Abstract
Introduction
Neonatal blood stream infections (BSI) are major cause of morbidity and mortality in developing countries. It is crucial to continuously monitor the local epidemiology of neonatal BSI to detect any changes in patterns of infection and susceptibility to various antibiotics.
Objective
To examine the etiology of BSI in two neonatal intensive care units (NICU) in the Republic of Georgia, a resource-poor country, and to determine antibiotic susceptibility of the isolated organisms.
Methods
Cross-sectional study among all septic infants was conducted in NICU of two pediatric hospitals in Tbilisi between 09/2003-09/2004.
Results
A total of 200 infants with clinical signs of sepsis were admitted in two NICUs. Of these, 126 (63%) had confirmed bacteremia. Mortality rate was 34%. A total of 98 (78%) of 126 recovered isolates were Gram-negative organisms, and 28 (22%) were Gram-positive. Klebsiella pneumoniae was the most common pathogen, accounting for 36 (29%) of 126 isolates, followed by Enterobacter cloacae – 19 (15%), and S. aureus – 15 (12%). The gram-negative organisms showed high degree of resistance to commonly used antibiotics such as ampicillin, amoxicillin/clavulanate, and comparatively low resistance to amikacin, ciprofloxacin, carbapenems, and gentamicin; 40% of S. aureus isolates were methicillin resistant (MRSA). In multivariate analysis only umbilical discharge was a significant risk factor for having positive blood culture at admission to NICU (PR=2.25, 95% CI 1.82-2.77).
Conclusions
Neonatal BSI was mainly caused by gram-negative organisms, which are developing resistance to commonly used antibiotics. Understanding the local epidemiology of neonatal BSI can lead to the development of better medical practices, especially more appropriate choices for empiric antibiotic therapy, and may contribute to improvement of infection control practices.
Keywords: blood stream infections, Republic of Georgia, neonatal
Introduction
The World Health Organization (WHO) estimates that 130 million infants are born each year. Of these, 8 million do not survive till their first birthday, and more than 10 million die before the age of five.1 It is estimated that neonatal deaths account for a third of global child mortality and that infections are a major cause of neonatal mortality.2 In the Republic of Georgia, the neonatal mortality rate in year 2000 was 25 per 1,000 live births, and infant mortality rate (under one year of age) was 41 per 1,000 live births in 20053
Neonatal infections can be acquired vertically through exposure to bacteria in the birth canal or by exposure after birth because of a lack of sanitation. Neonatal infections are primarily sepsis (blood stream infection [BSI]), meningitis, neonatal tetanus, omphalitis, and diarrhea. In areas where access to adequate maternal care is lacking, homebirths are the alternative and sanitary conditions can not be assured. These infections are attributed to failures in knowledge and training regarding basic infection control processes coupled with inadequate infrastructure, systems of care and resources.4
The rates of blood stream infections in neonates are 3-20 times higher in developing countries, and in some countries, approximately half of the patients in neonatal ICUs acquire an infection, and the case fatality rates may reach 52%.4,5 These numbers are likely a significant underestimate of the true burden of disease as many babies are born and may die outside of a hospital, and also within hospitals there may be lacking laboratory infrastructure for blood cultures. The high rates of neonatal infections in developing countries and the types of infections commonly identified such as bloodstream infections (BSI) with gram-negative rods, or Staphylococcus aureus infections, strongly suggest that lack of appropriate hygiene during labor and delivery, and postnatal care, are major contributors to infectious morbidity in newborns.6-10 The organisms responsible for neonatal sepsis vary across geographical boundaries and in time of onset. In addition, one organism or group of organisms may replace over time another as the leading cause of neonatal sepsis in a particular region. It is crucial to continuously monitor the local epidemiology of neonatal BSI to detect any changes in patterns of infection and susceptibility to various antibiotics.
The goal of this study was to examine the etiology of BSI in two neonatal intensive care units (NICU) in the Republic of Georgia, a resource-poor country; and to determine antibiotic susceptibility of the isolated organisms. Antibiotic use directed at specific pathogens along with improvements in infection control practices are two specific aims that better surveillance could impact.
