Abstract
Neuron-targeted, nucleic acid delivery systems are important technologies for realizing the potential of gene therapy for nervous system disorders. However, neurons are difficult cells to transfect using non-viral vectors due in part to the specific and unique delivery challenges present in these cells. We have investigated several bioactive peptides for their ability to assist in overcoming delivery barriers in mammalian cells. We summarize here our recent progress in developing and applying peptide-modified polycations for nucleic acid delivery. In addition, we present data demonstrating the potential of using multicomponent, peptide-modified polycations for nucleic acid delivery to neurons.
Keywords: Non-viral gene delivery, neurons, peptides, polyethylenimine
1. Introduction
Neurological disorders affect up to one billion people worldwide and are the cause of 12% of total global deaths [1]. Despite significant advances in understanding the molecular basis of these diseases, many prevalent neurological disorders have no effective cure due to the unavailability of drugs and/or delivery systems. Nucleic acid-based therapies are a relatively new class of drugs that can potentially treat neurological disease. Indeed, nucleic acid-based therapies have yielded promising disease reduction in animal models of amyotrophic lateral sclerosis [2, 3], Parkinson’s disease [4], Huntington’s disease [5], and spinocerebellar ataxia [6], to name a few. In addition, gene therapies are currently being evaluated in clinical trials for Parkinson’s disease and multiple sclerosis [7, 8].
Effective and specific gene delivery to target cells in the nervous system remains a major challenge in translating these technologies for clinical application. Most animal studies and clinical trials have utilized viral vectors due to their advantageous in vivo delivery efficiencies. However, synthetic (non-viral) vectors offer several merits compared with viral systems, including improved safety profiles, versatility in application, and relative ease in production. A recent review covers the development of synthetic systems for gene delivery to neurons, the key information-transmitting cells in the nervous system [9].
Non-viral gene delivery to neurons is particularly challenging, especially when vectors are administered to the neural projections, such as via intramuscular injection for motor neuron delivery. For successful nucleic acid delivery to the cell body, vectors must traverse a series of cellular barriers (Figure 1). Vectors must interact with the neuronal membrane, become internalized (typically into endocytic vesicles), undergo retrograde transport toward the cell nucleus, escape from vesicular compartments, and deposit the therapeutic in the desirable subcellular location (the nucleus for plasmids and the perinuclear cytoplasm for oligonucleotides and small, interfering RNA (siRNA)).
Figure 1.
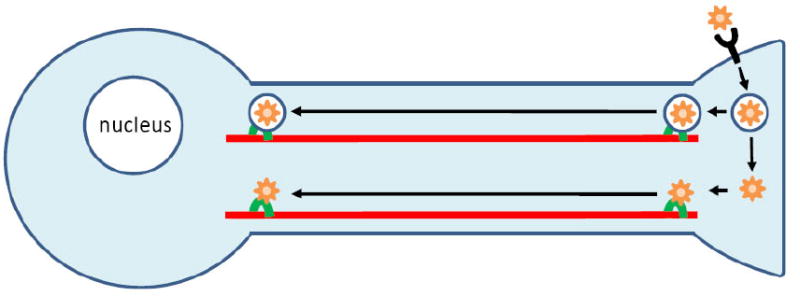
Neuron binding, uptake, retrograde transport, endosomal escape and nuclear localization of non-viral gene delivery vehicles.
Several intracellular trafficking studies conducted in neurons or neuron-like cells have highlighted unique delivery challenges presented by neurons that are not encountered in other mammalian cell types. Non-specific, electrostatic uptake of synthetic vectors is significantly reduced in differentiated, neuron-like cells compared to undifferentiated cells [10]. Furthermore, binding and internalization efficiencies of synthetic vectors are depressed at neurites compared with neuronal soma [11]. Vesicular escape by non-viral vectors in neurons is also extremely limited. Two hours after internalization into neurons, the release efficiency of polycation-based synthetic vectors from endocytic vesicles is low compared with adenoviral vectors (~15% compared with ~95%, respectively, as assessed using colocalization analysis of confocal microscopy images) [12]. Although retrograde transport of non-viral vectors residing within neuronal endocytic vesicles occurs [11, 12], any released vectors are likely to experience minimal motility in the cytoplasm due to limited diffusion of particles of this size range (~80-150 nm) [13, 14]. Viruses have been shown to overcome this limited cytoplasmic diffusion by hijacking the retrograde-biased motor protein dynein [15-19].
