Abstract
We have previously shown that Wnt5A and ROR2, an orphan tyrosine kinase receptor, interact to mediate melanoma cell motility. In other cell types, this can occur through the interaction of ROR2 with the cytoskeletal protein filamin A. Here, we found that filamin A protein levels correlated with Wnt5A levels in melanoma cells. Small interfering RNA (siRNA) knockdown of WNT5A decreased filamin A expression. Knockdown of filamin A also corresponded to a decrease in melanoma cell motility. In metastatic cells, filamin A expression was predominant in the cytoplasm, which western analysis indicated was due to the cleavage of filamin A in these cells. Treatment of nonmetastatic melanoma cells with recombinant Wnt5A increased filamin A cleavage, and this could be prevented by the knockdown of ROR2 expression. Further, BAPTA-AM chelation of intracellular calcium also inhibited filamin A cleavage, leading to the hypothesis that Wnt5A/ROR2 signaling could cleave filamin A through activation of calcium-activated proteases, such as calpains. Indeed, WNT5A knockdown decreased calpain 1 expression, and by inhibiting calpain 1 either pharmacologically or using siRNA, it decreased cell motility. Our results indicate that Wnt5A activates calpain-1, leading to the cleavage of filamin A, which results in a remodeling of the cytoskeleton and an increase in melanoma cell motility.
INTRODUCTION
Several recent studies have shown the upregulation of key metastatic markers during the progression of melanoma and have provided potential molecular targets (Bittner et al., 2000; Clark et al., 2000; Weeraratna, 2003; Smith et al., 2004). One such marker is Wnt5A, a member of the noncanonical Wnt pathway, which has been implicated in the pathogenesis of human cancer (Weeraratna et al., 2002; Kurayoshi et al., 2006; Fernandez-Cobo et al., 2007; MacLeod et al., 2007; Ripka et al., 2007). Activation of the Wnt5A pathway leads to the release of calcium from intracellular stores and to the upregulation of target proteins, such as protein kinase C (PKC). In melanoma, upregulation of Wnt5A increases cell invasion (Weeraratna et al., 2002). We have recently shown that this increased invasiveness is due, in part, to an epithelial-to-mesenchymal transition, accompanied by the upregulation of Snail and Vimentin (Dissanayake et al., 2007). These findings were consistent with our previous data that showed that Wnt5A overexpression can cause reorganization of the actin cytoskeleton (Weeraratna et al., 2002).
The actin cytoskeleton consists of pools of globular monomeric actin (G-actin) that can reversibly polymerize into filamentous actin (F-actin), thus resulting in alterations in the viscosity, elasticity, and mechanical resistance of the cell (Popowicz et al., 2006). Bundling of actin into filopodia (parallel bundles of actin filaments and associated proteins) is important for directional motility and adhesion of cells (Gupton and Gertler, 2007). Recently, filamin A, a scaffolding protein that is part of a family of nonmuscle actin-binding proteins, was shown to be required for filopodia formation, through actin reorganization (Nishita et al., 2006). Filamin A exists in a dimerized form, containing two hinge regions, which are putative target sites for calpain cleavage (Feng and Walsh, 2004). Calpains are calcium-dependent cysteineproteases involved in cytoskeleton remodeling and motility (Potter et al., 1998), and of the 13 known calpains, only calpains 1 and 2 are considered as ubiquitous calpains (Glading et al., 2002). Reduced cell migration and disruption of the actin cytoskeleton have been observed in calpaindeficient embryonic fibroblasts (Dourdin et al., 2001). Interestingly, calpain 3 has been shown to be dysregulated in melanoma, moving from the nuclei of non-neoplastic cells to the cytoplasm of malignant cells (Weeraratna et al., 2004).
Filamin A has been shown to mediate Wnt5A-induced cell migration through its interaction with the orphan tyrosine kinase receptor, ROR2 (Nishita et al., 2006). The suppression of either ROR2- or filamin A-induced filopodia inhibited cell migration mediated by Wnt5A. As Wnt5A has been shown to be an important mediator of melanoma progression, we investigated the relationship between filamin A and Wnt5A in metastatic melanoma.
RESULTS
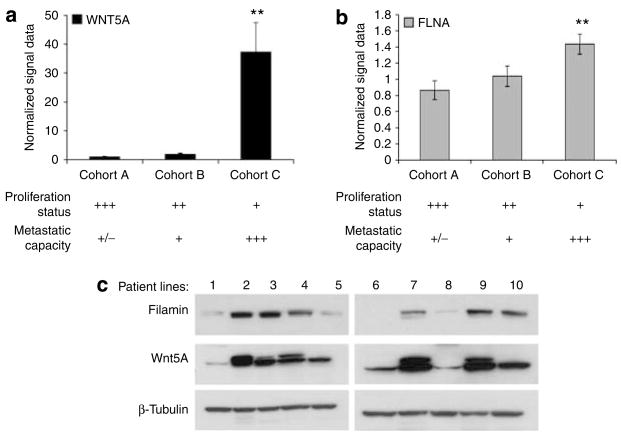
Filamin A expression is increased in Wnt5A-high melanoma cell lines
Highly metastatic melanoma cells show increased levels of Wnt5A and PO4-PKC (Weeraratna et al., 2002; Dissanayake et al., 2007). To determine if filamin A was elevated in melanoma cell lines with high Wnt5A and increased invasiveness, we examined the mRNA and protein levels of filamin A compared with Wnt5A in melanoma cell lines of differing metastatic potential. In a recent study, Hoek et al (2006) performed gene expression profiling studies on a large set of melanoma samples (known as the Mannheim data set). These samples were cell lines created from primary melanoma biopsies. Gene expression profiling data showed that these melanomas could be separated into three dominant groups of cell lines (cohorts A, B, and C). These groups could be characterized by their microphthalmia-associated transcription factor status, motility, and proliferation. Of these, cohort A and, to a lesser extent, Cohort B consisted of highly proliferative melanoma cells with low metastatic potential, and cohort C consisted of weakly proliferative cells with high metastatic potential. Analysis of the Mannheim data set showed that WNT5A is dramatically upregulated in cohort C as compared with cohorts A and B (Figure 1a). Bars shown are an average of all samples in each cohort. Filamin A (FLNA) mRNA was also increased in cohort C, although not as dramatically as WNT5A (Figure 1b). Western blot analysis was used to validate these observations in a second set of melanoma cell lines derived from patient samples, and showed a positive correlation between filamin A and Wnt5A protein expression, in 8 out of 10 cell lines (Figure 1c).
