Abstract
Transgenic expression of enhanced green fluorescent protein (eGFP) in myocardium can result in cardiac dysfunction and cardiomyopathy, presumably through toxic effects that disrupt normal cellular signaling. The multifunctional Ca2+ and calmodulin-dependent protein kinase II (CaMKII) is widely expressed in myocardium and CaMKII activity is increased in human and animal models of cardiomyopathy, so we hypothesized that increased CaMKII activity is important for cardiomyopathy due to transgenic expression of eGFP. Here we report that cardiomyocyte-delimited eGFP over-expression causes increased CaMKII activity that predicts left ventricular dilation and dysfunction. On the other hand, transgenic co-expression of a CaMKII inhibitory peptide with eGFP prevents eGFP-mediated left ventricular dilation and dysfunction. These findings suggest that increased CaMKII activity is a critical pathological signal in transgenic cardiomyopathy due to eGFP over-expression.
Keywords: calmodulin kinase II, cardiomyopathy, eGFP
1. Introduction
Transgenic over-expression models have played important roles in advancing understanding of myocardial physiology and diseases. The complete sequencing of the mouse genome, the relative ease of using mouse embryonic stem cells and identification of the highly specific α-myosin heavy chain promoter have contributed to the popularity of transgenic mouse models for studying myocardium. Although the many advantages of these transgenic models are self evident, one concern lies in the fact that protein over-expression can have unforeseen, sometimes toxic consequences in heart. Most transgenic models use wild type littermate controls, but wild type controls do not address the potential ‘non-specific’ effects of protein over-expression. Improved understanding of the cellular mechanisms responsible for transgenic cardiotoxicity may lend clarity to studies with myocardial transgenic models.
Transgenic over-expression of enhanced green fluorescent protein (eGFP) has been shown to cause cardiac dysfunction and chamber dilation by an unknown mechanism.1 The multifunctional Ca2+/calmodulin-dependent protein kinase II (CaMKII) is widely expressed in myocardium, and CaMKII activity is increased in patients and animal models of cardiomyopathy.2 On the other hand, CaMKII inhibition effectively protects against left ventricular (LV) dysfunction and dilation caused by myocardial infarction and catecholamine toxicity.3 We reasoned that CaMKII activity could also be important for transduction of LV dilation and dysfunction due to eGFP over-expression. We studied four lines of transgenic mice with α-myosin heavy chain promoter driven eGFP expression. These four lines of transgenic mice had a three fold range of eGFP expression. Two of the four transgenic lines had eGFP fused to a CaMKII inhibitory peptide (AC3-I), while the other two transgenic lines expressed eGFP fused to an inactive and scrambled version of AC3-I (AC3-C). These mice allowed us to dissect the effects of eGFP over-expression from CaMKII activity. Here we show that eGFP expression leads to increased CaMKII activity, and reduced LV fractional shortening. LV dilation and dysfunction due to eGFP over-expression were prevented by CaMKII inhibition. These data support a concept that increased CaMKII activity is a critical and specific pathological signal in cardiomyopathy due to transgenic eGFP over-expression.
2. Materials and Methods
2.1. Transgenic mice
We developed a genetic mouse model of cardiac CaMKII inhibition with α-myosin heavy chain promoter-directed myocardial enhanced GFP (eGFP) expression fused to a minigene encoding a CaMKII inhibitory peptide, auto-camtide-3-inhibitor (AC3-I). AC3-I specifically inhibits CaMKII by mimicking the autoinhibitory domain of CaMKII.4 Another transgenic mouse model was created with a minigene for eGFP and AC3-C, auto-camtide-3-control, a scrambled inactive from of AC3-I. eGFP was expressed in both AC3-I and AC3-C transgenic mice and was present throughout the cardiomyocytes. A detailed description of these transgenic mice was previously published.3 Two lines of AC3-I and two lines of AC3-C transgenic mice (designated L1 and L2) were developed. AC3-I and AC3-C male mice from L1 and L2 at 10 to 12-weeks old and wild type littermate controls (WT) were used for this study. Our previous studies described AC3-I L1 and AC3C L2.