We hypothesize that bioactive peptides can be integrated with synthetic delivery systems to result in neuron-targeted delivery vectors with high delivery efficiencies. Synthetic peptides have been successfully applied to enhance the efficiencies of several steps in the gene delivery pathway, including DNA condensation, cell binding, and endosomal escape, as reviewed elsewhere [20, 21]. In our laboratory, we have demonstrated the advantage of incorporating bioactive peptides into polycations for improved transfection to mammalian cells. Here, we review the application of various bioactive peptides for assisting in targeting, endosomal escape, and dynein binding of polycation vectors. In addition, we present new data demonstrating the synergistic effects of conjugating neuron targeting and endosomal escape peptides to polycation vectors for plasmid delivery to neuron-like cells.
2. Materials and Methods
2.1. PC-12 cell culture
PC-12 cells were obtained from ATCC (CRL-1721) and were maintained in growth medium (F-12K medium supplemented with 15% horse serum, 2.5% fetal bovine serum, and antibiotics) in a 37 °C, 5% CO2 environment. Medium was replaced every 2-3 days and cells were passaged when 60-80% confluent. Cells were detached by incubation with Trypsin-EDTA and resuspended in 1 mL of F-12K medium. In order to obtain a single cell suspension, cells were passed through a fire-polished glass pipette. For differentiation to a neuron-like phenotype, cells were plated on a poly-L-lysine coated flask in differentiation medium (F-12K medium supplemented with 1% horse serum, 100 ng/mL nerve growth factor, and antibiotics).
2.2. Polymer synthesis
2.2.1. Peptides
The L240 peptide (CFPFDTIVVDGADFVLHPSYFILRRRRKRFPYFFTDVRVAA) and the HGP peptide (LLGRRGWEVLKYWWNLLQYWSQELC) were synthesized and purified by GenScript Corporation (Scotch Plains, NJ). The Tet1 peptide (HLNILSTLWKYRC) was synthesized and purified by HPLC by Peptron (Daejeon, South Korea).
2.2.2. Tet1-PEI synthesis
Tet1-PEI was synthesized as described previously [22]. Briefly, branched polyethylenimine (PEI, MW 25k; Sigma, St. Louis, MO) was modified with 2 mole equivalents of N-succinimidyl 3-(2-pyridyldithio)-propionate (SPDP; Pierce, Rockford, IL) in DMF for 4 hours at room temperature. PEI was purified using a PD-10 column (GE Healthcare, Piscataway, NJ) and reacted with 2.4 mole equivalents of Tet1 peptide for 12 hours. Tet1-PEI was purified using a PD-10 column.
2.2.3. PEI-HGP and PEI-L240 syntheses
PEI-HGP and PEI-L240 were synthesized as described previously [23, 24]. In brief, branched PEI (25k) was modified with 5 mole equivalents of the heterobifunctional crosslinker, sulfo-succinimidyl 4-[p-maleimidophenyl]butyrate (sulfo-SMPB; Pierce) for 1 hour in 100 mM NaPO4 pH 7, 150 mM NaCl, 1 mM EDTA and purified using a PD-10 column. Lyophilized product was dissolved in DMF with 50 mM triethylamine (TEA) and reacted with 3-5 eq. HGP or L240 peptide for 24 hours. HCl was added to completely acidify PEI and free peptide was removed by extensive dialysis into water using a 10,000 MWCO membrane.
2.2.4. Tet1-PEI-HGP synthesis
Branched PEI modified with 5 eq. SPDP was prepared as described above. PEI was dissolved in DMF and reacted with 2 eq. HGP for 24 hours. Tet1 (2 eq.) was added and reacted for a further 24 hours. Polymer was purified by extensive dialysis into water using a 10,000 MWCO dialysis membrane.
2.2.5. Polymer characterization
PEI concentration was determined using a copper (II) acetate assay as described previously [25]. Peptide conjugation was determined by absorbance readings at 280 nm using a UV/vis spectrophotometer.
