Abstract
Retinopathy, a largely microvascular complication, affects over 80% of patients with diabetes for 20 years. The purpose of this study is to investigate the effect of diabetes on the activation of H-Ras, a small molecular weight G-protein that regulates cell fate, in the retinal microvessels. Microvessels were prepared from freshly isolated retina from streptozotocin diabetic rats or 30% galactose-fed rats by hypotonic lysis method. Ras activation was quantified by Raf-1 binding assay, and the activation of the signaling proteins, Raf-1 and mitogen activated protein (MAP) kinase, by quantifying their gene transcripts (RTPCR) and/or by protein expression (western blot). Two months of diabetes or experimental galactosemia activated H-Ras (Raf-binding assay) in the retinal microvessels by over 40% and 70% respectively compared to the values obtained from normal rat retinal microvessels. In the same diabetic rats the gene transcripts of H-Ras and its effector protein Raf-1 were elevated by 30% and 135% respectively with their protein expressions elevated by about 25% each, and this was paralleled by similar increases in the protein expressions of H-Ras and Raf-1 in experimentally galactosemic rats. Diabetes increased the gene expression of Ras-Raf-1 downstream signaling protein MAP kinase by over 2 fold, and that of nuclear transcriptional factor by 25–30%. This activation of H-Ras in retinal microvessels implies that its signaling pathway, in part, could be contributing to the microvascular pathology characteristic of diabetic retinopathy. Comparable activation of H-Ras and its signaling cascade in the retinal microvessels from experimentally galactosemic rats suggests that H-Ras activation is not due to insulin deficiency. Regulation of Ras function could provide important target in the complex approach to inhibit the pathogenesis of diabetic retinopathy.
Keywords: Diabetic retinopathy, G-Protein, H-Ras, Retina microvessels
Introduction
Ras proteins, members of a large family of small molecular weight guanosine triphosphate (GTP)/guanosine diphosphate (GDP)-binding G-proteins, serve as a ‘molecular switch’, that regulate cell fates by cycling between active GTP-bound and inactive GDP-bound conformations (Adjei, 2001; Leicht et al., 2007). GTP-bound Ras recruits a serine-threonine kinase Raf-1, a well-characterized downstream effector of Ras. Raf-1 is predominantly cytosolic, but, upon activation, is translocated to the plasma membrane, and activates downstream pathways resulting in phosphorylation of mitogen activated protein (MAP) kinases (Leicht, et al., 2007; Roberts and Der, 2007). Activation of H-Ras has been implicated in the pathogenesis of diabetic retinopathy; the therapy that inhibits apoptosis of retinal vascular cells and retinopathy in diabetic rats also inhibits increase in retinal Ras expression and mRNA levels (Kowluru et al., 2004). Further, activation of H-Ras and its downstream signaling pathway in diabetes appears to be under the control of superoxide, and overexpression of mitochondrial superoxide dismutase that prevents diabetic retinopathy also prevents H-Ras activation in the retina (Kowluru et al., 2007b; Kowluru and Kowluru, 2007).
Retinal capillary cells are lost selectively via apoptosis before other microvascular histopathology is evident in diabetic rodents, suggesting that the clinically silent initial phase of diabetic retinopathy consists of irreversible cellular events with late structural consequences (Kern and Engerman, 2001; Mizutani et al., 1996). Microvessels from rat retina have increased activity of protein kinase C (PKC) in diabetes compared to normal rats, and aminoguanidine treatment that inhibits retinopathy also inhibits increased retinal microvasculature PKC activity (Kern et al., 2000; Kowluru et al., 2000; Kowluru et al., 1998). In another animal model of diabetic retinopathy, experimental galactosemia that is induced by feeding normal rodents galactose-rich diet, increased PKC activity is also observed in the retinal microvessels (Kowluru, et al., 2000; Kowluru, et al., 1998).