2.2. Murine echocardiography
Transthoracic echocardiography was performed in conscious mice using previously published methods (Sonos 5500, Agilent, Andover, Mass).5
2.3. CaMKII activity
CaMKII activity of fresh ventricular homogenates was measured against a synthetic substrate (syntide 2), as described.6
2.4. eGFP concentration
We measured eGFP concentrations in whole heart homogenates using a fusion plate reader, as previously described.3
2.5. Statistics
The null hypothesis was rejected for P < 0.05. ANOVA and Bonferroni’s corrected t test for post hoc comparisons were used as appropriate. Correlation coefficients (R2) were derived from comparisons between CaMKII activities or eGFP concentrations and heart weight indexed to body weight, left ventricular (LV) fractional shortening and LV end diastolic dimension (LVEDD) using a best fit linear regression algorithm (Sigma Plot, Jandel Scientific).
3. Results
3.1. eGFP expression in transgenic mice
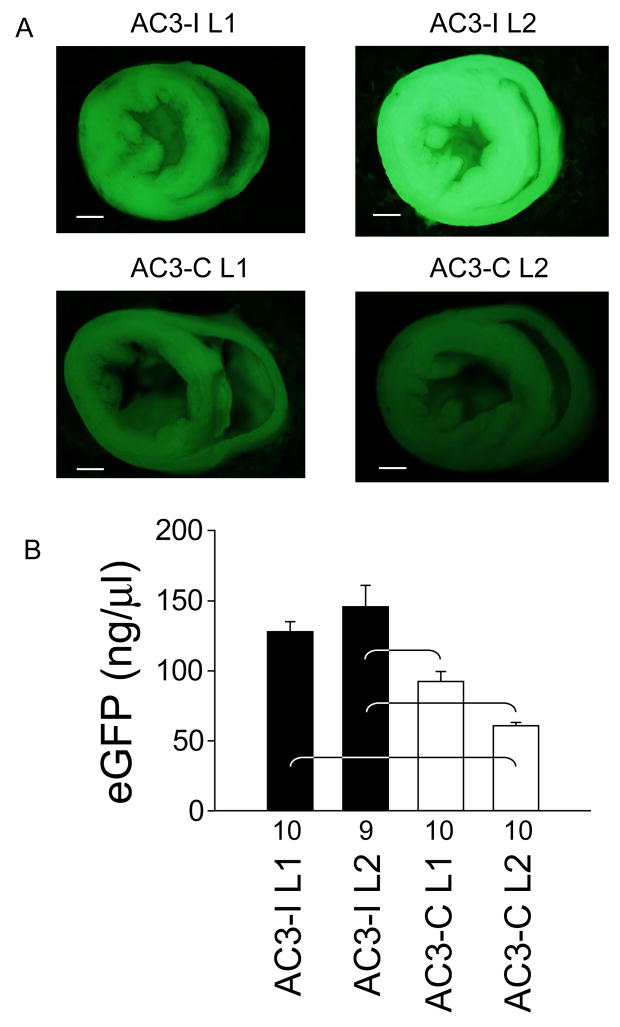
We first measured the concentrations of eGFP expressed in the hearts of the AC3-I and AC3-C transgenic mice. eGFP expression was readily apparent by direct fluorescence microscopy in all groups (Figure 1a), but line 2 AC3-I hearts had significantly more eGFP than AC3-C hearts (Figure 1b). These findings demonstrate a nearly three-fold range of eGFP expression between mice with and without myocardial CaMKII inhibition.
Figure 1. eGFP expression in AC3-I and AC3-C mice.
aTransverse hemi-sections of line 1 (L1) and line 2 (L2) whole hearts obtained from AC3-I and AC3-C mice under fluorescent illumination at 2X magnification. The exposure times were equal for all panels and the calibration bar equals 1 mm. b. Summary data for eGFP concentrations measured in whole heart homogenates. Numerals on the abscissa indicate the number of hearts studied in each group. eGFP concentrations were significantly different between groups (†p <0.001). The brackets indicate significant (P<0.05) post-hoc comparison differences.