2.3. Polyplex formulation and characterization
Polyplexes were formulated by adding an equal volume of polymer to nucleic acid at the desired ratio of polymer amine groups to nucleic acid phosphate groups (N/P ratio). The N/P ratio was calculated based on a PEI subunit of 43 g/mol and a DNA subunit of 330 g/mol. Polyplexes were incubated for 10 minutes at room temperature before use to allow for complete complexation. In order to verify that the amount of polymer was kept constant for each conjugate, complex formation was assayed using gel retardation at fine (0.5) N/P ratios. Hydrodynamic size and zeta potential were measured in triplicate using a ZetaPALS zeta potential and particle size analyzer (Brookhaven Instruments Corp., Holtsville, NY).
2.4. Plasmid transfection
Transfections were performed in replicates of 6. PC-12 cells were plated at 50,000 cells/well in 12-well plates and differentiated for 2 days with 100 ng/mL nerve growth factor (NGF). Polyplexes were formulated as described above using 1 μg of gWiz-luciferase plasmid DNA (Aldevron, Fargo, ND) in 20 μL for each sample. Cells were washed with phosphate-buffered saline, pH 7.4 (PBS) and were incubated with polyplex solution diluted in 180 μL F-12K media in a 37 °C, 5% CO2 environment for 4 hours. Cells were then washed once with PBS and incubated with differentiation media in a 37 °C, 5% CO2 atmosphere for an additional 48 hours. To collect lysate, cells were washed with PBS, lysed with 200 μL of reagent lysis buffer (Promega Corp., Madison, WI), and frozen at -80 °C. Lysate was collected and centrifuged at 14,000 g at 4 °C for 15 minutes. 20 μL of supernatant were assayed for luciferase expression using 100 μL of luciferase substrate (Promega Corp., Madison, WI). Luminescence was integrated for 1 second using a TECAN Safire2 microplate reader. Luciferase activity is reported in relative luminescence units normalized by mg protein (RLU/mg), as measured by a BCA Protein Assay Kit (Pierce).
2.5. siRNA transfection
siRNA targeting endogenous glyceraldehyde-3-phosphate dehydrogenase (siGAPDH) was purchased from Ambion (Austin, TX) and control siRNA targeting green fluorescent protein (siGFP) was synthesized by Dharmacon (Lafayette, CO) with the following sequences: 5’-GACGUAAACGGCCACAAGUUC-3’ (sense) and 5’-ACUUGUGGCCGUUUACGUCGC-3’ (antisense). PC-12 cells were seeded on a 24-well plate at 50,000 cells/well and differentiated for 6 days. Polyplexes were formulated as described above using 60 pmol siGAPDH or siGFP at an N/P ratio of 10. Each formulation was evaluated in triplicate wells. Polyplexes were diluted in 400 μL OptiMEM and incubated with cells for 5 hrs, after which the polyplex solution was removed and replaced with complete growth medium. 48 hours after transfection, total RNA was isolated using an RNeasy Mini Kit (Qiagen, Valencia, CA). 500 ng of RNA from each sample was reverse-transcribed using Omniscript RT (Qiagen) and random hexamers as primers (Operon, Huntsville, AB). Quantitative PCR was performed using a model 7300 Real Time PCR system (Applied Biosystems, Foster City, CA) following universal thermal cycling parameters. GAPDH expression levels were determined in 20 μL reactions using TaqMan Universal PCR Master Mix (Applied Biosystems) and a TaqMan gene expression assay for rat GAPDH, and were normalized by expression levels for beta-actin (TaqMan gene expression assay for rat ACTB). Relative GAPDH expression levels for each sample were determined based on a comparison with untreated control samples, and were calculated by the 2-ΔΔCT method [26].
2.6. Statistical analysis
Statistical significance was determined using the Student’s t-test. A p-value of less than 0.05 was considered as statistically significant.
3. Development and evaluation of peptide-modified PEI vectors
3.1. Neuron targeting
For neuron-specific delivery, it is desirable to target vehicles using ligand-receptor interactions in order to minimize secondary effects due to off-target delivery. To this end, several classes of neuron-specific ligands have been employed: neuropeptides, neurotrophins, and neurotoxins [9]. Of particular note is tetanus toxin (TeNT), which binds to peripheral neurons at their presynaptic terminals and is transported retrograde in motor neurons. The heavy chain of TeNT (TeNT Hc), which is responsible for TeNT binding [27], has been conjugated to PLL for neuron-specific uptake [28]. This approach is especially attractive because the TeNT Hc is internalized by neurons without associated toxicity or apparent activation of signaling pathways [29].
