Abstract
The polysaccharide capsule of Streptococcus pneumoniae is the main virulence factor, which makes the bacterium resistant to phagocytosis. Expression of capsular polysaccharide must be adjusted at different stages of pneumococcal infection, thus, their transcriptional regulation appears to be crucial. To get insight into the existence of regulatory mechanisms common to most serotypes, a bioinformatic analysis of the DNA region located upstream of the capsular locus was performed. With the exception of serotype 37, the capsular locus is located between dexB and aliA on the pneumococcal chromosome. Up to 26 different sequence organizations were found among pneumococci synthesizing their capsule through a Wzy-polymerase-dependent mechanism, mostly varying according to the presence/absence of distinct insertion elements. As a consequence, only ∼250 bp (including a 107 bp RUP_A element) was conserved in 86 sequences, although only a short (ca. 87 bp) region located immediately upstream of cpsA was strictly conserved in all the sequences analyzed. An exhaustive search for possible operator sequences was done. Interestingly, although the promoter region of serotype 3 isolates completely differs from that of other serotypes, most of the proteins proposed to regulate transcription in serotype 3 pneumococci were also predicted to function as possible regulators in non-serotype 3 S. pneumoniae isolates.
Key words: capsular polysaccharide, Streptococcus pneumoniae, transcriptional regulation, bioinformatic analysis, operator sequences
Streptococcus pneumoniae, or pneumococcus, is a significant human pathogen causing both mucosal, such as otitis media and pneumonia, and systemic diseases, including septicemia and meningitis. Pneumococcal capsular polysaccharide (CPS) is immunogenic and induces type-specific protective immunity.1 Although a 23-valent CPS vaccine and a heptavalent protein–polysaccharide conjugate vaccine (designed for pediatric use) are currently available, they are far from being satisfactory. At least 91 different CPS have been described to date in S. pneumoniae.2 The cap (or cps) cluster of S. pneumoniae (Supplementary Fig. S1), which appears to be organized as a single transcriptional unit (see below), is located between dexB and aliA (two genes that do not participate in capsule biosynthesis),3 with the notable exception of the serotype 37 CPS whose synthesis depends on a single protein encoded by a gene located far from the cps locus on the S. pneumoniae chromosome.4 At least 89 of the 91 pneumococcal CPS known to date appear to be synthesized by a Wzy-polymerase-dependent mechanism in which individual repeat units assembled on undecaprenyl phosphate on the inner face of the bacterial membrane are polymerized on the outer membrane surface.5 The synthesis of CPS of serotypes 3 and 37 is catalyzed by a single, membrane-bound glycosyltransferase (synthase) referred to as Cap3B/Cps3S, and Tts, respectively. In these two serotypes, the common sequences located at the 5′ end of all the other loci and that code for regulatory proteins either are not present (type 37) or are mutated and not transcribed (type 3) (Supplementary Fig. S1).4
One of the most striking features of the pneumococcal cps locus is its huge genetic divergence, since only a few genes are conserved among different clusters.6 These genes are located at the 5′ end of the cps locus and are known to be involved in the processing, regulation and export of CPS and, possibly in the attachment of the CPS to the cell wall.7 Remarkably, only the first gene of the cluster (cap/cpsA) is over 90% identical in all the gene clusters. A near consensus, functional promoter sequence (5′-TAGACA-17 nucleotides-TATAAT3’) (cpsp) has been identified 30 nucleotides upstream of the initiation codon of the cap/cpsA gene, and the transcription start point of the cap/cps operon has also been determined.8
Since the capsule makes the bacterium resistant to phagocytosis, maximal expression of CPS is essential for systemic virulence, although the capacity to regulate the amount of CPS also appears to be crucial, e.g. a reduced level of CPS is an absolute requirement for efficient nasopharyngeal colonization.9 It has been reported that the expression of some capsular genes was reduced when pneumococcal cells were treated with penicillin or vancomycin.10,11 Nevertheless, the existence of possible regulatory pathways for CPS biosynthesis, however, is basically unknown and controversial.12–17
We identified 115 different entries in the databases fulfilling the requirements established, i.e. the complete nucleotide sequence was available from the termination codon of dexB to the initiation codon of cpsA. As indicated in Supplementary Table S1, 26 different sequence organizations (SOs) were identified in this region. Sequence organization 10 (37 sequences) was by far the most frequent, and together with SO_1 (22 sequences), SO_2 (8 sequences) and SO_22 (18 sequences) accounted for close to 75% of all the SOs. Although, in most cases, the nucleotide sequence of only one isolate per serotype was available, it was noted that different strains with identical CPS may have different SOs, mostly varying according to the presence (or absence) of distinct insertion sequences (ISs) (Supplementary Fig. S2). Despite the appreciable polymorphism in the dexB–cpsA region, two major SO groups could be recognized: those containing an intact or truncated copy of IS630_Spn1 (from SO_1 to SO_21) (designated group I) and SO_22 to SO_26 (group II) lacking this IS and some additional fragments. IS630_Spn1 is a ∼0.9 kb element firstly reported by Oggioni and Claverys.18 When examining group I sequences, with the exception of SO_9, SO_12 and SO_21 that contain one