Abstract
CXC chemokines and their receptor, CXCR2, are important components of the hepatic inflammatory response to ischemia/reperfusion. However, direct effects of CXC chemokines on hepatocytes during this response have not been studied. Wild-type and CXCR2-/- mice were subjected to 90 minutes of partial hepatic ischemia followed by up to 96 hours of reperfusion. CXCR2-/- mice had significantly less liver injury at all reperfusion times compared to wild-type mice. Early neutrophil recruitment (12 hours) was diminished in CXCR2-/- mice, but within 24 hours was the same as wild-type mice. Hepatocyte proliferation and regeneration was accelerated in CXCR2-/- mice compared to wild-type mice. These effects were associated with increased activation of NF-κB and STAT3, despite there being no difference in the expression of proliferative factors such as TNFα, IL-6, and HGF. To establish whether the accelerated proliferation and regeneration observed in CXCR2-/- mice was due to effects on hepatocytes rather than just a generalized decrease in acute inflammatory injury, mice were treated with the CXCR2 antagonist, SB225002, after neutrophil recruitment and injury were maximal (24 hours after reperfusion). SB225002 treatment increased hepatocyte proliferation and regeneration in a manner identical to that observed in CXCR2-/- mice. Treatment of primary wild-type hepatocytes with MIP-2 showed that low concentrations protected against cell death whereas high concentrations induced cell death. These effects were absent in hepatocytes from CXCR2-/- mice.
Conclusion
The data suggest that hepatocyte CXCR2 regulates proliferation and regeneration after I/R injury and reveal important differences in the role of this receptor in liver regeneration and repair induced under different conditions that may be related to ligand concentration.
Keywords: liver injury, chemokines, regeneration, inflammation, proliferation
Ischemia/reperfusion (I/R) of the liver is a primary complication of liver resection surgery, transplantation, and trauma (1). Extended periods of hepatic ischemia and subsequent reperfusion can lead to liver injury and dysfunction through the initiation of a biphasic inflammatory response (2). The initial phase of this response is characterized by activation of Kupffer cells and their subsequent production and release of reactive oxygen species, leading to mild hepatocellular injury (3, 4). The later phase of injury is initiated by proinflammatory mediators released by activated Kupffer cells and hepatocytes. These mediators include the cytokines interleukin (IL)-12, tumor necrosis factor-α (TNFα) and IL-1 (5-8). The expression of these cytokines induces the production of CXC chemokines and endothelial cell adhesion molecules that facilitate the adhesion and transmigration of neutrophils from the vascular space into the hepatic parenchyma (9-12). These activated neutrophils then directly injure hepatocytes and vascular endothelial cells through their release of oxidants and proteases (13).
Previous studies have demonstrated that CXC chemokines mediate neutrophil infiltration during I/R injury of several organs including liver (10, 11). The receptor responsible for these effects is CXC chemokine receptor-2 (CXCR2). Blockade of either individual CXCR2 ligands (10, 11) or CXCR2 itself (14) prevents neutrophil infiltration and attenuates hepatic I/R injury. In addition, other studies have suggested that CXC chemokines may have direct effects on hepatocytes. CXCR2 has been shown to be expressed by hepatocytes (15), and in models of partial hepatectomy or acetaminophen toxicity, CXCR2 ligands were shown to promote hepatocyte proliferation and liver regeneration (16-18). In contrast, other studies have suggested that CXCR2 ligands are directly hepatotoxic (19, 20).
The manner in which the liver recovers and regenerates after a severe ischemic insult is not well characterized. Previous studies from our laboratory have shown that after prolonged (90 minutes) ischemia in mice, hepatocyte proliferation and cell cycle progression is initiated within 24-48 hours of reperfusion (21). Whether CXC chemokine signaling through CXCR2 regulates this reparative response is unknown. Therefore, we sought to determine whether deletion or antagonism of CXCR2 had any effect on the recovery from liver damage during hepatic I/R injury.
Methods
Hepatic I/R Injury Model
Male wild-type (Balb/c) and CXCR2-/- mice (on a Balb/c background) from Jackson Laboratory (Bar Harbor, ME) weighing 22-28 g were used in these experiments. This project was approved by the University of Cincinnati Animal Care and Use Committee and was in compliance with the National Institutes of Health guidelines. The animals underwent either sham surgery or I/R. Partial hepatic ischemia was induced as described previously (11). Sham control mice underwent the same protocol without vascular occlusion. Some mice were injected intra-peritoneally with 3% dimethylsulfoxide (DMSO) solution or 4 mg/kg SB225002 (N-(2-hydroxy-4-nitrophenyl)-N’-(2-bromophenyl)urea) (Calbiochem, San Diego, CA), a non-peptide antagonist of CXCR2 (22), dissolved in DMSO at 24 hours intervals starting after 24 hours of reperfusion.
Electrophoretic Mobility Shift Assay
Nuclear extracts of liver tissue were prepared by the method of Deryckere and Gannon (23), and analyzed by electrophoretic mobility shift assay (EMSA). Briefly, double-stranded consensus oligonucleotides to nuclear factor κB (NF-κB) (Promega, Madison, WI) were end-labeled with γ[32P]ATP (3,000 Ci/mmol at 10mCi/ml, Amersham, Arlington Heights, IL). Binding reactions (total volume 15 μl) containing equal amounts of nuclear protein extract (20 μg) and 35 fmols (∼50,000 cpm, Cherenkov counting) of oligonucleotide and were incubated at room temperature for 30 minutes. Binding reaction products were separated in a 4% polyacrylamide gel and analyzed by autoradiography.