Diabetic retinopathy is considered largely a microvascular complication (Klein, 1995; Kohner, 1989). However, the retina is a complex tissue with multiple cell types, and retinal cells, other than capillary cells, could contribute to the Ras activation seen in rat retina in diabetes. Isolated retinal capillary cells cultured in high glucose, though very critical in dissecting out any mechanism, may not truly represent capillary cells in diabetic conditions. To conclusively establish the role of H-Ras and its signaling pathway in the development of diabetic retinopathy, we have investigated the effect of diabetes on the activation of H-Ras and its signaling steps including Raf-1, MAP kinase, and nuclear transcriptional factor-kB (NF-kB) in the retinal microvessels isolated from diabetic rats. Since insulin is shown to increase farnesyltransferase activity (Stephens et al., 2001), to distinguish the effect of insulin deficiency from hyperglycemia we confirmed the major findings also in the retinal microvessels from experimentally galactosemic rat retina.
Methods
Rats
Male Wistar rats (200–220g BW) were randomly divided into normal, diabetic and experimentally galactosemic groups. Diabetes was introduced with streptozotocin injection (55mg/kg body weight) and insulin was administered to allow slow weight gain while maintaining hyperglycemia (blood glucose levels of 20–25 mmol/L). Experimental galactosemia was induced in normal rats by feeding them a diet supplemented with 30% galactose (Kern and Engerman, 1994; Kowluru, et al., 1998). Age-matched normal rats served as controls. Each group had 15–20 rats. The entire rat colony received fresh powder diet once every other week, and their food consumption was measured every week. The rats were weighed two times a week; average body weight of diabetic rats was 290 g, experimentally galactosemic rats 345g, and that of age-matched normal rats 370g. Glycated hemoglobin (GHb) was measured using affinity columns 2–3 days before termination of the experiment; average GHb values in diabetic, experimentally galactosemic and normal rats were about 12%, 7.7 %, 6% respectively. The rats were euthanized by an overdose of pentobarbital two months after initiation of the experiment, and the retina was isolated immediately. Microvessels were prepared from the fresh retina by hypotonic method. Treatment of animals conformed to the national Institutes of Health Principals of Laboratory Animal care, the Association for Research in vision and ophthalmology Resolution on the Use of Animals in Research, and the institutional guidelines.
Retinal microvessels
Freshly isolated whole retina was incubated in distilled water for one hour at 370C, and this was followed by 5 minutes incubation with DNase (2mg/ml). The retinal vasculature was isolated under a microscope by repetitive inspiration and ejection through Pasteur pipettes. Retinal blood vessels isolated by this method show a normal complement of nuclei and were devoid of nonvascular materials (Kern, et al., 2000; Kowluru, et al., 1998).
Ras activation assay
The effect of hyperglycemia (diabetes or experimental galactosemia) on H-Ras activation in retinal microvasculature was quantified by measuring the relative abundance of GTP-bound active form of H-Ras using a Raf-1 binding assay kit (Cytoskeleton, Denver, CO). Microvessel preparation (80μg protein) was incubated with Raf-1RBD, and the Raf-RBD/GTP-Ras complex was pulled down by glutathione affinity beads. To estimate the amount of activated Ras, the beads were re-suspended in Laemmli reducing sample buffer and boiled for 5 minutes. This was followed by performing western blots on the samples using Ras-Pan specific antibody that was supplied in the Raf-1 binding assay kit (Kowluru, et al., 2007b).
Quantitative PCR
The transcript levels of H-Ras, Raf-1, MAPK, NF-kB and iNOS were quantified in the microvessels by the real-time quantitative PCR (Q-RT-PCR) technique using the TaqMan Assays on Demand for the rat (Applied Biosystems, Foster City, CA), as routinely employed in our laboratory (Kowluru et al., 2007a; Kowluru et al., 2007c). RNA was isolated from the retinal microvessels with TRIZol reagent and was converted to cDNA using the High capacity cDNA reverse transcription kit with RNase inhibitor. Q-RT-PCR reactions were carried out in a total of 50–300ng cDNA templates in 96 well plates using ABI-7500 sequence detection system. Each sample was analyzed in triplicate. Data were normalized to the mRNA of the housekeeping gene beta-2-macroglobulin (B2M) in the same sample. The fold change in gene expression relative to normal was calculated using the ddCT method. The GenBank accession numbers and the amplicon length for the target and housekeeping genes used are provided in Table I.