or more IS in this region, it is evident that similarity in the vicinity of the cpsp region was restricted to a ca. 250 bp sequence (Supplementary Fig. S3). Two different regions can be distinguished in this sequence: (i) a 140 bp fragment that includes a 107 bp RUP_A sequence and a ∼34 bp sequence that resembles the insertion site of IS1381 (Fig. 1A) and (ii) a short (∼87 bp) region embracing the cpsp region (Fig. 1B) (indicated by a green rectangle in Supplementary Fig. S2). RUP_A is a highly repeated extragenic element of S. pneumoniae that is very similar to the inverted terminal repeats of IS630-Spn1 and might be trans-activated by transposase and promote sequence rearrangements.18 The possibility that RUP elements may serve as binding sites for regulatory proteins has been proposed.19 Interestingly, transcriptional start sites have been mapped in a RUP_C element located upstream of tts, the gene encoding the polysaccharide synthase responsible for the synthesis of serotype 37 CPS.20
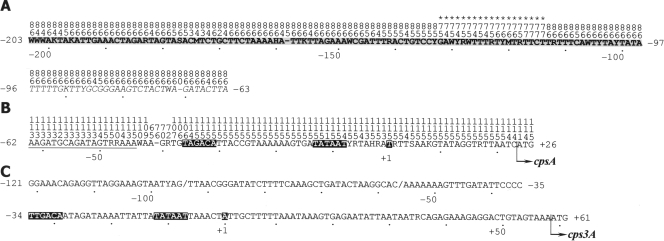
Figure 1.
Consensus sequences derived from alignments of strains synthesizing CPS through a Wzy-polymerase- (A and B) or a synthase-dependent mechanism (C). Eighty six (A), 115 (B) and four (C) sequences were aligned. The frequency of each nucleotide is indicated above the sequence, in vertical format. The position of the transcription initiation site is assigned +1 and other positions are numbered accordingly. (A) The RUP_A element is shaded and a sequence possibly related to some ISs is italicized. Asterisks indicate a region deleted in several strains. Abbreviations: H, A or C or T; K, G or T; M, A or C; R, A or G; S, C or G; W, A or T; Y, C or T. (B) The initiation codon of cpsA is indicated with an arrow. The −35, −10 and the transcription initiation site are indicated by white lettering on a black background. The underlined sequence corresponds to that tandemly duplicated in some strains. (C) Aligned nucleotide sequence of three serotype 3 strains. At positions −94 and −56, a slash indicates a T (or no nucleotide), or an A (or no nucleotide), respectively.
In sharp contrast with most of the group I sequences, group II sequences either lack RUP_A or it is separated from the cpsp region by intervening ISs (Supplementary Fig. S2). Consequently, the ∼87 bp region that contains the promoter of the cps gene cluster (Fig. 1B) turned out to be the only conserved sequence in all the S. pneumoniae isolates that synthesize their CPS through a Wzy-polymerase-dependent mechanism. However, on closer examination of the full alignment (Supplementary Fig. S3), the existence of potentially significant polymorphisms in this region was revealed. In addition to the T to C transition at position −8 in the unencapsulated R6 strain (AE008412), an identical mutation was found at position −11 in strains E294 (CR931699; serotype 33B), CSF/79 (CR931701; serotype 33D), SP18-BS74 (NZ_ABAE01000002; serotype 18C) and WCH18 (CR9316640; serotype 6B). All these mutations cause a change in the −10 region of cpsp from the consensus sequence TATAAT to TATAAC (in R6)21 or TACAAT (in the latter four strains) thereby potentially reducing the corresponding promoter strength. In particular, the reported reduced transcription of the cps genes associated with capsular polysaccharide formation in the unencapsulated strain R621,22 is likely attributable to a mutation in the −10 box of the R6 cps promoter from the consensus TATAAT to TATAAC (see above). Moreover, only the last four nucleotides of the −35 promoter box (TAGACA) were conserved among the 115 sequences examined (Supplementary Fig. S3). It should be noted that in all the sequences examined, 17 perfectly conserved nucleotides separate the −35 and −10 promoter boxes. The transcription initiation site (indicated as +1 in Fig. 1B) is also conserved in all the sequences analyzed.
The effect of intrinsic curvature upstream of a bacterial promoter on the efficiency of transcription was first reported in the early 1980s. To date, there are countless examples indicating the importance of a curved DNA sequence during steps of transcription, mainly in regulating the transcription initiation process.23 It has been recently shown that global transcription factors as well as several other transcriptional regulators have a significant tendency to regulate operons with curved DNA sequences in their upstream regulatory regions.24 Bendability/curvature propensity plots were constructed with the help of the Bend.it server (http://hydra.icgeb.trieste.it/dna/bend_it.html) using defaults parameters with the exception of a 20-nucleotide window size.25 We used the sequence of the 228 bp fragment (including the ATG initiation codon) located upstream of cpsA from the serotype 2 strain D39 (AE026 471) to predict its curvature. The curvature-propensity plot, constructed using DNase I-based trinucleotide parameters, shows four peaks around positions −125, −98, −43 and −17 of magnitudes ≥ 9.0 (data not shown). It should be underscored that the regions showing these potential curvatures occur in the 87 bp fragment containing cpsp, which is the only stretch common to all pneumococci that synthesize their CPS through a Wzy-dependent pathway (see above). A computer prediction of bendability of the above mentioned DNA fragment (AE026471) (data not shown) rendered at least seven conspicuous peaks (positions −175, −137, −128, −77, −57, −9, +5) with magnitudes ≥ 5.0. In addition, the bendability plot showed two long troughs (from positions −158 to −151 and −103 to −96; magnitudes ≤ 3.5) indicative of rigid segments. Interestingly, the latter rigid segment embraces a potential static curvature (centered at −98; see above) and is located near the 3′ end of the RUP_A element.
