Abstract
Background
Previous work has implicated noradrenergic beta-receptors in the consolidation and reconsolidation of conditioned fear. Less is known, however, about their role in fear expression and extinction. The beta-receptor blocker propranolol has been used clinically to reduce anxiety. Using an auditory fear conditioning task in rats, we assessed the effects of propranolol on the expression and extinction of two measures of conditioned fear: freezing and suppression of bar pressing.
Methods
One day after receiving auditory fear conditioning, rats were injected with saline, propranolol or peripheral blocker sotalol (both 10 mg/kg, ip). Twenty minutes after injection, rats were given either 6 or 12 extinction trials and were tested for extinction retention the following day. The effect of propranolol on the firing rate of neurons in prelimbic (PL) prefrontal cortex was also assessed.
Results
Propranolol reduced freezing by more than 50%, an effect that was evident from the first extinction trial. Suppression was also significantly reduced. Despite this, propranolol had no effect on the acquisition or retention of extinction. Unlike propranolol, the peripheral blocker sotalol did not affect fear expression, although both drugs significantly reduced heart rate. This suggests that propranolol acts centrally to reduce fear. Consistent with this, propranolol reduced the firing rate of PL neurons.
Conclusion
Propranolol reduced the expression of conditioned fear, without interfering with extinction learning. Reduced fear with intact extinction suggests a possible use for propranolol in reducing anxiety during extinction-based exposure therapies, without interfering with long-term clinical response.
Keywords: noradrenergic, heart rate, prelimbic, prefrontal, PTSD, anxiety disorder
Introduction
Emotional memories are associated with enhanced noradrenergic signaling via beta-receptors (1), and perturbations of the beta-noradrenergic system may contribute to the persistence of disturbing emotional memories (2). In general, studies of the noradrenergic system in memory formation have focused on post-training consolidation (3) and reconsolidation processes (4), but less is known about how norepinephrine affects the expression and extinction of learned fear.
Propranolol, a centrally acting beta-receptor antagonist, has been shown to reduce anxiety and fear. In humans, propranolol reduces acute stage fright (5), test anxiety (6) and contextual fear (7). In rats, propranolol dose-dependently decreases anxiety in an open field (8) and in a light-enhanced startle paradigm (9). Propranolol reduced the expression of conditioned startle responses in rats (10), but not conditioned freezing in mice (11). Additionally, there is evidence that both shock and conditioned fear stimuli evoke noradrenergic efflux throughout the cortex (12; 13; 14). The effect of propranolol on fear extinction has led to mixed results, showing no effect when given systemically (11) and impairment when infused into the medial prefrontal cortex (mPFC) (15).
These mixed findings prompted us to re-examine the effects of systemic propranolol on the expression and extinction of cued fear in an auditory fear conditioning task, using the same dosage as previous studies (10 mg/kg, ip). We administered propranolol to rats prior to extinction training and examined both freezing and bar-press suppression responses to a conditioned tone. The following day, we assessed retention of extinction. Our purpose was two-fold: 1) to assess the effects of propranolol on expression of conditioned fear, and 2) to assess the effects of propranolol on extinction memory. We also assessed the effect of systemic propranolol on the activity of neurons in the prelimbic region (PL) of mPFC, an area implicated in the expression of conditioned fear (16; 17; 18). Clarifying the effects of systemic propranolol on the expression and extinction of conditioned fear could have clinical significance, as extinction is the basis of exposure-based therapies for the treatment of anxiety disorders (19).
Methods and Materials
Subjects
A total of 131 male Sprague-Dawley rats (Hilltop Laboratory, Scottdale, PA, USA) weighing ~300 g were housed and handled as previously described (20). Rats were maintained on a 12 hr light/dark cycle and fed standard laboratory rat chow in a restricted manner (18 g/d) until they reached 85% of their free-feeding weight. Rats had free access to water throughout the experiment. Rats were individually housed and transported daily from the animal facility to a holding room in our laboratory during experimental phases. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Puerto Rico, in compliance with National Institute of Health (NIH) guidelines.
