Abstract
Increased phosphoinositide turnover was first identified as an early signal transduction event initiated by cell surface receptors that were linked to calcium signaling. Subsequently, the generation of inositol 1,4,5-trisphosphate by phosphoinositide-specific phospholipase C enzymes was defined as the major link between inositide turnover and the cytosolic Ca2+ rise in response to external stimulation. However, in the last decades, phosphoinositides have been emerging as major regulatory lipids involved in virtually every membrane-associated signaling process. Phosphoinositides regulate both the activity and the trafficking of almost all ion channels and transporters contributing to the maintenance of the ionic gradients that are essential for the proper functioning of all eukaryotic cells. Here we summarize the various means by which phosphoinositides affect ion channel functions with special emphasis on Ca2+ signaling and outline the principles that govern the highly compartmentalized roles of these regulatory lipids.
INTRODUCTION
The requirement of calcium ions for contractility of the heart was recognized by Sydney Ringer in 1883 marking the beginning of the development of our understanding of the role of Ca2+ in muscle contraction (see [1] for historical details). Calcium since became known as one of the most universal intracellular signaling molecules that regulates virtually every aspect of a cell’s life and death. These not only include rapid processes such as contraction and secretion but also long-term responses such as regulation of metabolic enzymes and ultimately gene expression. To act as an effective intracellular signal cytosolic Ca2+ concentration ([Ca2+]i) must be kept at a low (~100 nM) resting level, but also needs to rapidly rise to high levels (up to 10–100 μM) and quickly return to baseline. Therefore, the delicate control of cytoplasmic Ca2+ concentration has been a high priority during evolution. The source of Ca2+ for the [Ca2+]i increase, in most cases, is the extracellular fluid but cells can also use Ca2+ stored in organelles, a mechanism highly evolved in skeletal muscle. Rapid release of Ca2+ from intracellular stores [mostly the endoplasmic reticulum (ER)] is a general mechanism to rapidly elevate cytosolic Ca2+, but increased influx of Ca2+ is usually necessary to elicit a full biological response.
The mechanism of Ca2+ signal generation in so-called non-excitable tissues has become a center of interest when a group of hormones and neurotransmitters acting on cell surface receptors was found to activate cells without production of cAMP, the then recently discovered “second messenger” (see [2]). These stimuli termed “calcium-mobilizing agonists” were often linked with cGMP production and increased turnover of phosphatidylinositol (PtdIns) and both Ca2+ release and influx responses [3]. For a period, it was believed that the source of the internal Ca2+ release was the mitochondria, an organelle known for its ability to take up and release significant amounts of Ca2+ [4]. Two major discoveries have finally provided with an explanation of how the Ca2+ signal was generated. First, it was recognized that PtdIns(4,5)P2 breakdown by PLC in the plasma membrane (PM) is the first step in the increased turnover of PtdIns after hormonal stimulation [5, 6], and second, it was demonstrated that one of the products of this reaction, Ins(1,4,5)P3 was capable of releasing Ca2+ from non-mitochondrial internal Ca2+ stores [7]. With the finding of the Ins(1,4,5)P3 receptors (InsP3Rs) in the ER [8] and identifying them as Ca2+ release channels [9], the link between PtdIns turnover and Ca2+ release has been established.
Finding the mechanism responsible for the subsequent Ca2+ influx has proven to pose a greater challenge. In 1986, James Putney postulated that during stimulation of calcium mobilizing receptors, depletion of the ER Ca2+ stores was sufficient to activate Ca2+ influx without the need for any of the messengers formed by PLC action [10]. This mechanism has become known as store-operated Ca2+ entry (SOCE) and was believed not to depend on phosphoinositides other than indirectly through Ins(1,4,5)P3, a regulator of Ca2+ release from the ER. The nature of the channel responsible for SOCE remained elusive and for several years TRPC channels had been the most favored candidates [11]. TRP channels are non-specific cation channels first identified in Drosophila eye as the proteins responsible for a characteristic light-induced change in the membrane potential (transient receptor potential) in electric recordings from the eye [12]. After cloning of several similar channels from mammalian sources [13], research on TRP have dominated the field of SOCE [11]. However, the ion selectivity and I/V profile of TRP channels in electrical recordings did not match those of ICRAC, the electrophysiological correlate of SOCE previously identified in mast cells and T-cells [14, 15], both of which display massive SOCE, questioning whether TRP channels were responsible for the Ca2+ influx in these cells. The other unsolved question was the means by which the decreased luminal ER Ca2+ ([Ca2+]ER) is communicated to the PM to activate Ca2+ entry. The most accepted model termed “conformational coupling” assumed some sort of molecular proximity between the ER and the PM, where ER-resident proteins could regulate PM ion channels by direct interaction [16], although the existence of a diffusible messenger has been also considered [17]. The final answers to these questions were found recently when the ER proteins, STIM1 and -2, were discovered as the ER Ca2+ sensors and the Orai1/CRACM proteins as essential component of SOCE and expression of these two proteins reconstituted both ICRAC and SOCE (see[18–20]. However, it should be noted that SOCE may not be exclusively attributed to the Orai channels, as recent evidence suggests that STIM1 can also communicate to TRPC proteins [21, 22] and that elimination of either Orai1 or TRPC channels can decrease the native SOC pathway in some cells [23].
Although the link between SOCE and phosphoinositides has been firmly established (via Ins(1,4,5)P3 production) several studies suggested that a variety of other ion channels and transporters can also be regulated by PLC-coupled receptors and ultimately by membrane phosphoinositides (see [24, 25]). Therefore, the phosphoinositide-regulation of ion channel and membrane transport activities has emerged as a research topic parallel to the questions on SOCE and became an important new aspect of neuroscience and cell biology [26, 27]. A third thread of research converging on this subject matter originated from the questions of how newly synthesized channels are delivered to the PM and whether the channels were active within the internal membranes en route to their final destination in the PM (see [28]). Even more importantly, the removal and insertion of ion channels using internalization and recycling machineries of the cells, was recognized as a way of rapidly regulating the number of channels available in the PM. These processes linked ion channels to the general questions of cell biology namely membrane assembly and movements within the cells. In this review we will try to highlight some examples of the numerous distinct ways of regulation of ion channels by phosphoinositides. Because of the extensive literature in each of these topics, this review will not provide detailed in depth discussion available in several comprehensive reviews, but will attempt to emphasize the important overlaps between these otherwise disparate research areas that all relate to some aspect of Ca2+ signaling.
PHOSHOINOSITIDE REGULATION OF CALCIUM SIGNALING
Regulation of the SOCE pathway by phosphoinositides
With the recent discovery of the STIM and Orai/CRACM proteins, the basic mechanism by which Ca2+ depletion of the ER leads to Ca2+ influx has been largely uncovered [18–20]. As detailed in several recent reviews [19, 29, 30], the ER-resident single transmembrane protein STIM1 undergoes oligomerization upon Ca2+ unbinding in its Ca2+ sensing luminal EF-hand domain. This oligomerization induces an interaction between the ER-bound STIM1 protein and the Orai/CRACM channels located in the PM, leading to the opening of the latter and stabilization of ER-PM contact sites [31, 32]. STIM1 contains a polybasic sequence at its very C-terminus and its similarity to polybasic sequences found in other proteins that interact with negatively charged membrane phospholipids raised the possibility that STIM1-PM interactions are also facilitated by anionic phospholipids [31]. However, STIM1 constructs lacking the polybasic domain still can respond to Ca2+ depletion with oligomerization and activate Orai1-mediated Ca2+ influx indicating, that polybasic domains are not essential for SOCE [22, 33, 34].