Table I.
GenBank accession number and the amplicon length for the target and housekeeping genes used for Q-RT-PCR
| Gene | GenBank Accession No. | Amplicon length (bp) |
|---|---|---|
| H-Ras | XM_001061671 | 72 |
| Raf-1 | NM_012639 | 88 |
| MAPK | NM_031020 | 87 |
| NF-kB | XM_342346 | 63 |
| iNOS | NM_012611.2 | 77 |
| B2M | NM_012512.1 | 58 |
Western blotting
To confirm the effect of hyperglycemia on H-Ras and its signaling proteins in retinal microvasculature, 20μg protein was separated on a gradient polyacrylamide gel and transferred to nitrocellulose membranes. After blocking the membranes in 5% milk they were incubated with primary antibodies against H-Ras, Raf-1, NF-kB, inducible isoform of nitric oxide synthase, iNOS. The membranes were washed, and incubated with their respective secondary horse radish peroxidase-conjugated secondary antibodies, and developed using ECL-Plus western blotting detection kit (Amersham Biosciences, Piscataway, NJ). Kaleidoscope pre-stained molecular weight markers (Bio-Rad Laboratories, Hercules, CA) were run simultaneously on each gel. Equal loading among the lanes was ensured by stripping the membranes and measuring the expression of the house keeping protein β-actin in each sample. The band density was quantified using Un-Scan-It Gel digitizing software (Silk Scientific Inc, Orem, UT), and the values in the figures are presented as mean band intensity of the protein of interest adjusted by the intensity of β-actin in the same sample.
Statistical analysis
Values are reported as mean ± SD. The results were analyzed using one-way ANOVA followed by Fisher’s test. Similar conclusions were reached by nonparametric Kruskal-Wallis test followed by Mann-Whitney test for multiple group comparison.
Results
H-Ras is activated in retinal microvessels in hyperglycemia
Two months of diabetes or experimental galactosemia in rats activated H-Ras (as determined by Ras binding assay) in the microvasculature of the retina by 40% and 70% respectively (Figure 1a) compared to the microvasculature obtained from the age-matched normal rats. In the same diabetic rats, the gene expression of H-Ras was elevated by about 30% in the retinal microvessel (Figure 1b). This increase in mRNA levels of H-Ras was in concordance with the increase in protein expression of H-Ras; its protein expression was increased by about 25%–30% in retinal microvessels of diabetic or experimentally galactosemic rat retina compared to the normal rat retina (data not shown).
Figure 1.
Activation of H-Ras in retinal microvessels: a. GTP-bound Ras was quantified in retinal microvessels obtained from diabetic, experimentally galactosemic and age-matched normal rats by determining the amount of active GTP-bound Ras bound to Raf-1 using a pull-down assay. The data are representative of 6–7 rats in each group, and the histogram represents mean band intensity of H-Ras. b. mRNA levels of H-Ras were quantified in the microvessels by Q-RT-PCR, using the primers described in the method section. The values were normalized to that of the housekeeping gene, B2M, in the same sample. Each measurement was performed in triplicate using 5–8 rats in each group. The values obtained from normal rats are considered 100%. *P<0.05 compared to normal
Ras activation is paralleled by a similar increase in its effector protein-Raf-1
Figure 2a depicts that the protein expression of Raf-1, similar to H-Ras, is also activated by 25%–30% in the retinal microvessels of both diabetic rats and galactose-fed rats. Further, in the same diabetic rats, the gene expression of Raf-1 was elevated by 135% (Figure 2b).
Figure 2.