3.2. Preserved LV function, reduced hypertrophy and left ventricular dilation in mice with eGFP and AC3-I expression
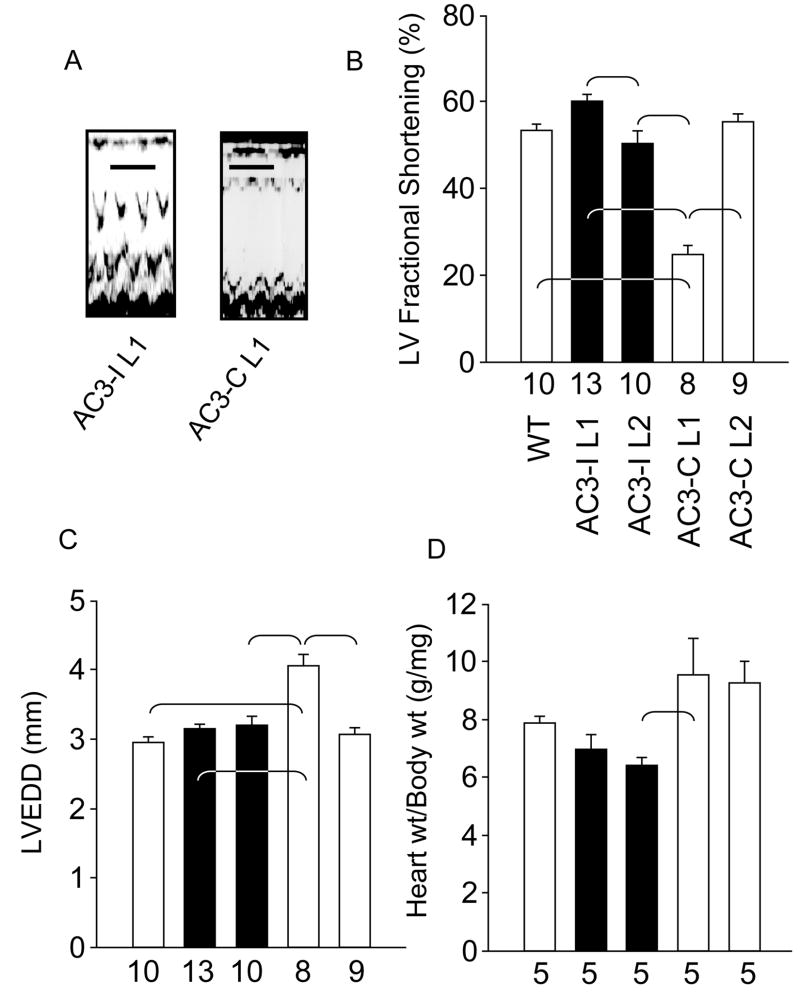
Both lines of AC3-I transgenic mice had LV function that was equivalent to WT (Fig 2a and b), despite expressing higher eGFP concentrations than AC3-C mice (Fig 1b). In comparison, AC3-C L1 showed significantly impaired LV fractional shortening (p < 0.001) compared to all other genotypes. AC3-C L2 mice, with lower eGFP expression, showed preserved LV contractility (Fig 2b). We measured heart weights and corrected them for body mass in order to assess possible LV hypertrophy. AC3-I mice and WT mice had equivalent heart weights, and AC3-I L2 mice had significantly (P<0.05) lower heart weights than AC3-C L1 mice. AC3-I hearts did not exhibit an increase in heart weight in step with increased eGFP expression (Fig 2d). Mice with eGFP expression can develop LV dilation1 and AC3-C L1 mice showed significant (P<0.001) LV dilation compared to all other genotypes (Fig 2c). In contrast, AC3-I L2 mice with the greatest eGFP expression did not develop LV dilation. These findings show that AC3-I mice are resistant to adverse consequences of eGFP over-expression in heart and so suggest the hypothesis that CaMKII activity is an important pathological signal element in eGFP cardiomyopathy.
