiforme invasive phenotype points to new therapeutic targets. Neoplasia. 2005;7:7–16. doi: 10.1593/neo.04535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutenberg A, Bruck W, Buchfelder M, Ludwig HC. Expression of tyrosine kinases FAK and Pyk2 in 331 human astrocytomas. Acta Neuropathol. 2004;108:224–230. doi: 10.1007/s00401-004-0886-3. [DOI] [PubMed] [Google Scholar]
- 19.Szulc J, Wiznerowicz M, Sauvain MO, Trono D, Aebischer P. A versatile tool for conditional gene expression and knockdown. Nat Methods. 2006;3:109–116. doi: 10.1038/nmeth846. [DOI] [PubMed] [Google Scholar]
- 20.Lipinski CA, Tran NL, Dooley A, Pang YP, Rohl C, Kloss J, et al. Critical role of the FERM domain in Pyk2 stimulated glioma cell migration. Biochem Biophys Res Commun. 2006;349:939–947. doi: 10.1016/j.bbrc.2006.08.134. [DOI] [PubMed] [Google Scholar]
- 21.Wiznerowicz M, Trono D. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J Virol. 2003;77:8957–8961. doi: 10.1128/JVI.77.16.8957-8961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipinski CA, Tran NL, Bay C, Kloss J, McDonough WS, Beaudry C, et al. Differential role of proline-rich tyrosine kinase 2 and focal adhesion kinase in determining glioblastoma migration and proliferation. Mol Cancer Res. 2003;1:323–332. [PubMed] [Google Scholar]
- 23.Zhao JH, Reiske H, Guan JL. Regulation of the cell cycle by focal adhesion kinase. J Cell Biol. 1998;143:1997–2008. doi: 10.1083/jcb.143.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slack-Davis JK, Martin KH, Tilghman RW, Iwanicki M, Ung EJ, Autry C, et al. Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem. 2007;282:14845–14852. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]
- 25.Shi Q, Hjelmeland AB, Keir ST, Song L, Wickman S, Jackson D, et al. A novel low-molecular weight inhibitor of focal adhesion kinase, TAE226, inhibits glioma growth. Mol Carcinog. 2007;46:488–496. doi: 10.1002/mc.20297. [DOI] [PubMed] [Google Scholar]
- 26.Kohno T, Matsuda E, Sasaki H, Sasaki T. Protein-tyrosine kinase CAKβ/PYK2 is activated by binding Ca2+/calmodulin to FERM F2 α2 helix and thus forming its dimer. Biochem J. 2008;410:513–523. doi: 10.1042/BJ20070665. [DOI] [PubMed] [Google Scholar]
- 27.Park SY, Avraham HK, Avraham S. RAFTK/Pyk2 activation is mediated by trans-acting autophosphorylation in a Src-independent manner. J Biol Chem. 2004;279:33315–33322. doi: 10.1074/jbc.M313527200. [DOI] [PubMed] [Google Scholar]
- 28.Archakov AI, Govorun VM, Dubanov AV, Ivanov YD, Veselovsky AV, Lewi P, et al. Protein–protein interactions as a target for drugs in proteomics. Proteomics. 2003;3:380–391. doi: 10.1002/pmic.200390053. [DOI] [PubMed] [Google Scholar]
- 29.Giese A, Loo MA, Tran N, Haskett D, Coons SW, Berens ME. Dichotomy of astrocytoma migration and proliferation. Int J Cancer. 1996;67:275–282. doi: 10.1002/(SICI)1097-0215(19960717)67:2<275::AID-IJC20>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Corcoran A, Del Maestro RF. Testing the “go or grow” hypothesis in human medulloblastoma cell lines in two and three dimensions. Neurosurgery. 2003;53:174–184. doi: 10.1227/01.neu.0000072442.26349.14. [DOI] [PubMed] [Google Scholar]
- 31.Gao CF, Xie Q, Su YL, Koeman J, Khoo SK, Gustafson M, et al. Proliferation and invasion: plasticity in tumor cells. Proc Natl Acad Sci USA. 2005;102:10528–10533. doi: 10.1073/pnas.0504367102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giannini C, Sarkaria JN, Saito A, Uhm JH, Galanis E, Carlson BL, et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro-oncol. 2005;7:164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]