Western Blot Analyses
Liver samples were homogenized in lysis buffer (10 mM HEPES, pH 7.9, 150 mM NaCl, 1 mM EDTA, 0.6% NP-40, 0.5 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotonin, 10 μg/ml soybean trypsin inhibitor, 1 μg/ml pepstatin) on ice. Homogenates were sonicated and centrifuged at 10,000 rpm to remove cellular debris. Protein concentrations of each sample were determined. Samples containing equal amounts of protein in equal volumes of sample buffer were separated in a denaturing 10% polyacrylimide gel and transferred to a 0.1 μm pore nitrocellulose membrane. Nonspecific binding sites were blocked with tris-buffered saline (TBS; 40 mM Tris, pH 7.6, 300 mM NaCl) containing 5% non-fat dry milk for 1 hour at room temperature. Membranes were then incubated with antibodies to phosphorylated signal transducers and activators of transcription-3 (p-STAT3) or β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) in TBS with 0.1% Tween 20 (TBST). Membranes were washed and incubated with secondary antibodies conjugated to horseradish peroxidase. Immunoreactive proteins were detected by enhanced chemiluminescence.
Blood and Tissue Analysis
Blood was obtained by cardiac puncture for analysis of serum alanine amino transferase (ALT) as an index of hepatocellular injury. Mesurements of serum ALT were made using a diagnosis kit by bioassay (Wiener Laboratories, Rosario, Argentina). Serum and tissue levels of TNFα, IL-6, macrophage inflammatory protein-2 (MIP-2) and keratinocyte chemokine (KC) were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN). Liver neutrophil accumulation was determined by myeloperoxidase (MPO) content and chloroacetate esterase staining. MPO content was assessed by methods described elsewhere (24). Chloroacetate esterase staining was performed on paraffin-embedded liver tissue using the Naphthol-ASD Chloroacetate Esterase Kit (Sigma, St. Louis, MO) according to the manufacturer’s instructions. Stained liver sections were scored as follows: (0) none, (1+) faint to moderate, (2+) moderate to strong, (3+) strong, (4+) very strong. For histological examination, liver tissues were fixed in 10% neutral-buffered formalin, processed and then embedded in paraffin prior to staining with hematoxylin and eosin.
Proliferating Cell Nuclear Antigen (PCNA) Staining
Immunohistochemical staining for PCNA was performed on paraffin-embedded liver tissue with anti-PCNA antibody using DakoCytomation ARK kit (Dako, Copenhagen, Denmark). Briefly, a three-step peroxidase method was performed according to the manufacturer’s instruction. PC-10 monoclonal antibody (Santa Cruz Biotechnology) was used at a dilution of 1:50, for 15 minutes at room temperature. The sections were counterstained with hematoxylin. Evaluation of PC-10 immunostaining was performed based on the percentage of positive nuclei of 400-600 hepatocytes from 4-6 highest positive fields at high power (400X), and was expressed as PCNA labeling index.
Primary Hepatocyte Studies
Hepatocytes from wild-type and CXCR2-/- mice were isolated as described elsewhere (25). Isolated hepatocytes in Williams media were distributed onto 96-well flat-bottomed plates (Trasadingen, Switzerland) at a concentration of 1.5 X 104 cells/200 μl/well, and incubated overnight to allow cell adherence. Media was changed and cells were incubated for 24 hours in Williams medium with or without recombinant murine MIP-2 or KC (Peprotech, Rocky Hill, NJ) at 1-10,000 ng/ml. Cytotoxicity was determined by lactate dehydrogenase (LDH) release according to the manufacturer’s instructions (Roche, Mannheim, Germany), and percent cell death was calculated. Hepatocyte apoptosis was determined by analyzing cell lysates for active caspase-3 activity by ELISA (R&D Systems, Minneapolis, MN). Hepatocyte proliferation was determined by DNA incorporation of 5-bromo-2’-deoxyuridine (BrdU) using the Biotrak™ cell proliferation ELISA system (GE Healthcare, Buckingham, UK). Data are expressed as a ratio compared with 0 ng/ml. All experiments were repeated at least twice to confirm these results.
Statistical Analysis
All data are expressed as mean ± standard error of the mean (SEM). Data were analyzed with a one-way analysis of variance with subsequent Student-Newman-Keuls test. Differences were considered significant when P<0.05.
Results
Signaling through CXCR2 Auguments Liver Injury after I/R
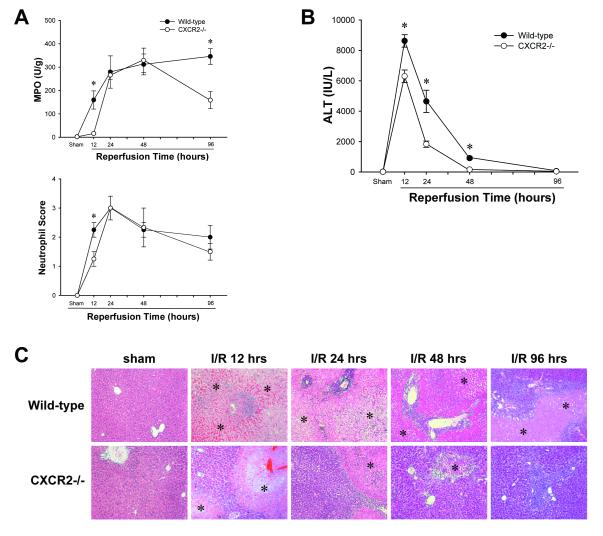
Because we and others have established the importance of CXCR2 ligands for acute neutrophil recruitment and injury after I/R in the liver (10, 11), we first assessed the response of wild-type and CXCR2-/- mice to ischemia with prolonged periods of reperfusion. As expected, after 12 hours of reperfusion there was significant neutrophil accumulation in wild-type mice, but not CXCR2-/- mice (Figure 1A). Interestingly, after 24 or 48 hours of reperfusion there was no difference in neutrophil accumulation. After 96 hours of reperfusion, there was significantly lower MPO content in the livers from CXCR2-/- mice compared to wild-type mice, but neutrophil scores were not different between these groups (Figure 1A).
Figure 1.