Tet1 is a 12-mer peptide, identified by phage display against the GT1b ganglioside, that displays similar binding characteristics to tetanus toxin [30]. This peptide was shown to be internalized and transported by neurons both in vitro and in vivo [31]. Park et al. demonstrated that neuron association of nonviral gene delivery vehicles is significantly increased when the vectors are modified with Tet1 peptide [22]. Tet1 peptide bearing a C-terminal cysteine was covalently linked to PEI using disulfide chemistry at ~0.6 Tet1 peptides per PEI and polyplexes were formed with plasmid DNA. These Tet1-targeted polyplexes exhibited similar physicochemical properties, including hydrodynamic size and surface charge, as untargeted polyplexes. The Tet1-targeted polyplexes also mediated significant increases in association with cultured neuron-like PC-12 cells and primary DRG cells compared to untargeted polyplexes (Figure 2). This association was successfully competed with a 1000-fold excess of free Tet1 peptide, suggesting a specific receptor-ligand interaction.
Figure 2.
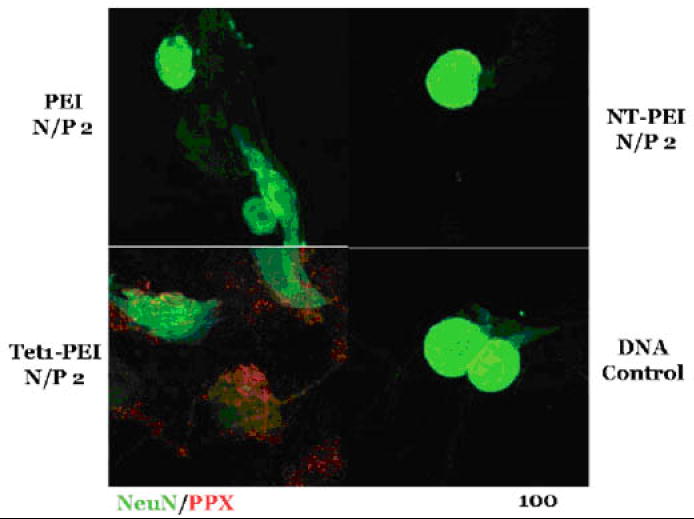
Binding of PEI-, neurotensin-PEI- (NT-PEI-), and Tet1-PEI-complexed Cy3-labeled DNA (red) to primary dorsal root ganglion cells. Polyplexes were incubated with cells for 1 hour at 37 °C. Neurons were stained with NeuN (green). (Figure from: Park et al. [22] Reproduced with permission from J Gene Med 9(8): 691-702. Copyright 2007 John Wiley & Sons Limited.)
3.2. Endosomal Release
After internalization via the endocytic pathway, it is necessary for non-viral gene delivery vehicles to exit the endosomes to avoid eventual lysosomal degradation. One strategy that has been employed is the use of protonatable cationic polymers, of which PEI has been the most widely used due to its efficacy [32]. PEI is hypothesized to mediate endosome rupture through a proton-sponge mechanism [33]. In brief, excess proton accumulation in endosomes due to the buffering capacity of protonatable polymers leads to counterion and water accumulation, resulting in osmolysis. However, this phenomenon is dependent on polymer concentration in the endosome, and high polymer concentrations in the endosome are required for optimal potency. Since high polymer concentrations can result in cell toxicity and since free polymer often becomes separated from polymer/DNA complexes in vivo, there is impetus to develop more potent mediators of endosomal escape.
Membrane-disruptive peptides have the potential to significantly increase the transfection efficiencies afforded by synthetic vehicles, and have thus been used widely in the development of non-viral gene delivery vehicles as reviewed elsewhere [21, 34, 35]. Our group has investigated three virally-derived peptides reported in the literature to have lytic activity: “AP6” from adenovirus protein VI [36], “HGP” from HIV-1 gp41 protein [37], and “L240” from papillomavirus L2 minor capsid protein [38]. Initial screens of these peptides to determine relative lytic activities were conducted using a liposomal dye release assay [36]. It was found that the HGP peptide mediated the most potent membrane disruption, which was 16-fold and 8-fold more potent than L240 and AP6 peptides, respectively, based on the percentage of dye released from liposomes (data not shown).