The annotated genome sequence of the S. pneumoniae strains TIGR4, R6 and D39 revealed the presence of, at least, 80 putative transcriptional regulatory genes, representing ∼4% of the complete genome. Unfortunately, in only few cases was the operator site identified. Putative operators were searched for using Fuzznuc (http://mobyle.pasteur.fr/cgi-bin/MobylePortal/portal.py?form=fuzznuc) and the consensus sequences reported in Fig. 1A and B allowing a maximum of two mismatches. Among the currently known (and putative) pneumococcal operators, only sequences similar to the binding sites of ComX1,26 CopY,27,28 MalR,29 GlnR30,31 or RitR32 were detected (Table 1). As a notable exception, it should be mentioned that none of the four potential binding sites for GlnR are located in the region common to all isolates whose CPS is synthesized through a Wzy-dependent pathway.
Table 1.
Binding sites of transcriptional regulators putatively involved in CPS biosynthesis of S. pneumoniae
| Organism | Transcriptional regulator (Accession no.) | Binding site | S. pneumoniae ortholog (Accession no.) | Identity/similarity (log10E value) | Sequence (5′ position)a |
|
|---|---|---|---|---|---|---|
| Wzy-dependentb | Synthase-dependent | |||||
| S. pneumoniae | ||||||
| ComX1 (Q8CM18) | TACGAATA | — | — | TACtAAgA (−75f) | TgaGAATA (+19f) | |
| TAacAATA (−152r) | TAttAATA (+25f) | |||||
| TAaGAAcA (−111r) | TAatAATR (+28f) | |||||
| TACGAtTA (−4r) | TAttAATA (+25r) | |||||
| CopY (Q8DQJ7) | KACAN2TGTA | — | — | TAgARTaGTA (−186f) | GAggACTGTA (+44f) | |
| TACACATcTg (−178f) | GACtGTaGTA (+47f) | |||||
| TAaAGATGcA (−63f) | ||||||
| aAaAGGTGTA (−44f) | ||||||
| TAtAATcGTA (−13f) | ||||||
| TAtAGGTGTt (+10f) | ||||||
| TACtAYTcTA (−177r) | ||||||
| cAgATGTGTA (−169r) | ||||||
| GACAAATtgA (−120r) | ||||||
| TgCATCTtTA (−54r) | ||||||
| TACACCTtTt (−35r) | ||||||
| TACgATTaTA (−4r) | ||||||
| aACACCTaTA (+19r) | ||||||
| MalR (P0A4T2) | AAACGTTTc | — | — | AAWCGaTT (−149f) | AAAaGTTT (−52f) | |
| AAAgGTgT (−43f) | AAACtaTT (−5f) | |||||
| AAtCGWTT (−142r) | AAACtTTT (−45r) | |||||
| AcACcTTT (−36r) | AAtaGTTT (+3r) | |||||
| GlnR (Q8DQX7) | TGTNAN7TNACA | — | — | TcTAAAAHATTKTTAgA (−166f) | TGaTATTCCCCTTGACA (−45f) | |
| TcTTATTTCATTTTACt (−115f) | TtTTAAATAAAGTGAgA (+7f) | |||||
| TcTAAMAATATTTTAgA (−150r) | TGaGAATATTAATAAtR (+19f) | |||||
| aGTAAAATGAAATAAgA (−99r) | TGTCAAGGGGAATAtCA (−29r) | |||||
| TcTCACTTTATTTAAaA (+23r) | ||||||
| YaTTATTAATATTCtCA (+35r) | ||||||
| RitR (Q04M91) | WNATTANW3RWYRR | — | — | ATATTATATTGAaAc (−201f) | TAATcAGTTTAACGG (−101f) | |
| TGATcAATTTGTCAt (−132f) | AGATaAAATTATTAt (−26f) | |||||
| TTAcTATATTtTTGG (−103f) | AAATTATTATATaAt (−21f) | |||||
| AAtaTAGTAAAATGA (−94r) | TTATTATATAATTAA (−18f) | |||||
| TAATTAAAcTATTGc (−10f) | ||||||
| AAgTgAGAATATTAA (+16f) | ||||||
| TGAgaATATTAATAA (+19f) | ||||||
| ATATTAATAAtgCAG (+24f) | ||||||
| TGATTACTTTccTAA (−96r) | ||||||
| TAATaATTTTATCtA (−13r) | ||||||
| TAATTATATAATaAt (−5r) | ||||||
| TTAaTtATATAATAA (−4r) | ||||||
| AGtTTAATTAtATAA (+1r) | ||||||
| TTATTtAAAAAgCAA (+16r) | ||||||
| ACtTTATTTAAAaAG (+19r) | ||||||
| AdcR (Q04I02) | TAACYRGTTAA | — | — | — | TAAtCAGTTtA (−91f) | |
| TAtCCcGTTAA (−83r) | ||||||
| S. pyogenes | ||||||