Effect of hyperglycemia on Raf-1 in retinal microvessels: a. Western blot for Raf-1 was performed in the microvessels by separating the proteins on a gradient polyacrylamide gel. The histogram represents the mean band intensity (from 4–5 rats in each group) of Raf-1 adjusted to the intensity of β actin in the same sample. b. Gene expression of Raf-1 was quantified by Q-RT-PCR, and the primers are provided in table I. Raf-1 gene expression in each sample was normalized to that of B2M in the same sample. Each measurement was performed in triplicate using 5–8 rats in each group. The values obtained from normal rats are considered 100%. *P<0.05 compared to normal
Diabetes modulates the signaling proteins that are regulated by H-Ras in retinal microvessels
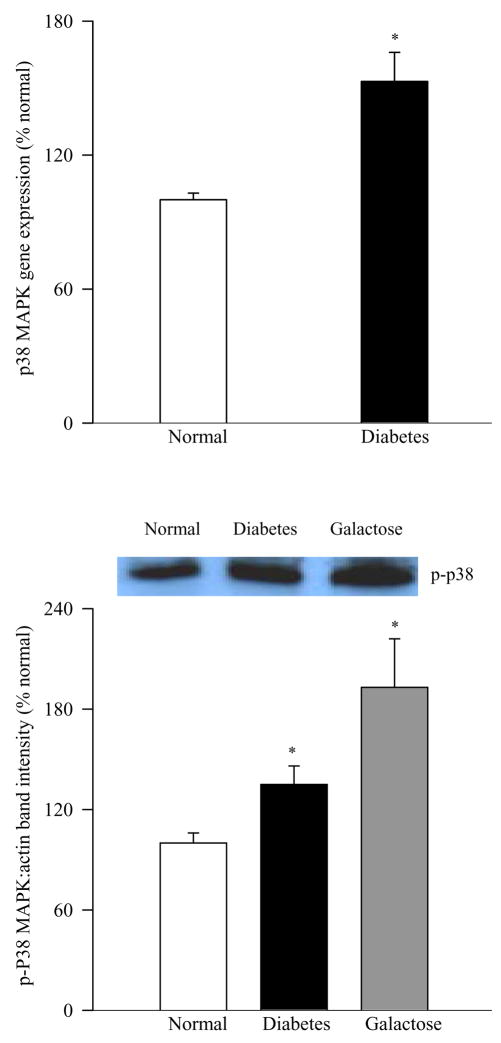
Activation of one of the major components of the signal transduction mediated by H-Ras, the MAP kinase pathway, was evaluated in the retinal microvessels by performing the western blot analysis of the phosphorylation of p38 MAP kinase. The expression of p-P38 was elevated by 50% in the retinal microvessels obtained from diabetic rats and by 100% in those obtained from experimentally galactosemic rats compared to the age-matched normal rats (Figure 3a). Parallel increase in gene expression of MAP kinase was also observed; mRNA levels of MAP kinase were 50% higher in the retinal microvessels of diabetic rats compared to normal rats (Figure 3b).
Figure 3.
Hyperglycemia-induced MAP kinase activation in retinal microvessels. (a) phosphorylation of p38 MAP kinase was determined by western blot using β-actin as a loading standard and each sample was run in duplicate, and the blots are representative of 4 or more rats in each group. The histogram represents the band density of p-P38 adjusted to the band density of β actin in the same sample, and the values obtained from normal rats are considered 100%. (b) Transcript expression of p38 MAP kinase was determined by Q-RT-PCR in the RNA from retinal microvessels obtained from normal and diabetic rats. Measurements were made in triplicate using RNA from 5–6 rats in each group. *P<0.05 compared to normal
Nuclear transcriptional factor and iNOS are activated in the retinal microvessels in hyperglycemia
Figure 4a shows that NF-kB, a ubiquitous transcriptional factor that is implicated in the pathogenesis of diabetic retinopathy, was activated by about 50% in the retinal microvessels of diabetic rats and also in experimentally galactosemic rats (as determined by the expression of its 65kD subunit). The gene expression of NF-kB was elevated by 25% in the retinal microvessels of diabetic rats (Figure 4b). In the same microvessels, diabetes increased the gene expression of iNOS by 130% compared to the microvessels from normal rats (Figure 4c). However, due to technical problems, we were unable to measure the gene expressions of some of the signaling molecules in the retinal microvessels prepared from the experimentally galactosemic rats.
Figure 4.