Figure 1. Filamin A expression is increased in primary melanoma cell lines with high Wnt5A expression.
(a) WNT5A and (b) FLNA mRNA expression in the three cohorts of the Mannheim data set. Cohorts A (n=19) and B (n=10) were considered low metastatic cell lines, whereas cohort C (n=16) was highly metastatic. Their metastasis rates inversely correlate with their proliferation as indicated in the table below the graphs. FLNA was significantly correlated with WNT5A (**P<0.01). Bars represent averages of all patients in each cohort. (c) Western blot analysis was performed on 10 melanoma patient cell lines to detect filamin A and Wnt5A, and confirmed that filamin A protein expression and Wnt5A expression are correlated at the protein level as well. β-Tubulin was used as a loading control.
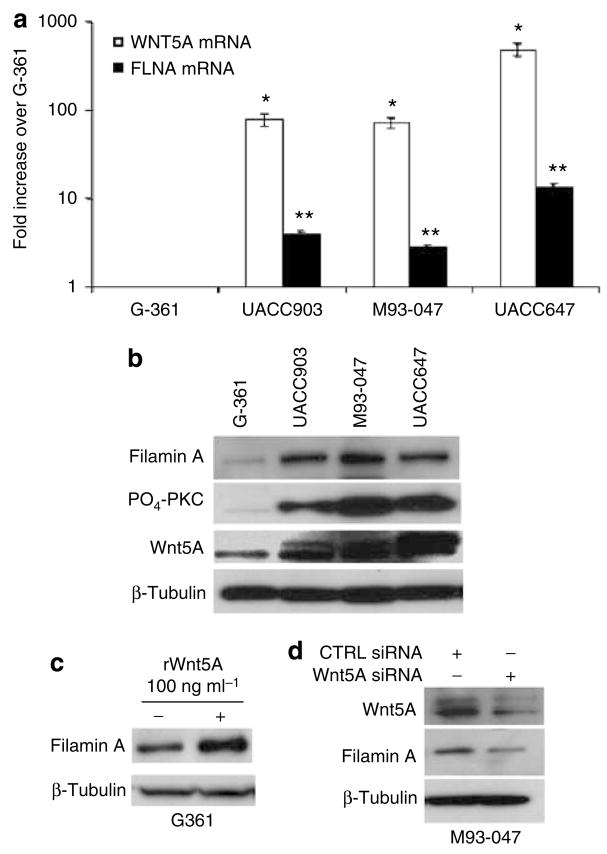
We have previously characterized a panel of melanoma cell lines on the basis of their Wnt5A status, PKC activity and invasive capacity determined that high Wnt5A and PKC correlate with increased metastatic capacity (Weeraratna et al., 2002; Dissanayake et al., 2007). We used real-time PCR to determine the correlation between FLNA mRNA and WNT5A mRNA. In these lines, the expression of FLNA mRNA was increased in lines with high WNT5A expression, and thus increased invasive capacity (Figure 2a). Filamin A protein expression was also increased in cells with Wnt5A and high levels of PO4-PKC (Figure 2b). Treatment of Wnt5A-low G-361 cells with recombinant Wnt5A led to an increase in filamin A protein levels (Figure 2c). To determine whether downregulation of Wnt5A expression affects the levels of filamin A, small interfering RNA (siRNA) targeted against WNT5A was used. Treatment of the high Wnt5A-expressing cell line M93-047 with WNT5A siRNA resulted in a significant decrease in filamin A protein levels, concomitant with a reduction in Wnt5A expression (Figure 2d). These results show a positive correlation between the levels of Wnt5A and filamin A in metastatic melanoma cells, but it is unclear if Wnt5A directly regulates filamin A, or if an intermediate step is required.
Figure 2. Wnt5A expression affects that of filamin A.
(a) Real-time PCR analysis of WNT5A and FLNA mRNA in G-361, UACC903, M93-047, and UACC647 cell lines, normalized to 18S levels. Error bars are represented as SEM, with values of *P<0.05 and **P<0.01. In G361 cells with low metastatic capacity, WNT5A and FLNA levels are low as compared with the other three metastatic cell lines. (b) Total protein was isolated for western blot analysis of filamin A, PO4-PKC, and Wnt5A in all cell lines. Again, G361 cells express lower levels of all of these proteins, but the expression of filamin A and Wnt5A can be increased significantly upon rWnt5A treatment. (c) High Wnt5A-expressing M93-047 cells were transfected with either control (CTRL) or WNT5A siRNA for 48 hours. (d) Total protein was isolated for western blot analysis of Wnt5A and filamin A expression, and showed decreases in filamin A occurring upon Wnt5A knockdown. β-Tubulin was used as a loading control.
