Abstract
Two small GTPase Rabs, Rab32 and Rab38, have recently been proposed to regulate trafficking of melanogenic enzymes to melanosomes in mammalian epidermal melanocytes; however, the exact molecular mechanism of Rab32/38-mediated transport of melanogenic enzymes has never been clarified, because no Rab32/38-specific effector has ever been identified. In this study, we screened for a Rab32/38-specific effector by a yeast two-hybrid assay using a guanosine triphosphate (GTP)-locked Rab32/38 as bait and found that VPS9-ankyrin-repeat protein (Varp)/Ankrd27, characterized previously as a guanine nucleotide exchange factor (GEF) for Rab21, functions as a specific Rab32/38-binding protein in mouse melanocyte cell line melan-a. Deletion analysis showed that the first ankyrin-repeat (ANKR1) domain functions as a GTP-dependent Rab32/38-binding domain, but that the N-terminal VPS9 domain (i.e., Rab21-GEF domain) does not. Small interfering RNA-mediated knockdown of endogenous Varp in melan-a cells caused a dramatic reduction in Tyrp1 (tyrosinase-related protein 1) signals from melanosomes but did not cause any reduction in Pmel17 signals. Furthermore, expression of the ANKR1 domain in melan-a cells also caused a dramatic reduction of Tyrp1 signals, whereas the VPS9 domain had no effect. Based on these findings, we propose that Varp functions as the Rab32/38 effector that controls trafficking of Tyrp1 in melanocytes.
INTRODUCTION
Melanosomes are specialized organelles that synthesize and store melanin pigments in pigment cells. The same as other organelles, a variety of membrane trafficking events are involved in the formation, maturation, and transport of melanosomes; thus, melanosomes are considered a good model system for analyzing organelle formation and transport (reviewed in Marks and Seabra, 2001; Raposo and Marks, 2007). Several distinct Rab-type small GTPases, which are conserved membrane trafficking proteins in all eukaryotic cells (reviewed in Pfeffer, 2001; Zerial and McBride, 2001), have been shown to be involved in the maturation and transport of melanosomes in mammalian epidermal melanocytes.
In general, Rab functions as a molecular switch by cycling between a guanosine diphosphate (GDP)-bound state and a GTP-bound state, and the GTP-bound active form promotes membrane traffic through interaction with a specific effector molecule (Grosshans et al., 2006; Schwartz et al., 2007). The best characterized Rab isoform in melanocytes is Rab27A, which is involved in melanosome transfer from microtubules to actin filaments through interaction with the melanocyte-specific effector Slac2-a/melanophilin (Fukuda et al., 2002; Wu et al., 2002; Kuroda et al., 2003; Hume et al., 2006; reviewed in Fukuda, 2005) and melanosome anchoring to the plasma membrane through interaction with another Rab27A effector, Slp2-a (Kuroda and Fukuda, 2004). Thus, dysfunctions of Rab27A cause pigment dilution in human type II Griscelli syndrome patients (Ménasché et al., 2000) and diluted coat color of the mouse ashen (Wilson et al., 2000) because of a defect in melanosome transport.
The diluted coat color of another mouse mutant, chocolate, has recently been shown to be caused by dysfunctions of Rab38, and targeting of tyrosinase-related protein 1 (Tyrp1) to melanosomes is impaired in chocolate melanocytes (Loftus et al., 2002). Rab38 is also involved in the trafficking of another melanogenic enzyme, tyrosinase, to melanosomes in skin melanocytes (Wasmeier et al., 2006) and to immature melanosomes in retinal pigment epithelial cells (Lopes et al., 2007). In addition, Rab32, a closely related isoform of Rab38, can compensate for the function of Rab38 in the trafficking of melanogenic enzymes in melanocytes (Wasmeier et al., 2006), and it also is involved in melanosome transport in Xenopus melanophores (Park et al., 2007). However, the molecular mechanism by which Rab32/38 regulate tyrosinase and/or Tyrp1 transport remains completely unknown, and no specific effector molecules for Rab32/38 have ever been identified in melanocytes. Because Rab32 can compensate for the dysfunction of Rab38 in melan-chocolate (cht) cell lines (Wasmeier et al., 2006), a putative effector molecule(s) for Rab38 in melanocytes must interact with both Rab32 and Rab38, the same as rabphilin interacts with both Rab3A and Rab27A (Kuroda et al., 2002; Fukuda, 2003; Fukuda et al., 2004), both of which regulate docking of hormone granules to the plasma membrane in neuroendocrine PC12 cells (Tsuboi and Fukuda, 2006). Wang et al. (2008) very recently reported that vacuolar protein sorting 9 (VPS9)-ankyrin-repeat protein (Varp; official gene symbol in National Center for Biotechnology Information: Ankrd27), originally identified as a guanine nucleotide exchange factor (GEF) for Rab21 (Zhang et al., 2006), interacted with Rab38 in COS-7 cells by overexpression study. However, neither Rab binding specificity of Varp (e.g., binding of Rab32) nor functional data on Varp in melanocytes was included in this report.
In this study, we screened for a dual effector for Rab32 and Rab38 and identified Varp as a Rab32- and Rab38-specific binding protein. We showed by deletion analysis that Rab32/38 specifically interacts with the first ankyrin repeat (referred to as ANKR1 below) and does not interact with the VPS9 domain, a putative Rab5 GEF domain (Horiuchi et al., 1997; Carney et al., 2006). We further showed that small interfering RNA (siRNA)-mediated knockdown of endogenous Varp or expression of the Rab32/38-binding domain (i.e., ANKR1) in cultured melanocytes induced disappearance of Tyrp1 signals from the cytoplasm. Based on our findings, we discuss the possible function of Varp in Tyrp1 trafficking to melanosomes in melanocytes.
MATERIALS AND METHODS
Materials
Horseradish peroxidase (HRP)-conjugated anti-FLAG tag (M2) mouse monoclonal antibody (mAb) and anti-FLAG tag antibody-conjugated agarose were obtained from Sigma-Aldrich (St. Louis, MO). HRP-conjugated anti-T7 tag mouse mAb and anti-T7 tag antibody-conjugated agarose were purchased from Merck Biosciences Novagen (Darmstadt, Germany). Anti-Tyrp1 mouse mAb (Ta99) was from ID Labs (London, ON, Canada). Anti-Tyrp1 goat polyclonal antibody and anti-actin goat polyclonal antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Pmel17 mouse mAb (HMB45) and Alexa 488/594/633-conjugated anti-mouse immunoglobulin G (IgG) goat antibody were from Dako North America (Carpinteria, CA) and Invitrogen (Carlsbad, CA), respectively. Anti-DsRed rabbit polyclonal antibody was from MBL (Nagoya, Japan). The lysosomal protease inhibitors E64d and pepstatin A and the proteasome inhibitor MG132 were from Peptide Institute (Osaka, Japan). All other reagents used in this study were analytical grade or the highest grade commercially available.