Nevertheless, the pivotal role of the luminal ER [Ca2+] in the control of SOCE together with the central importance of Ins(1,4,5)P3 in the regulation of Ca2+ release from the ER, tightly links SOCE to Ins(1,4,5)P3 formation (Fig. 1A). Any intervention that reduces the level of Ins(1,4,5)P3 – (such as inhibition of PLC, or depletion of its substrate, PtdIns(4,5)P2), immediately reduces SOCE provided that the ER Ca2+ uptake mechanism is functional. This was demonstrated recently when rapid elimination of the PM PtdIns(4,5)P2 led to a quick reversal of SOCE activation by a Ca2+-mobilizing agonist [35]. Similarly, several reports have shown that inhibition of the phosphatidylinositol 4-kinase(s) (PI4Ks) that supply the PM with PtdIns4P [and hence PtdIns(4,5)P2] leads to a depletion of PtdIns(4,5)P2 and a diminishing Ins(1,4,5)P3 generation in cells stimulated by Ca2+-mobilizing agonists (e.g. [36, 37] ultimately resulting in an inhibition of SOCE. These results, however, did not tell whether SOCE itself requires phosphoinositides. This question can only be studied if SOCE is activated without Ins(1,4,5)P3, namely by inhibition of the SERCA Ca2+ pump [by thapsigargin or the reversible inhibitor 2,5-di-(tert-butyl)-1,4-hydroquinone, TBHQ]. A few studies have investigated this question. Broad et al [38] has reported that high concentration of the PI3K inhibitors wortmannin (Wm) and LY294002 inhibited SOCE and endogenous ICRAC in rat basophilic leukemia cells. They showed that this inhibition was not caused by the lack of InsP3 or DAG and it correlated better with changes in PtdIns4P than in PtdIns(4,5)P2 levels [38]. These authors also showed that the PLC inhibitor, U73122 also inhibited the SOCE and ICRAC but only if added before its activation and the drug failed to close SOCE once it got activated. Rosado et al. also analyzed the effects of LY294002 on SOCE in platelets and concluded that the inhibitory effect was not due to depolarization [39]. The interpretation of the experiments is complicated by the fact that Wm at concentrations used in these studies (but not LY294002) also inhibits MLCK, and MLCK inhibitors have been shown to inhibit SOCE [40]. This question was recently reexamined in our group using overexpressed STIM1 and Orai1 proteins. We found that the movements of STIM1 were slightly impaired in cells acutely depleted in PtdIns(4,5)P2 but this had no impact on SOCE. However, PtdIns4P depletion either by using PI3K inhibitors at concentrations that inhibit type-III PI4Ks, or by PLC activation by agonists had a significant inhibitory effect on SOCE. These inhibitory effects correlated with changes in PtdIns4P rather than PtdIns(4,5)P2, and were not related to STIM1 movements. (Korzeniowski et al. submitted). These studies raise the possibility that Orai1 activation requires PtdIns4P generation. These findings are even more intriguing as the PI4K that is responsible for the generation of the PM pool of PtdIn4P is mostly ER localized in mammalian cells [41]. This makes the ER-PM contact sites stabilized by STIM1-Orai1 interaction a special cellular compartment potentially important for lipid transfer between the two membranes.
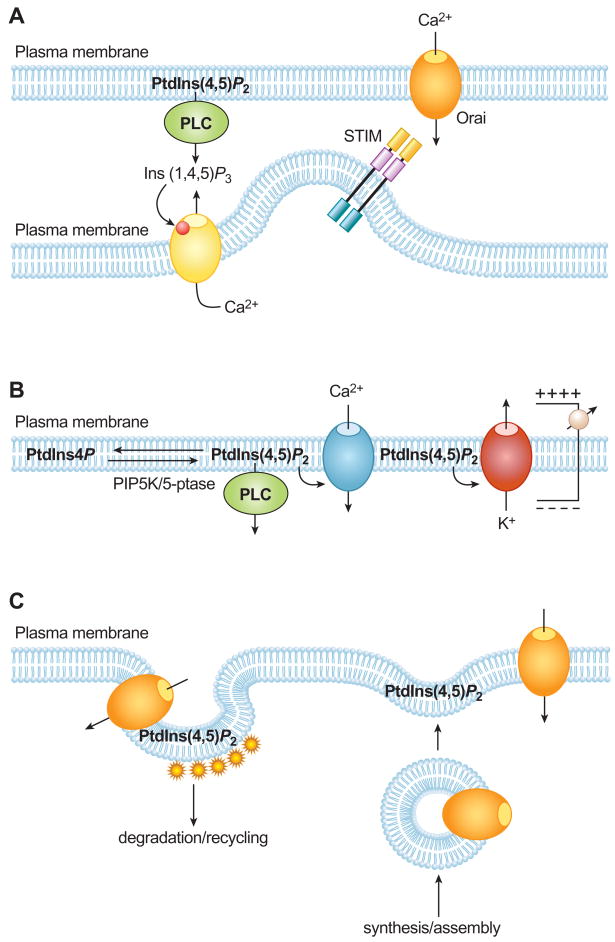
Figure 1.
Different forms of regulation of ion channels relevant to Ca2+ signaling. (A) In the canonical phosphoinositide signaling pathway, phospholipase C (PLC) activation from cell surface receptors leads to the generation of Ins(1,4,5)P3, which, in turn, releases Ca2+ from the endoplasmic reticulum (ER) Ca2+ stores via Ins(1,4,5)P3 receptor-channels. The decreased ER luminal Ca2+ is sensed by the ER resident STIM proteins that respond with oligomerization and interaction with PM (PM) Orai/CRACM channels at ER-PM junctional sites activating Ca2+ influx. This form of Ca2+ entry is indirectly linked to Ins(1,4,5)P3 production, but direct regulation of the Orai channels and possibly STIM interaction with the PM by phosphoinositides has also been proposed. (B) Direct regulation of ion channel (and transporter) activities by PM phosphoinositides. Here the lipid directly interacts with some molecular component of the channel usually by electrostatic interaction involving positively charged regions of the channel facing the cytoplasm. Phosphoinositide levels can change as a result of PLC activation, or due to a shift in the balance of 5-phosphatase and PIP 5-kinase ctivities. Channels with the highest affinity to inositides may not respond to decreasing inositide levels if they tightly hold on to their bound lipid, therefore, the same lipid change can evoke a variety of activity changes depending on the lipid affinities of the channels. Potassium channels are also known to be sensitive to phosphoinositide changes and because they have significant impact on the membrane potential, they can indirectly affect Ca2+ entry by changing the electrochemical driving force of Ca2+. This is especially important for voltage-gated Ca2+ channels or for the inwardly rectifying ICRAC channels. (C) Ion channels are also moved around within the cells as a result of membrane trafficking. The synthesis and assembly of channels usually occures in the ER and the channels are glycosylated in the Golgi from where they are heading their final destination, the PM. It is now believed that most channels are not active within the internal membranes, although there are examples suggesting otherwise. PtdIns(4,5)P2 is present in the PM and channels and transporters that require this lipid for activity gain functionality only upon insertion in the PM. In the opposite process, active channels can be removed from the PM by various interanlization routes, some clathrin-dependent, some –independent. Internalized channles are moved into recycling compartments from where they can recycle back to the membrane or head to degradation. All of these trafficking events are regulated by phosphoinositides.