HGP was evaluated as a mediator of endosomal release for non-viral gene delivery vehicles [23]. When HGP was conjugated to PEI (PEI-HGP) at ~2-3 peptide per PEI and complexed with DNA, it displayed significantly increased lytic activity in the liposomal dye release assay compared to free HGP peptide at matched concentrations (0.13 μM, 0.19 μM, and 0.26 μM for N/P ratios of 2, 3, and 4, respectively) and unmodified PEI complexes at the same concentrations (Figure 3A). In addition, when gene transfer efficiency was compared between PEI and PEI-HGP polyplexes in HeLa cells using the luciferase reporter system, PEI-HGP mediated significant increases over PEI at N/P ratios of 2 and 3 (Figure 3B). The intracellular distribution of polyplexes formed with labeled DNA was investigated with confocal microscopy [23]. It was found that DNA delivered with PEI exhibited punctate staining which was highly colocalized with vesicles labeled with Alexa Fluor 488-labeled 10,000 MW dextran, whereas DNA delivered with PEI-HGP exhibited diffuse staining that did not colocalize with vesicles, indicative of endosomal release.
Figure 3.
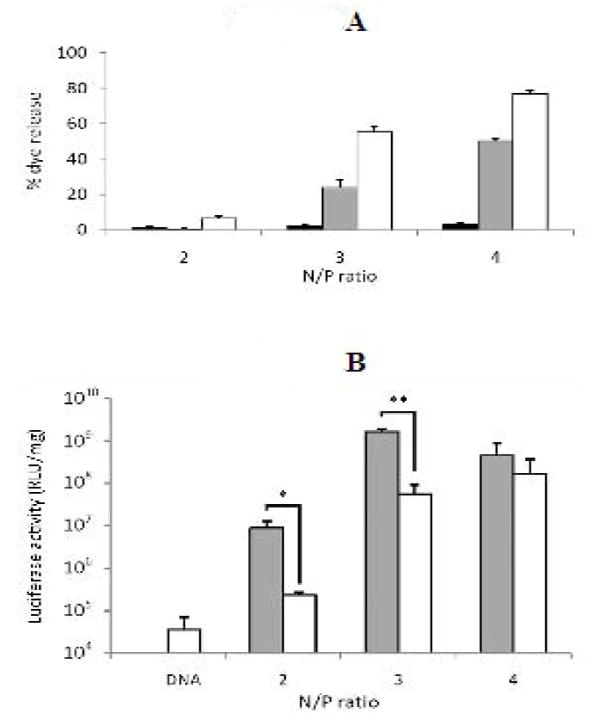
A. Percent dye release mediated by free HGP peptide (black bars), PEI polyplexes (grey bars), and PEI-HGP polyplexes (white bars). 100% dye release was achieved by solubilizing liposomes with a solution of Triton X-100. Results are reported as the mean ± SD of triplicate samples. (Figure from: Kwon et al. [23]. Reprinted in part with permission from Bioconj Chem 19(4):920-7. Copyright 2008 American Chemical Society.)
B. Luciferase reporter gene delivery to HeLa cells by PEI-HGP (grey bars) and PEI (white bars) polyplexes formulated at N/P ratios of 2, 3, and 4. Results are reported as the mean RLU/mg protein ± SD of triplicate samples. (*p<0.05, ** p<0.001) (Figure from: Kwon et al. [23]. Reprinted in part with permission from Bioconj Chem 19(4):920-7. Copyright 2008 American Chemical Society.)
3.3. Retrograde transport
The cell cytoplasm is a dense and crowded environment, resulting in severely restricted motility of macromolecules [39, 40]. Although some viruses have been shown to overcome diffusional barriers in the cytoplasm by trafficking on microtubule networks within intracellular vesicles [15, 41, 42], other viruses have been shown to directly utilize the dynein motor pathway to transport toward the cell nucleus [17-19, 43]. We and others have hypothesized that synthetic vectors might also be endowed with the ability to hitchhike on dynein motor complexes through the introduction of dynein-binding domains to the vectors [44-46]. To this end, we have investigated two potential peptides as dynein-binding peptides for intracellular vector attachment to motor assemblies.