Figure 2. AC3-I transgenic mice have preserved LV size and shortening.
a. Representative M-mode echocardiography tracings from AC3-I L1 and AC3-C L1 mice. Calibration bars indicate 200 ms. b. Summary data of left ventricular (LV) fractional shortening. Fractional shortening was significantly different between lines (P<0.001). The brackets indicate significant (P<0.05) post-hoc comparison differences in panels b–d. c. Summary data for LV end-diastolic diameter (LVEDD) measurements. LVEDD was significantly different between lines (P<0.001). d. Heart weight (wt) to body wt ratios were significantly different between groups (P=0.012). The number of animals studied is indicated by numerals on the abscissa.
3.3. CaMKII activity rather than eGFP expression predicts cardiomyopathic remodeling
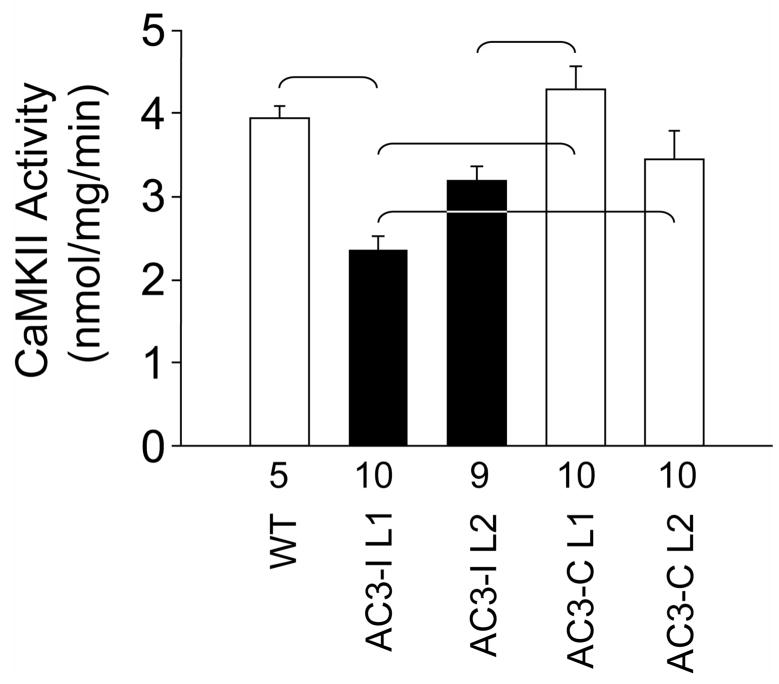
We performed CaMKII activity assays in cardiac homogenates from each of the individual transgenic lines (Fig 3). These studies showed that AC3-C L1 hearts had significantly higher (P<0.05) CaMKII activity compared to AC3-I L1 or AC3-I L2.
Figure 3. CaMKII activity in AC3-I and AC3-C heart homogenates.
CaMKII activity assayed from whole heart homogenates. CaMKII activity was significantly different (P<0.001) between groups. The brackets indicate significant (P<0.05) post-hoc comparison differences. The number of hearts studied is indicated by numerals on the abscissa.
Comparison of eGFP expression (Figure 1b) with CaMKII activity measurements (Figure 3) reveals that CaMKII activity increases in step with eGFP in the AC3-C mice, while CaMKII activity responses to eGFP expression are reduced in AC3-I hearts. Taken together, these results suggest that CaMKII inhibition can overcome adverse consequences of eGFP over-expression in heart.