Activation of NF-kB and gene expressions of NF-kB and iNOS: Activation of NF-kB was determined by measuring the expression of its 65kD subunit. The blots represent 4–5 rats in each group. mRNA of NF-kB (b) and iNOS (c) were determined by Q-RT-PCR using the primers provided in table I. The values presented are mean of 5–8 rats in each group. Normal rat gene transcripts are considered 100%. *P<0.05 compared to normal
Discussion
Diabetic retinopathy is regarded as a vascular disease that mainly affects microvasculature (Klein, 1995; Kohner, 1989), and the metabolic abnormalities in retinal microvessels, the major site of retinal histopathology, are very important. This is the first report showing that H-Ras, a protein that is important in converting signals from the cell membrane to the nucleus and regulating cellular processes, including apoptosis, is activated in the retinal microvessels obtained from two different animal models of diabetic retinopathy, diabetic rats and experimentally galactosemic rats. The major effector protein of H-Ras, Raf-1, was also activated in the same microvessel preparations resulting in the activation of downstream signaling pathway.
Retinal microvascular cells (both endothelial cells and pericytes) undergo accelerated apoptosis, and this capillary cell apoptosis precedes histological evidence of retinopathy in diabetes (Mizutani, et al., 1996; Kern, et al., 2000). Although isolated retinal microvessel preparations from the animals of diabetic retinopathy have not been extensively studied for the biochemical markers associated with the development of diabetic retinopathy, and the reason could be the limited tissue availability, our previous studies have shown that the activity of PKC is increased in the retinal microvessels in diabetes, and aminoguanidine (a therapy that prevents the development of vascular histopathology) can inhibit such increases (Kern, et al., 2000; Kowluru, et al., 2000). Others have reported significant increase in the levels of proapoptotic protein Bax in the microvessels prepared from the retina of diabetic patients compared to nondiabetic subjects suggesting that increased Bax could be important in the apoptosis of retinal capillary cells (Podesta et al., 2000). In addition, the levels of complement inhibitors CD55 and CD59 are decreased in retinal microvessels in diabetes (Dagher et al., 2004), and poly (ADP-ribose) polymerase are increased (Zheng et al., 2004). Our novel results showing that H-Ras and its signaling proteins that are activated in the retina in diabetes, are also activated in the microvessels isolated from the retina from two different animal models of diabetic retinopathy, diabetic rats and experimentally galactosemic rats, strongly suggest that H-Ras and its signaling pathway is important in the development of diabetic retinopathy.
Activation of H-Ras in the retina in diabetes can be prevented by the therapies that inhibit the development diabetic retinopathy (Kowluru, et al., 2004; Kowluru and Kowluru, 2007). In vitro studies using isolated retinal capillary cells have shown that the activation of H-Ras is one of the signaling steps involved in accelerated capillary cell apoptosis in diabetes, and inhibition of Ras-function, and genetic manipulation of functionally active H-Ras inhibit glucose-induced retinal capillary cell apoptosis (Kowluru, et al., 2004; Kowluru, et al., 2007b; Kowluru and Kowluru, 2007). Thus, the activation of H-Ras in the retinal microvessels in diabetes and in experimental galactosemia strongly implies that H-Ras has a critical role in the accelerated apoptosis of capillary cells that precedes the development of retinal vascular pathology characteristic of diabetic retinopathy.
Ras proteins exist either in an inactive GDP-bound form or in an active GTP-bound conformation. Guanine nucleotide exchange factors promote dissociation of GDP from the inactive Ras-GDP complex, allowing Ras proteins to bind GTP, and the activation requires posttranslational modification of Ras proteins including farnesylation. The process of farnesylation is catalyzed by farnesyltransferase (Appels, 2005). Insulin is shown to increase farnesyltransferase activity by promoting its phosphorylation (Draznin et al., 2000), and this can be inhibited by inhibiting endogenous insulin secretion (Stephens et al., 2001). Therefore, our results obtained from experimentally galactosemic rats are significant because these animals have similar circulating insulin levels as normal animals, but develop retinal abnormalities and histopathology similar to that seen in diabetic animals (Engerman and Kern, 1984; Kern and Engerman, 1994), and they suggest that the activation of H-Ras and its signaling pathway in diabetes is not due to insulin deficiency, but is the result of hyperglycemia per se.