FLNA knockdown reduces melanoma cell motility
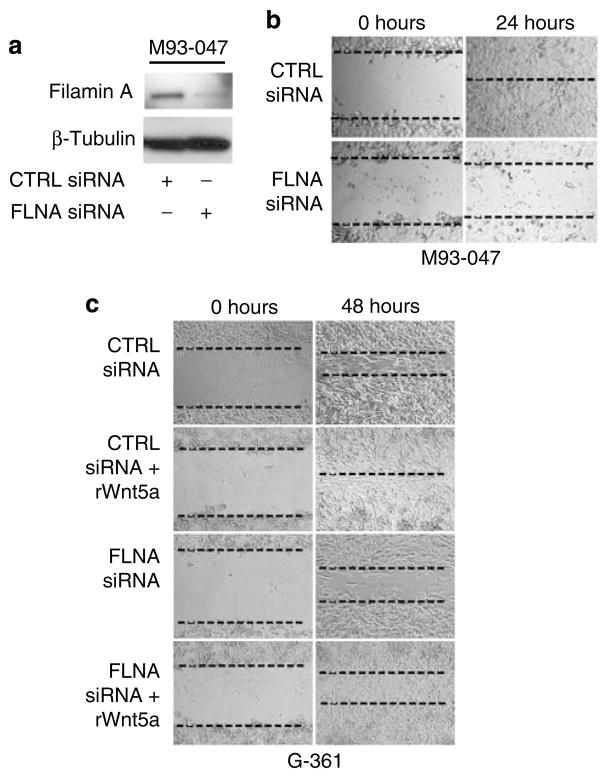
Filamin A has been shown to be essential for filopodia formation, an important event in the motility and migration of various cell types. To determine if a reduction in filamin A could lead to decreased motility in melanoma cells, cells were transfected with siRNA against FLNA, and knockdown of filamin A was observed at 48 hours (Figure 3a). After 48 hours of transfection with filamin A siRNA and negative control siRNA, cells were subjected to a wound-healing assay. In highly invasive M93-047 cells transfected with control siRNA, the wound closed within 24 hours. Knockdown of filamin A prevented closure of the wound (Figure 3b). Low Wnt5A-expressing G-361 cells, when transfected with negative-control siRNA, usually take more than 72 hours to completely heal a scratch wound (Figure 3c). When these negative control-siRNA transfected cells were treated with recombinant Wnt5A, the wound was completely healed by 48 hours (Figure 3c). Similar to negative control-siRNA-transfected cells, G-361 cells treated with FLNA siRNA alone also remained open at 48 hours. Subsequent treatment of FLNA siRNA-transfected cells with recombinant Wnt5A increased motility slightly, but did not fully rescue the effects of filamin A silencing (Figure 3c). Taken together, these results show an important role for filamin A in melanoma cell motility and Wnt5A-induced cell migration.
Figure 3. Filamin A is required for motility of melanoma cell lines.
(a) M93-047 cells were transfected with control or FLNA siRNA for 48 hours. Total protein was isolated for western blot analysis of filamin A. β-Tubulin was used as a loading control. (b) Highly metastatic M93-047 cells were transfected with control or FLNA siRNA for 48 hours and then subjected to a wound-healing assay for 24 hours. Images were taken until closure of the scratch wound (24 hours), and FLNA knockdown inhibited the motility of melanoma cells. (c) Low metastatic G-361 cells were transfected with control or FLNA siRNA for 48 hours, and then left untreated or treated with recombinant Wnt5A for the duration of the wound-healing assay (48 hours). Wnt5A treatment could not completely rescue the decrease in motility upon FLNA knockdown. Images were taken until closure of the scratch (48 hours).
Wnt5A affects the subcellular distribution of filamin A
One of the key steps in the acquisition of the metastatic phenotype is the reorganization of the cytoskeleton. Wnt5A overexpression has been shown to affect the expression and organization of cytoskeletal molecules, such as actin (Weeraratna et al., 2002). We used immunofluorescent analysis to determine whether Wnt5A expression could similarly affect the subcellular distribution of filamin A. In low Wnt5A-expressing cells (G-361), filamin A was primarily localized near the plasma membrane (Figure 4a), whereas in cells with higher levels of Wnt5A (M93-047 and UACC647), there was an increase in cytoplasmic filamin A (Figure 4a). Addition of recombinant Wnt5A to G-361 cells resulted in an increase in the expression of filamin A in the cytoplasm (Figure 4b).
Figure 4. Wnt5A increases filamin A cleavage.
(a) The expression and localization of filamin A (red) was examined in melanoma cell lines of differing metastatic potential using immunofluorescent confocal microscopy. Filamin A is found in a regular pattern around the periphery of the cell in G361 cells (low metastatic potential), but is more cytoplasmic and irregular in the cell lines with a higher metastatic potential. (b) Treatment of G-361 cells with recombinant Wnt5A for 16 hours resulted in a redistribution of filamin A to the cytoplasm of the cells akin to the lines with higher metastatic capacity. Scale bar=20 μm. (c) Total protein lysates from G-361, UACC903, M93-047, and UACC647 cell lines were analyzed by western blot for the presence of filamin A cleavage fragments. Filamin A cleavage fragments were identified as 190 kDa (N-terminal) and 90 kDa (C-terminal) bands, and cleavage was indeed increased in cell lines with higher metastatic capacity. β-Tubulin was used as a loading control. (d) G-361 cells were treated with increasing amounts of recombinant Wnt5A for 16 hours and total protein was isolated for western blot analysis of the 190 and 90 kDa N-terminal cleavage fragment. β-Tubulin was used as a loading control.