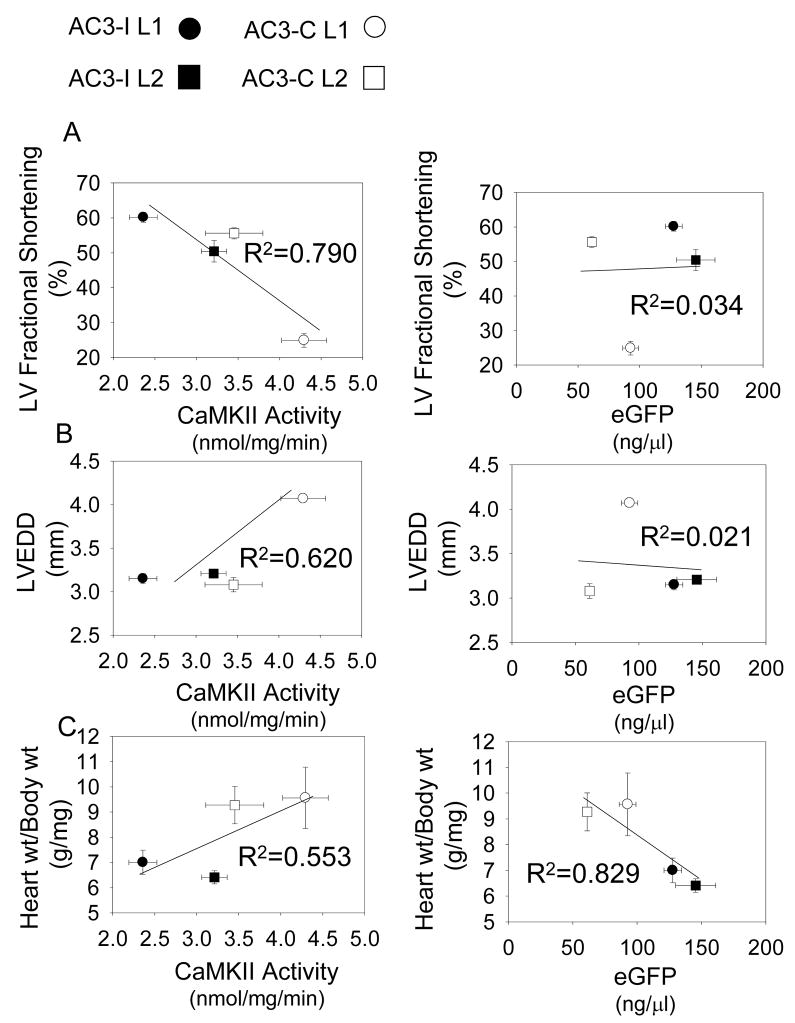
In order to understand if CaMKII activity or eGFP better predicted key cardiomyopathic phenotypes, we analyzed both of these parameters with reference to heart size and function (Figure 4). There was an inverse correlation between CaMKII activity and LV fractional shortening (R2 = 0.790) and a positive correlation (R2 = 0.620) between CaMKII activity and LV chamber diameter. On the other hand, there was no correlation between eGFP expression and LV fractional shortening (R2 =0.034) or LVEDD (R2 = 0.021). Surprisingly, there was an inverse correlation between eGFP and body weight adjusted heart weight (R2=0.829), while there was a positive correlation between body adjusted heart weight and CaMKII activity (R2=0.553).
Figure 4. CaMKII activity but not eGFP predict LV fractional shortening and LVEDD.
a. LV fractional shortening is inversely related to CaMKII activity (left panel), but not to eGFP (right panel). b. LVEDD is positively related to CaMKII activity (left panel), but not to eGFP (right panel). c. Heart weight adjusted to body weight did correlate with CaMKII activity (left panel), but was inversely related to eGFP (right panel). Data from individual groups is indicated as AC3-I L1 (black square), AC3-I L2 (black circle), AC3-C L1 (white square) and AC3-C L2 (white circle) in all panels. The correlations were performed on averaged data presented in previous figures: eGFP (Fig 1); LV fractional shortening (Fig 2b); LVEDD (Fig 2c); Heart wt/Body wt (Fig 2d); CaMKII activity (Fig 3).