Response of CXCR2-/- mice to ischemia/reperfusion. (A) Neutrophil accumulation was determined by liver content of myeloperoxidase (MPO) and scoring based on chloroesterase acetate staining. Data are mean ± SEM with n=4-15 per group. *P<0.05 compared with CXCR2-/- mice. (B) Liver injury was measured by serum levels of alanine aminotransferase (ALT). Data are mean ± SEM with n=4-15 per group. *P<0.05 compared with CXCR2-/- mice. (C) Liver histology. Normal hepatic architecture was observed in sham-operated wild-type mice and CXCR2-/- mice. After 12 or 24 hours of reperfusion, livers from wild-type mice had large areas of necrosis, whereas livers from CXCR2-/- mice showed less evidence of necrosis. After 48 hours of reperfusion, wild-type mice still had severe necrotic livers, whereas CXCR2-/- mice had significantly improved livers with small necrotic areas. After 96 hours of reperfusion, large necrotic areas remained in the livers of wild-type mice, whereas livers in CXCR2-/- mice were almost normal. Asterisks indicate necrotic areas. Original magnification was 50X.
More liver injury was observed in wild-type mice at virtually every time point measured. After ischemia and 12 or 24 hours of reperfusion, CXCR2-/- mice had significantly less liver injury than wild-type mice as determined by serum levels of ALT (Figure 1B). After 48 hours of reperfusion, serum ALT levels in CXCR2-/- mice returned to baseline values whereas levels in wild-type mice remained significantly elevated (Figure 1B). By 96 hours of reperfusion, serum ALT levels were normal in both groups. Histological examination confirmed our biochemical data. Both wild-type and CXCR2-/- mice undergoing sham surgery had normal liver architecture (Figure 1C). After 12 hours of reperfusion, both wild-type and CXCR2-/- mice had large areas of liver necrosis, but CXCR2-/- mice had less hepatocellular necrosis and little evidence of neutrophil accumulation (Figure 1C). However, after 24 and 48 hours of reperfusion, neutrophil accumulation in wild-type and CXCR2-/- mice was similar, but there was far less hepatocellular necrosis in CXCR2-/- mice (Figure 1C). After 96 hours of reperfusion, CXCR2-/- mice had nearly normal liver structure, except for a few small necrotic areas infiltrated with neutrophils (Figure 1C). In contrast, wild-type mice still had large necrotic areas with severe neutrophil infiltration.
CXCR2 Signaling does not Alter Cytokine Expression
The acute inflammatory response induced by I/R in the liver is driven primarily by TNFα and is regulated by IL-6 (6, 26). To determine if lack of CXCR2 expression altered either of these cytokines and therefore the injury response, we assessed serum levels of TNFα and IL-6 over the time-course of injury and recovery. No differences were observed in TNFα or IL-6 between wild-type and CXCR2-/- mice at any time point (Figure 2A). We next evaluated the expression of the two primary CXCR2 ligands in mice, MIP-2 and KC. Both chemokines were significantly increased in the serum and livers of CXCR2-/- mice compared to wild-type mice throughout the time-course of injury and recovery (Figures 2B and 2C). This suggests that signaling via CXCR2 provides negative feedback for chemokine production.
Figure 2.
Effects of CXCR2-deficiency on serum and liver production of cytokines and chemokines. Serum levels of (A) TNFα and IL-6, and (B) MIP-2 and KC were analyzed by ELISA during ischemia/reperfusion injury. (C) Liver levels of MIP-2 and KC were determined by ELISA. Data are mean ± SEM with n=4-15 per group. *P<0.05 compared with wild-type mice.
Enhanced Hepatocyte Proliferation in CXCR2-/- Mice after I/R
Because histopathologic analysis suggested that CXCR2-/- mice recovered faster from I/R injury, we evaluated hepatocyte proliferation after I/R. Liver sections were stained for PCNA, which is regarded as a marker of S phase entry. Sham-operated wild-type or CXCR2-/- mice had almost no PCNA-positive hepatocytes (Figure 4A). After 24 or 48 hours of reperfusion (48 hour data not shown), livers from wild-type mice had very few PCNA-positive hepatocytes, whereas significantly more PCNA-positive hepatocytes were detected in livers from CXCR2-/- mice (Figure 3A). After 96 hours of reperfusion, PCNA staining was increased in wild-type mice but was much more abundant in CXCR2-/- mice (Figure 3A). Figure 3B shows quantified PCNA data. The results suggest that the proliferative response after I/R injury occurs much earlier in CXCR2-/- mice than in wild-type mice. Because soluble factors, such as hepatocyte growth factor (HGF), are also important stimuli for hepatocyte proliferation, we measured HGF protein levels in the liver. No significant difference was observed in HGF expression between wild-type mice and CXCR2-/- mice (data not shown).
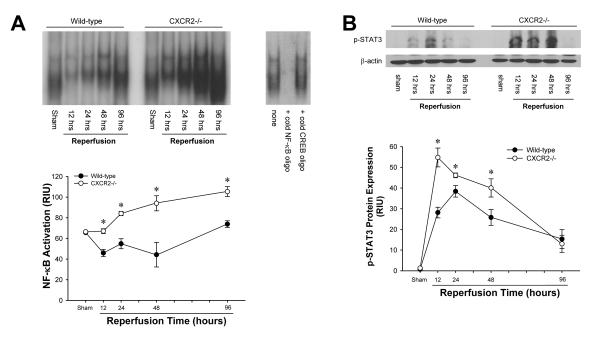
Figure 4.
Liver activation of (A) NF-κB and (B) STAT3 after ischemia/reperfusion in wild-type and CXCR2-/- mice. For NF-κB, nuclear extracts were analyzed by EMSA. Liver nuclear extracts from mice undergoing sham surgery were subjected to competition assay with cold NF-κB or CREB oligonucleotides. For STAT3, phosphorylated STAT3 was detected by Western blot with β-actin staining as a control. Results were quantitated by image analysis of autoradiograms. Data are mean ± SEM with n=4-6 per group. *P<0.05 compared with wild-type mice.
Figure 3.
Hepatocyte proliferation after ischemia/reperfusion injury in wild-type and CXCR2-/- mice. (A) Hepatocyte proliferation was determined by immunohistochemical staining for proliferating cell nuclear antigen (PCNA). Livers from sham-operated wild-type mice or CXCR2-/- mice had very few PCNA-positive hepatocytes. After 24 hours of reperfusion, livers from wild-type mice still had few PCNA-positive hepatocytes, whereas PCNA-positive hepatocytes were detected in some CXCR2-/- mice. After 96 hours of reperfusion, PCNA-positive hepatocytes were increased in livers from wild-type mice. However, more PCNA-positive hepatocytes were detected in CXCR2-/- mice. Original magnification was 400X. (B) Quantitative analysis of PCNA labeling. Data are mean ± SEM with n=4-12 per group. *P<0.05 compared with wild-type mice.