Regulation of Ca2+ transport by the plasma membrane phosphoinositides
The first reports on a direct role of membrane PtdIns(4,5)P2 on a calcium transport system was the finding of a stimulatory effect of acidic phospholipids on the PM Ca2+ pump that was similar to those elicited by calmodulin or limited proteolysis [42]. Similarly, purified sarcoplasmic reticulum Ca2+ ATPase was shown to contain tightly associated phosphoinositides suggesting a possible regulatory interaction between the Ca2+ pump and these phospholipids [43]. However, fewer studies addressed whether the lipid regulation shown in isolated membranes or purified proteins does play a role in an intact cell system. Our studies using Wm at concentrations that inhibit type-III PI 4-kinases in combination with agonist-induced PLC activation showed that cells depleted in PtdIns(4,5)P2 still can reduce their [Ca2+]i very effectively with Ca2+ extrusion [44]. On the other hand, LY294002 was shown to inhibit Ca2+ extrusion in platelets at concentrations that also inhibit PI 4-kinases and perhaps other enzymes [45].
Although the effect of anionic phospholipids on the Na+/Ca2+ exchange activity in reconstituted systems have been described earlier [46], Donald Hilgemann was the first to demonstrate that the effects of ATP on the Na+/Ca2+ exchanger (NCX1) and on KATP potassium channels in giant excised patches of the heart were mediated by the synthesis of anionic phospholipids, mainly PtdIns(4,5)P2 [47]. However, the changes in phosphoinositides in the heart are not particularly robust after hormonal influences and it is still puzzling whether Na+/Ca2+ exchange activities are controlled by changing PtdIns(4,5)P2 levels or the high PtdIns(4,5)P2 of the PM is required as a permissive environment restricting the transport activity of the NCX1 protein to the PM [48]. It is noteworthy, though, that Ca2+-calmodulin has a strong stimulatory effect on ATP-induced PtdIns(4,5)P2 (but not PtdIns4P) synthesis in crude cardiac membranes [49] and osmotically-induced shrinkage increases both PtdIns4P and PtdIns(4,5)P2 in several cell types [49]. However, whether PtdIns(4,5)P2 levels change during contraction and relaxation in the heart is yet to be determined.
The reversal of the inactivation (termed run-down) of the Na+/Ca2+ exchanger or the KATP potassium channel in excised macropatches by PtdIns(4,5)P2 and ATP (via synthesis of PtdIns(4,5)P2)[47] has established an experimental paradigm that was followed by a large number of studies revealing the PtdIns(4,5)P2 requirement of several ion channels [24, 25]. These included potassium channels of a great variety (see below) as well as calcium conductive channels, such as the voltage-gated P/Q [50] and N-type [25, 51] Ca2+ channels as well as several members of the TRP family of non-selective cation channels [52]. Recently this list was extended to some of the ligand-gated channels such as the NMDA receptors [53] and the ATP-gated P2X4 [54]. In almost all of these studies the question was raised whether PtdIns(4,5)P2 is merely a requirement for the proper functioning of the channels or it is actually regulated by PtdIns(4,5)P2 changes that occur during activation of PLC-coupled receptor mechanisms. This question has been unequivocally answered in some cases, such as the M-current (mediated by the KCNQ potassium channels) [55, 56] or the cold and menthol sensitive TRPM8 channels [57, 58]. The activity of the KCNQ channels follows very tightly the changes in PM PtdIns(4,5)P2 so much so that it can be used as a PtdIns(4,5)P2 sensor [55]. However, there are other examples where the role of PtdIns(4,5)P2 is more controversial, being reported as inhibitory as well as a stimulatory factor. For example, an apparently opposing effects of PtdIns(4,5)P2 are thought to depend on the levels of the lipid in the case of P/Q channels. It was proposed that a high affinity lipid binding site site stabilizes the channel, whereas binding of PtdIns(4,5)P2 to a putative lower affinity site would switch the channel into a “reluctant” mode [51]. In another example, TRPV1 channels were shown to be sensitized by PLC-coupled agonists apparently via reduction of PtdIns(4,5)P2 [59] but the same lipid was found to be required for the recovery of TRPV1 channels from desensitization [60]. These seemingly contradictory findings were resolved when it was shown that PtdIns(4,5)P2 depletion contributes to the desensitization of the TRPV1 channels at high capsaicin concentration, but at low capsaicin doses PtdIns(4,5)P2 inhibited the same channels [61]. The inositol lipid regulation of TRPM8 and TRPV1 channels are discussed in detail in this issue by Tibor Rohacs.
The mechanism of direct inositol lipid regulation of these Ca2+ conductive channels remains elusive (Fig. 1B). Almost all of these channels contain clusters of basic residues in their membrane-adjacent regions facing the cytosol or within their C-terminal tails. In some TRP channels there are sequences identified as “half PH domains” that can form intermolecular PH domains potentially binding phosphoinositides [62], but lipid regulation was also mapped to the TRP domains of their cytoplasmic tails [57, 63]. An interesting and common feature of the PtdIns(4,5)P2 regulation of many potassium and TRP channels is that the lipid alters the interaction of the channels with other specific regulators. In several instances this regulator is calmodulin, which competes with PtdIns(4,5)P2 such as in TRPC6, TRPV1, voltage-gated Ca2+ channels and KCNQ potassium channels [64]. Other examples include PtdIns(4,5)P2 modulation of the interaction of Kir3 potassium channels with βγ-subunits [65], or the PtdIns(4,5)P2-mediated decrease of the apparent ATP affinity of KATP channels [66]. The direct interaction of phosphoinositides with ion channels whether regulatory or permissive is a fascinating research area and adds to the complexity by which phosphoinositide changes can influence the ionic homeostasis of the cells.
Regulation of the removal or insertion of channels in the plasma membrane by phosphoinositides
Delivery of ion channels and transporters from a reserve pool found in subplasmalemmal vesicles could be a mechanism by which the number of channels can be rapidly increased in the PM. Conversely, channels can be rapidly moved from the membrane by endocytosis to decrease their number in the membrane (Fig. 1C). In addition, the constitutive trafficking of Ca2+ channel subunits to the site of their assembly is important to supply the PM with functional channels. Regulation of membrane trafficking and fusion events, therefore, can indirectly influence ion fluxes through the membrane. Since phosphoinositides play important roles in both exo- and endocytosis [67], they provide an additional means to channel regulation.
PI3K-dependent trafficking of voltage-gated Ca2+ channels to the PM has been recently reported in myoblasts and COS-7 cells [68]. This is mediated by PtdIns(3,4,5)P3 induced Akt activation and phosphorylation on a Ser residue of the β2a subunit of the N-type Ca2+ channels [68]. An IGF1-induced PI3K-dependent increase of high voltage-activated Ca2+ channels in cerebellar neurons has also been described [69]. Another well-documented example of regulated channel insertion into the PM is the epithelial sodium channel (ENaC) (even though this is not directly related to Ca2+ signaling), a channel that also is regulated by PtdIns(4,5)P2 once in the PM [70]. ENaC channels rapidly incorporate into the apical membrane by a Rho and PIP 5-kinase-dependent mechanism [71], whereas their removal from the membrane and degradation is inhibited by a PI3K-mediated mechanism [72].