Ras in its inactive form exists in the GDP-bound, but upon activation it becomes GTP-bound, and Ras-GTP recruits a serine–threonine kinase Raf-1, a well-characterized downstream effector of Ras (Adjei, 2001; Leicht, et al., 2007). Raf-1 is predominantly cytosolic, but upon activation is translocated to the plasma membrane, and is subsequently phosphorylated (Leicht, et al., 2007; Roberts and Der, 2007). We have shown that Raf-1 expression is increased in the retina in diabetes (Kowluru, et al., 2004; Kowluru and Kowluru, 2007). Here we provide exciting data showing that the same microvessels that have activated H-Ras also have activated Raf-1, suggesting that retinal microvessels are the site of diabetes-induced H-Ras mediated Raf-1 activation. In support, our recent studies have shown that Raf-1 kinase inhibition prevents retinal endothelial cells from glucose-induced increased apoptosis (Rayappa and Kowluru, 2007). Further, activation of Raf-1 mediated by growth factors is implicated in the regulation of proliferation and migration in many cell types, including endothelial cells (Culmsee et al., 2006). The levels of growth factors, including VEGF and IGF, are elevated in the retina early in the pathogenesis of diabetic retinopathy (Duh and Aiello, 1999); and could account, in part, for the activation of microvasculature Raf-1 in diabetes.
One of the major components of the signal transduction mediated by Ras is the MAP kinase pathway (Leicht, et al., 2007; Roberts and Der, 2007). In diabetic rodents MAP kinase activity and its gene expression are increased in the retina (Joussen et al., 2001; Joussen et al., 2002; Kowluru and Kowluru, 2007). MAP kinase pathway is considered important in mediating hepatocyte growth factor induced retinal vascular permeability that is impaired in diabetes (Clermont et al., 2006). Our recent study has shown that MAP kinase is phosphorylated in the retina of diabetic mice and in retinal endothelial cells incubated in high glucose, and overexpression of MnSOD or inhibition of Raf-1 kinase inhibits such increased phosphorylation (Kowluru and Kowluru, 2007; Rayappa and Kowluru, 2007). Activation of MAP kinase in the microvasculature of the retina in diabetes and also in experimental galactosemic rats strengthens the role of H-Ras mediated pathway in the development of this microvascular complication of diabetes.
NF-kB, a redox sensitive nuclear transcriptional factor important in regulating expression of genes that are critical for the regulation of apoptosis and inflammation, is activated in the retina in diabetes (Lenardo and Baltimore, 1989; Kowluru et al., 2003). It is localized in sub-retinal membranes and in microvessels (Hammes et al., 1999). In vitro experiments have shown that NF-kB is activated in both retinal pericytes and endothelial cells incubated in high glucose (Kowluru, et al., 2003). NF-kB activation in endothelial cells is considered as a key step in the signaling pathway by which high glucose induces apoptosis (Du et al., 2002). Overexpression of constitutionally active H-Ras vectors in retinal endothelial cells further activates NF-kB in high glucose conditions (Kowluru, et al., 2007b), and inhibition of H-Ras function and Raf-1 kinase prevents glucose-induced activation of NF-kB (Kowluru, et al., 2004; Rayappa and Kowluru, 2007). Our exciting data presented here showing the activation of NF-kB in the microvessels of the retina further strengthens the role of NF-kB in the development of diabetic retinopathy.
Activated NF-kB binds to nuclear DNA and up-regulates the production of iNOS (Schreck et al., 1991). iNOS is considered to play an important role in the development of diabetic retinopathy; and is linked to leukostasis and blood retinal breakdown, the abnormalities that are observed early in the development of retinopathy (Leal et al., 2007). Mice deficient in iNOS are protected from diabetes-induced retinal histopathology (Zheng et al., 2007). Elevated levels of nitric oxide in the retina in diabetes and experimental galactosemia are considered to be in part via upregulation of iNOS (Kowluru 2003; Zheng et al., 2007). Inhibitors of Ras function or Raf-1 kinase inhibit diabetes-induced increase in nitric oxide levels in retinal endothelial cells (Kowluru, et al., 2004; Rayappa and Kowluru, 2007). Increased expression of iNOS in the retinal microvessels of diabetic rats observed in the present study strongly suggests that iNOS has a critical role in the development of diabetic retinopathy.