Activation of NF-κB and STAT3 is Increased in CXCR2-/- Mice after I/R
Because NF-κB and STAT3 are known to promote hepatocyte proliferation (27-29), we assessed the activation of these two factors. In wild-type mice, NF-κB activation (as measured by EMSA) was significantly decreased after 12 hours of reperfusion and recovered to the basal line after 96 hours of reperfusion. Interestingly, NF-κB activation was significantly increased in CXCR2-/- mice compared to wild-type mice at all reperfusion time points measured (Figure 4A). STAT3 activation, as determined by STAT3 phosphorylation, was markedly increased within 12 hours of reperfusion and was maximal after 24 hours in wild-type mice (Figure 4B). STAT3 activation was significantly higher in CXCR2-/- mice compared to wild-type mice after 12, 24 and 48 hours of reperfusion (Figure 4B). After 96 hours of reperfusion, there was no difference in STAT3 activation between wild-type and CXCR2-/- mice.
Antagonism of CXCR2 Augments Liver Proliferation and Recovery Despite Fulminant Inflammatory Injury
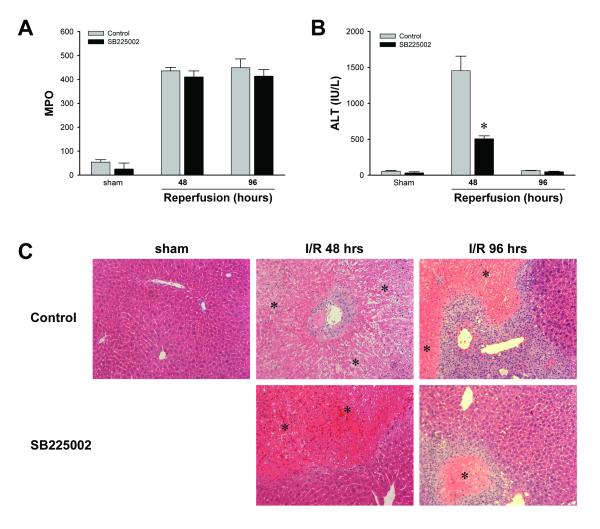
Our experiments show that CXCR2-/- mice have less neutrophil accumulation and liver injury than wild-type mice (Figure 1). These experiments do not eliminate the possibility that the accelerated regeneration and recovery observed in CXCR2-/- mice might be due to the reduced acute neutrophil accumulation and injury in these mice. Therefore, we examined whether the reduced liver injury and accelerated liver regeneration in CXCR2-/- mice during the late phase of reperfusion was a result of reduced neutrophil accumulation and injury or elimination of a direct hepatoxic effect of CXC chemokines. To do this, wild-type mice were injected intraperitoneally with 3% DMSO (control) or 4 mg/kg SB225002, a non-peptide antagonist of CXCR2, 24 hours after of reperfusion and every 24 hours thereafter. Since neutrophil accumulation in the liver peaks by 24 hours, administration of SB225002 at this time does not alter acute neutrophil recruitment. Treatment with SB225002 had no effect on neutrophil accumulation after 48 or 96 hours of reperfusion (Figure 5A). However, SB225002 significantly reduced liver injury after 48 hours of reperfusion, as measured by serum ALT (Figure 5B). After 96 hours of reperfusion, ALT levels in both groups returned to baseline values. Histopathologic analysis revealed that control mice undergoing sham surgery had normal hepatic architecture (Figure 5C). However, control mice after 48 and 96 hours of reperfusion had severe hepatocellular necrosis and neutrophilic infiltrates (Figure 5C). After 48 or 96 hours of reperfusion, livers from mice treated with SB225002 showed high levels of neutrophil infiltration but reduced areas of necrosis (Figure 5C). These results suggested that CXC chemokines have direct hepatotoxic effects that are independent of neutrophil infiltration.
Figure 5.
Effects of SB225002 on hepatic ischemia/reperfusion injury. Mice were injected intra-peritoneally with 3% dimethylsulfoxide solution (control) or 4 mg/kg SB225002 after 24 hours of reperfusion and every 24 hours thereafter. (A) Neutrophil accumulation was determined by liver content of MPO. Data are mean ± SEM with n=4 per group. (B) Liver injury was measured by serum levels of ALT. Data are mean ± SEM with n=4-6 per group. *P<0.05 compared with control. (C) Liver histology. Sham-operated mice displayed normal hepatic architecture. After 48 hours of reperfusion, livers from control mice had large areas of necrosis. Livers from mice treated with SB225002 had less necrosis. After 96 hours of reperfusion, livers from control mice had large areas of necrosis. However livers from mice treated with SB225002 had far fewer and smaller necrotic areas. Asterisks indicate necrotic areas. Original magnification was 50X.
Blockade of CXCR2 Promotes Hepatocyte Proliferation after I/R in Association with Increased NF-κB and STAT3 Activation
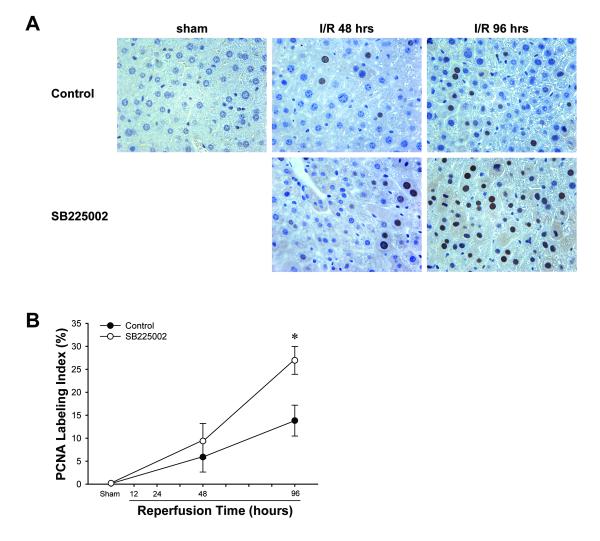
We next assessed if treatment with SB225002 had any effect on hepatocyte proliferation after I/R. Immunohistochemical staining for PCNA revealed that sham-operated control mice had almost no PCNA-positive hepatocytes (Figure 6A). After 48 hours of reperfusion, PCNA staining was increased in both control and SB225002-treated mice, but no differences were seen between the groups (Figure 6A). In contrast, PCNA staining after 96 hours of reperfusion was significantly greater in mice treated with SB225002 compared to control mice (Figure 6A). Quantification of PCNA data revealed a significant increase in PCNA staining after 96 hours of reperfusion (Figure 6B).