Fear Conditioning
Fear conditioning was carried out in standard operant chambers (Coulbourn Instruments, Whitehall, PA) located inside sound-attenuating boxes (Med Associates, Burlington, VT) in an isolated testing room. Further details regarding the apparatus have been previously described (20). Prior to fear conditioning, rats were trained in the operant chambers to press a bar for food pellets (Bioserve, Inc, Frenchtown, NJ) on a variable interval reinforcement schedule (VI-60). Bar pressing was used to maintain a constant level of activity against which freezing could reliably be measured. Following bar-press training, rats were fear conditioned. On day 1, rats were presented with five non-reinforced tones (4 kHz; 78 dB; 30 sec; Habituation) followed by seven tones paired with footshock (0.55 mA, 0.5 sec; Conditioning). After conditioning, rats were assigned to two groups that were matched for levels of freezing across conditioning. On day 2, rats were injected with either saline or propranolol (10 mg/kg; i.p.) 20 minutes prior to extinction training. Separate groups of rats were given either complete extinction (12 nonreinforced tone presentations) or partial extinction (6 nonreinforced tone presentations). On day 3, extinction tones were given to test for retention of extinction memory. In all phases of the experiment, the intertrial interval was variable, with an average of 3 min. Forty-eight hours after testing (day 5), a subgroup of rats that had received partial extinction were given 6 additional extinction trials, followed by two unsignaled shocks (0.55 mA). On day 6, rats were tested for reinstatement of conditioned fear. In a separate experiment, rats were administered sotalol (10 mg/kg; i.p.), a beta-receptor antagonist that does not cross the blood-brain barrier (21), 20 min prior to 12 extinction trials in order to assess the role of central vs. peripheral receptors in fear expression and extinction.
Open Field and Heart Rate Testing
To test the effects of propranolol on locomotor activity, rats were given injections of saline or propranolol (10 mg/kg, i.p.) 20 min prior to testing in an open field. Grid lines drawn on the floor of the arena (91.5 × 91.5 × 61 cm) divided it into a peripheral region (within 15.25 cm of the walls) and central region (61 × 61 cm) of approximately equal area. The number of line crosses and time spent in the central region were scored by an observer blind with respect to experimental groups.
Resting heart rate was acquired from anesthetized rats using an electrocardiogram (ECG) monitor (EC 60, Silogic International Limited, Mitcham, Surrey, UK). Rats were anesthetized with ketamine (80 mg/kg)/xylazine (5 mg/kg; i.p.) and connected to the ECG. Baseline heart rates were recorded, after which rats received an injection of either propranolol (10 mg/kg; i.p.) or sotalol (10 mg/kg; i.p.). Ten minutes post-injection, heart rate was again recorded. During each session, heart rates were sampled every 10 seconds for 1 minute and averaged.
Behavioral Analysis
Throughout all phases of the fear conditioning experiments, we used two measures of conditioned fear: percent of time spent freezing (22) and suppression of bar pressing (23; 20). Freezing is defined as the absence of all movement except respiration and was quantified from digital videos during each tone presentation using commercial software (FreezeScan, Clever Systems, Reston, VA). Rats failing to show greater than 20% freezing (averaged over conditioning trials 4–7) were excluded, which resulted in the exclusion of 14/90 rats. Bar-press suppression ratios were determined by comparing pressing during the tone to pressing prior to the tone as follows: suppression ratio = (pretone rate − tone rate)/(pretone rate + tone rate). A value of 0 indicates no suppression of bar pressing (no fear), whereas a value of 1 indicates complete suppression (high fear). We also analyzed the rate of bar pressing prior to the first extinction trial to observe any differences in motivation to bar press for food. Freezing, bar-press suppression, activity in the open field and bar press rate, were analyzed using Student’s t-test or repeated-measures ANOVA. Heart rate changes were analyzed using confidence interval of the mean and Student’s t test.