Yeast Two-Hybrid Screening
cDNAs of the GTP-fixed mutants Rab32(Q83L)ΔCys and Rab38(Q69L)ΔCys were subcloned into the pGBD-C1 vector as described previously (Fukuda et al., 2008). Each Rab construct was used to screen oligo(dT)-primed mouse testis and mouse embryo mixed cDNA libraries according to the manufacturer's instructions (Clontech, Mountain View, CA), and 1 × 108 colonies were investigated. After DNA sequencing and database searching, the Rab binding specificity of positive clones was further investigated by using the panel of 60 different putatively GTP-locked Rabs (simply referred to as GTP-locked Rabs below) essentially as described previously (Itoh et al., 2006). The yeast strain, medium, culture conditions, and transformation protocol used are described in James et al. (1996). The materials used for the two-hybrid assay in this study were as follows: yeast strain pJ69-4A; plasmid pGAD-C1 (or pAct2) for expression of activating domain fusion protein; plasmid pGBD-C1 for expression of DNA-binding domain fusion protein; and a synthetic complete medium lacking adenine, histidine, leucine, and tryptophan (SC-AHLW, 0.67% yeast nitrogen base without amino acids, 2% glucose, 2% Bacto agar, 0.01% uracil, 0.0125% lysine, and 0.01% methionine) for the selection medium.
Plasmid Constructions
pGBD-C1 vector harboring GTP-fixed mutants of Rab1-43 lacking the C-terminal geranylgeranylation site was prepared as described previously (Itoh et al., 2006; Fukuda et al., 2008). Putatively nucleotide-free and/or GDP-locked mutants of Rab (simply referred to as nucleotide free Rabs below) were produced by replacement of a conserved threonine (or serine) with asparagine by conventional or two-step polymerase chain reaction (PCR) techniques as described previously (Fukuda et al., 1995, 2008). The sequences of the mutagenic oligonucleotides used are available from the authors on request. The following nucleotide-free Rab mutants were used in this study: Rab1A(S25N), Rab1B(S22N), Rab2A(S20N), Rab2B(S20N), Rab3A(T36N), Rab3B(T36N), Rab3C(T44N), Rab3D(T36N), Rab4A(S27N), Rab4B(S22N), Rab5A(S34N), Rab5B(S48N), Rab5C(S35N), Rab6A(T27N), Rab6B(T27N), Rab6C(T27N), Rab7(T22N), Rab8A(T22N), Rab8B(T22N), Rab9A(S21N), Rab9B(S21N), Rab10(T23N), Rab11A(S25N), Rab11B(S25N), Rab12(T55N), Rab13(T22N), Rab14(S25N), Rab15(T22N), Rab17(T33N), Rab18(S22N), Rab19(T31N), Rab20(T19N), Rab21(T31N), Rab22A(S19N), Rab22B(S19N), Rab23(S23N), Rab24(T21N), Rab25(T26N), Rab26(S81N), Rab27A(T23N), Rab27B(T23N), Rab28(T26N), Rab29(T21N), Rab30(T23N), Rab32(T37N), Rab33A(T50N), Rab33B(T47N), Rab34(T66N), Rab35(S22N), Rab36(T71N), Rab37(T43N), Rab38(T23N), Rab39A(S22N), Rab39B(S22N), Rab40A(S28N), Rab40B(G28N), Rab40C(G28N), Rab41(T30N), Rab42(T22N), and Rab43(T23N). Because Rab40B and Rab40C lack the conserved threonine (or serine) residue and its position is occupied by glycine, we used Rab40B(G28N) and Rab40C(G28N), respectively, as the nucleotide-free mutants in this study. The nucleotide-free Rab mutants were subcloned into the pGAD-C1 vector.
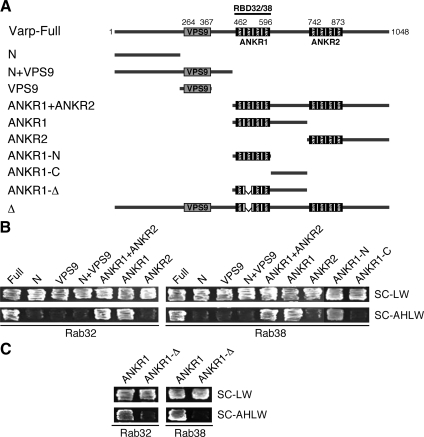
cDNA of mouse Varp was amplified from Marathon-Ready mouse brain cDNA (Clontech) by PCR using the following oligonucleotides with a restriction enzyme site (underlined) or a stop codon (bold) as described previously (Fukuda et al., 1999): 5′-GGATCCATGGCTCTATATGATGAAGA-3′ (Met primer, sense) and 5′-CTAAGACTGAGGAACACTCG-3′ (stop primer, antisense). The nucleotide sequence reported in this article has been submitted to the GenBank/EBI Data Bank with an accession AB455262. Truncated mutants of Varp (see Figure 3A) were prepared by conventional PCR using specific pairs of oligonucleotides, and their sequences are available from the authors on request. Varp-Full contains amino acid residues 1-1048 of Varp; Varp-N, residues 1-250; Varp-N+VPS9, residues 1-450; Varp-VPS9, residues 251-450; Varp-ANKR1+ANKR2, residues 451-1048; Varp-ANKR1, residues 451-729; Varp-ANKR2, residues 730-1048; Varp-ANKR1-N, residues 451-600; and Varp-ANKR1-C, residues 601-729. These truncated Varp mutants were subcloned into the pGAD-C1, pGBD-C1, pGEX-4T-3 (GE Healthcare, Chalfont St. Giles, Buckinghamshire, United Kingdom), pmStrawberry-C1 (pStr-C1) (Shaner et al., 2004), and/or pEF-T7 tag expression vector (Fukuda et al., 1999). A mutant Varp that lacks a second repeat (amino acid residues 495-527) in the ANKR1 (named Δ; see Figure 3A) was similarly prepared by PCR using a specific pair of oligonucleotides, and their sequences are also available from us on request. The mutant Varp-Δ (or ANKR1-Δ) was subcloned into the pGAD-C1 and/or pStr-C1 vectors. pEF-FLAG-Rab32 and -Rab38 were prepared as described previously (Fukuda, 2003).
Figure 3.
Mapping of the Rab32/38-binding site in Varp. (A) Schematic representation of Varp and its truncated mutants used in this study. Varp contains an N-terminal VPS9 domain and C-terminal tandem ankyrin repeats (named ANKR1 and ANKR2). (B) ANKR1 functions as the Rab32/38-binding domain of Varp. (C) A Rab32/38-binding-defective mutant of Varp ANKR1 (named ANKR1-Δ) that lacks a second repeat in the ANKR1 (see (A)). Yeast cells containing pGAD plasmid expressing Varp mutants and pGBD plasmid expressing GTP-locked Rab32 (left) or Rab38 (right) were streaked on SC-LW (top) and SC-AHLW (bottom) and incubated at 30°C.