This observation raised the question of whether the cytoplasmic localization of filamin A upon Wnt5A treatment could be due to its cleavage by cellular proteases. To investigate this possibility, cell lysates were analyzed using commercial monoclonal antibodies that recognize either the N- or C-terminal domain of filamin A. Filamin A contains two hinge regions (Gorlin et al., 1990), and cleavage at hinge 1 generates a 190 kDa fragment that can be detected using an antibody that recognizes the N-terminal of this protein. Cleavage at hinge 2 results in a ~90 kDa fragment, which can be detected with an antibody directed against the C-terminal. In low-Wnt5A, nonmetastatic G361 cells, there is little cleavage at either hinge 1 or hinge 2 as compared with the highly metastatic lines (Figure 4c). However, treatment of G-361 cells with recombinant Wnt5A increased the cleavage of filamin A in a dose-dependent manner (Figure 4d). These results indicate that cleavage of filamin A occurs at both hinge regions and is inducible by Wnt5A, which may explain the intracellular redistribution of filamin A in response to Wnt5A treatment.
Cleavage of filamin A is mediated by calpain 1 in melanoma cell lines
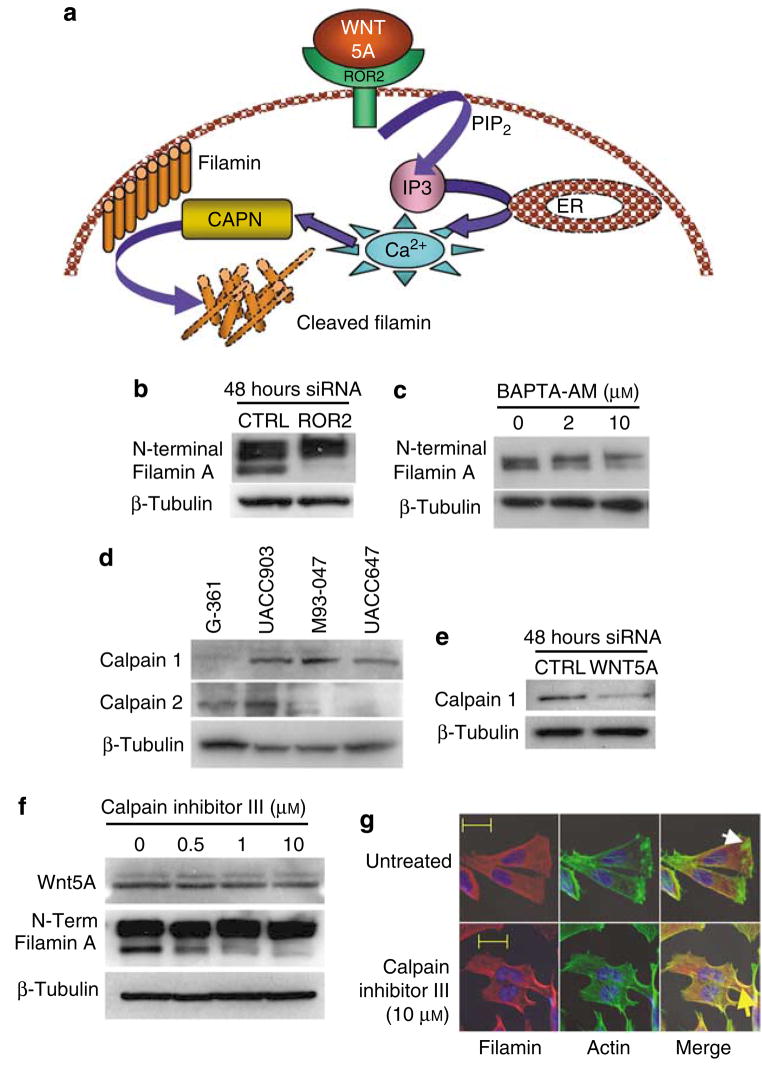
Calpains are calcium-dependent proteases that can cleave filamin A at both hinge regions (Loy et al., 2003). At present, there are 13 known human calpains (CAPN1–7, 9–11, 13, and 15), but only calpains 1 and 2 are considered as ubiquitous (Glading et al., 2002). We show here that Wnt5A promotes cleavage of filamin A. It has been reported previously that Wnt5A can increase the intracellular concentrations of calcium (reviewed by Kühl et al., 2000). In addition, we have recently shown that Wnt5A requires ROR2 to activate the Ca2+/PKC pathway and mediate the motility of melanoma cells (O’Connell et al., unpublished data). We hypothesized that Wnt5A signaling through ROR2 increases calcium release from the endoplasmic reticulum, thereby activating calpain, which in turn cleaves filamin (Figure 5a). We tested the first step in this hypothesis by knocking down ROR2 using siRNA. ROR2 knockdown in Wnt5A-high cells resulted in a decrease in filamin A cleavage (Figure 5b). The use of 1,2- Bis(2-aminophonoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis-(acetoxymethylester) (BAPTA-AM) as a calcium chelator in these cells also decreased filamin cleavage (Figure 5c), implying that both ROR2 and calcium are required for Wnt5A-mediated filamin A cleavage, potentially through the activation of calpains. To investigate this possibility, endogenous mRNA levels of several calpains were determined in low and high Wnt5A-expressing melanoma cell lines using real-time PCR (data not shown). CAPN1 and 2 were most abundantly expressed at the mRNA level. Western analysis showed that, of these, only CAPN1 protein levels correlated with Wnt5A expression (Figure 5d), and that the knockdown of WNT5A resulted in a decrease in CAPN1 (Figure 5e).
Figure 5. Calpain 1 plays a role in Wnt5A-mediated cleavage of filamin A.