Multi-channel Unit Recording
A separate group of rats were surgically implanted with recording electrodes that consisted of drivable bundles of 16 microwires (22 μm, Stablohm 650; California Fine Wire) as previously described (24). Electrodes were aimed at the prelimbic cortex (PL), located 2.9 mm anterior, 0.6 mm lateral and 4.0 mm ventral from bregma. Following surgery, rats were allowed 6 days to recover. Rats were then acclimated to recording procedures in the same chambers as in the behavioral experiments, and electrodes were driven in increments of 44 μm until single units were isolated using principle components analysis and template matching (Offline Sorter, Plexon Inc., Dallas, TX). Once cells in PL were well isolated, we assessed the effects of injections of saline or propranolol (10 mg/kg; i.p.) on spontaneous activity while rats were in the operant chamber pressing for food. Ten min sessions of spontaneous activity were recorded at four time-points: 10 min prior to and 30 min after saline injection, and 10 min prior to and 30 min after propranolol injection. Firing rates before and after injections were compared using a Wilcoxon matched-pairs test. After recording the four sessions at a given location, the electrode drive was advanced in 80 μm steps until new cells were found, and the experiment was repeated. Spike trains were analyzed with NeuroExplorer (NEX Technologies, Littleton, MA) to obtain firing rate and bursting. Bursts were defined as three or more successive spikes in which the first interspike interval was less than 25 ms and subsequent intervals were less than 50 ms (24). At the conclusion of the experiment, lesions were made at the tip of the recording wires by passing an anodal current of 25 μA for 18 s. Rats were then perfused with 10% buffered formalin and the brains were removed to mark the microlesions with a blue reaction of 6% ferrocyanide while fixing the tissue in 30% sucrose/10% buffered formalin. Locations of lesions were reconstructed onto coronal drawings adapted from (25) from 40 μm Nissl-stained sections.
Results
Propranolol reduces fear expression without affecting extinction memory
We first examined the effects of propranolol given prior to extinction training on cued fear expression and subsequent extinction learning. Rats were injected with saline (n=10) or propranolol (n=12) 20 minutes prior to extinction training. Reduced freezing under propranolol was evident from the first extinction training trial (Sal, 31%; Prop, 11%; t(20)=2.55; p=0.02), indicating reduced fear expression (see Figure 1). Across the extinction session, propranolol-treated rats maintained significantly lower levels of both freezing (F(1,20)=12.49, p=0.002) and bar press suppression (F(1,20)=6.42, p=0.02) compared to saline-treated rats. The smaller effect in suppression versus freezing was likely due to ceiling levels of suppression in controls. Despite this reduction in fear expression, propranolol-treated rats exhibited normal extinction learning, as evidenced by a progressive decrease in bar-press suppression throughout the extinction session. The following day, when tested drug-free, saline- and propranolol-treated rats showed similar low levels of freezing (Sal, 7%; Prop, 3%; t(20)=1.27; p=0.22) and bar-press suppression (Sal, 0.41; Prop, 0.37; t(20)=0.28; p=0.78), indicating that although propranolol reduced fear expression during extinction training it did not impair extinction learning.
Figure 1.
Propranolol reduces fear expression but does not impair extinction learning. Systemic injections of propranolol (arrow) prior to complete extinction on day 2 led to a significant decrease in fear expression as measured by (A) percent freezing and (B) bar-press suppression. On day 3 both groups recalled extinction similarly. Data shown in blocks of 2 trials. (conditioning: Cond)
Although the previous experiment showed that propranolol did not impair extinction retention, both saline- and propranolol-treated rats showed little freezing during the extinction recall test. With floor levels of freezing, it is not possible to detect if propranolol-treated rats exhibited less fear than controls. Enhanced recall of extinction might be expected if, for example, propranolol interfered with reconsolidation of the fear memory on day 2 (4). To address this issue, we repeated the experiment using partial extinction training (6 non-reinforced tone presentations), resulting in moderate levels of freezing during the drug-free test, thereby allowing us to detect any enhancement of extinction (see Figure 2). As in the previous experiment, rats were injected with saline or propranolol 20 minutes prior to extinction training (Sal, n=15; Prop, n=14). Again, propranolol significantly reduced freezing during the first extinction trial (Sal, 47%; Prop, 24%; t(27)=2.12; p=0.04), and reduced both freezing (F(1,27)=6.54, p=0.02) and bar-press suppression (F(1,27)=13.16, p=0.001) throughout extinction training. The following day, however, propranolol-treated rats showed the same level of freezing (Sal, 31%; Prop, 23%; t(27)=0.66; p=0.52) and suppression (Sal, 0.74; Prop, 0.68; t(27)=0.53; p=0.60) as controls. Furthermore, when given unsignaled footshocks, propranolol-treated rats showed similar levels of fear reinstatement to controls in both freezing (Sal, 26%; Prop, 30%; t(27)=−0.44; p=0.66) and suppression (Sal, 0.82; Prop, 0.77; t(27)=0.36; p=0.71) measurements. Thus, propranolol neither facilitated extinction nor blocked reconsolidation of fear under these conditions.