Methods
Study design and patient population
The study had a prospective cross-sectional design. All infants admitted to the NICU of two pediatric hospitals (Hospital A and Hospital B) in Tbilisi, capital city of the Republic of Georgia, between September 1, 2003 and September 1, 2004 were enrolled in the study. Hospital's A NICU is comprised of 6 beds with an average of 400 admissions per year; Hospital's B NICU has 8 beds and averages over 540 admissions per year. The admissions to these two NICUs include children born at other hospitals and transferred to these facilities as both hospitals serve as regional referral NICUs. Eligibility criteria included age of eight weeks or younger, and having clinical manifestations of generalized infection (neonatal sepsis). The study was approved by the Institutional Review Board (IRB) of each hospital in Tbilisi, University at Albany, State University of New York, and by Emory University, Atlanta IRB.
Definitions
Clinical criteria of sepsis were the following: temperature >38°C or <36°C, heart rate >20 breaths/min, white blood cell count ≥12,000/mm or <4,000/mm, or >10% of immature cells, inflammatory response syndrome (SIRS). Blood stream infection (BSI) was defined as infection confirmed by blood culturxe. Low birth weight was defined as weight of ≤2,500 grams. Premature membrane rupture was defined as rupture of membranes before the onset of labor.
Laboratory Methods
Two blood samples per patient were drawn at least 30 minutes apart from each other. The blood cuture system Hemoline performance disphasic medium (BioMerieux, Marcy l'Etoile, France) were used for the recovery of pathogens. Blood culture bottles were incubated for seven days at 37°C and visually inspected daily to detect positive growth. Positive cultures were Gram stained and subcultured to sheep blood agar, chocolate agar, MacConkey agar and Columbia colistin–nalidixic acid agar as per routine bacteriologic guidelines. Isolates of bacteria were identified by conventional biochemical and serological methods. Confirmation of species identification was performed by API technique (API-System, BioMerieux, La Balmes-les Grottes, France) whenever there were identification discrepancies. Isolation of Bacillus spp., Corynebacterium spp., and coagulase-negative staphylococci recovered from a single culture were considered as contaminants. A BSI was defined as isolation of at least one positive peripheral-blood culture, except cases of infection with coagulase-negative staphylococci, for which isolation of two positive blood cultures was required.
Susceptibility testing
The antibiotic susceptibility for isolated pathogens was determined and met all the recommendations of the National Committee of Clinical Laboratory Standards breakpoint values.11 Antimicrobial susceptibility testing of isolated pathogens to clinically used antimicrobials was performed by using the Kirby Bauer disk diffusion method and ATB susceptibility systems (BioMerieux La Balmes-les Grottes, France).
Data collection and statistical analysis
Medical records of infants were reviewed and clinical information was abstracted. Patient data was recorded on a standardized data collection form. A survey presented to mothers of admitted infants provided demographic and health behavior information. Data was entered in Microsoft Excel 2000 database (Microsoft Corp., Redmond, WA), and analyzed using SAS software version 9.1 (SAS Institute, Cary NC). Frequencies for demographic data and clinical characteristics were reported. The differences in distribution of categorical factors were assessed using Mantel-Haenzel chi-square test or Fisher's Exact Test as appropriate. Prevalence ratios with 95% confidence intervals for risk factors of having positive blood culture were estimated with univariate log-binomial regression models using PROC GENMOD in SAS. Initial multivariate log-binomial regression model included risk factors significantly associated with positive blood culture in univariate analysis as well as potential confounders. Backward elimination was used to derive final model. A p-value ≤ 0.05 was considered statistically significant.