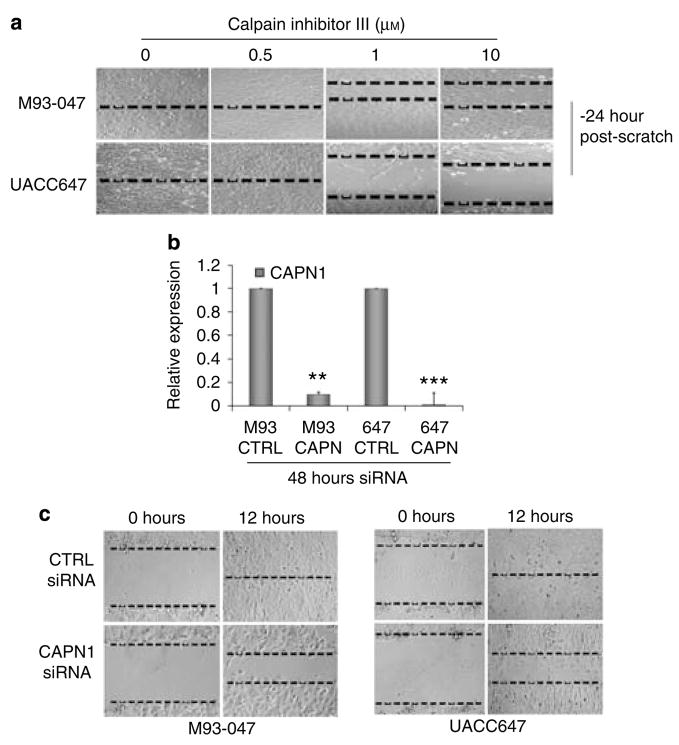
(a) Schematic hypothesis of Wnt5A-mediated filamin A cleavage: Wnt5A binds to ROR2 causing the intracellular release of calcium, which can increase calpain activity, resulting in an increase in filamin A cleavage. (b) SiRNA knockdown of ROR2 prevents filamin A cleavage in Wnt5A-high cells. (c) BAPTA chelation of intracellular calcium decreases the levels of filamin A cleavage in a dose-dependent manner. (d) Calpain 1, but not calpain 2, protein expression correlates with that of Wnt5A and, thus, to increased invasive potential. (e) Knockdown of Wnt5A decreases calpain 1 expression. (f) M93-047 cells were treated with increasing concentrations of calpain inhibitor III. Western blot analysis of Wnt5A and filamin A N-terminal cleavage (190 kDa) were performed, and showed that filamin A cleavage is attenuated in the presence of calpain inhibitor. β-Tubulin was used as a loading control. (g) Treatment of M93-047 cells with 10 μM of calpain inhibitor III results in a redistribution of filamin A (red staining) from the cytosol to the periphery of the cell. In calpain-inhibitor-treated cells, filamin A also colocalizes with actin (green). Scale bar=20 μm. All experiments shown here were performed with multiple Wnt5A-high cell lines, but in the interest of space, only M93-047 data is shown.
Next, we investigated the effect of a cell-permeable calpain inhibitor (calpain inhibitor III) on the cleavage of filamin A in Wnt5A-high cells. Treatment of cells with calpain inhibitor for 24 hours caused a marked reduction in filamin A fragmentation (Figure 5f). Wnt5A levels were unaffected as would be expected, if calpain signaling is truly downstream of Wnt5A. To determine whether cellular redistribution of filamin A could result from the inhibition of calpain activity, indirect immunofluorescent analyses were performed using the N-terminal filamin antibody. Cells were costained for F-actin using Phalloidin. Filamin A immunoreactivity was observed throughout the cytoplasm of the cell, and actin was polymerized at the leading edge of these highly invasive cells (Figure 5g). Furthermore, there was almost no colocalization of actin and filamin A (Figure 5g, white arrow, Figure S1A). Upon pretreatment of these cells with calpain inhibitor III, filamin A clearly colocalized with cortical actin at the cell periphery (Figure 5g, yellow arrow, Figure S1B), confirming that calpain cleavage is necessary for the cytoplasmic redistribution of filamin A. Taken together, these data indicate that Wnt5A signaling through ROR2 activates calcium and is required for the expression and activation of CAPN1, and the subsequent cleavage of filamin A.
Calpain inhibition reduces motility in melanoma cell lines
To determine if the cleavage of filamin A was necessary for melanoma cell motility, wound healing assays were performed in the presence or absence of calpain inhibitor III for 24 hours. The results clearly showed a dose-dependent reduction in motility in the two Wnt5A-high lines M93-047 and UACC647 (Figure 6a). SiRNA-mediated gene silencing was then used to specifically target CAPN1. This resulted in a significant inhibition of CAPN1 expression as compared with negative control siRNA (Figure 6b). Knockdown of CAPN1 expression delayed wound closure by several hours in both M93-047 and UACC647 cell lines, when compared with cells transfected with control siRNA (Figure 6c).
Figure 6. Inhibition of CAPN1 decreases motility in highly metastatic melanoma cell lines.
(a) M93-047 and UACC647 cells were treated with increasing amount of calpain inhibitor III, then subjected to a wound-healing assay for 24 hours. Calpain inhibition decreased the motility of melanoma cells. (b) M93-047 and UACC647 cells were treated with CTRL or CAPN1 siRNA for 48 hours. Total RNA was isolated and real-time PCR analysis of CAPN1 expression was performed. Levels were normalized to 18S expression. Error bars are represented as SEM, with values of **P<0.01. (c) M93-047 and UACC647 cells were treated with CTRL or CAPN1 siRNA for 48 hours, then subjected to a wound-healing assay for 12 hours. Calpain 1 inhibition by siRNA inhibited the motility of melanoma cells.