Figure 2.
Propranolol does not facilitate extinction learning or erase fear memory. Injection of propranolol (arrow) prior to partial extinction on day 2 led to a significant decrease in fear expression as measured by (A) percent freezing and (B) bar-press suppression. There were no differences between groups in further extinction sessions on day 3 and day 5. Two unpaired shocks on day 5 reinstated fear on day 6 equally in both groups. Data shown in blocks of 2 trials. (conditioning: Cond; extinction: Ext; reinstatement: Reinst; day 2: D2)
Propranolol-induced fear reductions are not due to non-specific behavioral effects, and are mediated centrally
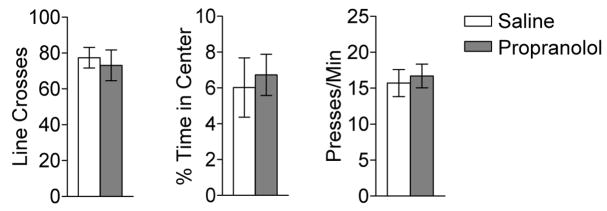
To examine non-specific effects of propranolol that might account for the observed reduction in fear expression, we evaluated its effects on locomotion and anxiety in an open field (Sal, n=8; Prop, n=8) as well as on motivation to bar press for food (Sal, n=25; Prop, n=26; see Figure 3). Propranolol had no effect on spontaneous locomotion as measured by the number of line crosses in the open field (Sal, 77 crosses; Prop, 73 crosses; t(14)=0.41; p=0.69). In addition, anxiety was not affected, as both groups spent a similar percentage of time in the center of the open field (Sal, 6.0%; Prop, 6.7%; t(14)= −0.35; p=0.73). Propranolol also had no effect on the rate of bar pressing for food prior to the first extinction trial (Sal, 16 presses/min; Prop, 17 presses/min; t(49)= −0.39; p=0.70). Collectively, these results indicate that the reduction in freezing observed after propranolol administration was not due to non-specific effects such as changes in anxiety levels, locomotor behavior or motivation to bar press.
Figure 3.
(A) Propranolol does not alter spontaneous locomotion (left), anxiety in an open field (center), or rate of bar-pressing for food reward (right).
Because propranolol acts both centrally and peripherally, it is possible that the reduction in fear was caused by reduced feedback from the peripheral nervous system, e.g., cardiovascular responses (26). To assess whether reduced fear expression by propranolol is centrally or peripherally mediated, we repeated the experiment with the noradrenergic beta-receptor antagonist sotalol, which does not cross the blood-brain barrier (27; 21). We injected rats with saline or sotalol (10 mg/kg) 20 minutes prior to receiving 12 extinction trials (Sal, n=11; Sot, n=14; see Figure 4). Sotalol-treated rats did not differ from the saline-treated rats in levels of freezing during the first extinction trial (Sal, 28%; Sot, 31%; t(23)= −0.33; p=0.74), nor during the rest of the extinction session as indicated by freezing (F(1,23)=0.37; p=0.54) and bar-press suppression (F(1,23)=0.71; p=0.41). The following day, sotalol- and saline-treated rats showed similar extinction retrieval, as measured by freezing (Sal, 6.9%; Sot, 4.9%; t(23)=0.25; p=0.80) and bar-press suppression (Sal, 0.54; Sot, 0.44; t(23)=0.72; p=0.48). Thus when confined to the periphery, beta blockers do not reduce conditioned fear expression.