Results
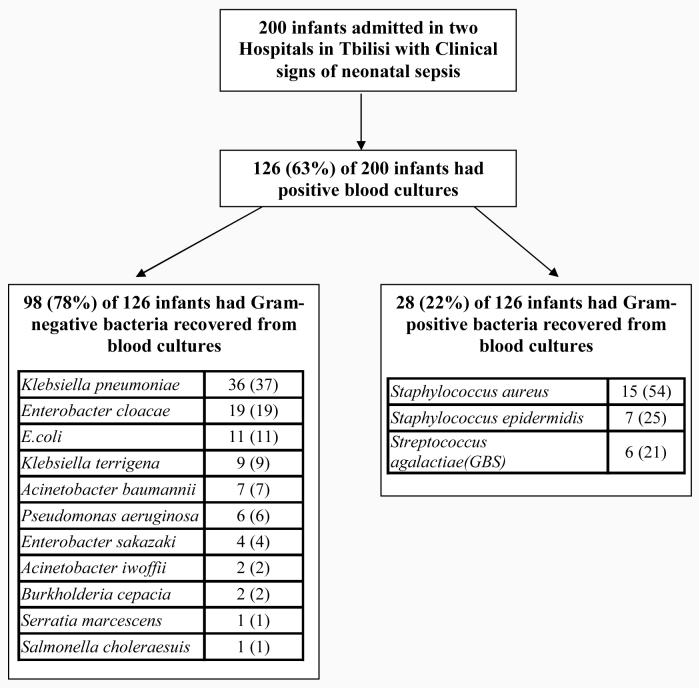
During one year study period a total of 200 infants with clinical signs of sepsis were admitted to two NICUs. Of these, 200 infants 112 (56%) were female, and 88 (44%) were male. The median age at admission of infants to the NICU was 4 days (range 1-43 days). The average length of stay in the NICU prior to sepsis episode was 6 days (range 2-30 days). All 200 septic newborns were treated with broad-spectrum antibiotics during the current hospitalization. For 73% of admitted infants, treatment with antibiotics was started in the first 48 hours of life. Of 200 infants with clinical criteria of sepsis 126 (63%) had a confirmed BSI (Figure 1).
Figure 1.
Study enrollment chart and pathogens recovered from blood cultures of 126 infants
The demographic and clinical characteristics of 200 admitted infants and their mothers are shown in Table 1. Infants with positive blood cultures were significantly more likely to have been more than seven days old (PR=1.52, 95% CI 1.24-1.85), had umbilical discharge (PR=2.25, 95% CI 1.82-2.77), and had mothers with positive Hepatitis B surface antibodies (anti-HBs) (PR=1.55, 95% CI 1.28-1.89), compared to infants with negative blood cultures. In multivariate analysis only umbilical discharge was a significant risk factor for having positive blood culture at admission to NICU (PR=2.25, 95% CI 1.82-2.77). Overall mortality rate was 34% (68 of 200 infants died).
Table 1.
Demographic and clinical characteristics of infants with clinical sepsis (N=200) and their mothers
| Variable | Overall (N=200) n (%) |
Positive blood culture (N=126) n (%) |
Negative blood culture (N=74) n (%) |
PR* (95% CI) | P value | |
|---|---|---|---|---|---|---|
|
Demographic and clinical characteristics of infants |
||||||
| Gender | ||||||
| Male | 88 (44) | 59 (47) | 29 (39) | 1.12 (0.91-1.38) | ||
| Female | 112 (56) | 67 (53) | 45 (61) | 1.00 | 0.29 | |
| Age at NICU admission | ||||||
| ≥7 days | 72 (36) | 58 (46) | 14 (19) | 1.52 (1.24-1.85) | ||
| < 7 days | 128 (64) | 68 (54) | 60 (81) | 1.00 | <.001 | |
| Birth weight, grams | ||||||
| ≤ 2500 | 40 (20) | 26 (21) | 14 (19) | 1.04 (0.80-1.34) | 0.77 | |
| > 2500 | 160 (80) | 100 (79) | 60 (81) | 1.00 | ||
| Apgar score | ||||||
| ≤6 | 64 (32) | 44 (35) | 20 (27) | 1.14 (0.92-1.41) | 0.25 | |
| > 6 | 136 (68) | 82 (65) | 54 (73) | 1.00 | ||
| Umbilical Discharge | ||||||
| Yes | 76 (38) | 73 (58) | 3 (4) | 2.25 (1.82-2.77) | <0.001† | |
| No | 124 (62) | 53 (42) | 71 (96) | 1.00 | ||
| Mothers' characteristics | ||||||
| Mother's Age | ||||||
| ≤ 18 | 32 (16) | 21 (17) | 11 (15) | 1.05 (0.80-1.38) | 0.74 | |
| > 19 | 168 (84) | 105 (83) | 63 (85) | 1.00 | ||
| Residence | ||||||
| Rural | 84 (42) | 52 (41) | 32 (43) | 0.97 (0.78-1.21) | 0.79 | |
| Urban | 116 (58) | 74 (59) | 42 (57) | 1.00 | ||
| Education | ||||||
| High school or lower | 121 (61) | 74 (59) | 47 (64) | 0.93 (0.75-1.15) | 0.50 | |
| College or higher | 79 (39) | 52 (41) | 27 (36) | 1.00 | ||
| Marriage status | ||||||
| Not married | 14 (7) | 9 (7) | 5 (7) | 1.02 (0.68-1.53) | 1.00† | |