The following pairs of nucleotides with a 19-base target site (site 1 in bold) and a nine-base loop (in italics) were used to generate short hairpin RNA (shRNA) expression plasmids against mouse Varp mRNA: Varp shRNA(+) primer (5′-GATCCAAGGAAACAGGATTAAGCTAGTTCAAGAGACTAGCTTAATCCTGTTTCCTTTTTTTTGGAAA-3′) and Varp shRNA(−) primer (5′-AGCTTTTCCAAAAAAGGAAACAGGATTAAGCTAGTCTCTTGAACTAGCTTAATCCTGTTTCCG-3′). The pairs of nucleotides were mixed, denatured at 94°C for 2 min, annealed at 72°C for 1 min, gradually cooled to 4°C for 2 h, and then subcloned into the BamHI/HindIII site of the pSilencer 2.1-U6 neo (Ambion, Austin, TX), which expresses shRNA under the control of the mouse U6 promoter. The plasmid obtained was referred to as pSilencer-Varp site 1 (unless otherwise stated, pSilencer-Varp means pSilencer-Varp site 1. pSilencer-Varp site 2 (target site: 5′-CAGACTGAAGGAAACACCA-3′) was similarly constructed. The knockdown efficiency of pSilencer-Varp site 1/2 was evaluated in COS-7 cells (see Figure 6A, right) as described previously (Kuroda and Fukuda, 2004).
Figure 6.
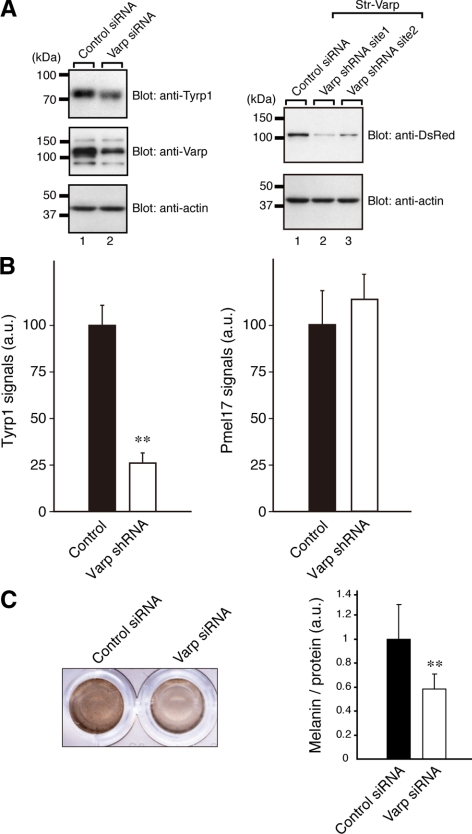
Reduced expression of Tyrp1 in Varp-deficient melanocytes. (A) Reduced expression of Tyrp1 and Varp in siVarp-treated melanocytes as revealed by immunoblotting (left panel). Cell lysates of melan-a cells treated with either siVarp or control siRNA (∼20 μg) were subjected to 10% SDS-polyacrylamide gel electrophoresis (PAGE) followed by immunoblotting with anti-Varp specific antibody (1/100 dilution), anti-Tyrp1 antibody (1/200 dilution), and anti-actin antibody (1/400 dilution). Right, efficiency and specificity of shRNA targeted against Varp were shown. Str-Varp was expressed in COS-7 cells together with Varp shRNA or a control vector. Cell lysates were subjected to 10% SDS-PAGE followed by immunoblotting with anti-DsRed antibody (1/100 dilution) and anti-actin antibody. The positions of the molecular mass markers (× 10−3) are shown on the left. (B) Reduced expression of Tyrp1 in Varp shRNA-expressing melanocytes as revealed by immunofluorescence analysis. Note that expression of Tyrp1 in Varp-deficient cells dramatically reduced, whereas expression of Pmel17 had no effect. The bars represent the means ± SE of data from three independent dishes (n > 50). **p < 0.01, Student's unpaired t test. (C) Reduced melanin content in Varp siRNA-expressing melanocytes. Melan-a cells expressing Varp siRNA (right) and control siRNA (left) were harvested, and their melanin content was assayed by measuring optical density at 490 nm. The bars represent the means ± SD of data from triplicate experiments. **p < 0.01, Student's unpaired t test.
Antibody Production
Glutathione transferase (GST)-Varp-N (amino acid residues 1-250), GST-Rab32, and GST-Rab38 were prepared by standard protocols (Kuroda and Fukuda, 2005). Japanese White rabbits were immunized with the purified GST-Varp-N, GST-Rab32, or GST-Rab38, and specific antibodies were affinity purified by exposure to antigen-bound Affi-Gel 10 beads (Bio-Rad Laboratories, Hercules, CA) as described previously (Fukuda and Mikoshiba, 1999).
Immunofluorescence Analysis
The immortal mouse melanocyte cell line melan-a, derived from a black mouse (generous gift of Dorothy C. Bennett, St. George's Hospital Medical School, London, United Kingdom) was cultured on glass-bottomed dishes (35-mm dish; MatTek, Ashland, MA) (Bennett et al., 1987; Kuroda et al., 2003). Transfection of pStr-C1-Varp, pEGFP-C1-Rab, pSilencer-Varp, or a control vector (pStr-C1, pEGFP-C1, or pSilencer alone, respectively) was achieved by using FuGENE 6 (Roche Molecular Biochemicals, Mannheim, Germany) according to the manufacturer's instructions. Two days after transfection, cells were fixed in 4% paraformaldehyde, permeabilized with 0.3% Triton X-100, stained with anti-Tyrp1 mouse mAb (1/100 dilution) or anti-Pmel17 mouse mAb (1/100 dilution), and then visualized with anti-mouse Alexa Fluor 488/594/633 IgG, and examined for fluorescence with a confocal fluorescence microscope (FluoView; Olympus, Tokyo, Japan) as described previously (Kuroda et al., 2003). Exposure to the proteasome inhibitor (10 μM MG132) or lysosomal protease inhibitors (10 μg/ml E64d and 10 μg/ml pepstatin A) was performed 6 h before fixation. The images were processed with Photoshop software (CS3; Adobe Systems, Mountain View, CA). To quantitatively measure Tyrp1 and Pmel17 signals, the images of the green fluorescent protein (GFP)-expressing cells (i.e., Varp shRNA-expressing cells) or the Str-Varp–expressing cells were captured at random (n > 50 from three independent dishes) with the confocal microscope, and fluorescent signals of Tyrp1 or Pmel17 were quantified with MetaMorph software (Molecular Devices, Sunnyvale, CA).