| CovR/CsrR (Q8P2J8) | DDHHATTARAR | CsrR (Q8DR53) | 45/68 (−49) | TTATATTgAAA (−198f) | GGTTAggAAAG (−112f) | |
| TGAAAcTAGAR (−192f) | AGTAATYAGtt (−103f) | |||||
| TGCTtcTAAAA (−170f) | ATCTtTTcAAA (−83f) | |||||
| HATTkTTAGAA (−159f) | TGATAcTaAGG (−70f) | |||||
| GTCTAcTAAgA (−78f) | TGACAaTAGAt (−33f) | |||||
| AGATAcTtAAA (−70f) | AATAgaTAAAA (−29f) | |||||
| GATAcTTAAAG (−69f) | TAAAATTAttA (−23f) | |||||
| AGATAgTgAAA (−54f) | AATTATTAtAt (−20f) | |||||
| GATAgTgAAAA (−53f) | ATATAaTtAAA (−13f) | |||||
| AGACATTAccG (−35f) | TATAATTAAAc (−12f) | |||||
| TTACcgTAAAA (−30f) | TGCTtTTtAAA (+3f) | |||||
| TACCgTaAAAA (−29f) | TTTAAaTAAAG (+8f) | |||||
| TTTCAaTAtAA (−188r) | TAAAgTgAGAA (+14f) | |||||
| TACTATTctAG (−177r) | GAATATTAAtA (+22f) | |||||
| ATDTtTTAGAA (−157r) | TATTAaTAAtR (+25f) | |||||
| GGACAgTyAAA (−133r) | TAATAaTRcAG (+28f) | |||||
| GAACATgAcAA (−114r) | ||||||
| TGAAAYaAGAA (−106r) | ||||||
| TAAAATgAAAt (−101r) | ||||||
| GTAAAaTgAAA (−100r) | ||||||
| ATATAgTAAAA (−95r) | ||||||
| GTATcTTAGtA (−65r) | ||||||
| ATACATTgAAc (+12r) | ||||||
| S. agalactiae | ||||||
| RovS (Q8E447) | AWAAWVHTDAWN6/7 WTKWWAMDWAK | SPD_0939 | 52/73 (−76) | — | ATAtAATTAAACTATTGCTTTTTAAATAa (−13f) | |
| B. subtilis | ||||||
| CitT (O34534) | WWCAAA | RpsI (A5MLP7)d | 27/50 (−17) | TTgAAA (−193f) | AACAgA (−118f) | |
| TACAcA (−178f) | TTCAAA (−78f) | |||||
| TTCtAA (−167f) | TACtAA (−67f) | |||||
| TAgAAA (−153f) | cACAAA (−59f) | |||||
| ATCAAt (−130f) | AAaAAA (−55f) | |||||
| TACtAA (−75f) | AAaAAA (−54f) | |||||
| AAaAAA (−46f) | ATaAAA (−24f) | |||||
| TAaAAA (−24f) | ATtAAA (−8f) | |||||
| AAaAAA (−23f) | TTtAAA (+8f) | |||||
| TTCAAt (+3f) | AAtAAA (+12f) | |||||
| TTCAAt (−189r) | TRCAgA (+34f) | |||||
| AACAAt (−153r) | AACtRA (−93r) | |||||
| TTCtAA (−149r) | TTgAAA (−74r) | |||||
| gTCAAA (−138r) | ATCAAA (−42r) | |||||
| gACAAA (−120r) | TAaAAA (+11r) | |||||
| ATgAAA (−105r) | TTaAAA (+12r) | |||||
| AcCAAA (−88r) | TTtAAA (+13r) | |||||
| AACcAA (−87r) | ||||||
| DeoR (P39140) | TTCAAT | Spr0228 (Q8DRC3)d | 28/50 (−30) | aTCAAT (−130f) | TTCAAa (−78f) | |
| TTCAWT (−109f) | TTaAAT (+9f) | |||||
| TTCAAT (+3f) | TTtAAT (−3r) | |||||
| TTCAAT (−189r) | ||||||
| TTCAcT (−45r) | ||||||
| GerE (P11470) | RWWTRGGYN2YY | RR03 (Q8DR45) | 49/67 (−6)e | GATTtGaCTGTC (−145f) | AATTAaaCTATT (−9f) | |
| ATTTGacTGTCC (−144f) | GAAaAGaTATCC (−76r) | |||||
| GTATAGGTRTTa (+10f) | ||||||
| AAATcGWTTTCT (−141r) | ||||||
| cTTTAaGTATCT (−59r) | ||||||
| GATTAtaTCACT (−7r) | ||||||
| CcpA (P25144) | WTGNAANCGNWN2CW | CcpA (Q97NM1) | 54/74 (−96) | TaGAAAWCGATTTrA (−152f) | TTcAAAGCtGATACT (−78f) | |
| ATaTAATCGTAAGaT (−14f) | ||||||
| gTyAAATCGWTTTCT (−138r) | ||||||
| Spo0A (P06 534) | TGTCGAA | RR09 (Q8DQN8) | 38/62 (−10) | TaTtGAA (−195f) | TtTCaAA (−79f) | |
| TGTaGAc (−38f) | TGTaGtA (+50f) | |||||
| TGTtcAA (+1f) | TaTCaAA (−41r) | |||||
| TtTaGAA (−161r) | TGTCaAg (−29r) | |||||
| TtTCtAA (−148r) | ||||||
| aGTCaAA (−137r) | ||||||
| TGaCaAA (−119r) | ||||||
| TGTCtAc (−31r) | ||||||
af and r indicate whether the sequence corresponds to the forward or reverse sequence of that included in the EMBL database (AF026471 or Z47210), respectively. Unless otherwise stated, a maximum of two mismatches were allowed.