| Married | 186 (93) | 117 (93) | 69 (93) | 1.00 | ||
| Tobacco use | ||||||
| Yes | 13 (7) | 8 (6) | 5 (7) | 0.98 (0.63-1.52) | 1.00† | |
| No | 187 (93) | 118 (94) | 69 (93) | 1.00 | ||
| Syphilis (TP antibody test) |
||||||
| Seropositive | 13 (7) | 10 (8) | 3 (4) | 1.24 (0.90-1.70) | 0.38† | |
| Seronegative | 187 (93) | 116 (92) | 71 (96) | 1.00 | ||
| Hepatitis B surface antibodies (anti-HBs) |
||||||
| Positive | 68 (34) | 56 (44) | 12 (16) | 1.55 (1.28-1.89) | <0.001 | |
| Negative | 132 (66) | 70 (56) | 62 (84) | 1.00 | ||
| Anti-HCV antibodies (ELISA) |
||||||
| Positive | 10 (5) | 7 (6) | 3 (4) | 1.12 (0.73-1.70) | 0.75† | |
| Negative | 190 (95) | 119 (94) | 71 (96) | 1.00 | ||
|
Pregnancy and delivery characteristics |
||||||
| First child | ||||||
| Yes | 41 (21) | 26 (21) | 15 (20) | 1.01 (0.78-1.31) | 0.95 | |
| No | 159 (79) | 100 (79) | 59 (80) | 1.00 | ||
| Prenatal Care | ||||||
| No | 24 (12) | 16 (13) | 8 (11) | 1.07 (0.79-1.45) | 0.69 | |
| Yes | 176 (88) | 110 (87) | 66 (89) | 1.00 | ||
| Premature delivery | ||||||
| Yes | 29 (15) | 21 (17) | 8 (11) | 1.18 (0.91-1.52) | 0.26 | |
| No | 171 (86) | 105 (83) | 66 (89) | 1.00 | ||
| Premature Membrane Rupture |
||||||
| Yes | 24 (12) | 12 (10) | 12 (16) | 0.77 (0.51-1.17) | 0.16 | |
| No | 176 (88) | 114 (90) | 62 (84) | 1.00 | ||
| Type of delivery | ||||||
| Caesarean section | 36 (18) | 23 (18) | 13 (18) | 1.02 (0.77-1.34) | 0.90 | |
| Vaginal | 164 (82) | 103 (82) | 61 (82) | 1.00 | ||
Prevalence ratio (PR) for comparison of infants with positive blood culture to the infants with negative blood culture;
Fisher's Exact Test
Laboratory Results
The microorganisms identified in blood cultures of 126 infants are shown in Figure 1. A total of 98 (78%) of 126 recovered isolates were Gram-negative organisms, and 28 (22%) isolates of 126 were Gram-positive. Klebsiella pneumoniae was the most common pathogen, accounting for 36 (29%) of 126 isolates, followed by Enterobacter cloacae – 19 (15%). The most common gram-positive microorganism was S. aureus – 15 (12%) of 126 isolates. Streptoccoccus agalactiae (GBS) was isolated from only 6 (5%) infants.
Susceptibility testing results are shown in Tables 2 and 3. Klebsiella species revealed high susceptibility to imipenem (98%), amikacin (98%), and ciprofloxacin (96%) and cephalosporins. Enterobacter isolates were susceptible to amikacin (96%), ciprofloxacin (96%), imipenem (96%), but less so to the cephalosporins. Forty percent of S. aureus isolates were methicillin resistant (MRSA) and all were susceptible to vancomycin. E.coli strains were suceptible to ciprofloxacin (91%), carbapenems (91% to imipenem), cefepime (91%), and cextriaxone (91%) but only 45% susceptible to amoxicillin and 64% to trimethoprim sulfamethoxazole. Acinetobacter baumannii showed decreased susceptibility to carbapenems (86% to imipenem) and amikacin (86%). P. aeruginosa revealed decreased susceptibility to cefepime (83%), ceftazidime (83%), carbapenems (83% to imipenem), piperacillin/tazobactam (83%), ciprofloxacin (83%). Susceptibility of P. aeruginosa was low to gentamicin (33%).