There are other documented examples of the phosphoinositide-regulation of trafficking of TRP channels [28]. A curious stimulatory effect of PLCγ1 expression on the activity of TRPC3 channels (which did not require the PLC activity of the protein) has been described [73]. This was attributed to the enhanced surface expression of the channels aided by an intermolecular PH domain formation between the 1/2 PH-domains of PLCγ1 and the other half within the intracellular tails of TRPC channels and the presumed inositol lipid binding of the hybrid PH domain [62]. It has been shown recently that growth factor stimulation enhances the insertion of TRPC5 channels from vesicular pools by a PI3K and Rac1 mediated mechanism that also involves PIP5KIα [74]. Similarly, EGF-stimulated insertion of TRPC4 channels into the PM was also reported. Channels of other TRP subfamilies, including TRPV2 and TRPV1 were also shown to translocate to the PM following NGF or IGF-1 stimulation [75, 76]. One of the most prominent examples of channel regulation by insertion into and removal from the PM is found in Drosophila eye, where the TRP-like, or TRPL channel was shown to undergo a light-induced internalization into a storage compartment from which the channel gets reinserted to the photoreceptor membrane during recovery in the dark [77, 78].
Several studies have shown the removal of ion channels from the PM as a way of reducing channel activity. The Kir1.1 K+ channels (also known as ROMK) in the kidney are down regulated by clathrin-dependent endocytosis during dietary potassium restriction by a mechanism involving the endocytic adaptor protein, ARH and the “NPXY” internalization motif present in these channels [79]. Overexpression of PI 4-kinase and PIP 5-kinase significantly decreased rather than increased the current densities of NXC1, and a targeted expression of PI4KIIα in the heart of transgenic mice resulted in a large decrease of NXC1 current density without a change in expression levels and an increased rate of clathrin-mediated endocytosis [80]. These data strongly suggested that the effects of phosphoinositides on the endocytosis and recycling of these channels are dominant during long-term experiments.
These examples demonstrate that the acute regulation of channel activity in the PM by phosphoinositide changes is complemented with phosphoinositide control of the trafficking and distribution of these channels between the various membranes, adding a new level of complexity to the control of Ca2+ influx into the cell.
Regulation of Ca2+ influx by phosphoinositides indirectly by the changing membrane potential
The amount of Ca2+ entering a cell through various Ca2+ conductive channels is primarily determined by the open and closed state of the channel pores. Activation and inactivation of voltage gated Ca2+ channels are clearly regulated by the membrane potential, but even channels that are not gated by voltage are sensitive to the membrane potential as the amount of Ca2+ flowing through them depends on the electrochemical gradient of Ca2+. This is especially true for the ICRAC channels that show a pronounced inward rectification and hence this form of Ca2+ entry is very sensitive to depolarization [81]. It is because of this feature of Ca2+ entry why phosphoinositide-regulation of the various potassium channels is relevant to Ca2+ signaling. Potassium channels of a great variety show a wide range of functions. Inwardly rectifying K+ (Kir) channels regulate the pattern of firing of action potentials, contribute to metabolic regulation (via control of insulin secretion) or determine potassium homeostasis (ROMK). A large family of two-pore K+ channels serve as “background channels” determining the basal membrane potential in many neurons and neuroendocrine tissues [82], while members of the KCNQ family of K+ channels underlie the M-current (KCNQ2, -3, -5) found in neurons as well as the I(Ks) current (KCNQ1) of cardiac tissues [83]. As outlined above, all these K+ channels show a dependence and/or regulation by PM phosphoinositides that have been discussed in many recent reviews and will not be further detailed here [84]. However, it is important to keep in mind that phosphoinositide changes can have profound influence on both the basal membrane potential or on the shape of action potentials via their influence on these K+ channels, which, in turn, affects Ca2+ signaling (Fig. 1B). Such an indirect effect should not be mistaken with the direct regulation of Ca2+ conductive channels by phosphoinositides.
ORGANIZATION OF LOCALIZED CHANGES AND MICRODOMAINS IN PHOSPHOINOSITIDE REGULATION
The extent to which phosphoinositide regulation is compartmentalized is being increasingly recognized. The PM has long been considered as the relevant site of polyphosphoinositide synthesis and PLC-mediated hydrolysis from a Ca2+ signaling standpoint. The only question regarding compartmentalization was how the ER-synthesized PtdIns reaches the PM to serve as precursor for polyphosphoinositide synthesis leading to the identification of the PI transfer proteins [85, 86]. This view has suddenly changed when the yeast PI 3-kinase, Vps34p and its lipid product, PtdIns3P were identified as key regulators of vacuolar sorting in yeast [87] and endocytic trafficking in mammalian cells [88]. This was followed by the surprising localization of several PI 4-kinase and phosphoinositide phosphatase isoforms in the Golgi and endocytic compartments [89–91] challenging our understanding of how PtdIns4P is made and reaches the PM in mammalian cells [92]. Therefore, when thinking of compartmentalization, most of us refer to the unique phosphoinositides composition of various intracellular membranes. While this view is certainly valid and important in defining the inositide signature of internal membranes, it is becoming evident that further compartmentalization of phosphoinositides takes place even within the PM, Golgi or any membrane within the cell.
Some of these compartments can be physically unique, such as the apparent enrichment of phosphoinositides in detergent resistant membrane domains often referred to as “rafts” [93]. Pike and Casey [94] suggested that agonist-sensitive phosphoinositides are associated with caveolin-rich rafts in A431 cells. Indeed, the type II PI 4-kinase was found in and purified from detergent resistant (though non-caveolar) membrane fractions [95]. Similarly, several important signaling molecules related to phosphoinositide synthesis and hydrolysis are present in “rafts” [96, 97] leading to the general notion that phosphoinositides are mostly “raft” associated. However, “raft” is a term that is not linked to any specific membrane (although most believe it is part of the PM) and thorough attempts using the recently available phosphoinositide visualization tools could not confirm that PtdIns(4,5)P2 are enriched in “rafts” in the PM [98]. Moreover, the majority of the PI4KIIα enzyme, mentioned above, has been localized to the TGN and endosomal membrane network as opposed to the PM [89–91], although, undoubtedly this activity is present in the PM (the best example being the red blood cell membrane). Nevertheless, the possibility still remains that some phsophoinositide regulation is linked to “rafts” and in this context it is relevant that among the Ca2+ conductive channels, the TRPC1 channels are enriched in caveoli [99] and are responsible to the cyclodextrin sensitivity of SOCE in some cell types [100].