Discussion
Cardiomyopathy and calcium signaling
Cellular calcium dysregulation is a consistent and general finding in structural heart disease. Many calcium-activated signaling molecules, including CaMKII, have been shown to cause cardiomyopathy. The two most important clinical phenotypes in patients with cardiomyopathy are myocardial dysfunction and sudden death. CaMKII over-expression is sufficient to cause heart failure marked by LV hypertrophy, dilation and premature sudden death in mice.7 Over-expression of a constitutively active form of calcineurin, a Ca2+ and calmodulin-activated phosphatase, induces a severe cardiomyopathy.8 Calcineurin over-expression also induces CaMKII over-expression and interbreeding calcineurin mice with AC3-I mice allowed us to separately test the role of calcineurin and CaMKII in determining cardiomyopathy phenotypes, because the calcineurin x AC3-I interbred mice had increased calcineurin expression but normal levels of CaMKII. CaMKII inhibition in the calcineurin over-expressing mice reduced arrhythmias, prolonged survival and improved mechanical function.9 Our current study benefits from a mouse model where eGFP and CaMKII activity are separately controlled. Only the AC3-C L1 mice had severe cardiomyopathy, based on heart weights or echocardiographically measured LV function, indicating that the range of eGFP expression in the two lines of AC3-C mice was sufficient to produce significant differences in these parameters. It appears that certain stress signals, such as calcineurin and eGFP, can trigger cardiomyopathic responses and that CaMKII is an important contributor to these effects. Interestingly, eGFP expression correlated negatively with body adjusted heart weight. This finding is consistent with the concept that increased CaMKII activity is required for hypertrophic effects of eGFP.
Elevated CaMKII activity is required for this model of eGFP cardiomyopathy
As was previously reported, α-MHC promoter driven eGFP over-expression causes decreased LV fractional shortening, hypertrophy and increased LVEDD.1 However, we show that in our models it is CaMKII activity rather than eGFP expression that predicts the severity of these phenotypes. There was a dose-related eGFP myocardial depressant effect in the control (AC3-C) mice but this relation was not evident in AC3-I mice with chronic CaMKII inhibition, despite the presence of similar or higher concentrations of eGFP. Our findings suggest that eGFP over-expression activates a CaMKII-dependent signaling pathway that leads to cardiomyopathy. Activation of CaMKII by eGFP over-expression further suggests the hypothesis that other cardiomyopathies marked by protein over-expression might also activate CaMKII. On the other hand, cardiomyopathy is a complex, multifactorial process and our findings allow for the possibility that CaMKII inhibition protects the heart from eGFP over-expression indirectly, by interactions with other signaling pathways.
Cellular responses to protein over-expression
The underlying mechanism of eGFP cardiomyopathy remains incompletely understood. One possibility is cellular toxicity due to protein accumulation, as is seen in human infiltrative cardiomyopathies. Robust α-myosin heavy chain promoter-driven eGFP expression, such as was seen in our transgenic mice, has the potential to result in overcrowding with adverse effects such as disruption of cellular signaling and exertion of significant stress on the intracellular trafficking system.10, 11 In addition, eGFP has the potential to evoke immune responses that could potentially contribute to cardiomyopathy phenotypes.12, 13
Is there a role for fluorescent marker proteins in transgenic models?
Apart from eGFP cardiomyopathy, another model of cardiac transgenesis utilizing α-myosin heavy chain promoter-driven tetracycline transactivator has also been shown to cause dilated cardiomyopathy.14 On the other hand, many transgenic models use fluorescent reporter proteins without apparent adverse effects. For example, the enhanced yellow fluorescent protein (eYFP) has been shown to be a useful marker that does not affect pancreatic islet cell function.15 eGFP has been shown to affect the behavior of connexin 43,16, 17 while other studies have suggested that eGFP can be fused with ion channels without altering their function or localization.18 Taken together, this and other studies highlight the importance of careful consideration in designing animal disease models with intracellular marker proteins.