Table 2.
Results of susceptibility testing for Gram-negative isolates (N=92*).
| Antimicrobials |
Klebsiella spp. (N=45), n (%) susceptible |
Enterobacter spp. (N=23), n (%) susceptible |
E.coli (N=11), n (%) susceptible |
Acinetobacter baumannii (N=7), n (%) susceptible |
Pseudomonas spp. (N=6), n (%) susceptible |
|---|---|---|---|---|---|
| Beta-lactam | |||||
| Ampicillin/ Sulbactam |
*** | *** | *** | 5 (71) | *** |
| Amoxicillin | 1 (2) | 1 (4) | 5 (45) | *** | *** |
| Amoxicillin clavulanate |
34 (76) | *** | 7 (64) | *** | *** |
| Ticarcillin/ Clavulanate |
37 (82) | 13 (57) | 8 (73) | 4 (57) | 5 (83) |
| Piperacillin/ Tazobactam |
38 (84) | 15 (65) | 10 (91) | 4 (57) | 5 (83) |
| Cefazolin | 34 (76) | *** | *** | *** | *** |
| Cefepime | 40 (89) | 19 (83) | 10 (91) | 4 (57) | 5 (83) |
| Cefotaxime | 38 (84) | 16 (70) | 10 (91) | 2 (29) | 5 (83) |
| Ceftazidime | 37 (82) | 15 (65) | 10 (91) | 4 (57) | 5 (83) |
| Ceftriaxone | 40 (89) | 15 (65) | 10 (91) | 2 (29) | 5 (83) |
| Imipenem | 44 (98) | 22 (96) | 10 (91) | 6 (86) | 5 (83) |
| Non-beta-lactam | |||||
| Amikacin | 44 (98) | 22 (96) | 10 (91) | 6 (86) | 4 (67) |
| Gentamicin | 40 (89) | 21 (91) | 9 (82) | *** | 2 (33) |
| Tobramycin | 43 (96) | 21 (91) | 10 (91) | 5 (71) | 3 (50) |
| Ciprofloxacin | 43 (96) | 22 (96) | 10 (91) | 5 (71) | 5 (83) |
| Moxifloxacin | 43 (96) | 22 (96) | 10 (91) | 5 (71) | 5 (83) |
| Tetracycline | 34 (76) | 16 (70) | 8 (73) | 4 (57) | *** |
| TMS-SMX | 22 (49) | 15 (65) | 7 (64) | 3 (43) | *** |
Note.
Susceptibilities for Acinetobacter iwoffi (N=2), Burkholderia cepacia (N=2), Serratia marcescens (N=1), Salmonella choleraesuis (N=1) were not tested because of small numbers of isolates.
not tested for susceptibility to this antibiotic/antimicrobial.
TMS-SMX - Trimethoprim sulfamethoxazole
Table 3.
Results of susceptibility testing for Gram-positive isolates (N=28).
| Antimicrobials |
S.aureus (N=15), n (%) susceptible |
S.epidermidis (N=7), n (%) susceptible |
S.agalactiae (N=6), n (%) susceptible |
|---|---|---|---|
| Beta-lactam | |||
| Oxacillin | 9 (60) | 3 (43) | *** |
| Amoxicillin | 5 (33) | 1 (14) | *** |
| Amoxicillin clavulanate |
6 (40) | 3 (43) | *** |
| Piperacillin/ Tazobactam |
11 (73) | 3 (43) | *** |
| Cefotaxime | *** | *** | 5 (83) |
| Ceftriaxone | 13 (87) | 6 (86) | 5 (83) |
| Imipenem | 14 (93) | 7 (100) | 6 (100) |
| Meropenem | 14 (93) | 7 (100) | 6 (100) |
| Non-beta-lactam | |||
| Ciprofloxacin | 13 (87) | 4 (57) | 5 (83) |
| Moxifloxacin | 13 (87) | 4 (57) | 5 (83) |
| Clindamycin | 11 (73) | 2 (29) | 4 (67) |
| Erythromicin | 10 (67) | 2 (29) | 3 (50) |
| Tetracycline | 9 (60) | 5 (71) | 4 (67) |
| Rifampin | 11 (73) | 6 (86) | 5 (83) |
| SMX-TM | 13 (87) | 3 (43) | *** |
| Vancomycin | 15 (100) | 7 (100) | 5 (83) |
Note.