Figure 6.
Effects of SB225002 on hepatocyte proliferation after ischemia/reperfusion injury. (A) Hepatocyte proliferation was determined by immunohistochemical staining for proliferating cell nuclear antigen (PCNA). Mice were injected intra-peritoneally with 3% dimethylsulfoxide solution (control) or 4 mg/kg SB225002 after 24 hours of reperfusion and every 24 hours thereafter. Livers from sham-operated mice had almost no PCNA-positive hepatocytes. After 48 hours of reperfusion, PCNA staining was similar between control and SB225002-treated mice. After 96 hours of reperfusion, there were increased numbers of PCNA-positive hepatocytes in livers from control mice. However, there were far more PCNA-positive hepatocytes in SB225002-treated mice. Original magnification was 400X. (B) Quantitative analysis of PCNA labeling. Data are mean ± SEM with n=4 per group. *P<0.05 compared with control mice.
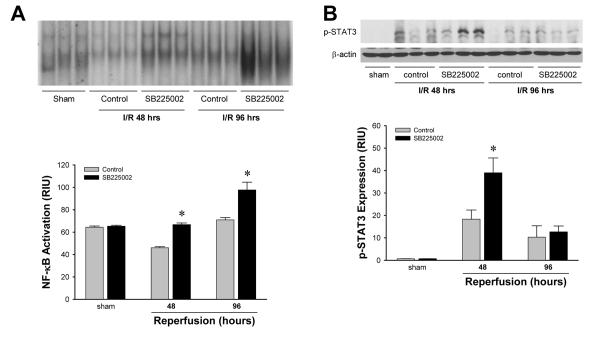
Analysis of NF-κB and STAT3 activation revealed that treatment with SB225002 had similar effects as knock-out of CXCR2. EMSA analysis of liver nuclear extracts demonstrated that treatment with SB225002 increased activation of NF-κB compared to the vehicle control after 48 and 96 hours of reperfusion (Figure 7A). Western blot analysis showed that SB225002 also increased STAT3 activation 48 hours after reperfusion (Figure 7B). After 96 hours of reperfusion STAT3 phosphorylation was similar between the two groups.
Figure 7.
Effects of SB225002 on liver activation of (A) NF-κB and (B) STAT3 after ischemia/reperfusion. Mice were injected intra-peritoneally with 3% dimethylsulfoxide solution (control) or 4 mg/kg SB225002 after 24 hours of reperfusion and every 24 hours thereafter. For NF-κB, nuclear extracts were analyzed by EMSA. Liver nuclear extracts from mice undergoing sham surgery were subjected to competition assay with cold NF-κB or CREB oligonucleotides. For STAT3, phosphorylated STAT3 was detected by Western blot with β-actin staining as a control. Results were quantitated by image analysis of autoradiograms. Data are mean ± SEM with n=3-6 per group. *P<0.05 compared with wild-type mice.
CXCR2 Signaling can be Hepatoprotective or Hepatotoxic Depending on Ligand Concentration
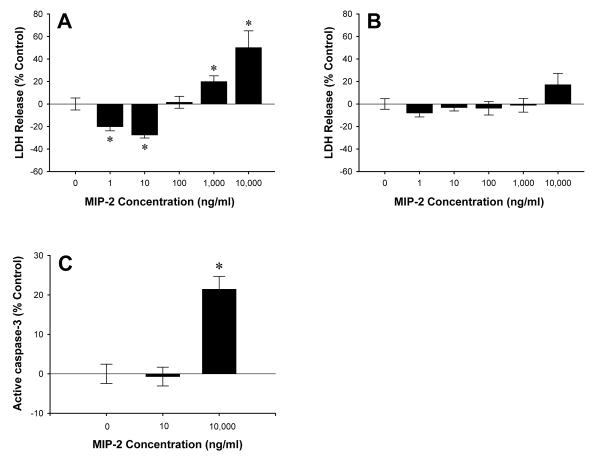
Previous studies have shown that treatment of primary hepatocytes with the CXCR2 ligand, MIP-2, induces proliferation (16). Given our current findings suggesting that signaling via CXCR2 is harmful after I/R, we examined whether signaling through CXCR2 may directly affect hepatocyte cell death. Primary mouse hepatocytes were cultured in the presence or absence of 1-10,000 ng/ml of MIP-2 and hepatotoxicity was determined by release of LDH. As shown in Figure 8A, low concentrations of MIP-2 (<100 ng/ml) protected wild-type hepatocytes against cell death, whereas high concentrations of MIP-2 (>100 ng/ml) induced cell death. These effects were mediated by CXCR2, as hepatocytes from CXCR2-/- mice showed no significant differences at any dose of MIP-2 (Figure 8B). To determine if MIP-2 may also affect hepatocyte apoptosis, active caspase-3 activity was assessed after treatment with 10 or 10,000 ng/ml of MIP-2. Only the high dose of MIP-2 (10,000 ng/ml) induced a significant increase in apoptosis (Figure 8C).
Figure 8.
Effects of CXCR2 signaling hepatocyte cell death. Primary mouse hepatocytes from wild-type (A) and CXCR2-/- (B) mice were treated with MIP-2 (0-10,000 ng/ml) for 24 hours. Cell death was determined by release of LDH. (C) Active caspase-3 levels after MIP-2 treatment (0, 10, 10,000 ng/ml) were measured by ELISA. Data are mean ± SEM with n=7-22 replicates per group. *P<0.05 compared with 0 ng/ml.