Acknowledgments
This work was funded by NIH R01 HL 079031, R01 HL 62494, and R01 HL 70250 and the University of Iowa Research Foundation. Dr Khoo was funded by grant 9920117V from the American Heart Association (SE Affiliate).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huang W, Aramburu J, Douglas PS, Izumo S. Transgenic expression of green fluorescence protein can cause dilated cardiomyopathy. Nat Med. 2000;6:482–83. doi: 10.1038/74914. [DOI] [PubMed] [Google Scholar]
- 2.Zhang T, Brown JH. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc Res. 2004;63:476–86. doi: 10.1016/j.cardiores.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 3.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–17. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 4.Braun AP, Heist EK, Schulman H. Inhibition of a mammalian large conductance, calcium-sensitive K+ channel by calmodulin-binding peptides. J Physiol (London) 2000;527:479–92. doi: 10.1111/j.1469-7793.2000.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rottman JN, Ni G, Khoo M, Wang Z, Zhang W, Anderson ME, et al. Temporal changes in ventricular function assessed echocardiographically in conscious and anesthetized mice. J Am Soc Echocardiogr. 2003;16:1150–57. doi: 10.1067/S0894-7317(03)00471-1. [DOI] [PubMed] [Google Scholar]
- 6.Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble RW, et al. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circ. 2002;106:1288–93. doi: 10.1161/01.cir.0000027583.73268.e7. [DOI] [PubMed] [Google Scholar]
- 7.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr, Bers DM, et al. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–19. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 8.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–28. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khoo MS, Li J, Singh MV, Yang Y, Kannankeril P, Wu Y, et al. Death, cardiac dysfunction, and arrhythmias are increased by calmodulin kinase II in calcineurin cardiomyopathy. Circ. 2006;114:1352–59. doi: 10.1161/CIRCULATIONAHA.106.644583. [DOI] [PubMed] [Google Scholar]
- 10.Ellis RJ. Macromolecular crowding: an important but neglected aspect of the intracellular environment. Curr Opin Struct Biol. 2001;11:114–19. doi: 10.1016/s0959-440x(00)00172-x. [DOI] [PubMed] [Google Scholar]
- 11.Martin J, Hartl FU. The effect of macromolecular crowding on chaperonin-mediated protein folding. Proc Natl Acad Sci U S A. 1997;94:1107–12. doi: 10.1073/pnas.94.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenzweig M, Connole M, Glickman R, Yue SP, Noren B, DeMaria M, et al. Induction of cytotoxic T lymphocyte and antibody responses to enhanced green fluorescent protein following transplantation of transduced CD34(+) hematopoietic cells. Blood. 2001;97:1951–59. doi: 10.1182/blood.v97.7.1951. [DOI] [PubMed] [Google Scholar]
- 13.Stripecke R, Carmen VM, Skelton D, Satake N, Halene S, Kohn D. Immune response to green fluorescent protein: implications for gene therapy. Gene Ther. 1999;6:1305–12. doi: 10.1038/sj.gt.3300951. [DOI] [PubMed] [Google Scholar]
- 14.McCloskey DT, Turnbull L, Swigart PM, Zambon AC, Turcato S, Joho S, et al. Cardiac transgenesis with the tetracycline transactivator changes myocardial function and gene expression. Physiol Genomics. 2005;22:118–26. doi: 10.1152/physiolgenomics.00016.2005. [DOI] [PubMed] [Google Scholar]
- 15.Nyqvist D, Mattsson G, Kohler M, Lev–Ram V, Andersson A, Carlsson PO, et al. Pancreatic islet function in a transgenic mouse expressing fluorescent protein. J Endocrinol. 2005;186:333–41. doi: 10.1677/joe.1.06204. [DOI] [PubMed] [Google Scholar]
- 16.Sekar RB, Kizana E, Smith RR, Barth AS, Zhang Y, Marban E, et al. Lentiviral vector mediated expression of GFP or Kir2.1 alters the electrophysiology of neonatal rat ventricular myocytes without inducing cytotoxicity. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.00477. [DOI] [PubMed] [Google Scholar]
- 17.Kizana E, Chang CY, Cingolani E, Ramirez-Correa GA, Sekar RB, Abraham MR, et al. Gene transfer of connexin43 mutants attenuates coupling in cardiomyocytes: novel basis for modulation of cardiac conduction by gene therapy. Circ Res. 2007;100:1597–604. doi: 10.1161/CIRCRESAHA.106.144956. [DOI] [PubMed] [Google Scholar]
- 18.Lopatin AN, Makhina EN, Nichols CG. Novel tools for localizing ion channels in living cells. Trends Pharmacol Sci. 1998;19:395–408. doi: 10.1016/s0165-6147(98)01238-3. [DOI] [PubMed] [Google Scholar]