- not tested for susceptibility to this antibiotic/antimicrobial.
SMX-TM - Trimetoprim sulfametoxazol.
Discussion
Since gaining independence in 1991, the ex-Soviet republic of Georgia has suffered severe socio-economic deterioration and civil unrest that has significantly affected the healthcare system.12-14 For example, blood cultures were rarely trusted in the standardized care of febrile or septic patients due to the questionable quality of affordable reagents and inability to process specimens using developed country standards. Instead, with a lack of microbial susceptibility data, patients are treated empirically with broad spectrum antibiotics. Infection control practices are also underdeveloped and are known to contribute to the risk of antibiotic resistance. Our study is the first published study from Georgia to report the bacterial etiology of neonatal sepsis, and to identify the antimicrobial susceptibility of these organisms.
It is known that the organisms associated with neonatal infection change over time and are different in different geographic areas thus reinforcing the need for local microbiological surveillance to guide empiric antimicrobial therapy and to recognize local epidemics and ultimately to help with overall quality improvement of neonatal care. Neonatal sepsis in our studied population was caused in 78% of cases by gram-negative bacteria (Klebsiella pneumonia, Enterobacter spp., E. coli, etc). Klebsiella pneumoniae was the most commonly isolated pathogen (29%) thus implicating these infections are hospital acquired versus maternally transmitted. Forty percent of S. aureus isolates were MRSA. These data are consistent with other reviews of neonatal sepsis in developing countries where gram negative infections are more frequently isolated, especially Klebsiella, than are gram-negative organisms.4,15 A review by Zaidi4 demonstrated that of 11, 471 bloodstream samples throughout the developing world 60% BSI were caused by gram-negative organisms with Klebsiella pneumoniae accounting for 23%, Pseudomonas 7%, Acinetobacter 3.5% and other gram-negatives 14%. It is well recognized that GBS is an uncommon pathogen in neonatal sepsis in many parts of the world while it is the most common bacterial pathogen associated with early-onset neonatal sepsis in developed countries.15
The patterns and rates of resistance for the gram negative organisms and for MRSA in this study are surprisingly high and cause a great deal of concern with respect to infection control practices and antibiotic prescribing practices. Relatively high resistance of P. aeruginosa strains against cephalosporins (cefepime and ceftazidime) was observed and Acinetebacter spp. demonstrated extensive resistance again implicating that the transmission of these organisms is likely hospital acquired. Enterobacter infections are a known surrogate in the NICU for nosocomial transmission.6 Also, the gram-negatives Klebsiella, Pseudomonas, and Acinetobacter are “water-bugs” and can cause common-source outbreaks because they can live in multi-use medication vials, soap and inadequately processed equipment.4 The current standard practice of using empiric ampicillin and gentamicin for suspected neonatal sepsis needs further critical appraisal as this combination has historically been used to target GBS, E. coli and Listeria. However, outside the developed world these are often not the offending organisms and this standard regimen may not be active in the face of growing drug resistance seen primarily among gram-negative organisms.
The epidemiology of BSI in neonatal intensive care units in Georgia has not been previously reported in the literature, nor any hospital based BSI data. The extent of hospital acquired infections in Georgia and other former Soviet states is not well known. There is a paucity of data in the medical literature on hospital acquired infections in the former Soviet states. A recent observational study of postoperative infection rates revealed that 17% of operations in Georgia were complicated by infections.16 Understanding the epidemiology of BSI is a key component in decreasing of neonatal mortality. This knowledge can lead to the development of better medical practices, especially more appropriate choices for empiric antibiotic therapy.
The lack of culture driven antimicrobial therapy and limited, consistent infection control practices is likely responsible for the resistant gram negative BSI in this study. Next steps to further evaluate the scope of this problem include improved surveillance of hospital acquired infections, education regarding appropriate antibiotic prescribing and developing standard infection control practices. If this problem is not confronted directly, an epidemic of drug resistant hospital acquired infections in Georgia, and likely other former Soviet republics, could be developing.