Figure 4.
Peripheral beta blocker sotalol does not reduce fear expression or impair extinction learning. Systemic injections of sotalol (arrow) prior to complete extinction on day 2 did not decrease fear expression as measured by (A) percent freezing and (B) bar-press suppression. On day 3, both groups recalled extinction similarly. Data shown in blocks of 2 trials. (C) Propranolol and sotalol both reduced heart rate from baseline levels in anesthetized rats. Rates were assessed prior to, and 10 min after, injection of drugs. Propranolol significantly reduced heart rate below baseline (94%, *p<0.05). Similarly, sotalol significantly reduced heart rate below baseline (87%, **p<0.01). (conditioning: Cond; heart rate: HR)
To confirm that both sotalol and propranolol had similar peripheral actions, we monitored heart rate in a separate group of anesthetized rats (Prop, n=10; Sot, n=10; see Figure 4C). Injection of propranolol (10 mg/kg) significantly reduced heart rate relative to baseline (94%, p<0.05 ), as did injection of sotalol (10 mg/kg) (87%, p<0.01). Thus, although sotalol and propranolol have similar peripheral actions, only the centrally-acting propranolol was effective in reducing fear expression.
Propranolol reduces firing rate of prelimbic neurons
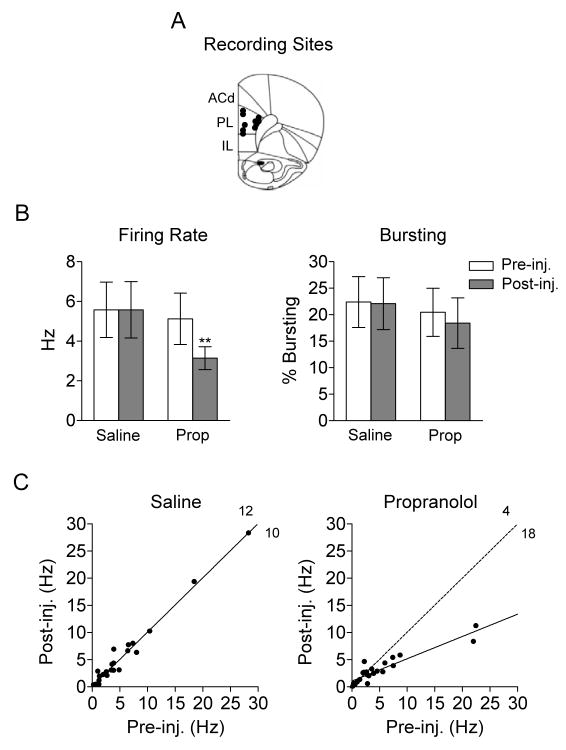
We have recently shown that activity in the prelimbic cortex (PL) is necessary for the expression of conditioned fear (16; 18). Thus, we examined the effect of propanolol on spontaneous activity of individual PL neurons (see Figure 5). Spontaneous activity was recorded prior to and after injection of saline or propranolol (10 mg/kg). A total of 22 neurons from 5 rats were maintained across all four 10 min recording sessions (Pre-Sal, Post-Sal, Pre-Prop, Post-Prop). Propranolol significantly reduced the spontaneous firing rate of PL neurons, from 5.2 Hz to 3.2 Hz (Wilcoxon matched-pairs test: Z=2.94, p=0.003). There was no effect on high-frequency bursting (t(21)=0.41, p=0.68). The response of individual neurons to injections of saline and propranolol are shown in scatter plots in Figure 5C. Unlike saline, propranolol reduced the firing rate of the majority of neurons (Fisher exact test; p=0.013). Taken together, these results suggest that reduced fear expression by propranolol could be due to a decrease in PL excitability.
Figure 5.
Propranolol reduces spontaneous firing rate of prelimbic (PL) neurons in awake rats. (A) Unit recording sites in PL (IL: infralimbic cortex; ACd: dorsal anterior cingulate cortex). (B) Systemic propranolol decreased spontaneous firing rate (**p<0.01), but not bursting, in PL neurons. (C) The firing rates of individual neurons before and after injection are shown. Unlike saline, injection of propranolol decreased the firing rate of the majority of neurons (p<0.05). Solid lines in each plot represent the linear regression; dotted line in the propranolol plot represents the linear regression of the saline results. (propranolol: Prop; injection: inj.)