Regardless of whether inositides are segregated in physically definable compartments, strong evidence suggests that phosphoinositides are functionally compartmentalized. On the one hand, this is a result of the interaction of phosphoinositides, such as PtdIns(4,5)P2 with multiple effector proteins (Fig. 2A). These protein-phosphoinositide interactions display a variety of affinities and dissociation rates. This implies that during activation of a PLC or phosphoinositide phosphatase enzyme, the decreasing free PtdIns(4,5)P2 level will not be uniformly followed by a similar decreases in the amount of PtdIns(4,5)P2 bound to various interacting molecules. Proteins that bind PtdIns(4,5)P2 with high affinity and slow dissociation rate may not “sense” immediately the decreasing lipid levels while those from which the lipid rapidly dissociates will “read” the free lipid changes very closely. This affinity-driven functional compartmentalization was shown to play a role for example in the differential inositide sensitivities of two-pore K+ channels [101]. Since the lateral diffusion of free phosphoinositides is fast, it is not possible to generate a sharp lipid gradient in any particular membrane without association of the lipids with proteins with slower lateral mobility. The dissociation rate of the lipid from the binding protein (the effector) together with the lateral mobility of the latter will determine how far the lipid can diffuse on the “back” of an effector molecule. This has been elegantly analyzed recently using pleckstrin homology (PH) domains with various affinities to PtdIns(4,5)P2 [102]. This mechanism, however, alone is probably not sufficient to generate functional inositide gradients. What is also needed is the highly localized production (or elimination) of the lipids in the close vicinity of the effector molecule. This model assumes that the inositol lipid kinases (and/or phosphatase) are in close association with the effector molecule (for this review, an ion channel) that is to be regulated by the lipid. This arrangement has several advantages: the localized lipid change can control the channel without any “spilling” effect on neighboring proteins, and the lipid binding specificity of the channel is less critical for the regulation. The specificity is provided by the kinase and phosphatase that is tightly associated with the signaling complex. Although this signaling organization represents the highest degree of specificity and is most likely widely used in biology, it is the most difficult to demonstrate experimentally. Such highly localized lipid changes dedicated to specific effector molecules will probably evade detection by the widely used GFP-PH domain lipid binding tools unless the probe originates from the very effector molecule itself. There are already few examples of the use of ion channels as reporters of phosphoinositide changes [55]. This organization explains why inositide kinases or phosphatases with apparently identical localization and biochemical activity can assume non-redundant functions. Recent examples are the distinct roles of two PIP 5-kinase isoforms, both localized in phagocytic cups, in actin polymerization and phagocytosis [103], or the non-redundant role of PI 4-kinase IIIβ and PI4KIIα, both localized to the Golgi, in supporting the transfer function of the ceramide transfer protein [104]. Although this level of specificity has not been clearly demonstrated for Ca2+ conductive channels there are some indications that such specific control mechanisms also exists in ion channel regulation. Interaction of ion channels with enzymes that control phosphoinositides has been reported. One of the TRP channels, TRPM7 (TRP-PLIK) was identified as an interactor with the C-terminal tail of PLCβ1 [105]. Subsequently it was shown that TRPM7 associates with PLCβ1 and is regulated by PLC-activating agonists [106], although the direction of regulation remains controversial [107]. In another example, proteomic analysis of proteins associated with the purinergic P2X7 channel has identified PI4KIIIα as part of the channel-associated complex [108]. The recently described voltage-regulated inositide 5-phosphatase isolated from ciona intestinalis (ci-VSP) also suggests that rapid localized changes in phosphoinositide levels regulated by voltage could be directly linked to ion channel function [109].
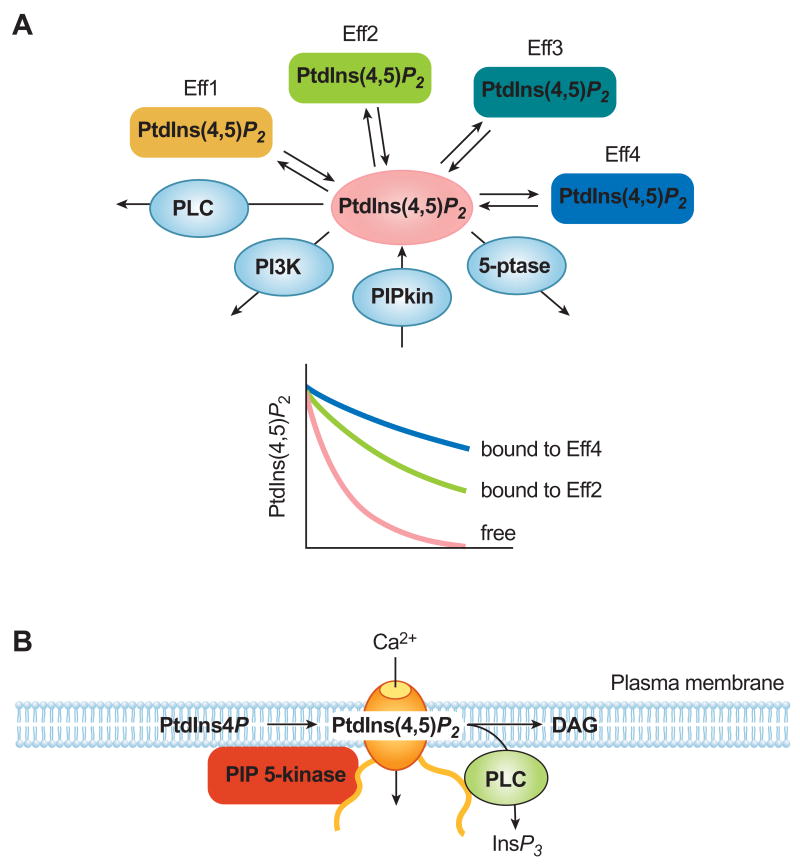
Figure 2.
Principles governing the compartmentalized regulation of ion channels (or other effectors) by phosphoinositides. (A) PtdIns(4,5)P2 in the plasma membrane exists in free form but is also bound to several proteins regulated by this lipid (effectors, Eff1-4). These could be channels, transporters, actin binding proteins, clathrin adaptors, enzymes and several other proteins. The enzymes that produce and convert these lipids [including PIP 5-kinases (PIPkin), phosphoinositide 5-phosphatases (5-ptase), Class I PI 3-kinases (PI3K) and phospholipase C (PLC) enzymes] act on the unbound fraction (in pink) which is in a dynamic equilibrium with the protein bound fractions (Eff1-4). Changes in the level of free PtdIns(4,5)P2 are reflected differently in the pools bound to the various effectors depending on their rates of dissociation. This represents a functional compartmentalization that does not necessarily mean a phisical segregation but may result in different metabolic turnover rates. (B) Theoretical example of the regulation of an effector (ion channel) by a phosphoinositide dedicated to the protein. Here the associated kinase or phosphatase ensures that the phosphoinositide is not diffusing away from the effector and does not contribute to the larger “shared” pool of the inositol lipid in question.
Unfortunately, it is extremely difficult to analyze the existence and the importance of direct interaction of inositide kinases or phosphatases with ion channels. RNAi-mediated depletion, or expression of dominant negative versions of these enzymes, has grave consequences on the vesicular trafficking of the cells likely altering the distribution of these channels. Interruption of the association between the two proteins or acute inhibition of the enzymes might be a better approach to analyze the significance of these protein-protein interactions leading to localized delivery of phosphoinositides to ion channels.