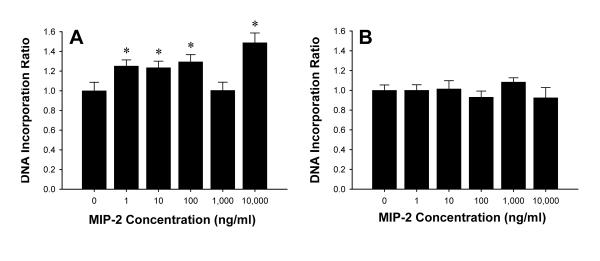
To evaluate the effects of MIP-2 on hepatocyte proliferation, DNA incorporation assays were conducted. MIP-2 stimulated hepatocyte proliferation at virtually every dose tested (Figure 9A). These effects appeared to be mediated specifically by CXCR2 as no increases in proliferation were observed in hepatocytes from CXCR2-/- mice (Figure 9B).
Figure 9.
Effects of CXCR2 on hepatocyte proliferation. Primary mouse hepatocytes from wild-type (A) and CXCR2-/- (B) mice were treated with MIP-2 (0-10,000 ng/ml) for 24 hours. Cell proliferation was determined by DNA incorporation. Data are mean ± SEM with n=8-22 replicates per group. *P<0.05 compared with 0 ng/ml.
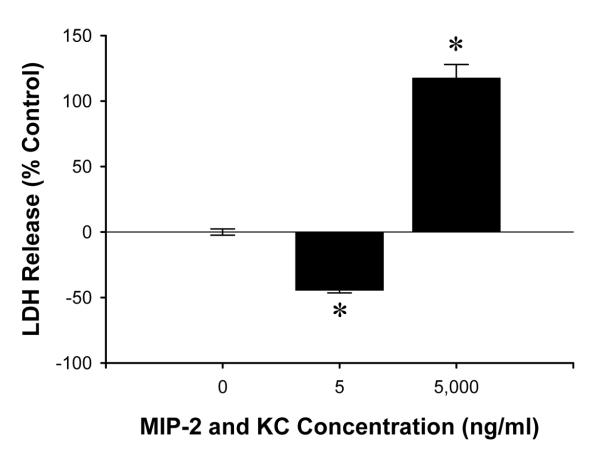
Finally, because there are a number of ligands for CXCR2 which may have overlapping function, we assessed the effects of combined stimulation of wild-type hepatocytes with MIP-2 and KC. There appeared to be synergistic effects as cell death was significantly reduced by treatment of hepatocytes with 5 ng/ml each of MIP-2 and KC (Figure 10). The reduction in cell death was nearly twice that observed with 10 ng/ml of only MIP-2 (Figure 8A). Likewise, treatment of hepatocytes with 5,000 ng/ml each of MIP-2 and KC resulted in nearly twice the amount of cell death as 10,000 ng/ml of MIP-2 (Figure 10).
Figure 10.
Effects of combined MIP-2 and KC treatment on hepatocyte cell death. Primary hepatocytes from wild-type mice were treated with both MIP-2 and KC at the same time (0, 5, 5,000 ng/ml each) for 24 hours. Data are mean ± SEM with n=14-35 replicates per group. *P<0.05 compared with 0 ng/ml.
Discussion
The present study is the first to examine the effects of CXCR2 ligands on liver recovery after I/R injury. The most significant finding of this report is the demonstration that signaling through CXCR2 is detrimental to hepatocyte proliferation and regeneration in this model. This is in stark contrast to studies in models of partial hepatectomy or acetaminophen toxicity in which CXCR2 was found to promote hepatocyte proliferation and liver regeneration (16-18). While the precise mechanism of the divergent effects of CXCR2 ligands on liver regeneration between I/R and partial hepatectomy or acetaminophen models is unclear, it is plausible that the differences are related to the amount of CXC chemokines produced during these insults. Our lab has found that levels of MIP-2 and KC are increased 3-fold and 5-fold, respectively, 48 hours after 70% hepatectomy (S. Kuboki and A.B. Lentsch, unpublished data), which is consistent with previous studies in rats (16). In models of acetaminophen toxicity, MIP-2 and KC levels increase no more than 2.5-fold 48 hours after challenge (17). In contrast, our data show that after ischemia and 48 hours of reperfusion MIP-2 and KC levels are 50-fold and 25-fold higher, respectively, than sham-operated controls. Adenoviral-mediated overexpression of KC in the liver (>100-fold) results in massive hepatocellular necrosis within 48 hours (20). Collectively, these studies suggest that moderate increases in CXCR2 ligands, as occurs after partial hepatectomy or acetaminophen toxicity may promote liver regeneration, whereas much larger increases in expression of CXCR2 ligands, as occurs after I/R injury, may be hepatotoxic and/or oppose hepatocyte proliferation and regeneration. Our in vitro data support this concept. We found that low concentrations of the CXCR2 ligand, MIP-2, had hepatoprotective effects in hepatocytes from wild-type mice whereas high concentrations induced significant cytotoxicity. These divergent effects were absent in hepatocytes isolated from CXCR2-/- mice, indicating that CXCR2 may mediate both protective and cytotoxic signaling. Finally, we found that when hepatocytes were treated with both MIP-2 and KC, the effects were far greater than just MIP-2 at equal concentrations, suggesting that multiple CXCR2 ligands may induce additive or synergistic effects through the receptor.
Alternatively, the divergent effects of CXCR2 signaling on liver regeneration between partial hepatectomy and I/R may be related to the impact of the insult on the hepatocyte. After partial hepatectomy, the remaining liver is comprised of almost entirely normal hepatocytes. Conversely, after I/R all hepatocytes are exposed to an injurious stimulus and those that survive are highly stressed. It has been reported that CXC chemokines greatly enhance acute liver injury, whereas their hepatotoxic effects were less in healthy hepatocytes (19). Therefore, CXC chemokines might be mitogenic to normal healthy hepatocytes, but hepatotoxic to injured hepatocytes. Further study is needed to reveal the differences in CXC chemokine behavior under different conditions.