Discussion
We examined the effects of the noradrenergic beta-receptor antagonist propranolol on the expression and extinction of cued fear conditioning. Propranolol significantly reduced fear expression, as measured by freezing and bar-press suppression. Extinction learning, however, was unaffected by propranolol, as evidenced by normal acquisition and recall of extinction. Propranolol-induced reductions of fear could not be attributed to effects on locomotion, motivation to press for food, or anxiety. Propranolol’s effects appear to be mediated centrally, as the peripheral beta-adrenergic antagonist sotalol had no effect on fear expression. Consistent with this, propranolol reduced the activity of neurons in PL.
Propranolol-induced reduction in the expression of cued fear generally agrees with prior findings in other conditioning procedures. The same dose of propranolol reduced expression of fear-potentiated startle (10) and tone-induced freezing (28) in rats, as well as contextual freezing in mice (29). Cain et al (2004) observed accelerated extinction under propranolol, but because freezing to the first extinction tone was not reduced, they concluded that propranolol did not impair expression of conditioned fear. In contrast, we observed a significant reduction in freezing from the first extinction tone onward, consistent with decreased expression of fear. Thus, differences in species used or experimental parameters could account for the variability in the effects of propranolol on fear expression.
Despite previous reports that central infusions of propranolol can impair extinction (30; 15), we observed no impairment of extinction consolidation after systemic injections of propranolol, in agreement with Cain and colleagues (2004). Furthermore, with partial extinction training, we observed that propranolol did not facilitate extinction consolidation. Thus, at the dose used here, pre-extinction propranolol did not alter extinction learning or retention. The apparent discrepancy with local infusion studies could be due to differences in the concentration of propranolol that reaches structures such as the prefrontal cortex with systemic vs. localized administration.
Although our study was not designed to identify the site of action of propranolol in the brain, we observed a significant reduction in the spontaneous firing rate of PL neurons after systemic propranolol injections. Reduced excitability in PL would be expected to reduce tone-evoked responses. Several lines of evidence implicate PL in expression of conditioned fear. Pharmacological inactivation of PL reduces tone-evoked freezing (17; 18), and electrical stimulation of PL has the opposite effect (16). In addition, tone responsiveness of PL neurons increases during auditory fear conditioning (31; 32). Thus, propranolol may act by blocking norepinephrine-induced increases in PL activity during high-fear states. Propranolol could also reduce the activity of afferents to PL, such as the basolateral amygdala (33). Either way, reduced activity of PL neurons is consistent with the hypothesis that propranolol reduces activity in fear expression circuits.
The ability of propranolol to reduce fear expression without interfering with extinction learning suggests that propranolol might be useful as an adjunct to exposure-based cognitive-behavioral therapy for anxiety disorders. The stress associated with repeated exposure to fear-inducing stimuli is thought to contribute to dropout rates in these therapies (34). Reducing excessive stress during the exposure session with propranolol could make these therapies more tolerable, especially for patients with high-fear reactions. While propranolol does not facilitate extinction of fear like other adjuncts (35), neither does it impair extinction. Thus, the reduction in fear would not interfere with the clinical effectiveness of the therapy. This differs from benzodiazepines, which can reduce fear expression, but lead to a return of fear via a state-dependent learning effect (36).
In humans, propranolol has traditionally been used to reduce performance anxiety in musicians (37), but its effects on other types of anxiety have been mixed. Propranolol successfully reduced anxiety in dental phobics (38), and avoidance behavior in panic disorder patients (39). Other studies, however, showed no effect of propranolol on subjective anxiety in phobics (40), or expression of cued fear conditioning (7). It is interesting to note that propranolol improves cognitive ability under stressful conditions (6; 41), suggesting potential use in therapies that combine exposure and cognitive therapies.