DISCUSSION
We have shown previously that Wnt5A increases melanoma metastasis through increases in PKC and affects cytoskeletal reorganization (Weeraratna et al., 2002; Dissanayake et al., 2007). We have also shown recently that Wnt5A requires ROR2 to signal effectively, and the knockdown of ROR2 can abrogate Wnt5A-mediated melanoma cell motility (O’Connell et al., unpublished data). In osteoblasts, ROR2 interacts physically with filamin A (Flanagan et al., 2001). We show here that Wnt5A signaling, through ROR2, can increase filamin A expression. This interaction requires a mechanism involving the activation of calcium-activated proteases, specifically calpain 1, and to our knowledge this is unreported previously. Wnt5A can increase both PKC activity and the levels of intracellular calcium (Kühl et al., 2000). We have shown previously that other calcium-activated proteins (such as calmodulin-activated kinase II) can be increased upon increased Wnt5A signaling (Dissanayake et al., 2007). Calpains are a family of calcium-dependent, non-lysosomal cysteine proteases that cleave cytoskeletal proteins, such as filamin A (Potter et al., 1998; Feng and Walsh, 2004). The correlation between calpain 1 and Wnt5A protein expression suggests that calpain 1 is a Wnt5A-inducible protein whose upregulation may be responsible for the increased levels of cleaved filamin A in response to Wnt5A signaling. We propose that in nonmetastatic melanoma cells with little Wnt5A signaling, where the levels of calcium are low, calpain 1 signaling is minimal, whereas upon activation of the Wnt5A/ROR2 signaling pathway, calcium influx increases and calpain 1 becomes activated, resulting in the subsequent cleavage of filamin A. This is supported by our data indicating that filamin A cleavage can be inhibited by ROR2 siRNA, as well as by chelation of calcium by BAPTA-AM. Furthermore, we show that WNT5A knockdown decreases calpain 1 expression, and filamin A cleavage and expression.
Blocking calpain activity reduces melanoma cell motility, possibly due to the lack of filamin A cleavage and ensuing intracellular redistribution. These results support earlier findings that calpain-mediated cleavage of cytoskeletal proteins is essential for motility and invasiveness. For example, it has been reported that calpains are required for normal osteoclast function and that reduction of calpains leads to a decrease in motility (Marzia et al., 2006). In renal cell cancer, calpain 1 expression has been shown to correlate with advanced disease and poorer outcome (Braun et al., 1999). In melanoma, specifically, calpains have been shown to be important in the degradation of p27kip1, the loss of which is associated with poorer outcome for melanoma (Delmas et al., 2003). Another interesting observation is that calpains are involved also in regulating melanin biosynthesis, potentially through the degradation of tyrosinase (Ohguchi et al., 2005). The reason that this is particularly intriguing is that recent data from our laboratory suggest that Wnt5A overexpression results in a decrease in both melanin production and the expression of many markers associated with melanogenesis (Dissanayake et al., 2008). Although we show that at least some of these markers are regulated through Wnt5A/PKC activation of STAT3, and subsequent STAT3-mediated inhibition of the transcription factors PAX3 and SOX10, not all melanogenic proteins are under the control of these factors. It is probable that the Wnt5A-mediated activation of calpain-1, and the subsequent degradation of other melanogenic proteins, may also play a complementary role in the regulation of melanogenesis. As many of the proteins involved in melanogenesis (for example, MART-1, GP100) can act as antigens for tumor-infiltrating immune cells (Kawakami et al., 1998), the downregulation of these antigens by tumor cells may result in increased tumor aggression.
To our knowledge, the findings that filamin A expression correlates with levels of Wnt5A in melanoma cell lines and that a reduction of filamin A decreases the metastatic potential of these cells have not been reported previously. Furthermore, Wnt5A can induce calpain-dependent cleavage of filamin A, an essential step in promoting cell motility and invasiveness in melanoma cell lines. Targeting Wnt5A in conjunction with any of these downstream markers could have significant implications for melanoma therapy.
MATERIALS AND METHODS
Analysis of the Mannheim data set
The Mannheim data is publicly available from NCBI’s gene expression omnibus (www.ncbi.nlm.nih.gov/geo) under GEO Series accession no. GSE4845. This data set was generated from microarray analysis of 45 primary cultures of melanoma biopsies, using Affymetrix HGU133 microarray chips. Analysis of the data set for expression of WNT5A and FLNA was performed using a Student’s two-tailed t-test (assuming equal variance) to determine statistical significant between cohorts.
Cell culture
Cells were cultured in T-25 tissue culture flasks and expanded in T-75 flasks (Corning, Corning, NY). UACC903, M93-047, UACC647, and Franklin Square cell lines were maintained in RPMI (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen), 100Uml−1 penicillin and streptomycin, and 4mM L-glutamine. G-361 cells were maintained in McCoy’s (Invitrogen) supplemented with 10% fetal bovine serum, 100Uml−1 penicillin and streptomycin, and 4mM L-glutamine. All cell lines were cultured at 37°C in 5% CO2, and the medium was replaced every 2–3 days as needed.
Wnt5A and calpain inhibitor treatment
For experiments requiring treatment, cells were incubated with 50–200 ng ml−1 of recombinant mouse Wnt5A (R&D Systems, Minneapolis, MN) for 16 hours or 0.5, 1, or 10 μM of calpain inhibitor III (Sigma Aldrich, St. Louis, MO) for 24 hours.
siRNA transfection
Cells were transfected with high performance (HP)-validated negative control (CTRL), FLNA, and CAPN1 siRNA (200 nmol, Qiagen, Valencia, CA) using Lipofectamine (Invitrogen) for 48 hours as described previously (Dissanayake et al., 2007). Sequences for siRNA were as follows: CTRL: sense, r(UUCUCCGAACGUGUCG UCACGU)dTdT; antisense, r(ACGUGACACGUUCGGAGAA)dTdT; FLNA: sense, r(GGAAGAAGAUCCAGCAGAA)dTdT; antisense, r(UUCUGCUGGAUCUUCUUCC)dGdG; CAPN1: sense, r(CCACG GAACUGCUGUCAAA)dTdT; antisense, r(UUUGACAGCAGUUCC GUGG)dGdG.