Another interesting finding of our study is the nature in which CXCR2 regulates neutrophil recruitment following I/R. Consistent with previous studies utilizing neutralizing antibodies to CXC chemokines (10, 11), the lack of CXCR2 blocked early neutrophil recruitment (<12 hours). Surprisingly, neutrophil recruitment was similar between wild-type and CXCR2-/- mice after 24 and 48 hours of reperfusion. This suggests that CXCR2-mediated neutrophil recruitment is relevant only during the acute inflammatory phase of injury and not for the more chronic reparative phase. These studies also confirm earlier studies regarding the contributions of neutrophils to acute liver injury after I/R (4, 30). CXCR2-/- mice had significantly less liver injury than wild-type mice. This finding represents a confounding variable in our studies with CXCR2-/- mice. Could it be that the improved recovery observed in CXCR2-/- mice was simply due to reduced initial injury? Our studies with the CXCR2 antagonist, SB225002, provide strong evidence against this possibility. In wild-type mice, we administered SB225002 after 24 hours of reperfusion, a time at which neutrophil recruitment to the liver was already maximal. Treatment with SB225002 not only reduced hepatocellular necrosis, but accelerated hepatocyte proliferation and ultimate regenerative repopulation of the liver. Combined with our studies in CXCR2-/- mice and our in vitro studies with primary hepatocytes, these data demonstrate that signaling through CXCR2 in hepatocytes after I/R is directly hepatotoxic and delays proliferation and regeneration.
The precise mechanism by which CXCR2 limits hepatocyte proliferation and regeneration after I/R remains to be elucidated. However, our current data suggest that deletion or blockade of CXCR2 signaling is associated with increased activation of NF-κB and STAT3. Both NF-κB and STAT3 are critical for proper liver regeneration (27-29). Primary stimuli for activation of NF-κB and STAT3 in the liver are TNFα and IL-6. However, we detected no differences in TNFα or IL-6 expression between wild-type and CXCR2-/-. Expression of HGF, a primary mediator of hepatocyte proliferation, was also similar in wild-type mice and CXCR2-/- mice. Therefore, it seems likely that signals transduced through CXCR2 impinge on upstream components of the NF-κB and STAT3 pathways, such as kinases regulating IκBα and/or STAT3 phosphorylation, to limit activation of these transcription factors. However, it should be noted that the current data do not demonstrate any direct connection between CXCR2 signaling pathways and NF-κB or STAT3. It is also interesting that in CXCR2-/- mice or SB225002-treated mice that STAT3 activation was found to be augmented during late injury and early recovery phases (12-48 hours after reperfusion). This increased activation occurs during the initial period of increased hepatocyte proliferation. This could suggest that STAT3 plays an important role in the early stages of liver recovery and regeneration after ischemia/reperfusion.
CXCR1 is another receptor for CXC chemokines that has some functional similarities with CXCR2 (31). For many years, it was thought that rodents did not express a homolog of human CXCR1. However, the murine homolog to CXCR1 was recently discovered (32, 33). While any potential role of CXCR1 in liver injury and repair is currently unknown, it is tempting to speculate as to how this receptor might augment or oppose the function of CXCR2 in hepatocytes. Clearly, this will be an important area of research in the near future.
In summary, the present study demonstrates that CXCR2 is an important regulator of hepatocyte proliferation and regeneration after I/R injury. These data reveal important differences in the role of this receptor in liver regeneration and repair induced by different stimuli and suggest that ligand concentration may be an important factor in these differences. These data highlight the importance of future studies aimed at delineating the mechanism by which CXCR2 signal transduction affects activation of transcription factors such as NF-κB and STAT3. Our data, combined with previous studies, suggest that CXCR2 is a rational therapeutic target that may be relevant to a wide variety of liver insults and diseases.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health grants AG025881 and DK56029 to A.B.L.
Abbreviations
- I/R
ischemia/reperfusion
- IL
interleukin
- TNFα
tumor necrosis factor-α
- CXCR2
CXC chemokine receptor-2
- DMSO
dimethylsulfoxide
- NF-κB
nuclear factor κB
- TBS
tris-buffered saline
- STAT3
signal transducers and activators of transcription-3
- ALT
alanine amino transferase
- MIP-2
macrophage inflammatory protein-2
- KC
keratinocyte chemokine
- ELISA
enzyme-linked immunosorbent assay
- MPO
myeloperoxidase
- PCNA
proliferating cell nuclear antigen
- LDH
lactate dehydrogenase
- BrdU
5-bromo-2’-deoxyuridine
- SEM
standard error of the mean
- HGF
hepatocyte growth factor.