CONCLUDING REMARKS
Postulating and revealing the connection between phosphoinositides and calcium signaling [2, 110] have been one of the greatest milestones in the last 50 years in signal transduction research. Yet, none of us who witnessed these developments could have foreseen the complexity, depth and variety of inositide-regulation of cellular Ca2+ homeostasis. This short summary highlighted three aspects of Ca2+ channel regulation, namely the classical link between PLC-mediated Ins(1,4,5)P3 production, Ca2+ release and the store-operated Ca2+ entry, the direct effect of PM phosphoinositides on channel gating, and the regulation of channel trafficking by phosphoinositides. This, however, is an artificial separation, because these regulatory means are closely interrelated and work in concert all the time. Appreciating this complexity is crucial for the right interpretation of our experimental data. Often the only way to change phosphoinositides is to express or knock down the enzymes that either form them (the kinases) or eliminate them (the phosphatases). However, these manipulations require a long time period before the effects can be analyzed. During this time the assembly, trafficking or elimination of the channels may have a more important and lasting effects on apparent channel activity than the actual change in the phosphoinositides in the membrane. This is one of the many reasons why development of specific inositide kinase inhibitors is so desirable and also why we and others have been working on methods by which inositides can be changed within the intact cell acutely in a matter of seconds [56, 111, 112]. These new tools should facilitate our understanding on the importance of each of the inositide-dependent regulatory processes for each of the channels in a real cellular setting. Such studies are still needed because the fundamental question of what makes these lipids so versatile and universally adaptable signaling cues remains to be answered.
Acknowledgments
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller DJ. Sydney Ringer; physiological saline, calcium and the contraction of the heart. J Physiol. 2004;555:585–7. doi: 10.1113/jphysiol.2004.060731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michell RH. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975;415:81–147. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- 3.Michell RH. Inositol lipids and their role in receptor function: history and general principles. In: Putney JW Jr, editor. Phosphoinositides and Receptor Mechanisms. Alan R. Liss, Inc; New York: 1986. pp. 1–24. [Google Scholar]
- 4.Pozzan T, Magalhaes P, Rizzuto R. The comeback of mitochondria to calcium signalling. Cell Calcium. 2000;28:279–83. doi: 10.1054/ceca.2000.0166. [DOI] [PubMed] [Google Scholar]
- 5.Creba JA, Downes CP, Hawkins PT, Brewster G, Michell RH, Kirk CJ. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983;212:733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berridge MJ. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyze polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983;212:849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–68. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 8.Spät A, Bradford PG, McKinney JS, Rubin RP, Putney JW. A saturable receptor for 32P-inositol-1,4,5-trisphosphate in hepatocytes and neutrophils. Nature. 1986;319:514–516. doi: 10.1038/319514a0. [DOI] [PubMed] [Google Scholar]
- 9.Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- 10.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.Parekh AB, Putney JW., Jr Store-operated calcium channels. Phys Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 12.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–23. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 13.Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L. Trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 1996;85:661–671. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]
- 14.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 15.Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T-lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci USA. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berridge M. Conformational coupling: a physiological calcium entry mechanism. Sci STKE. 2004;2004:pe33. doi: 10.1126/stke.2432004pe33. [DOI] [PubMed] [Google Scholar]
- 17.Randriamampita C, Tsien RY. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993;364:809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- 18.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–7. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 19.Putney JW., Jr Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here) Cell Calcium. 2007;42:103–10. doi: 10.1016/j.ceca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soboloff J, Spassova MA, Dziadek MA, Gill DL. Calcium signals mediated by STIM and Orai proteins--a new paradigm in inter-organelle communication. Biochim Biophys Acta. 2006;1763:1161–8. doi: 10.1016/j.bbamcr.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 21.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–10. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 22.Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell. 2008;32:439–48. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MS, Zeng W, Yuan J, Shin DM, Worley P, Muallem S. Native store-operated Ca2+ influx requires the channel function of Orai1 and TRPC1. J Biol Chem. 2009 doi: 10.1074/jbc.M808097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE. 2001;2001:RE19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- 25.Gamper N, Shapiro MS. Regulation of ion transport proteins by membrane phosphoinositides. Nat Rev Neurosci. 2007;8:921–34. doi: 10.1038/nrn2257. [DOI] [PubMed] [Google Scholar]
- 26.Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–95. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cayouette S, Boulay G. Intracellular trafficking of TRP channels. Cell Calcium. 2007;42:225–32. doi: 10.1016/j.ceca.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Luik RM, Lewis RS. New insights into the molecular mechanisms of store-operated Ca2+ signaling in T cells. Trends Mol Med. 2007;13:103–7. doi: 10.1016/j.molmed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Hewavitharana T, Deng X, Soboloff J, Gill DL. Role of STIM and Orai proteins in the store-operated calcium signaling pathway. Cell Calcium. 2007;42:173–82. doi: 10.1016/j.ceca.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci U S A. 2007;104:9301–6. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–13. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Lu J, Xu P, Xie X, Chen L, Xu T. Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J Biol Chem. 2007;282:29448–56. doi: 10.1074/jbc.M703573200. [DOI] [PubMed] [Google Scholar]
- 34.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–43. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varnai P, Toth B, Toth DJ, Hunyady L, Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 Complex. J Biol Chem. 2007;282:29678–90. doi: 10.1074/jbc.M704339200. [DOI] [PubMed] [Google Scholar]
- 36.Nakanishi S, Catt KJ, Balla T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc Natl Acad Sci USA. 1995;92:5317–5321. doi: 10.1073/pnas.92.12.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willars GB, Nahorski SR, Challiss RA. Differential regulation of muscarinic acetylcholine receptor-sensitive polyphosphoinositide pools and consequences for signaling in human neuroblastoma cells. J Biol Chem. 1998;273:5037–46. doi: 10.1074/jbc.273.9.5037. [DOI] [PubMed] [Google Scholar]