bSequences corresponding to those common to all strains are indicated with a gray background. Mismatches are indicated by lowercase lettering.
cThis sequence is a subset of the CcpA binding site.
dOnly one mismatch was allowed.
eSimilarity restricted to part of the protein (from residue 145 to 197 of the pneumococcal protein and 12 to 64 of that of B. subtilis).
We also searched conserved regions for additional operator sequences using proven and putative binding sites from related streptococci (from published works; see Supplementary Table S2) and Bacillus subtilis (from the database of transcriptional regulation in B. subtilis; DBTBS).33 Among the reported binding sites for transcriptional regulators, only those with a clear pneumococcal ortholog were considered. Many potential Streptococcus pyogenes CovR/CsrR binding sites were found34 and the locations of potential operators for five different B. subtilis transcriptional regulators were also determined (Table 1). Furthermore, we also examined the conserved regions for the presence of direct and/or inverted repeats but, although inconclusive at the moment, these searches suggested that other binding sequences are present in this promoter.
As reviewed elsewhere,4 the promoter region of serotype 3 S. pneumoniae isolates completely differs from that of other serotypes. Three different serotype 3 isolates of S. pneumoniae were completely sequenced between dexB and the ATG initiation codon of cps3A (Supplementary Table S1) and two SOs were found, differing only in terms of the length of the IS630_Spn1 element (Supplementary Fig. S4). The three isolates showed ≥ 95% nucleotide sequence identity between the deleted copy of cpsD and the ATG initiation codon of cps3A. This was also true for the serotype 3 strain WU2 (Accession no. U66846 and U15171). Downstream of the deleted copy of cpsD, three pseudogenes were detected: an internal fragment (83 bp) of wchA, a gene putatively encoding the initial sugar transferase from a serotype 20 S. pneumoniae strain; the so-called orf5 (491 bp) putatively coding for a membrane protein (corresponding to Spr1830 in the genome of strain R6) and a 403 bp fragment showing 86% identity to IS1548. A ca. 180 bp DNA fragment containing the serotype 3 promoter (cps3p) was located between IS1548 and cps3A. Polymorphism was only found at four positions in the three serotype 3 isolates that were aligned (Supplementary Fig. S3C).
The curvature-propensity plot of the region containing cps3p showed two prominent peaks around positions −61 and −18. Further, two peaks of bendability ≥ 5.0 were predicted at positions −35 and −66 whereas rigid segments (bendability ≤ 3.5) appeared to span positions −49 to −45, −14 to −11, and −2 to +8 (data not shown).
We also searched the ca. 180 bp sequence upstream of cps3A (Fig. 1C) for potential operators as for the other serotypes (Table 1). Interestingly, with the exceptions of AdcR and RovS, the proteins proposed to regulate cps transcription in serotype 3 pneumococci were also predicted to function as possible regulators in non-serotype 3 S. pneumoniae isolates, which might indicate the existence of common regulatory mechanism in otherwise divergent sequences. Nevertheless, the potential relevance of this finding remains to be determined.
As already mentioned, experiments aimed to demonstrate that CPS biosynthesis is transcriptionally regulated have yielded conflicting results. Notwithstanding, in an elegant electron microscopy study on cultured epithelial cells neither serotype 3 pneumococci in close contact with the host cell membrane nor invading pneumococci exhibited a visible capsular structure, whereas pneumococci not in close contact with the host membrane had a typical capsule. Moreover, S. pneumoniae cells expressed CPS in the lungs of infected mice, whereas bacteria in contact with lung epithelial tissue showed a drastic reduction in the density of the CPS layer.35
Recently, in vitro serotype-dependent expression of cpsA in transparent variants of S. pneumoniae has been observed, i.e. the serotypes/groups associated with invasive infections tend to express more cpsA than those frequently isolated from carriers.17 Unfortunately, in that report, the number of isolates examined was insufficient for a reliable comparison of cpsA expression among different clones of the same serotype (or serogroup).