In conclusion this is the first report showing the importance of activation of H-Ras and its downstream signaling pathway in retinal microvessels in diabetes, and the conclusions are strengthened by the exciting results from experimental galactosemic rats. Our studies strongly support the role of H-Ras in the development of retinopathy in diabetes and elucidate the possible mechanism involved. This is expected to have immense clinical implications because regulation of H-Ras activation in the retinal microvasculature could help inhibit the development of retinopathy in diabetes.
Acknowledgments
This study was supported in part by grants from the National Institutes of Health, the Juvenile Diabetes Research Foundation, the Thomas Foundation, and Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adjei AA. J Natl Cancer Inst. 2001;93:1062–1074. doi: 10.1093/jnci/93.14.1062. [DOI] [PubMed] [Google Scholar]
- Appels NM, Beijnen JH, Schellens JH. Oncologist. 2005;10:565–578. doi: 10.1634/theoncologist.10-8-565. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Gasser E, Hansen S, Tonn JC, Wagner E, Goldbrunner R. Brain Res. 2006;1125:147–154. doi: 10.1016/j.brainres.2006.09.065. [DOI] [PubMed] [Google Scholar]
- Dagher Z, Park YS, Asnaghi V, Hoehn T, Gerhardinger C, Lorenzi M. 2004 doi: 10.2337/diabetes.53.9.2404. [DOI] [PubMed] [Google Scholar]
- Clermont AC, Cahill M, Salti H, Rook SL, Rask-Madsen C, Goddard L, Wong JS, Bursell D, Bursell SE, Aiello LP. Invest Ophthalmol Vis Sci. 2006;47:2701–2708. doi: 10.1167/iovs.05-0071. [DOI] [PubMed] [Google Scholar]; Diabetes. 53:2404–2411. doi: 10.2337/diabetes.53.9.2404. [DOI] [PubMed] [Google Scholar]
- Draznin B, Miles P, Kruszynska Y, Olefsky J, Friedman J, Golovchenko I, Stjernholm R, Wall K, Reitman M, Accili D, Cooksey R, McClain D, Goalstone M. Endocrinology. 2000;141:1310–1306. doi: 10.1210/endo.141.4.7411. [DOI] [PubMed] [Google Scholar]
- Du X, Stocklauser-Farber K, Rosen P. Free Radic Biol Med. 1999;27:752–763. doi: 10.1016/s0891-5849(99)00079-9. [DOI] [PubMed] [Google Scholar]
- Du Y, Smith MA, Miller CM, Kern TS. J Neurochem. 2002;80:771–779. doi: 10.1046/j.0022-3042.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- Duh E, Aiello LP. Diabetes. 1999;48:1899–1906. doi: 10.2337/diabetes.48.10.1899. [DOI] [PubMed] [Google Scholar]
- Engerman RL, Kern TS. Diabetes. 1984;33:97–100. doi: 10.2337/diab.33.1.97. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Hoerauf H, Alt A, Schleicher E, Clausen JT, Bretzel RG, Laqua H. Invest Ophthalmol Vis Sci. 1999;40:1855–1859. [PubMed] [Google Scholar]
- Joussen AM, Huang S, Poulaki V, Camphausen K, Beecken WD, Kirchhof B, Adamis AP. Invest Ophthalmol Vis Sci. 2001;42:3047–3057. [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Tsujikawa a, Qin W, Qaum T, Xu Q, Moromizato Y, Bursell SE, Wiegand SJ, Rudge J, Ioffe E, Yancopoulos GD, Adamis AP. Am J Pathol. 2002;160:1683–1693. doi: 10.1016/S0002-9440(10)61115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern TS, Engerman RL. Curr Eye Res. 1994;13:863–867. doi: 10.3109/02713689409015087. [DOI] [PubMed] [Google Scholar]
- Kern TS, Engerman RL. Diabetes. 2001;50:1636–1642. doi: 10.2337/diabetes.50.7.1636. [DOI] [PubMed] [Google Scholar]