- 38.Broad LM, Braun FJ, Lievremont JP, Bird GS, Kurosaki T, Putney JW., Jr Role of the phospholipase C-inositol 1,4,5-trisphosphate pathway in calcium release-activated calcium current and capacitative calcium entry. J Biol Chem. 2001;276:15945–52. doi: 10.1074/jbc.M011571200. [DOI] [PubMed] [Google Scholar]
- 39.Rosado JA, Sage SO. Phosphoinositides are required for store-mediated calcium entry in human platelets. J Biol Chem. 2000;275:9110–3. doi: 10.1074/jbc.275.13.9110. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe H, Tran QK, Takeuchi K, Fukao M, Liu MY, Kanno M, Hayashi T, Iguchi A, Seto M, Ohashi K. Myosin light-chain kinase regulates endothelial calcium entry and endothelium-dependent vasodilation. Faseb J. 2001;15:282–4. doi: 10.1096/fj.00-0587fje. [DOI] [PubMed] [Google Scholar]
- 41.Balla A, Ju Kim Y, Varnai P, Szentpetery Z, Knight Z, Shokat KM, Balla T. Maintenance of Hormone-sensitive Phosphoinositide Pools in the Plasma Membrane Requires Phosphatidylinositol 4-Kinase III{alpha} Mol Biol Cell. 2007;19:711–721. doi: 10.1091/mbc.E07-07-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niggli V, Adunyah ES, Carafoli E. Acidic phospholipids, unsaturated fatty acids, and limited proteolysis mimic the effect of calmodulin on the purified erythrocyte Ca2+-ATPase. J Biol Chem. 1981;256:8588–92. [PubMed] [Google Scholar]
- 43.Varsanyi M, Tolle HG, Heilmeyer MG, Jr, Dawson RM, Irvine RF. Activation of sarcoplasmic reticular Ca2+ transport ATPase by phosphorylation of an associated phosphatidylinositol. Embo J. 1983;2:1543–8. doi: 10.1002/j.1460-2075.1983.tb01621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakanishi S, Catt KJ, Balla T. Inhibition of agonist-stimulated inositol 1,4,5-trisphosphate production and calcium signaling by the myosin light chain kinase inhibitor, wortmannin. J Biol Chem. 1994;269:6528–6535. [PubMed] [Google Scholar]
- 45.Rosado JA, Sage SO. Regulation of plasma membrane Ca2+-ATPase by small GTPases and phosphoinositides in human platelets. J Biol Chem. 2000;275:19529–35. doi: 10.1074/jbc.M001319200. [DOI] [PubMed] [Google Scholar]
- 46.Vemuri R, Philipson KD. Phospholipid composition modulates the Na+-Ca2+ exchange activity of cardiac sarcolemma in reconstituted vesicles. Biochim Biophys Acta. 1988;937:258–68. doi: 10.1016/0005-2736(88)90248-9. [DOI] [PubMed] [Google Scholar]
- 47.Hilgemann DW, Ball R. Regulation of cardiac Na+, Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- 48.Nasuhoglu C, Feng S, Mao Y, Shammat I, Yamamato M, Earnest S, Lemmon M, Hilgemann DW. Modulation of cardiac PIP2 by cardioactive hormones and other physiologically relevant interventions. Am J Physiol Cell Physiol. 2002;283:C223–34. doi: 10.1152/ajpcell.00486.2001. [DOI] [PubMed] [Google Scholar]
- 49.Hilgemann DW. On the physiological roles of PIP(2) at cardiac Na+ Ca2+ exchangers and K(ATP) channels: a long journey from membrane biophysics into cell biology. J Physiol. 2007;582:903–9. doi: 10.1113/jphysiol.2007.132746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu L, Bauer CS, Zhen XG, Xie C, Yang J. Dual regulation of voltage-gated calcium channels by PtdIns(4,5)P2. Nature. 2002;419:947–52. doi: 10.1038/nature01118. [DOI] [PubMed] [Google Scholar]
- 51.Michailidis IE, Zhang Y, Yang J. The lipid connection-regulation of voltage-gated Ca(2+) channels by phosphoinositides. Pflugers Arch. 2007;455:147–55. doi: 10.1007/s00424-007-0272-9. [DOI] [PubMed] [Google Scholar]
- 52.Rohacs T, Nilius B. Regulation of transient receptor potential (TRP) channels by phosphoinositides. Pflugers Arch. 2007;455:157–68. doi: 10.1007/s00424-007-0275-6. [DOI] [PubMed] [Google Scholar]
- 53.Michailidis IE, Helton TD, Petrou VI, Mirshahi T, Ehlers MD, Logothetis DE. Phosphatidylinositol-4,5-bisphosphate regulates NMDA receptor activity through alpha-actinin. J Neurosci. 2007;27:5523–32. doi: 10.1523/JNEUROSCI.4378-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bernier LP, Ase AR, Chevallier S, Blais D, Zhao Q, Boue-Grabot E, Logothetis D, Seguela P. Phosphoinositides regulate P2X4 ATP-gated channels through direct interactions. J Neurosci. 2008;28:12938–45. doi: 10.1523/JNEUROSCI.3038-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horowitz LF, Hirdes W, Suh BC, Hilgemann DW, Mackie K, Hille B. Phospholipase C in living cells: activation, inhibition, Ca2+ requirement, and regulation of M current. J Gen Physiol. 2005;126:243–62. doi: 10.1085/jgp.200509309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suh BC, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006;314:1454–7. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- 58.Daniels RL, Takashima Y, McKemy DD. Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate. J Biol Chem. 2009;284:1570–82. doi: 10.1074/jbc.M807270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 60.Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:4835–4843. doi: 10.1523/JNEUROSCI.1296-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J Neurosci. 2007;27:7070–80. doi: 10.1523/JNEUROSCI.1866-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Rossum DB, Patterson RL, Sharma S, Barrow RK, Kornberg M, Gill DL, Snyder SH. Phospholipase Cgamma1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature. 2005;434:99–104. doi: 10.1038/nature03340. [DOI] [PubMed] [Google Scholar]
- 63.Nilius B, Owsianik G, Voets T. Transient receptor potential channels meet phosphoinositides. Embo J. 2008;27:2809–16. doi: 10.1038/emboj.2008.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell. 2007;25:491–503. doi: 10.1016/j.molcel.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 66.Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–4. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- 67.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–7. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 68.Viard P, Butcher AJ, Halet G, Davies A, Nurnberg B, Heblich F, Dolphin AC. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat Neurosci. 2004;7:939–946. doi: 10.1038/nn1300. [DOI] [PubMed] [Google Scholar]
- 69.Blair LA, Marshall J. IGF-1 modulates N and L calcium channels in a PI 3-kinase-dependent manner. Neuron. 1997;19:421–9. doi: 10.1016/s0896-6273(00)80950-2. [DOI] [PubMed] [Google Scholar]
- 70.Butterworth MB, Edinger RS, Frizzell RA, Johnson JP. Regulation of the epithelial sodium channel by membrane trafficking. Am J Physiol Renal Physiol. 2009;296:F10–24. doi: 10.1152/ajprenal.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pochynyuk O, Medina J, Gamper N, Genth H, Stockand JD, Staruschenko A. Rapid translocation and insertion of the epithelial Na+ channel in response to RhoA signaling. J Biol Chem. 2006;281:26520–7. doi: 10.1074/jbc.M603716200. [DOI] [PubMed] [Google Scholar]
- 72.Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–78. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 73.Patterson RL, van Rossum DB, Ford DL, Hurt KJ, Bae SS, Suh PG, Kurosaki T, Snyder SH, Gill DL. Phospholipase C-gamma is required for agonist-induced Ca2+ entry. Cell. 2002;111:529–541. doi: 10.1016/s0092-8674(02)01045-0. [DOI] [PubMed] [Google Scholar]
- 74.Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- 75.Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol. 1999;1:165–70. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- 76.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. Embo J. 2005;24:4211–23. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bahner M, Frechter S, Da Silva N, Minke B, Paulsen R, Huber A. Light-regulated subcellular translocation of Drosophila TRPL channels induces long-term adaptation and modifies the light-induced current. Neuron. 2002;34:83–93. doi: 10.1016/s0896-6273(02)00630-x. [DOI] [PubMed] [Google Scholar]