We should point out that the catabolite repressor protein CcpA (also denoted RegM) appears to be involved in transcriptional activation of the cps operon in the serotype 2 strain D39.36 Besides, it has been reported that a ccpA mutant of the serotype 4 strain TIGR4 was drastically attenuated for infection of the lung and colonization of the nasopharynx.37 Extracellular glucose concentrations might positively regulate the level of CPS biosynthesis. Thus, the glucose concentration is normally very low (< 1 mM) in healthy nasopharyngeal secretions,38 and a reduced amount of CPS is required for optimal attachment of the pneumococcus to epithelial cells.9 In contrast, invading pneumococci encounter high glucose concentrations (5.4 mM) in the blood stream of healthy individuals39 where maximum CPS biosynthesis is most needed. Although no data are available on serotype 3 pneumococci, it should be underscored that the hasABC operon, which is involved in the synthesis of the hyaluronic acid capsule of S. pyogenes and is phylogenetically related to the pneumococcal cps3ABC operon,40 is significantly down-regulated in a ΔccpA mutant.41
Recent studies have also examined the role in virulence of several proven pneumococcal transcriptional regulators using microarrays. Kloosterman et al.31 used a glnR mutant and did not observe a differential expression of the capsular locus, suggesting that the binding sites identified here (Table 1) might not be relevant. However, it has been reported that RitR represses the expression of cps2N, a gene involved in the biosynthesis of dTDP-rhamnose, one of the sugar nucleotides required for the biosynthesis of type 2 CPS.32 Interestingly, binding sites for RitR have been found in the cpsp region (Table 1). It should be kept in mind, that the S. pneumoniae orphan response regulator RitR is very similar to the streptococcal global regulator CovR (also designated as CsrR) and there is conclusive evidence showing that CovR is a global regulator that either represses (S. pyogenes, Streptococcus suis) or upregulates (Streptococcus agalactiae) CPS biosynthesis.42–44 Putative CovR-like operator sequences are located in the region containing the capsular promoter.
Although there is no experimental data on the existence of functional promoters (different of cpsp) located in the intergenic regions of the capsular locus, their possible presence and function should be mentioned. For example, an enhanced biosynthesis of the UDP-glucose dehydrogenase Cps2K has been observed in a D39 ΔcodY mutant.45 A close examination of the 129 bp long region located between cps2H and cps2I (both genes located upstream of cps2K) revealed the existence of a putative promoter (5′-TAGTTG-18 nucleotides-TATTTT-3′) and, further upstream, of an imperfect palindrome (AATTTTtAGAgAATT) quite similar to the consensus binding site of CodY (AATTTTCWGAAAATT).46 Whether these sequences are relevant in vivo requires further studies.
In summary, this study provides some of the sequence data needed to pave the way for systematically identifying the regulatory pathways of CPS biosynthesis in S. pneumoniae.
Supplementary Data
Supplemental data are available online at www.dnaresearch.oxfordjournals.org
Funding
This work was supported by a grant from the Dirección General de Investigación Científica y Técnica (SAF2006-00390). CIBER de Enfermedades Respiratorias (CIBERES) is an initiative of ISCIII.
Acknowledgements
The authors wish to thank P. García and R. López for helpful comments and critical reading of the manuscript, A. Burton for revising the English version and E. Cano for skilful technical assistance.
Footnotes
Edited by Katsumi Isono
References
- 1.López R. Pneumococcus: the sugar-coated bacteria. Int. Microbiol. 2006;9:179–190. [PubMed] [Google Scholar]
- 2.Park I. H., Pritchard D. G., Cartee R., Brandao A., Brandileone M. C. C., Nahm M. H. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 2007;45:1225–1233. doi: 10.1128/JCM.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentley S. D., Aanensen D., Mavroidi A., et al. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2006;2:e31. doi: 10.1371/journal.pgen.0020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López R., García E. Recent trends on the molecular biology of pneumococcal capsules, lytic enzymes, and bacteriophage. FEMS Microbiol. Rev. 2004;28:553–580. doi: 10.1016/j.femsre.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Yother J. In: The Pneumococcus. Tuomanen E. I., Mitchell T. J., Morrison D. A., Spratt B. G., editors. Washington, DC: American Society for Microbiology Press; 2004. pp. 30–48. [Google Scholar]
- 6.Aanensen D. M., Mavroidi A., Bentley S. D., Reeves P. R., Spratt B. G. Predicted functions and linkage specificities of the products of the Streptococcus pneumoniae capsular biosynthetic loci. J. Bacteriol. 2007;189:7856–7876. doi: 10.1128/JB.00837-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadioglu A., Weiser J., Paton J. C., Andre P. W. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 2008;6:288–301. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- 8.Muñoz R., Mollerach M., López R., García E. Molecular organization of the genes required for the synthesis of type 1 capsular polysaccharide of Streptococcus pneumoniae: formation of binary encapsulated pneumococci and identification of cryptic dTDP-rhamnose biosynthesis genes. Mol. Microbiol. 1997;25:79–92. doi: 10.1046/j.1365-2958.1997.4341801.x. [DOI] [PubMed] [Google Scholar]