Acknowledgements
Funding. This research was supported in part by the New York State International Training and Research Program grants, 1D43TW007384-01, 2D43TW000233-11, NIH Fogarty International Center, and National Institutes of Health/Fogarty International Center grants D43 TW007124 and D43 TW01042.
Footnotes
Conflict of Interest. There was no conflict of interest for all authors.
References
- 1.WHO, Neonatal and Perinatal Mortality . Country, Regional and Global Estimates. World Health Organization; 2006. [Google Scholar]
- 2.Lawn JE, Cousens S, Darmstadt GL, Paul V, Martines J. Why are 4 million newborn babies dying every year? Lancet. 2004;364(9450):2020. doi: 10.1016/S0140-6736(04)17511-9. [DOI] [PubMed] [Google Scholar]
- 3.2008 Web-site: http://www.unicef.org/infobycountry/georgia_statistics.html. Accessed March 12.
- 4.Zaidi AK, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA. Hospital-acquired neonatal infections in developing countries. Lancet. 2005;365(9465):1175–88. doi: 10.1016/S0140-6736(05)71881-X. [DOI] [PubMed] [Google Scholar]
- 5.Allegranzi B, Pittet D. Healthcare-associated infection in developing countries: simple solutions to meet complex challenges. Infect Control Hosp Epidemiol. 2007;28(12):1323–7. doi: 10.1086/521656. [DOI] [PubMed] [Google Scholar]
- 6.Hervas JA, Ballesteros F, Alomar A, Gil J, Benedi VJ, Alberti S. Increase of Enterobacter in neonatal sepsis: a twenty-two-year study. Pediatr Infect Dis J. 2001;20(2):134–40. doi: 10.1097/00006454-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Al-Zwaini EJ. Neonatal septicaemia in the neonatal care unit, Al-Anbar governorate, Iraq. East Mediterr Health J. 2002;8(45):509–14. [PubMed] [Google Scholar]
- 8.Ahmed AS, Chowdhury MA, Hoque M, Darmstadt GL. Clinical and bacteriological profile of neonatal septicemia in a tertiary level pediatric hospital in Bangladesh. Indian Pediatr. 2002;39(11):1034–9. [PubMed] [Google Scholar]
- 9.Aurangzeb B, Hameed A. Neonatal sepsis in hospital-born babies: bacterial isolates and antibiotic susceptibility patterns. J Coll Physicians Surg Pak. 2003;13(11):629–32. [PubMed] [Google Scholar]
- 10.Gastmeier P, Groneberg K, Weist K, Rüden H. A cluster of nosocomial Klebsiella pneumoniae bloodstream infections in a neonatal intensive care department: Identification of transmission and intervention. Am J Infect Control. 2003;31(7):424–30. doi: 10.1067/mic.2003.70. [DOI] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards . Performance standards for antimicrobial susceptibility testing. National Committee for Clinical Laboratory Standards; Wayne, PA: 1999. (NCCLS approved standard M100-S9). [Google Scholar]
- 12.Collins C. The Georgian healthcare system: Is it reaching the WHO health system goals? International Journal of Health Planning and Management. 2006;21:297–312. doi: 10.1002/hpm.853. [DOI] [PubMed] [Google Scholar]
- 13.Balabanova D, McKee M, Pomerleau J, Rose R, Haerpfer C. Health service utilization in the former Soviet Union: evidence from eight countries. Health Serv Res. 2004;39(6 Pt 2):1927–50. doi: 10.1111/j.1475-6773.2004.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamkrelidzae A, et al. Healthcare Systems in Transition. 2. Vol. 4. European Observatory on Health Care Systems; Georgia: 2002. [Google Scholar]
- 15.Stoll BJ. The global impact of neonatal infection. Clin Perinatol. 1997;24(1):1–21. [PubMed] [Google Scholar]
- 16.Brown S, Kurtsikashvili G, Alonso-Echanove J, Ghadua M, Ahmeteli L, Bochoidze T, Shushtakashvili M, Eremin S, Tsertsvadze E, Imnadze P, O'Rourke E. Prevalence and predictors of surgical site infection in Tbilisi, Republic of Georgia. J Hosp Infect. 2007;66(2):160–6. doi: 10.1016/j.jhin.2007.03.007. [DOI] [PubMed] [Google Scholar]