- 78.Meyer NE, Joel-Almagor T, Frechter S, Minke B, Huber A. Subcellular translocation of the eGFP-tagged TRPL channel in Drosophila photoreceptors requires activation of the phototransduction cascade. J Cell Sci. 2006;119:2592–603. doi: 10.1242/jcs.02986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fang L, Kim B-Y, Wade JB, Welling PA. Molecular mechanism of ROMK channel endocytosis. Faseb J. 2008;22:1158.9. [Google Scholar]
- 80.Yaradanakul A, Feng S, Shen C, Lariccia V, Lin MJ, Yang J, Kang TM, Dong P, Yin HL, Albanesi JP, Hilgemann DW. Dual control of cardiac Na+ Ca2+ exchange by PIP(2): electrophysiological analysis of direct and indirect mechanisms. J Physiol. 2007;582:991–1010. doi: 10.1113/jphysiol.2007.132712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sarkadi B, Tordai A, Gardos G. Membrane depolarization selectively inhibits receptor-operated calcium channels in human T (Jurkat) lymphoblasts. Biochim Biophys Acta. 1990;1027:130–40. doi: 10.1016/0005-2736(90)90076-z. [DOI] [PubMed] [Google Scholar]
- 82.Patel AJ, Lazdunski M, Honore E. Lipid and mechano-gated 2P domain K(+) channels. Curr Opin Cell Biol. 2001;13:422–8. doi: 10.1016/s0955-0674(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 83.Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 84.Suh BC, Hille B. Regulation of KCNQ channels by manipulation of phosphoinositides. J Physiol. 2007;582:911–6. doi: 10.1113/jphysiol.2007.132647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cockcroft S, Carvou N. Biochemical and biological functions of class I phosphatidylinositol transfer proteins. Biochim Biophys Acta. 2007;1771:677–91. doi: 10.1016/j.bbalip.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 86.Mousley CJ, Tyeryar KR, Vincent-Pope P, Bankaitis VA. The Sec14-superfamily and the regulatory interface between phospholipid metabolism and membrane trafficking. Biochim Biophys Acta. 2007;1771:727–36. doi: 10.1016/j.bbalip.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993;260:88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- 88.Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- 89.Balla A, Tuymetova G, Barshishat M, Geiszt M, Balla T. Characterization of type II phosphatidylinositol 4-kinase isoforms reveals association of the enzymes with endosomal vesicular compartments. J Biol Chem. 2002;277:20041–2050. doi: 10.1074/jbc.M111807200. [DOI] [PubMed] [Google Scholar]
- 90.Wang YJ, Wang J, Sun HQ, Martinez M, Sun YX, Macia E, Kirschhausen T, Albanesi JP, Roth MG, Yin HL. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 2003;114:299–310. doi: 10.1016/s0092-8674(03)00603-2. [DOI] [PubMed] [Google Scholar]
- 91.Minogue S, Waugh MG, De Matteis MA, Stephens DJ, Berditchevski F, Hsuan JJ. Phosphatidylinositol 4-kinase is required for endosomal trafficking and degradation of the EGF receptor. J Cell Sci. 2006;119:571–81. doi: 10.1242/jcs.02752. [DOI] [PubMed] [Google Scholar]
- 92.Balla A, Balla T. Phosphatidylinositol 4-kinases; old enzymes with emerging functions. Trends Cell Biol. 2006;16:351–361. doi: 10.1016/j.tcb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 93.Shaikh SR, Edidin MA. Membranes are not just rafts. Chem Phys Lipids. 2006;144:1–3. doi: 10.1016/j.chemphyslip.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 94.Pike LJ, Casey L. Localization and turnover of phosphatidylinositol 4,5-bisphosphate in caveolin-enriched membrane domains. J Biol Chem. 1996;271:26453–26456. doi: 10.1074/jbc.271.43.26453. [DOI] [PubMed] [Google Scholar]
- 95.Minogue S, Anderson JS, Waugh MG, dosSantos M, Corless S, Cramer R, Hsuan JJ. Cloning of a human type II phosphatidylinositol 4-kinase reveals a novel lipid kinase family. J Biol Chem. 2001;276:16635–16640. doi: 10.1074/jbc.M100982200. [DOI] [PubMed] [Google Scholar]
- 96.Aman MJ, Ravichandran KS. A requirement for lipid rafts in B cell receptor induced Ca(2+) flux. Curr Biol. 2000;10:393–6. doi: 10.1016/s0960-9822(00)00415-2. [DOI] [PubMed] [Google Scholar]
- 97.Rodriguez R, Matsuda M, Storey A, Katan M. Requirements for distinct steps of phospholipase Cgamma2 regulation, membrane-raft-dependent targeting and subsequent enzyme activation in B-cell signalling. Biochem J. 2003;374:269–80. doi: 10.1042/BJ20021778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Rheenen J, Achame EM, Janssen H, Calafat J, Jalink K. PIP2 signaling in lipid domains: a critical re-evaluation. Embo J. 2005;24:1664–73. doi: 10.1038/sj.emboj.7600655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brazer SC, Singh BB, Liu X, Swaim W, Ambudkar IS. Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J Biol Chem. 2003;278:27208–15. doi: 10.1074/jbc.M301118200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pani B, Ong HL, Liu X, Rauser K, Ambudkar IS, Singh BB. Lipid rafts determine clustering of STIM1 in endoplasmic reticulum-plasma membrane junctions and regulation of store-operated Ca2+ entry (SOCE) J Biol Chem. 2008;283:17333–40. doi: 10.1074/jbc.M800107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lopes CM, Rohacs T, Czirjak G, Balla T, Enyedi P, Logothetis DE. PIP2 hydrolysis underlies agonist-induced inhibition and regulates voltage gating of two-pore domain K+ channels. J Physiol. 2005;564:117–129. doi: 10.1113/jphysiol.2004.081935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hammond GR, Sim Y, Lagnado L, Irvine RF. Reversible binding and rapid diffusion of proteins in complex with inositol lipids serves to coordinate free movement with spatial information. J Cell Biol. 2009;184:297–308. doi: 10.1083/jcb.200809073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mao YS, Yamaga M, Zhu X, Wei Y, Sun HQ, Wang J, Yun M, Wang Y, Di Paolo G, Bennett M, Mellman I, Abrams CS, De Camilli P, Lu CY, Yin HL. Essential and unique roles of PIP5K-gamma and -alpha in Fcgamma receptor-mediated phagocytosis. J Cell Biol. 2009;184:281–96. doi: 10.1083/jcb.200806121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Toth B, Balla A, Ma H, Knight ZA, Shokat KM, Balla T. Phosphatidylinositol 4-kinase IIIbeta regulates the transport of ceramide between the endoplasmic reticulum and Golgi. J Biol Chem. 2006;281:36369–77. doi: 10.1074/jbc.M604935200. [DOI] [PubMed] [Google Scholar]
- 105.Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–7. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- 106.Runnels LW, Yue LX, Clapham DE. The TRPM7 channel ois inactivated by PIP2 hydrolysis. Nat Cell Biol. 2002;15:370–378. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- 107.Langeslag M, Clark K, Moolenaar WH, van Leeuwen FN, Jalink K. Activation of TRPM7 channels by phospholipase C-coupled receptor agonists. J Biol Chem. 2007;282:232–9. doi: 10.1074/jbc.M605300200. [DOI] [PubMed] [Google Scholar]
- 108.Kim M, Jiang LH, Wilson HL, North RA, Surprenant A. Proteomic and functional evidence for a P2X7 receptor signalling complex. Embo J. 2001;20:6347–58. doi: 10.1093/emboj/20.22.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–43. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- 110.Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- 111.Varnai P, Thyagarajan B, Rohacs T, Balla T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J Cell Biol. 2006;175:377–82. doi: 10.1083/jcb.200607116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–61. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]