- 9.Magee A. D., Yother J. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect. Immun. 2001;69:3755–3761. doi: 10.1128/IAI.69.6.3755-3761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas W., Kaushal D., Sublett J., Obert C., Tuomanen E. I. Vancomycin stress response in a sensitive and a tolerant strain of Streptococcus pneumoniae. J. Bacteriol. 2005;187:8205–8210. doi: 10.1128/JB.187.23.8205-8210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers P. D., Liu T. T., Barker K. S., et al. Gene expression profiling of the response of Streptococcus pneumoniae to penicillin. J. Antimicrob. Chemother. 2007;59:616–626. doi: 10.1093/jac/dkl560. [DOI] [PubMed] [Google Scholar]
- 12.Ogunniyi A. D., Giammarinaro P., Paton J. C. The genes encoding virulence-associated proteins and the capsule of Streptococcus pneumoniae are upregulated and differentially expressed in vivo. Microbiology. 2002;148:2045–2053. doi: 10.1099/00221287-148-7-2045. [DOI] [PubMed] [Google Scholar]
- 13.Orihuela C. J., Radin J. N., Sublett J. E., Gao G., Kaushal D., Tuomanen E. I. Microarray analysis of pneumococcal gene expression during invasive disease. Infect. Immun. 2004;72:5582–5596. doi: 10.1128/IAI.72.10.5582-5596.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeMessurier K. S., Ogunniyi A. D., Paton J. C. Differential expression of key pneumococcal virulence genes in vivo. Microbiology. 2006;152:305–311. doi: 10.1099/mic.0.28438-0. [DOI] [PubMed] [Google Scholar]
- 15.Mahdi L. K., Ogunniyi A. D., LeMessurier K. S., Paton J. C. Pneumococcal virulence gene expression and host cytokine profiles during pathogenesis of invasive disease. Infect. Immun. 2008;76:646–657. doi: 10.1128/IAI.01161-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall-Stoodley L., Nistico L., Sambanthamoorthy K., et al. Characterization of biofilm matrix, degradation by DNase treatment and evidence of capsule downregulation in Streptococcus pneumoniae clinical isolates. BMC Microbiol. 2008;8:173. doi: 10.1186/1471-2180-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hathaway L. J., Bättig P., Mühlemann K. In vitro expression of the first capsule gene of Streptococcus pneumoniae, cpsA, is associated with serotype-specific colonization prevalence and invasiveness. Microbiology. 2007;153:2465–2471. doi: 10.1099/mic.0.2006/005066-0. [DOI] [PubMed] [Google Scholar]
- 18.Oggioni M. R., Claverys J. P. Repeated extragenic sequences in procaryotic genomes: a proposal for the origin and dynamics of the RUP element in Streptococcus pneumoniae. Microbiology. 1999;145:2647–2653. doi: 10.1099/00221287-145-10-2647. [DOI] [PubMed] [Google Scholar]
- 19.Hoskins J., Alborn W. E., Jr, Arnold J., et al. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 2001;183:5709–5717. doi: 10.1128/JB.183.19.5709-5717.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llull D., García E., López R. Tts, a processive β-glucosyltransferase of Streptococcus pneumoniae, directs the synthesis of the branched type 37 capsular polysaccharide in pneumococcus and other Gram-positive species. J. Biol. Chem. 2001;276:21053–21061. doi: 10.1074/jbc.M010287200. [DOI] [PubMed] [Google Scholar]
- 21.Lanie J. A., Ng W. -L., Kazmierczak K. M., et al. Genome sequence of Avery's virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J. Bacteriol. 2007;189:38–51. doi: 10.1128/JB.01148-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko K. S., Park S., Oh W. S., et al. Comparative analysis of growth-phase-dependent gene expression in virulent and avirulent Streptococcus pneumoniae using a high-density DNA microarray. Mol. Cells. 2006;21:82–88. [PubMed] [Google Scholar]
- 23.Pérez-Martín J., Rojo F., de Lorenzo V. Promoters responsive to DNA bending: a common theme in prokaryotic gene expression. Microbiol. Rev. 1994;58:268–290. doi: 10.1128/mr.58.2.268-290.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olivares-Zavaleta N., Jáuregui R., Merino E. Genome analysis of Escherichia coli promoter sequences evidences that DNA static curvature plays a more important role in gene transcription than has previously been anticipated. Genomics. 2006;87:329–337. doi: 10.1016/j.ygeno.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Vlahoviček K., Kajan L., Pongor S. DNA analysis servers: plot.it, bend.it, model.it and IS. Nucleic Acids Res. 2003;31:3686–3687. doi: 10.1093/nar/gkg559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo P., Morrison D. A. Transient association of an alternative sigma factor, ComX, with RNA polymerase during the period of competence for genetic transformation in Streptococcus pneumoniae. J. Bacteriol. 2003;185:349–358. doi: 10.1128/JB.185.1.349-358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Portmann R., Poulsen K. R., Wimmer R., Solioz M. CopY-like copper inducible repressors are putative ‘winged helix’ proteins. Biometals. 2006;19:61–70. doi: 10.1007/s10534-005-5381-3. [DOI] [PubMed] [Google Scholar]