These siRNAs are designed with unequal stabilities of the 3′ and 5′ bases, to reduce the risk of off-target effects. Details can be found at the Qiagen website: http://www1.qiagen.com/products/genesilencing/hponguardsirnadesign.aspx.
Wound-healing assay
Cells were seeded at 1×105 cells per well in 24-well plates coated with fibronectin (BD Biosciences, San Jose, CA). Once confluent, a 200 μl tip (PGC Scientifics) was heat-sealed, and one horizontal and one vertical scratch was made. Images of the same field were taken at 0, 12, 24, 48, and 72 hours, as required, on a light microscope (Zeiss) using phased light at a gain of 1.
Immunofluorescence
Cells were seeded at 5×105 cells per well (G-361) and 3×105 cells per well (UACC903 and M93-047) into one-well chamber slides (Lab-Tek, Campbell, CA) and incubated overnight at 37°C, 5% CO2, in a humidified environment. Cells were fixed in ice-cold 95% methanol for 20minutes at room temperature, washed in phosphate-buffered saline, and blocked using sterile-filtered blocking buffer (0.2% Triton X-100, 0.2% BSA, 0.2% casein, 0.2% gelatin, and 0.02% sodium azide) for 1 h at room temperature. Filamin A primary antibody (1:100, RDI, Concord, MA) was added in blocking buffer and slides were incubated overnight at 4°C. Cells were then washed in phosphate-buffered saline and treated with Alexa Fluor-555 secondary antibody (1:1,000, Invitrogen) in blocking buffer for 1 h at room temperature. For F-actin staining, cells were fixed in 4% paraformaldehyde and stained with Phalloidin-488 (Invitrogen). Next, cells were washed in phosphate-buffered saline and mounted in Prolong Gold antifade reagent containing 4,6-diamidino-2-phenylindole (Invitrogen) and cured for 24 hours at room temperature in the dark. Images were taken using a Zeiss Meta 510 confocal microscope.
RNA extraction, cDNA synthesis, and real-time PCR
RNA was extracted using Trizol (Invitrogen) and an RNeasy Mini kit (Qiagen), and from 1 μg of RNA, cDNA was synthesized as described previously (Dissanayake et al., 2007). Gene expression was quantified using the SYBR green method of real-time PCR and mRNA levels were compared with standard curves and normalized to 18S mRNA following the absolute quantitation method. PCRs were performed in triplicate with Universal 18S primers (Ambion, Austin, TX) or 100 nM of each gene-specific primer. Primers are included in Table S1. Real-time PCR was performed on an ABI Prism 7,300 sequence detection system using the following conditions: 50°C for 2 minutes, 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute.
SDS-PAGE and western blotting
Cells were lysed as described previously (Dissanayake et al., 2007). A total of 50 μg of protein was run on a 4–20% Tris-glycine gel (Invitrogen), transferred, and blocked as described previously (Dissanayake et al., 2007). Primary antibodies were incubated overnight at 4°C as follow: anti-filamin A (1:1,000, RDI), N-terminal anti-filamin A (1:1,000, Chemicon, Billerica, MA), C-terminal anti-filamin A (1:1,000, Chemicon), biotin-conjugated anti-Wnt5A (1:100, R&D Systems), PO4-PKC (1:1,000, Cell Signaling, Danvers, MA), and β-tubulin (1:1,000, Cell Signaling). Membranes were washed in TBST, and the appropriate secondary antibody was added in 5% milk/TBST for 1 h at room temperature with agitation. Membrane was washed in TBST and developed using ECL plus (Amersham Biosciences, GE Healthsciences, Piscataway, NJ).
Densitometry
Images were saved as Tiff files, inverted, and integrated density was analyzed. Densitometry was performed using ImageJ software (NIH) and values were normalized to β-tubulin.
Statistical analysis
A Student’s t-test was performed when required in at least three independent experiments. Significance was designated as *P<0.05 and **P<0.01.
Supplementary Material
Figure S1. Colocalization of actin and filamin A in M93-047 cells.
Table S1. Primer sequences used for real-time PCR.
Acknowledgments
We thank Liqun Jiang for generously providing calpain inhibitor III. This research was supported by the Intramural Research Program (IRP) of the National Institute on Aging (NIA), National Institutes of Health (NIH). K.S.H. was supported by the Swiss National Foundation (3100A0-103671) and Oncosuisse (OCS-01927-08-2006).