Binding Assays
Plasmids (pEF-T7-Varp and pEF-FLAG-Rab) were transfected into COS-7 cells (3 × 105 cells/6-cm dish, the day before transfection) by using Lipofectamine Plus (Invitrogen) according to the manufacturer's notes. Coimmunoprecipitation assays using anti-FLAG tag antibody-conjugated agarose were performed as described previously (Fukuda and Kanno, 2005). Direct interaction between T7-Varp and GST-Rab32/38 and endogenous interaction between Varp and Rab32/38 also were performed as described previously (Fukuda and Kanno, 2005).
siRNA-mediated Knockdown of Varp in Melan-a Cells
siRNA against the site 1 (see above) of mouse Varp (5′-GGAAACAGGAUUAAGCUAGdTdT-3′; sense) was chemically synthesized by Nippon EGT (Toyama, Japan). Transfection of the Varp siRNA or control siRNA into melan-a cells was achieved by using Lipofectamine RNAiMax (Invitrogen) according to the manufacturer's instructions. At 48 h after transfection, the cells were harvested and lysed. An aliquot of total cell lysates was analyzed by immunoblotting using anti-Varp antibody, anti-Tyrp1 antibody, and anti-actin antibody. Immunoreactive bands were visualized by enhanced chemiluminescence.
Melanin Assay
Melanin assay was performed essentially as described previously (Wasmeier et al., 2006). In brief, melan-a cells were solubilized in 50 mM HEPES-KOH, pH 7.2, 150 mM NaCl, 1% Triton X-100, and protease inhibitors. Pigment was pelleted by centrifugation at 17,400 × g for 20 min at 4°C, and the pellet was allowed to dissolve in 1 M NaOH/10% dimethyl sulfoxide for 30 min at 100°C. Melanin content was measured as optical density at 490 nm and normalized to protein content.
RESULTS
Identification of Varp as a Rab32/38-specific Binding Protein
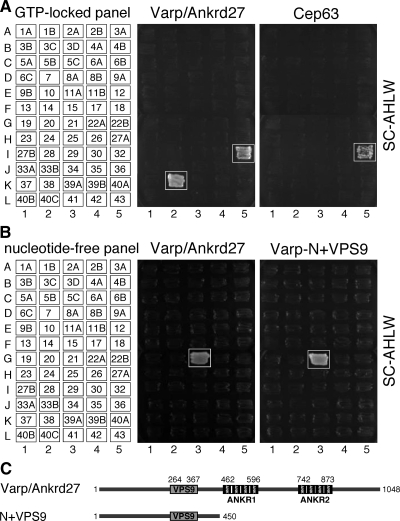
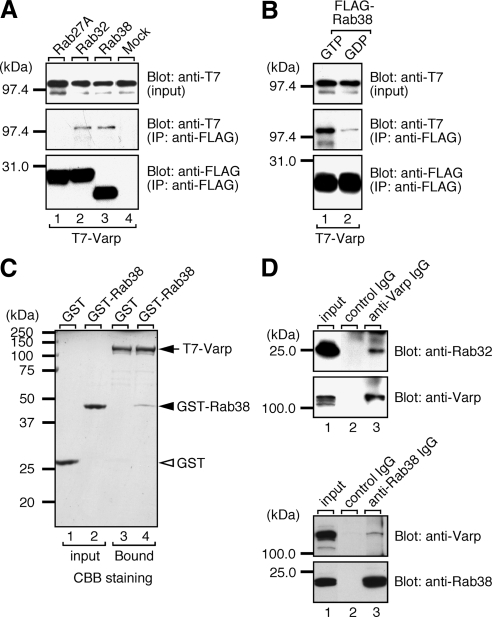
To identify a Rab32/38-specific effector molecule(s) that functions in melanocytes, we screened mouse cDNA libraries by yeast two-hybrid assay by using the GTP-fixed form of Rab32 and Rab38 as bait (see Materials and Methods for details). Although no positive clone was obtained for Rab38, we succeeded in obtaining two positive clones for Rab32, a Varp clone and a centrosomal protein 63 (Cep63) clone. Their Rab binding specificity was further investigated by a yeast two-hybrid assay with a panel of 60 different GTP-locked Rabs (Itoh et al., 2006; Fukuda et al., 2008). As shown in Figure 1A (boxed), Varp specifically interacted with two phylogenetically related Rabs, Rab32 and Rab38, whereas Cep63 recognized Rab32 alone. However, because the interaction between Cep63 and Rab32 was not observed in mammalian cells (data not shown), we concluded that the Cep63-Rab32 interaction observed in yeast cells was a false positive. By contrast, Varp did interact with both Rab32 and Rab38 (Figure 2A, middle, lanes 2 and 3) in COS-7 cells, but not with Rab27A (another melnaosome-resident Rab). The interaction between Varp and Rab32/38 is GTP-dependent (Figure 2B and Supplemental Figure S1A; Wang et al., 2008) and direct (lane 4 in Figure 2C and Supplemental Figure S1B). We further confirmed the endogenous interaction between Varp and Rab32/38 in melan-a cells by coimmunoprecipitation assay using anti-Varp specific antibody (Figure 2D). We therefore focused on Varp as a candidate Rab32/38-specific effector for subsequent analysis.
Figure 1.
Rab binding specificity of Varp as revealed by yeast two-hybrid panels. (A) Specific interaction of Varp-Full with the GTP-fixed form of Rab32 and Rab38, and of Cep63 with the GTP-fixed form of Rab32. Yeast cells containing pGBD plasmid expressing GTP-locked Rab protein (positions indicated in the left panels) and pAct2 plasmid expressing Varp (or Cep63) protein were streaked on SC-AHLW and incubated at 30°C. Positive patches are boxed. (B) Specific interaction of Varp-Full and Varp-N+VPS9 with the nucleotide-free form of Rab21. Yeast cells containing pGBD plasmid expressing Varp protein and pGAD plasmid expressing nucleotide-free Rab (positions indicated in the left panels) were streaked on SC-AHLW and incubated at 30°C. Positive patches are boxed. (C) Schematic representation of Varp and its truncated mutant Varp-N+VPS9 used in A and B. Varp contains an N-terminal VPS9 domain and C-terminal tandem ankyrin repeats (named ANKR1 and ANKR2).
Figure 2.
Varp interacts with Rab32 and Rab38 in mammalian cells. (A) Varp interacts with Rab32 and Rab38, but not with Rab27A, in the presence of 0.5 mM guanosine 5′-O-(3-thio)triphosphate (GTPγS). T7-tagged Varp-Full and FLAG-tagged Rab27A/32/38 were expressed in COS-7 cells, and their associations were analyzed by coimmunoprecipitation assay using anti-FLAG tag antibody-conjugated agarose beads as described previously (Fukuda and Kanno, 2005). Coimmunoprecipitated T7-Varp-Full (middle) and immunoprecipitated FLAG-Rabs (bottom) were detected with HRP-conjugated anti-T7 tag antibody and HRP-conjugated anti-FLAG tag antibody, respectively. Input means 1/80 volume of the reaction mixture used for immunoprecipitation (top). (B) GTP-dependent interaction between Varp and Rab38. Binding assay was performed in the presence of 0.5 mM GTPγS (lane 1) or 1 mM GDP (lane 2) as described in A. (C) Direct interaction between Varp and Rab38. Agarose beads coupled with T7-Varp (arrow) were incubated with purified GST-Rab38 (lane 4, closed arrowhead) or GST alone (lane 3, open arrowhead), and bound proteins were detected with Coomassie Brilliant Blue R-250. Input means 1/25 volume of the reaction mixture (lanes 1 and 2). (D) Endogenous interaction between Varp and Rab32/38 in melan-a cells. The positions of the molecular mass markers (× 10−3) are shown on the left.