- 28.Reyes A., Leiva A., Cambiazo V., Méndez M. A., González M. Cop-like operon: structure and organization in species of the lactobacillale order. Biol. Res. 2006;39:87–93. doi: 10.4067/s0716-97602006000100010. [DOI] [PubMed] [Google Scholar]
- 29.Nieto C., Espinosa M., Puyet A. The maltose/maltodextrin regulon of Streptococcus pneumoniae. Differential promoter regulation by the transcriptional repressor MalR. J. Biol. Chem. 1997;272:30860–30865. doi: 10.1074/jbc.272.49.30860. [DOI] [PubMed] [Google Scholar]
- 30.Doroshchuk N. A., Gel'fand M. S., Rodionov D. A. Regulation of nitrogen metabolism in gram-positive bacteria. Mol. Biol. (Mosk.) 2006;40:919–926. [PubMed] [Google Scholar]
- 31.Kloosterman T. G., Hendriksen W. T., Bijlsma J. J. E., et al. Regulation of glutamine and glutamate metabolism by GlnR and GlnA in Streptococcus pneumoniae. J. Biol. Chem. 2006;281:25097–25109. doi: 10.1074/jbc.M601661200. [DOI] [PubMed] [Google Scholar]
- 32.Ulijasz A. T., Andes D. R., Glasner J. D., Weisblum B. Regulation of iron transport in Streptococcus pneumoniae by RitR, an orphan response regulator. J. Bacteriol. 2004;186:8123–8136. doi: 10.1128/JB.186.23.8123-8136.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sierro N., Makita Y., de Hoon M., Nakai K. DBTBS: a database of transcriptional regulation in Bacillus subtilis containing upstream intergenic conservation information. Nucleic Acids Res. 2008;36:D93–D96. doi: 10.1093/nar/gkm910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Federle M. J., Scott J. R. Identification of binding sites for the group A streptococcal global regulator CovR. Mol. Microbiol. 2002;43:1161–1172. doi: 10.1046/j.1365-2958.2002.02810.x. [DOI] [PubMed] [Google Scholar]
- 35.Hammerschmidt S., Wolff S., Hocke A., Rosseau S., Müller E., Rohde M. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect. Immun. 2005;73:4653–4667. doi: 10.1128/IAI.73.8.4653-4667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giammarinaro P., Paton J. C. Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae. Infect. Immun. 2002;70:5454–5461. doi: 10.1128/IAI.70.10.5454-5461.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iyer R., Baliga N. S., Camilli A. Catabolite control protein A (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae. J. Bacteriol. 2005;187:8340–8349. doi: 10.1128/JB.187.24.8340-8349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Philips B., Meguer J. -X., Redman J., Baker E. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med. 2003;29:2204–2210. doi: 10.1007/s00134-003-1961-2. [DOI] [PubMed] [Google Scholar]
- 39.Baker E. H., Clark N., Brennan A. L., et al. Hyperglycemia and cystic fibrosis alter respiratory fluid glucose concentrations estimated by breath condensate analysis. J. Appl. Physiol. 2007;102:1969–1975. doi: 10.1152/japplphysiol.01425.2006. [DOI] [PubMed] [Google Scholar]
- 40.Llull D., López R., García E. Genetic bases and medical relevance of capsular polysaccharide biosynthesis in pathogenic streptococci. Curr. Mol. Med. 2001;1:475–491. doi: 10.2174/1566524013363618. [DOI] [PubMed] [Google Scholar]
- 41.Shelburne S. A., III, Keith D., Horstmann N., et al. A direct link between carbohydrate utilization and virulence in the major human pathogen group A Streptococcus. Proc. Natl Acad. Sci. USA. 2008;105:1698–1703. doi: 10.1073/pnas.0711767105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graham M. R., Smoot L. M., Migliaccio C. A. L., et al. Virulence control in group A Streptococcus by a two-component gene regulatory system: global expression profiling and in vivo infection modeling. Proc. Natl Acad. Sci. USA. 2002;99:13855–13860. doi: 10.1073/pnas.202353699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamy M. -C., Zouine M., Fert J., et al. CovS/CovR of group B streptococcus: a two-component global regulatory system involved in virulence. Mol. Microbiol. 2004;54:1250–1268. doi: 10.1111/j.1365-2958.2004.04365.x. [DOI] [PubMed] [Google Scholar]
- 44.Pan X., Ge J., Li M., et al. The orphan response regulator CovR: a globally negative modulator of virulence in Streptococcus suis serotype 2. J. Bacteriol. 2009 doi: 10.1128/JB.01309-08. doi:10.1128/JB.01309–01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hendriksen W. T., Bootsma H. J., Estevão S., et al. CodY of Streptococcus pneumoniae: link between nutritional gene regulation and colonization. J. Bacteriol. 2008;190:590–601. doi: 10.1128/JB.00917-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.den Hengst C. D., van Hijum S. A. F. T., Geurts J. M. W., Nauta A., Kok J., Kuipers O. P. The Lactococcus lactis CodY regulon: identification of a conserved cis-regulatory element. J. Biol. Chem. 2005;280:34332–34342. doi: 10.1074/jbc.M502349200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.