References
- 1.Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169–173. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 2.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 3.Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by Kupffer cells and priming of neutrophils during reperfusion after hepatic ischemia. Free Radic Res Commun. 1991;15:277–284. doi: 10.3109/10715769109105223. [DOI] [PubMed] [Google Scholar]
- 4.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 5.Wanner GA, Ertel W, Muller P, Hofer Y, Leiderer R, Menger MD, Messmer K. Liver ischemia and reperfusion induces a systemic inflammatory response through Kupffer cell activation. Shock. 1996;5:34–40. doi: 10.1097/00024382-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA., Jr. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85:1936–1943. doi: 10.1172/JCI114656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki S, Toledo-Pereyra LH. Interleukin 1 and tumor necrosis factor production as the initial stimulants of liver ischemia and reperfusion injury. J Surg Res. 1994;57:253–258. doi: 10.1006/jsre.1994.1140. [DOI] [PubMed] [Google Scholar]
- 8.Lentsch AB, Yoshidome H, Kato A, Warner RL, Cheadle WG, Ward PA, Edwards MJ. Requirement for interleukin-12 in the pathogenesis of warm hepatic ischemia/reperfusion injury in mice. Hepatology. 1999;30:1448–1453. doi: 10.1002/hep.510300615. [DOI] [PubMed] [Google Scholar]
- 9.Colletti LM, Cortis A, Lukacs N, Kunkel SL, Green M, Strieter RM. Tumor necrosis factor up-regulates intercellular adhesion molecule 1, which is important in the neutrophil- dependent lung and liver injury associated with hepatic ischemia and reperfusion in the rat. Shock. 1998;10:182–191. doi: 10.1097/00024382-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Colletti LM, Kunkel SL, Walz A, Burdick MD, Kunkel RG, Wilke CA, Strieter RM. Chemokine expression during hepatic ischemia/reperfusion-induced lung injury in the rat. The role of epithelial neutrophil activating protein. J Clin Invest. 1995;95:134–141. doi: 10.1172/JCI117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC. Hepatology. 1998;27:1172–1177. doi: 10.1002/hep.510270440. [DOI] [PubMed] [Google Scholar]
- 12.Kato A, Gabay C, Okaya T, Lentsch AB. Specific role of interleukin-1 in hepatic neutrophil recruitment after ischemia/reperfusion. Am J Pathol. 2002;161:1797–1803. doi: 10.1016/S0002-9440(10)64456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- 14.Bertini R, Allegretti M, Bizzarri C, Moriconi A, Locati M, Zampella G, Cervellera MN, et al. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc Natl Acad Sci U S A. 2004;101:11791–11796. doi: 10.1073/pnas.0402090101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bone-Larson CL, Hogaboam CM, Evanhoff H, Strieter RM, Kunkel SL. IFN-gamma-inducible protein-10 (CXCL10) is hepatoprotective during acute liver injury through the induction of CXCR2 on hepatocytes. J Immunol. 2001;167:7077–7083. doi: 10.4049/jimmunol.167.12.7077. [DOI] [PubMed] [Google Scholar]
- 16.Colletti LM, Green M, Burdick MD, Kunkel SL, Strieter RM. Proliferative effects of CXC chemokines in rat hepatocytes in vitro and in vivo. Shock. 1998;10:248–257. doi: 10.1097/00024382-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Hogaboam CM, Bone-Larson CL, Steinhauser ML, Lukacs NW, Colletti LM, Simpson KJ, Strieter RM, et al. Novel CXCR2-dependent liver regenerative qualities of ELR-containing CXC chemokines. FASEB J. 1999;13:1565–1574. doi: 10.1096/fasebj.13.12.1565. [DOI] [PubMed] [Google Scholar]
- 18.Ren X, Carpenter A, Hogaboam C, Colletti L. Mitogenic properties of endogenous and pharmacological doses of macrophage inflammatory protein-2 after 70% hepatectomy in the mouse. Am J Pathol. 2003;163:563–570. doi: 10.1016/S0002-9440(10)63684-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stefanovic L, Brenner DA, Stefanovic B. Direct hepatotoxic effect of KC chemokine in the liver without infiltration of neutrophils. Exp Biol Med (Maywood) 2005;230:573–586. doi: 10.1177/153537020523000809. [DOI] [PubMed] [Google Scholar]
- 20.Stefanovic L, Stefanovic B. Mechanism of direct hepatotoxic effect of KC chemokine: sequential activation of gene expression and progression from inflammation to necrosis. J Interferon Cytokine Res. 2006;26:760–770. doi: 10.1089/jir.2006.26.760. [DOI] [PubMed] [Google Scholar]
- 21.Barone S, Okaya T, Rudich S, Petrovic S, Tenrani K, Wang Z, Zahedi K, et al. Distinct and sequential upregulation of genes regulating cell growth and cell cycle progression during hepatic ischemia-reperfusion injury. Am J Physiol Cell Physiol. 2005;289:C826–835. doi: 10.1152/ajpcell.00629.2004. [DOI] [PubMed] [Google Scholar]
- 22.White JR, Lee JM, Young PR, Hertzberg RP, Jurewicz AJ, Chaikin MA, Widdowson K, et al. Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J Biol Chem. 1998;273:10095–10098. doi: 10.1074/jbc.273.17.10095. [DOI] [PubMed] [Google Scholar]
- 23.Deryckere F, Gannon F. A one-hour minipreparation technique for extraction of DNA-binding proteins from animal tissues. Biotechniques. 1994;16:405. [PubMed] [Google Scholar]
- 24.Schierwagen C, Bylund-Fellenius AC, Lundberg C. Improved method for quantification of tissue PMN accumulation measured by myeloperoxidase activity. J Pharmacol Methods. 1990;23:179–186. doi: 10.1016/0160-5402(90)90061-o. [DOI] [PubMed] [Google Scholar]
- 25.Kuboki S, Shin T, Huber N, Eismann T, Galloway E, Schuster R, Blanchard J, et al. Peroxisome proliferator-activated receptor-gamma protects against hepatic ischemia/reperfusion injury in mice. Hepatology. 2008;47:215–224. doi: 10.1002/hep.21963. [DOI] [PubMed] [Google Scholar]
- 26.Camargo CA, Jr., Madden JF, Gao W, Selvan RS, Clavien PA. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology. 1997;26:1513–1520. doi: 10.1002/hep.510260619. [DOI] [PubMed] [Google Scholar]
- 27.Luedde T, Trautwein C. Intracellular survival pathways in the liver. Liver Int. 2006;26:1163–1174. doi: 10.1111/j.1478-3231.2006.01366.x. [DOI] [PubMed] [Google Scholar]
- 28.Cressman DE, Diamond RH, Taub R. Rapid activation of the Stat3 transcription complex in liver regeneration. Hepatology. 1995;21:1443–1449. [PubMed] [Google Scholar]
- 29.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 30.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- 31.Murphy PM. Neutrophil receptors for interleukin-8 and related CXC chemokines. Semin Hematol. 1997;34:311–318. [PubMed] [Google Scholar]
- 32.Fu W, Zhang Y, Zhang J, Chen WF. Cloning and characterization of mouse homolog of the CXC chemokine receptor CXCR1. Cytokine. 2005;31:9–17. doi: 10.1016/j.cyto.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Moepps B, Nuesseler E, Braun M, Gierschik P. A homolog of the human chemokine receptor CXCR1 is expressed in the mouse. Mol Immunol. 2006;43:897–914. doi: 10.1016/j.molimm.2005.06.043. [DOI] [PubMed] [Google Scholar]