ANKR1 Domain of Varp Functions as a Rab32/38-binding Domain
Mouse Varp consists of 1048 amino acids and contains an N-terminal single VPS9 domain and C-terminal two ankyrin repeat domains (named the ANKR1 domain and ANKR2 domain) (Figure 3A). To identify the Rab32/38-binding site of Varp, a series of deletion mutants of Varp was produced (Figure 3A) and their binding to Rab32/38 was investigated by the two-hybrid assay as described above (Figure 3B). The results indicated that the ANKR1 domain alone is necessary and sufficient for binding of Rab32 and Rab38. Although the VPS9 domain of Varp has recently been shown to interact with the GDP-bound form of Rab21 (Wang et al., 2008) and to exhibit Rab21 GEF activity (Zhang et al., 2006), neither Rab32 nor Rab38 bound the N-terminal VPS9 domain under our experimental conditions. Consistent with the report by Wang et al. (2008), Varp also interacted with the nucleotide-free (i.e., constitutive negative) mutant of Rab21, but not with any of the other 59 nucleotide-free Rabs, including Rab32 and Rab38 (Figure 1B, boxed). We therefore concluded that Varp interacts with the GDP-bound/nucleotide-free form of Rab21 via the N-terminal VPS9 domain and with the GTP-bound form of Rab32/38 via the C-terminal ANKR1 domain.
Colocalization between Varp and Rab32/38 in Melanocytes
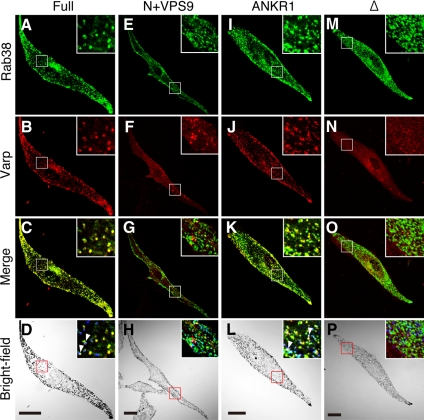
We next attempted to determine whether Varp colocalizes with Rab32/38 in cultured melanocytes. We initially attempted to determine the subcellular localization of endogenous Varp by using anti-Varp specific antibody, but because it was impossible to use the antibody for an immunofluorescence analysis, we were unable to determine the localization of endogenous Varp in melan-a cells. To overcome this problem, GFP-tagged Rab38 (or Rab32) and monomeric strawberry (Str)-tagged Varp were coexpressed in melan-a cells, and their subcellular localization was analyzed by confocal fluorescence microscopy. Consistent with a previous observation (Wasmeier et al., 2006), GFP-Rab32 and GFP-Rab38 were targeted to melanosomes in addition to the perinuclear region (Figure 4A and Supplemental Figure S2A). It should be noted that Varp overlapped well with Rab32/38 (yellow dots in Figure 4C and Supplemental Figure S2C) and Tyrp1 (Supplemental Figure S3, A, B, and D), whereas colocalization of Varp with melanosomes is limited in the peripheral region of the cell (Supplemental Figure S3, A, C, and E). This colocalization must be mediated by the C-terminal ANKR1 domain of Varp, because the ANKR1 domain alone is sufficient to cause colocalization with Rab32/38 (Figure 4, I–L, and Supplemental Figure S2, I–L), and no colocalization was observed between Rab32/38 and the N-terminal half of Varp, which contains the VPS9 domain (Figure 4, E–H, and Supplemental Figure S2, E–H). To further determine whether Rab32/38–Varp interaction is required for their colocalization in melanocytes, we produced a Rab32/38-binding-defective mutant of Varp (named Varp-Δ; also see Figure 3C). As expected, no colocalization between Rab32/38 and Varp-Δ was observed in melan-a cells (Figure 4, M–P, and Supplemental Figure S2, M–P). We therefore concluded that colocalization between Rab32/38 and Varp in melanocytes is mediated by a direct interaction between Rab32/38 and Varp.
Figure 4.
Colocalization of Varp and Rab38 in melanocytes. Melan-a cells transiently expressing GFP-Rab38 and Str-Varp (A–D), GFP-Rab38 and Str-Varp-N+VPS9 (E–H), GFP-Rab38 and Str-Varp-ANKR1 (I–L), or GFP-Rab38 and Str-Varp-Δ (M–P). Note that Varp-Full and Varp-ANKR1, but not Varp-N+VPS9 or Varp-Δ, colocalized well with Rab38 (yellow in C and K). The insets show magnified views of the boxed area. Only a few Varp- and Rab38-positive signals colocalized with melanosomes (pseudocolored in blue; arrowheads) in the perinuclear region (but see Supplemental Figure S3; colocalization between Varp and melanosomes were observed in the cell periphery). Bars, 20 μm.
Functional Involvement of Varp in Tyrp1 Trafficking in Melanocytes
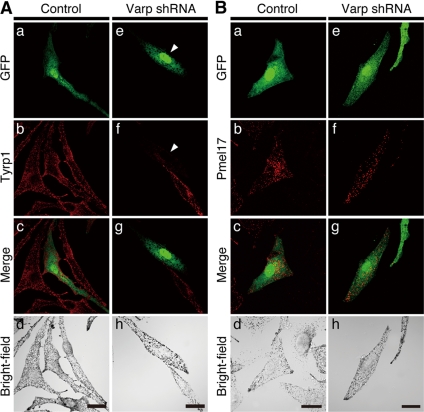
Finally, because Varp is endogenously expressed in melan-a cells (Figure 2D) and Rab32/38 have been shown to be involved in the trafficking of melanogenic enzymes (Wasmeier et al., 2006), we investigated the effect of knockdown of endogenous Varp on trafficking of Tyrp1 by expressing specific shRNA against mouse Varp (Figure 5). As shown in Figures 5A and 6B, there was a dramatic reduction in Tyrp1 signals in the Varp-knockdown cells (arrowhead), especially at the cell periphery, without affecting melanosome distribution, whereas no change in the level of Tyrp1 signals was observed in control GFP-expressing (i.e., control pSilencer-expressing) cells. By contrast, expression of Pmel17/GP100, a premelanosomal integral membrane protein (Theos et al., 2005), was unaffected by the knockdown of Varp (Figure 5B). Because similar results were obtained for the independent shRNA (site 2; Supplemental Figure S4), the observed effects (Figure 5A) are unlikely to be attributable to an off target effect. Reduced expression of Varp and Tyrp1 was further confirmed by immunoblot analysis (Figure 6A, left). Because there did not seem to be any difference in the melanin content of the Varp-knockdown cells and control cells under a light microscope when they were examined (Figure 5), we harvested the cells, which had been treated with either Varp siRNA or control siRNA for 48 h, and we compared their melanin content by measuring optical density at 490 nm. As shown in Figure 6C, there was a clear reduction in the melanin content of the Varp siRNA-treated cells, in comparison with the control siRNA-treated cells.