Our rodent findings suggest that propranolol may be useful if given prior to exposure to traumatic stimuli to reduce the expression of fear responses during extinction-based therapies. Given the conflicting reports in the rodent and human literatures, however, additional studies are needed to determine if propranolol reduces learned fear in healthy humans and anxiety patients while leaving extinction learning intact.
Acknowledgments
This research was supported by NIH grants MH058883 and GM008224 to GJQ, the University of Puerto Rico School of Medicine (RCMI, G12-RR03051), and a Career Opportunity in Research (COR) Education and Training Scholarship (T34-MH019134) to JRR. The authors thank Dimaris Merced and Alexis Florian Ayala for assistance with behavioral experiments, as well as Dr. Jamie Peters and Demetrio Sierra-Mercado for assistance with the single unit recording. We also thank Dr. Kevin Corcoran and Dr. Anthony Burgos-Robles for providing helpful comments on the manuscript.
Footnotes
Financial Disclosure
Jose Rodriguez-Romaguera, Francisco Sotres-Bayon, Devin Mueller and Gregory J. Quirk have no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 2.Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- 3.Hatfield T, McGaugh JL. Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiol Learn Mem. 1999;71:232–239. doi: 10.1006/nlme.1998.3875. [DOI] [PubMed] [Google Scholar]
- 4.Debiec J, LeDoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Brantigan CO, Brantigan TA, Joseph N. Effect of beta blockade and beta stimulation on stage fright. Am J Med. 1982;72:88–94. doi: 10.1016/0002-9343(82)90592-7. [DOI] [PubMed] [Google Scholar]
- 6.Faigel HC. The effect of beta blockade on stress-induced cognitive dysfunction in adolescents. Clin Pediatr (Phila) 1991;30:441–445. doi: 10.1177/000992289103000706. [DOI] [PubMed] [Google Scholar]
- 7.Grillon C, Cordova J, Morgan CA, Charney DS, Davis M. Effects of the beta-blocker propranolol on cued and contextual fear conditioning in humans. Psychopharmacology (Berl) 2004;175:342–352. doi: 10.1007/s00213-004-1819-5. [DOI] [PubMed] [Google Scholar]
- 8.Angrini M, Leslie JC, Shephard RA. Effects of propranolol, buspirone, pCPA, reserpine, and chlordiazepoxide on open-field behavior. Pharmacol Biochem Behav. 1998;59:387–397. doi: 10.1016/s0091-3057(97)00457-7. [DOI] [PubMed] [Google Scholar]
- 9.Walker DL, Davis M. Light-enhanced startle: further pharmacological and behavioral characterization. Psychopharmacology (Berl) 2002;159:304–310. doi: 10.1007/s002130100913. [DOI] [PubMed] [Google Scholar]
- 10.Davis M, Redmond DE, Jr, Baraban JM. Noradrenergic agonists and antagonists: effects on conditioned fear as measured by the potentiated startle paradigm. Psychopharmacology (Berl) 1979;65:111–118. doi: 10.1007/BF00433036. [DOI] [PubMed] [Google Scholar]
- 11.Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassens G, Roffman M, Kuruc A, Orsulak PJ, Schildkraut JJ. Alterations in brain norepinephrine metabolism induced by environmental stimuli previously paired with inescapable shock. Science. 1980;209:1138–1140. doi: 10.1126/science.7403874. [DOI] [PubMed] [Google Scholar]
- 13.Hugues S, Garcia R, Lena I. Time course of extracellular catecholamine and glutamate levels in the rat medial prefrontal cortex during and after extinction of conditioned fear. Synapse. 2007;61:912–916. doi: 10.1002/syn.20448. [DOI] [PubMed] [Google Scholar]
- 14.Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- 15.Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blum S, Hebert AE, Dash PK. A role for the prefrontal cortex in recall of recent and remote memories. Neuroreport. 2006;17:341–344. doi: 10.1097/01.wnr.0000201509.53750.bc. [DOI] [PubMed] [Google Scholar]