- Kern TS, Tang J, Mizutani M, Kowluru R, Nagraj R, Lorenzi M. Invest Ophthalmol Vis Sci. 2000;41:3972–3978. [PubMed] [Google Scholar]
- Klein R. Diabetes Care. 1995;18:258–268. doi: 10.2337/diacare.18.2.258. [DOI] [PubMed] [Google Scholar]
- Kohner EM. Br Med Bull. 1989;45:148–173. doi: 10.1093/oxfordjournals.bmb.a072309. [DOI] [PubMed] [Google Scholar]
- Kowluru RA. Diabetes. 2003;52:818–823. doi: 10.2337/diabetes.52.3.818. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Engerman RL, Kern TS. Curr Eye Res. 2000;21:814–819. doi: 10.1076/ceyr.21.4.814.5545. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Jirousek MR, Stramm LE, Farid NA, Engerman RL, Kern TS. Diabetes. 1998;47:464–469. doi: 10.2337/diabetes.47.3.464. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Kanwar M, Chang P-S, Zhang J-P. Arch Ophthalmol. 2007a Under editorial review. [Google Scholar]
- Kowluru RA, Koppolu P, Chakrabarti S, Chen S. Free Radic Research. 2003;37:1169–1180. doi: 10.1080/10715760310001604189. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Kowluru A, Chakrabarti S, Khan Z. Diabetes. 2004;53:775–783. doi: 10.2337/diabetes.53.3.775. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Kowluru A, Kanwar M. Mol Cell Biochem. 2007b;296:69–76. doi: 10.1007/s11010-006-9299-z. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Menon B, Gierhart D. Inves Ophthalmol Vis Sci. 2007c doi: 10.1167/iovs.07-0764. In press. [DOI] [PubMed] [Google Scholar]
- Kowluru V, Kowluru RA. Mol Vis. 2007;13:602–610. [PMC free article] [PubMed] [Google Scholar]
- Leal EC, Manivannan A, Hosoya K, Terasaki T, Cunha-Vaz J, Ambrósio AF, Forrester JV. Invest Ophthalmol Vis Sci. 2007;48:5257–5265. doi: 10.1167/iovs.07-0112. [DOI] [PubMed] [Google Scholar]
- Leicht DT, Balan V, Kaplun A, Singh-Gupta V, Kaplun L, Dobson M, Tzivion G. Biochim Biophys Acta. 2007;1773:1196–1212. doi: 10.1016/j.bbamcr.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo MJ, Baltimore D. Cell. 1989;58:227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Mizutani M, Kern TS, Lorenzi M. J Clin Invest. 1996;97:2883–2890. doi: 10.1172/JCI118746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podesta F, Romeo G, Liu WH, Krajewski S, Reed JC, Gerhardinger C, Lorenzi M. Am J Pathol. 2000;156:1025–1032. doi: 10.1016/S0002-9440(10)64970-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayappa S, Kowluru RA. Int J Biomed Sci. 2007 under editorial review. [PMC free article] [PubMed] [Google Scholar]
- Roberts PJ, Der CJ. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- Schreck R, Rieber P, Baeuerle PA. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens E, Thureen PJ, Goalstone ML, Anderson MS, Leitner JW, Hay WW, Draznin B. Am J Physiol Endocrinol Metab. 2001;281:E217–223. doi: 10.1152/ajpendo.2001.281.2.E217. [DOI] [PubMed] [Google Scholar]
- Zheng L, Du Y, Miller C, Gubitosi-Klug RA, Kern TS, Ball S, Berkowitz BA. Diabetologia. 2007;50:1987–1996. doi: 10.1007/s00125-007-0734-9. [DOI] [PubMed] [Google Scholar]
- Zheng L, Szabo C, Kern TS. Diabetes. 2004;53:2960–2967. doi: 10.2337/diabetes.53.11.2960. [DOI] [PubMed] [Google Scholar]