Figure 5.
(A and B) Effect of the Varp shRNA on Tyrp1 staining and distribution in melan-a cells. Melan-a cells were transfected with a control vector (a–d) or Varp shRNA expression vector (e–h) together with the GFP expression vector as a transfection marker. Cells were immunostained with anti-Tyrp1 antibody (A) or anti-Pmel17 antibody (B) and visualized with Alexa Fluor 594 secondary antibody. Bright-field images show the melanosome distribution in the cells. Bars, 20 μm. Note that siRNA-mediated knockdown of Varp dramatically reduced Tyrp1-staining (arrowheads in A) but did not reduce Pmel17-staining, in melan-a cells.
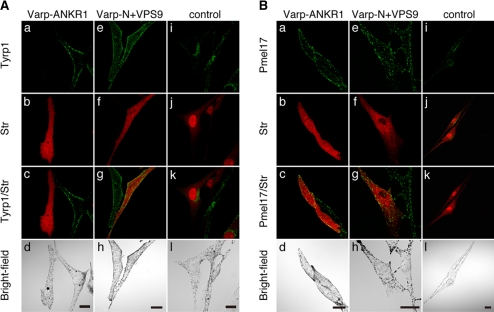
To further investigate the involvement of Varp in Rab32/38-dependent Tyrp1 trafficking, we used the Rab32/ 38-binding domain of Varp (i.e., ANKR1) as a dominant-negative construct in an attempt to block the function of Rab32/38 by interrupting the interaction between endogenous Rab32/38 and Varp. As expected, expression of the ANKR1 domain similarly caused a drastic reduction in Tyrp1 signals, whereas expression of the N-terminal VPS9 domain (N+VPS9) or the control Rab32/38-binding-defective ANKR1-Δ mutant had virtually no effect on Tyrp1 staining or its distribution (Figures 7A, 8, A and B, and Supplemental Figure S5). The same as in Varp-knockdown cells, expression of Pmel17 was unaffected in ANKR1-expressing cells (Figures 7B and 8B). Therefore, Varp is likely to be involved together with Rab32/38 in the Tyrp1 trafficking in melanocytes.
Figure 7.
Effect of the Varp-ANKR1 domain on Tyrp1 staining and distribution in melan-a cells. Melan-a cells transiently expressing Str-Varp-ANKR1 (a–d), Str-Varp-N+VPS9 (e–h), or control Str alone (i–l) were immunostained with anti-Tyrp1 antibody (A) or anti-Pmel17 antibody (B) and visualized with Alexa Fluor 488 secondary antibody. Bright-field images show the melanosome distribution in the cells. Note that expression of Varp-ANKR1, but not of Varp-N+VPS9, caused the dramatic reduction in Tyrp1 signals (A), whereas no effect was observed for Pmel17 signals (B). Bars, 20 μm.
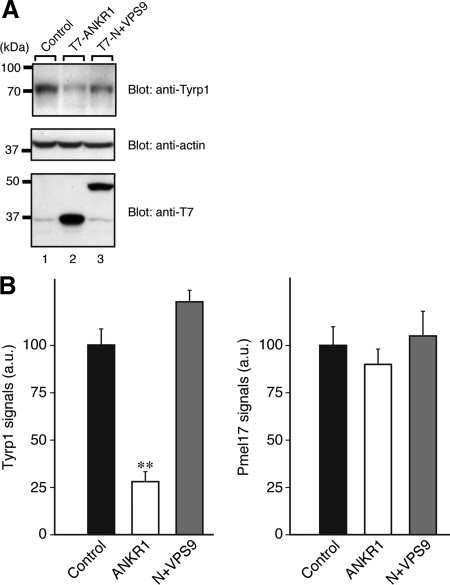
Figure 8.
Reduced expression of Tyrp1 in Varp-ANKR1-expressing melanocytes. (A) Reduced expression of Tyrp1 in Varp-ANKR1–expressing melanocytes as revealed by immunoblotting. Cell lysates of B16-F1 cells expressing either T7-Varp-ANKR1 or T7-Varp-N+VPS9 (∼30 μg) were subjected to 10% SDS-PAGE followed by immunoblotting with anti-Tyrp1 antibody (1/200 dilution), anti-actin antibody (1/400 dilution), and anti-T7 tag antibody (1/5000 dilution). The positions of the molecular mass markers (× 10−3) are shown on the left. (B) Reduced expression of Tyrp1 in Varp-ANKR1–expressing melanocytes as revealed by immunofluorescence analysis. Note that expression of Tyrp1 in Varp-ANKR1–expressing cells dramatically reduced, whereas expression of Pmel17 had no effect. Expression of Tyrp1 in Varp-N+VPS9–expressing cells was slightly increased in comparison with the control cells, but this was not statistically significant under our experimental conditions. The bars represent the means ± SE of data from three independent dishes (n > 50). **p < 0.01, Student's unpaired t test.
DISCUSSION
It has previously been reported that Rab32 and Rab38 redundantly regulate the trafficking of melanogenic enzymes to melanosomes (Wasmeier et al., 2006); however, no Rab32/38-specific effector that functions in melanocytes had ever been identified. In the present study, we succeeded in identifying Varp as a Rab32/38-specific binding protein. While this manuscript was being prepared for publication, Varp was reported to interact with Rab38 in COS-7 cells by another group (Wang et al., 2008). The present study, however, considerably extends the study by Wang et al. (2008) in the following respects. First, we precisely determined the Rab binding specificity of Varp by a yeast two-hybrid assay using a panel of 60 different GTP-locked Rabs and demonstrated that Varp specifically interacts with the GTP-locked Rab32 and Rab38, but not with any of the other 58 Rabs tested (Figure 1A), via the ANKR1 domain. Second, we demonstrated that Varp (or Varp-Rab32/38 complex) is endogenously expressed in melan-a cells (Figures 2D and 6A) and that GFP-Rab32/38 and Str-Varp are well colocalized in these cells (Figure 4 and Supplemental Figure S2). Third, we for the very first time demonstrated that knockdown of endogenous Varp in melan-a cells by a specific shRNA or expression of the Rab32/38-binding domain of Varp in melan-a cells causes a dramatic reduction in endogenous Tyrp1 signals (Figures 5A, 6B, 7A, and 8B), but no reduction in Pmel17 signals (Figures 5B, 6B, 7B, and 8B), strongly suggesting that Varp together with Rab32/38 is involved in trafficking of Tyrp1 to melanosomes in melanocytes.