- 18.Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cahill SP, Foa EB, Hembree EA, Marshall RD, Nacash N. Dissemination of exposure therapy in the treatment of posttraumatic stress disorder. J Trauma Stress. 2006;19:597–610. doi: 10.1002/jts.20173. [DOI] [PubMed] [Google Scholar]
- 20.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahlof C. Studies on beta-adrenoceptor mediated facilitation of sympathetic neurotransmission. Acta Physiol Scand Suppl. 1981;500:1–147. [PubMed] [Google Scholar]
- 22.Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- 23.Estes WK, Skinner BF. Some quantitative properties of anxiety. J Exp Psychol. 1941;29:390–400. [Google Scholar]
- 24.Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Amsterdam: Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- 26.Nakano J, Kusakari T. Effect of beta adrenergic blockade on the cardiovascular dynamics. Am J Physiol. 1966;210:833–837. doi: 10.1152/ajplegacy.1966.210.4.833. [DOI] [PubMed] [Google Scholar]
- 27.Arendt RM, Greenblatt DJ, deJong RH, Bonin JD, Abernethy DR. Pharmacokinetics, central nervous system uptake, and lipid solubility of propranolol, acebutolol, and sotalol. Cardiology. 1984;71:307–314. doi: 10.1159/000173684. [DOI] [PubMed] [Google Scholar]
- 28.Morris RW, Westbrook RF, Killcross AS. Reinstatement of extinguished fear by beta-adrenergic arousal elicited by a conditioned context. Behav Neurosci. 2005;119:1662–1671. doi: 10.1037/0735-7044.119.6.1662. [DOI] [PubMed] [Google Scholar]
- 29.Ouyang M, Thomas SA. A requirement for memory retrieval during and after long-term extinction learning. Proc Natl Acad Sci U S A. 2005;102:9347–9352. doi: 10.1073/pnas.0502315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav Neurosci. 2005;119:1496–1510. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- 32.Vidal-Gonzalez I, Corcoran KA, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Converging pharmacological, microstimulation, and physiological evidence that the prelimbic prefrontal cortex mediates expression of learned fears in the rat. Neuropsychopharmacology. 2006;31:S161. [Google Scholar]
- 33.Buffalari DM, Grace AA. Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of alpha-2 and beta receptor activation. J Neurosci. 2007;27:12358–12366. doi: 10.1523/JNEUROSCI.2007-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choy Y, Fyer AJ, Lipsitz JD. Treatment of specific phobia in adults. Clin Psychol Rev. 2007;27:266–286. doi: 10.1016/j.cpr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Davis M, Myers KM, Chhatwal J, Ressler KJ. Pharmacological treatments that facilitate extinction of fear: relevance to psychotherapy. NeuroRx. 2006;3:82–96. doi: 10.1016/j.nurx.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouton ME, Kenney FA, Rosengard C. State-dependent fear extinction with two benzodiazepine tranquilizers. Behav Neurosci. 1990;104:44–55. doi: 10.1037//0735-7044.104.1.44. [DOI] [PubMed] [Google Scholar]
- 37.Tyrer P. Current status of beta-blocking drugs in the treatment of anxiety disorders. Drugs. 1988;36:773–783. doi: 10.2165/00003495-198836060-00006. [DOI] [PubMed] [Google Scholar]
- 38.Liu HH, Milgrom P, Fiset L. Effect of a beta-adrenergic blocking agent on dental anxiety. J Dent Res. 1991;70:1306–1308. doi: 10.1177/00220345910700091401. [DOI] [PubMed] [Google Scholar]
- 39.Ravaris CL, Friedman MJ, Hauri PJ, McHugo GJ. A controlled study of alprazolam and propranolol in panic-disordered and agoraphobic outpatients. J Clin Psychopharmacol. 1991;11:344–350. [PubMed] [Google Scholar]
- 40.Fagerstrom KO, Hugdahl K, Lundstrom N. Effect of beta-receptor blockade on anxiety with reference to the three-systems model of phobic behavior. Neuropsychobiology. 1985;13:187–193. doi: 10.1159/000118186. [DOI] [PubMed] [Google Scholar]
- 41.Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ. Beta-adrenergic modulation of cognitive flexibility during stress. J Cogn Neurosci. 2007;19:468–478. doi: 10.1162/jocn.2007.19.3.468. [DOI] [PubMed] [Google Scholar]