Abbreviations
- PKC
protein kinase C
- siRNA
small interfering RNA
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–40. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- Braun C, Engel M, Seifert M, Theisinger B, Seitz G, Zang KD, et al. Expression of calpain I messenger RNA in human renal cell carcinoma: correlation with lymph node metastasis and histological type. Int J Cancer. 1999;84:6–9. doi: 10.1002/(sici)1097-0215(19990219)84:1<6::aid-ijc2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–5. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- Delmas C, Aragou N, Poussard S, Cottin P, Darbon JM, Manenti S. MAP kinase-dependent degradation of p27Kip1 by calpains in choroidal melanoma cells. Requirement of p27Kip1 nuclear export. J Biol Chem. 2003;278:12443–51. doi: 10.1074/jbc.M209523200. [DOI] [PubMed] [Google Scholar]
- Dissanayake SK, Olkhanud PB, O’Connell MP, Carter A, French AD, Camilli TC, et al. Wnt5A regulates expression of tumor-associated antigens in melanoma via changes in signal transducers and activators of transcription 3 phosphorylation. Cancer Res. 2008;68:10205–14. doi: 10.1158/0008-5472.CAN-08-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayake SK, Wade M, Johnson CE, O’Connell MP, Leotlela PD, French AD, et al. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem. 2007;282:17259–71. doi: 10.1074/jbc.M700075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourdin N, Bhatt AK, Dutt P, Greer PA, Arthur JS, Elce JS, et al. Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts. J Biol Chem. 2001;276:48382–48388. doi: 10.1074/jbc.M108893200. [DOI] [PubMed] [Google Scholar]
- Feng Y, Walsh CA. The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat Cell Biol. 2004;6:1034–8. doi: 10.1038/ncb1104-1034. [DOI] [PubMed] [Google Scholar]
- Fernandez-Cobo M, Zammarchi F, Mandeli J, Holland JF, Pogo BG. Expression of Wnt5A and Wnt10B in non-immortalized breast cancer cells. Oncol Rep. 2007;17:903–7. [PubMed] [Google Scholar]
- Flanagan LA, Chou J, Falet H, Neujahr R, Hartwig JH, Stossel TP. Filamin A, the Arp2/3 complex, and the morphology and function of cortical actin filaments in human melanoma cells. J Cell Biol. 2001;155:511–7. doi: 10.1083/jcb.200105148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glading A, Lauffenburger DA, Wells A. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 2002;12:46–54. doi: 10.1016/s0962-8924(01)02179-1. [DOI] [PubMed] [Google Scholar]
- Gorlin JB, Yamin R, Egan S, Stewart M, Stossel TP, Kwiatkowski DJ, et al. Human endothelial actin-binding protein (ABP-280, nonmuscle filamin): a molecular leaf spring. J Cell Biol. 1990;111:1089–105. doi: 10.1083/jcb.111.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupton SL, Gertler FB. Filopodia: the fingers that do the walking. Sci STKE. 2007;2007:re5. doi: 10.1126/stke.4002007re5. [DOI] [PubMed] [Google Scholar]
- Hoek KS, Schlegel NC, Brafford P, Sucker A, Ugurel S, Kumar R, et al. Metastatic potential of melanomas defined by specific gene expression profiles with no BRAF signature. Pigment Cell Res. 2006;19:290–302. doi: 10.1111/j.1600-0749.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Robbins PF, Wang RF, Parkhurst M, Kang X, Rosenberg SA. The use of melanosomal proteins in the immunotherapy of melanoma. J Immunother. 1998;21:237–46. doi: 10.1097/00002371-199807000-00001. [DOI] [PubMed] [Google Scholar]
- Kühl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16:279–83. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, et al. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66:10439–48. doi: 10.1158/0008-5472.CAN-06-2359. [DOI] [PubMed] [Google Scholar]
- Loy CJ, Sim KS, Yong EL. Filamin-A fragment localizes to the nucleus to regulate androgen receptor and coactivator functions. Proc Natl Acad Sci USA. 2003;100:4562–7. doi: 10.1073/pnas.0736237100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod RJ, Hayes M, Pacheco I. Wnt5A secretion stimulated by the extracellular calcium-sensing receptor inhibits defective Wnt signaling in colon cancer cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G403–11. doi: 10.1152/ajpgi.00119.2007. [DOI] [PubMed] [Google Scholar]
- Marzia M, Chiusaroli R, Neff L, Kim NY, Chishti AH, Baron R, et al. Calpain is required for normal osteoclast function and is down-regulated by calcitonin. J Biol Chem. 2006;281:9745–54. doi: 10.1074/jbc.M513516200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishita M, Yoo SK, Nomachi A, Kani S, Sougawa N, Ohta Y, et al. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5A-induced cell migration. J Cell Biol. 2006;175:555–62. doi: 10.1083/jcb.200607127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohguchi K, Akao Y, Nozawa Y. Involvement of calpain in melanogenesis of mouse B16 melanoma cells. Mol Cell Biochem. 2005;275:103–7. doi: 10.1007/s11010-005-1081-0. [DOI] [PubMed] [Google Scholar]
- Popowicz GM, Schleicher M, Noegel AA, Holak TA. Filamins: promiscuous organizers of the cytoskeleton. Trends Biochem Sci. 2006;31:411–9. doi: 10.1016/j.tibs.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Potter DA, Tirnauer JS, Janssen R, Croall DE, Hughes CN, Fiacco KA, et al. Calpain regulates actin remodeling during cell spreading. J Cell Biol. 1998;141:647–62. doi: 10.1083/jcb.141.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripka S, Konig A, Buchholz M, Wagner M, Sipos B, Kloppel G, et al. WNT5A—target of CUTL1 and potent modulator of tumor cell migration and invasion in pancreatic cancer. Carcinogenesis. 2007;28:1178–87. doi: 10.1093/carcin/bgl255. [DOI] [PubMed] [Google Scholar]
- Smith AP, Weeraratna AT, Spears JR, Meltzer PS, Becker D. SAGE identification and fluorescence imaging analysis of genes and transcripts in melanomas and precursor lesions. Cancer Biol Ther. 2004;3:104–9. doi: 10.4161/cbt.3.1.661. [DOI] [PubMed] [Google Scholar]
- Weeraratna AT. Serial analysis of gene expression (SAGE): advances, analysis and applications to pigment cell research. Pigment Cell Res. 2003;16:183–9. doi: 10.1034/j.1600-0749.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- Weeraratna AT, Becker D, Carr KM, Duray PH, Rosenblatt KP, Yang S, et al. Generation and analysis of melanoma SAGE libraries: SAGE advice on the melanoma transcriptome. Oncogene. 2004;23:2264–74. doi: 10.1038/sj.onc.1207337. [DOI] [PubMed] [Google Scholar]
- Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, et al. Wnt5A signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–88. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Colocalization of actin and filamin A in M93-047 cells.
Table S1. Primer sequences used for real-time PCR.