Because it has recently been proposed that Tyrp1 is transported to melanosomes through endosomes (Raposo and Marks, 2007), it would be fascinating if, in addition to Rab32/38, endosomal Rab21, which is activated by Varp (Zhang et al., 2006), is also involved in Tyrp1 trafficking in melanocytes. In the present study, however, we were unable to obtain any evidence that Rab21 is involved in Tyrp1 trafficking, first, because expression of the N-terminal VPS9 domain of Varp had no effect on Tyrp1 staining or its distribution (Figure 7A, e–h); and, second, expression of constitutive active/negative mutants of Rab21 or specific Rab21 shRNA had no effect on Tyrp1 expression (Supplemental Figure S6). These observations collectively indicate that Rab21 is unlikely to be directly involved in Tyrp1 trafficking in melanocytes.
What is the function of Varp in the melanosomal localization of Tyrp1 in melanocytes? Because melanogenic enzymes have recently been suggested to be mistargeted and degraded in Rab32/38-deficient melanocytes (Wasmeier et al., 2006), the most likely function of Varp is to control trafficking of Tyrp1 (e.g., transport from an endoplasmic reticulum [ER] to the Golgi, transport from endosomes or the trans-Golgi network, tethering, docking, and/or fusion of Tyrp1-containing vesicles to melanosomes) together with Rab32/38. In the absence of Varp, Tyrp1 is not correctly targeted to melanosomes, and mistargeted Tyrp1 (or untransported Tyrp1 in the ER) may be ubiquitinated (Ando et al., 2004, 2007; Kageyama et al., 2004) and degraded by proteasomes (e.g., ER-associated degradation), because our preliminary experiments showed that treatment of Varp-knockdown cells (or GFP-ANKR1–expressing cells) with a proteasome inhibitor, MG132, but not with lysosomal protease inhibitors (E64d and pepstatin A), restored Tyrp1 staining (Supplemental Figure S7Af). However, treatment of cells with proteasome inhibitors also affected the morphology of the cell (i.e., change into extended and filamentous shape), which precludes us to determine the exact localization of mistargeted proteins by immunofluorescence analysis (Supplemental Figure S7A, e–h). Further work is necessary to determine the exact mechanism of reduction in Tyrp1 levels in the absence of Varp.
In summary, we have identified Varp as a Rab32/38-specific binding protein in melanocytes and demonstrated that knockdown of endogenous Varp or expression of a dominant-negative construct (i.e., ANKR1) in melanocytes greatly attenuates the signals of a melanogenic enzyme, Tyrp1. So far as we know, Varp is the first candidate for a downstream target of Rab32/38 in melanocytes that is required for proper melanosomal localization of Tyrp1.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Dorothy C. Bennett for kindly donating melan-a cells and members of the Fukuda Laboratory for valuable discussions. This work was supported in part by the Ministry of Education, Culture, Sports, and Technology (MEXT) of Japan (to M. F. and T. I.), by the Global COE Program (Basic & Translational Research Center for Global Brain Science) from MEXT of Japan (to N. O. and M. F.), by the Kato Memorial Bioscience Foundation (to M. F.), and by the Cosmetology Research Foundation (to T. I.).
Abbreviations used:
- ANKR
ankyrin-repeat
- GEF
guanine nucleotide exchange factor
- GFP
green fluorescent protein
- GST
glutathione transferase
- HRP
horseradish peroxidase
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- Tyrp1
tyrosinase-related protein 1
- Str
monomeric strawberry
- Varp
VPS9-ankyrin-repeat protein
- VPS9
vacuolar protein sorting 9.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-12-1161) on April 29, 2009.
REFERENCES
- Ando H., Watabe H., Valencia J. C., Yasumoto K., Furumura M., Funasaka Y., Oka M., Ichihashi M., Hearing V. J. Fatty acids regulate pigmentation via proteasomal degradation of tyrosinase: a new aspect of ubiquitin-proteasome function. J. Biol. Chem. 2004;279:15427–15433. doi: 10.1074/jbc.M313701200. [DOI] [PubMed] [Google Scholar]
- Ando H., Kondoh H., Ichihashi M., Hearing V. J. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J. Invest. Dermatol. 2007;127:751–761. doi: 10.1038/sj.jid.5700683. [DOI] [PubMed] [Google Scholar]
- Bennett D. C., Cooper P. J., Hart I. R. A line of nontumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int. J. Cancer. 1987;39:414–418. doi: 10.1002/ijc.2910390324. [DOI] [PubMed] [Google Scholar]
- Carney D. S., Davies B. A., Horazdovsky B. F. Vps9 domain-containing proteins: activators of Rab5 GTPases from yeast to neurons. Trends Cell Biol. 2006;16:27–35. doi: 10.1016/j.tcb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Distinct Rab binding specificity of Rim1, Rim2, rabphilin, and Noc 2, identification of a critical determinant of Rab3A/Rab27A recognition by Rim2. J. Biol. Chem. 2003;278:15373–15380. doi: 10.1074/jbc.M212341200. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Versatile role of Rab27 in membrane trafficking: focus on the Rab27 effector families. J. Biochem. 2005;137:9–16. doi: 10.1093/jb/mvi002. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Mikoshiba K. A novel alternatively spliced variant of synaptotagmin VI lacking a transmembrane domain: implications for distinct functions of the two isoforms. J. Biol. Chem. 1999;274:31428–31434. doi: 10.1074/jbc.274.44.31428. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Kanno E. Analysis of the role of Rab27 effector Slp4-a/granuphilin-a in dense-core vesicle exocytosis. Methods Enzymol. 2005;403:445–457. doi: 10.1016/S0076-6879(05)03039-9. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Kanno E., Mikoshiba K. Conserved N-terminal cysteine motif is essential for homo- and heterodimer formation of synaptotagmins III, V, VI, and X. J. Biol. Chem. 1999;274:31421–31427. doi: 10.1074/jbc.274.44.31421. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Kojima T., Aruga J., Niinobe M., Mikoshiba K. Functional diversity of C2 domains of synaptotagmin family: mutational analysis of inositol high polyphosphate binding domain. J. Biol. Chem. 1995;270:26523–26527. doi: 10.1074/jbc.270.44.26523. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Kuroda T. S., Mikoshiba K. Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: implications of a tripartite protein complex for melanosome transport. J. Biol. Chem. 2002;277:12432–12436. doi: 10.1074/jbc.C200005200. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Kanno E., Yamamoto A. Rabphilin and Noc2 are recruited to dense-core vesicles through specific interaction with Rab27A in PC12 cells. J. Biol. Chem. 2004;279:13065–13075. doi: 10.1074/jbc.M306812200. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Kanno E., Ishibashi K., Itoh T. Large scale screening for novel Rab effectors reveals unexpected broad Rab binding specificity. Mol. Cell. Proteomics. 2008;7:1031–1042. doi: 10.1074/mcp.M700569-MCP200. [DOI] [PubMed] [Google Scholar]
- Grosshans B. L., Ortiz D., Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc. Natl. Acad. Sci. USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi H., Lippé R., McBride H. M., Rubino M., Woodman P., Stenmark H., Rybin V., Wilm M., Ashman K., Mann M., Zerial M. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell. 1997;90:1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Hume A. N., Tarafder A. K., Ramalho J. S., Sviderskaya E. V., Seabra M. C. A coiled-coil domain of melanophilin is essential for Myosin Va recruitment and melanosome transport in melanocytes. Mol. Biol. Cell. 2006;17:4720–4735. doi: 10.1091/mbc.E06-05-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Satoh M., Kanno E., Fukuda M. Screening for target Rabs of TBC (Tre-2/Bub2/Cdc16) domain-containing proteins based on their Rab-binding activity. Genes Cells. 2006;11:1023–1037. doi: 10.1111/j.1365-2443.2006.00997.x. [DOI] [PubMed] [Google Scholar]
- James P., Halladay J., Craig E. A. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama A., Oka M., Okada T., Nakamura S., Ueyama T., Saito N., Hearing V. J., Ichihashi M., Nishigori C. Down-regulation of melanogenesis by phospholipase D2 through ubiquitin proteasome-mediated degradation of tyrosinase. J. Biol. Chem. 2004;279:27774–27780. doi: 10.1074/jbc.M401786200. [DOI] [PubMed] [Google Scholar]
- Kuroda T. S., Ariga H., Fukuda M. The actin-binding domain of Slac2-a/melanophilin is required for melanosome distribution in melanocytes. Mol. Cell. Biol. 2003;23:5245–5255. doi: 10.1128/MCB.23.15.5245-5255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda T. S., Fukuda M. Rab27A-binding protein Slp2-a is required for peripheral melanosome distribution and elongated cell shape in melanocytes. Nat. Cell Biol. 2004;6:1195–1203. doi: 10.1038/ncb1197. [DOI] [PubMed] [Google Scholar]
- Kuroda T. S., Fukuda M. Identification and biochemical analysis of Slac2-c/MyRIP as a Rab27A-, myosin Va/VIIa-, and actin-binding protein. Methods Enzymol. 2005;403:431–444. doi: 10.1016/S0076-6879(05)03038-7. [DOI] [PubMed] [Google Scholar]
- Kuroda T. S., Fukuda M., Ariga H., Mikoshiba K. The Slp homology domain of synaptotagmin-like proteins 1–4 and Slac2 functions as a novel Rab27A binding domain. J. Biol. Chem. 2002;277:9212–9218. doi: 10.1074/jbc.M112414200. [DOI] [PubMed] [Google Scholar]
- Loftus S. K., Larson D. M., Baxter L. L., Antonellis A., Chen Y., Wu X., Jiang Y., Bittner M., Hammer J. A., III., Pavan W. J. Mutation of melanosome protein RAB38 in chocolate mice. Proc. Natl. Acad. Sci. USA. 2002;99:4471–4476. doi: 10.1073/pnas.072087599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes V. S., Wasmeier C., Seabra M. C., Futter C. E. Melanosome maturation defect in Rab38-deficient retinal pigment epithelium results in instability of immature melanosomes during transient melanogenesis. Mol. Biol. Cell. 2007;18:3914–3927. doi: 10.1091/mbc.E07-03-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M. S., Seabra M. C. The melanosome: membrane dynamics in black and white. Nat. Rev. Mol. Cell Biol. 2001;2:738–748. doi: 10.1038/35096009. [DOI] [PubMed] [Google Scholar]
- Ménasché G., Pastural E., Feldmann J., Certain S., Ersoy F., Dupuis S., Wulffraat N., Bianchi D., Fischer A., Le Deist F., de Saint Basile G. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat. Genet. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- Park M., Serpinskaya A. S., Papalopulu N., Gelfand V. I. Rab32 regulates melanosome transport in Xenopus melanophores by protein kinase A recruitment. Curr. Biol. 2007;17:2030–2034. doi: 10.1016/j.cub.2007.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 2001;11:487–491. doi: 10.1016/s0962-8924(01)02147-x. [DOI] [PubMed] [Google Scholar]
- Raposo G., Marks M. S. Melanosomes: dark organelles enlighten endosomal membrane transport. Nat. Rev. Mol. Cell Biol. 2007;8:786–797. doi: 10.1038/nrm2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. L., Cao C., Pylypenko O., Rak A., Wandinger-Ness A. Rab GTPases at a glance. J. Cell Sci. 2007;120:3905–3910. doi: 10.1242/jcs.015909. [DOI] [PubMed] [Google Scholar]
- Shaner N. C., Campbell R. E., Steinbach P. A., Giepmans B.N.G., Palmer A. E., Tsien R. Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Theos A. C., Truschel S. T., Raposo G., Marks M. S. The Silver locus product Pmel17/gp100/Silv/ME 20, controversial in name and in function. Pigment Cell Res. 2005;18:322–336. doi: 10.1111/j.1600-0749.2005.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T., Fukuda M. Rab3A and Rab27A cooperatively regulate the docking step of dense-core vesicle exocytosis in PC12 cells. J. Cell Sci. 2006;119:2196–2203. doi: 10.1242/jcs.02962. [DOI] [PubMed] [Google Scholar]
- Wang F., Zhang H., Zhang X., Wang Y., Ren F., Zhang X., Zhai Y., Chang Z. Varp interacts with Rab38 and functions as its potential effector. Biochem. Biophys. Res. Commun. 2008;372:162–167. doi: 10.1016/j.bbrc.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Wasmeier C., Romao M., Plowright L., Bennett D. C., Raposo G., Seabra M. C. Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes. J. Cell Biol. 2006;175:271–281. doi: 10.1083/jcb.200606050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. M., Yip R., Swing D. A., O'Sullivan T. N., Zhang Y., Novak E. K., Swank R. T., Russell L. B., Copeland N. G., Jenkins N. A. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc. Natl. Acad. Sci. USA. 2000;97:7933–7938. doi: 10.1073/pnas.140212797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. S., Rao K., Zhang H., Wang F., Sellers J. R., Matesic L. E., Copeland N. G., Jenkins N. A., Hammer J. A., III. Identification of an organelle receptor for myosin-Va. Nat. Cell Biol. 2002;4:271–278. doi: 10.1038/ncb760. [DOI] [PubMed] [Google Scholar]
- Zerial M., McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zhang X., He X., Fu X. Y., Chang Z. Varp is a Rab21 guanine nucleotide exchange factor and regulates endosome dynamics. J. Cell Sci. 2006;119:1053–1062. doi: 10.1242/jcs.02810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.