The dynein motor is a multiprotein complex composed of heavy, intermediate, and light chain polypeptide constituents with roles in motor, scaffolding and proposed cargo recognition, respectively [47]. Dynein light chains include LC8, Tctex-1/rp3 and roadblock. A reported peptide consensus motif for cargo binding to LC8 found in several viral proteins that bind to LC8 is: (K/R)XTQT [48]. This peptide sequence was selected for further evaluation as a potential dynein-binding peptide. It was found that, while this peptide motif binds to free LC8, the peptide was not capable of binding to dynein-associated LC8 [45]. Recent studies have confirmed that a cargo protein with the KXTQT motif competes with dynein intermediate chain for the same binding groove in LC8 [49].
Structural studies with the Tctex-1 protein suggest that the cargo interacting domains of Tctex-1 reside at a distance from the proposed DIC binding domain of Tctex-1 [50]. Therefore, Tctex-1 is another promising target for a dynein-binding peptide. Two recent publications have reported that the C-terminus of the human papillomavirus (HPV) L2 minor capsid protein mediates endosomal escape and dynein motor interactions [43, 51]. Various domains of the L2 minor capsid protein were evaluated for membrane lysis and dynein binding ability [24]. The optimal sequence, based on the 40 C-terminal amino acids of the HPV L2 protein (denoted L240), that exhibited high dynein binding and membrane lytic behavior was conjugated to PEI at ~3 peptides per polymer. The resulting polymer, PEI-L240, was shown to transfect HeLa cells with ~20-fold higher transfection efficiency than the unmodified polymer. The mechanism of increased delivery efficiency was investigated using mutant L240 peptides, and found to include contributions from both endosomal release and dynein-binding properties [24].
3.4. Nucleic acid delivery to cultured, neuron-like cells using peptide-modified vectors
The aforementioned studies with PEI-HGP and PEI-L240 constructs were conducted in HeLa cells due to their ease of culture and transfection sensitivity. In this work, the peptide-modified polymers PEI-HGP and PEI-L240 were evaluated for their ability to deliver both plasmid and siRNA to neuron-like PC-12 cells. In addition, a vector modified with both the neuron-targeting Tet1 peptide and the endosomal release HGP peptide (Tet1-PEI-HGP) was synthesized to probe for possible synergistic effects on transfection efficiency.
3.4.1. Polyplex size and zeta potential
The hydrodynamic sizes and zeta potentials of polyplexes containing plasmid DNA and siRNA were determined by dynamic light scattering (Figure 4). Our studies have shown that formulations with siRNA require high N/P ratios for siRNA activity to be observed (unpublished results). Therefore, polyplexes were formulated at an N/P ratio of 10 with siRNA and at an N/P ratio of 3 with plasmid DNA. Hydrodynamic sizes and zeta potentials were similar for the peptide-polymer series (~100-120 nm for siRNA and ~110-160 nm for plasmid DNA). Zeta potential measurements for polyplexes formulated with siRNA (N/P of 10) were positive (~ +30 mV), which was expected since complexes were formulated with a large excess of polycation. Zeta potential measurements for polyplexes formulated with plasmid DNA at an N/P ratio of 3 were near neutral since there was only a slight excess of polycation at this charge ratio. However, DNA was fully complexed at this N/P ratio, as demonstrated by gel retardation studies (data not shown).
Figure 4.
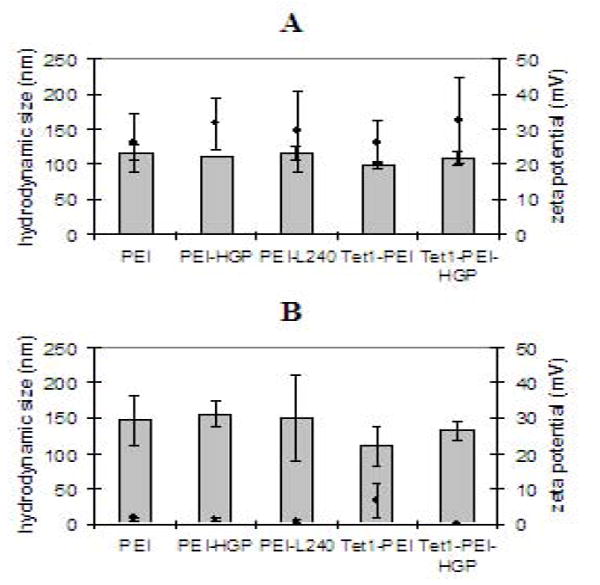
Hydrodynamic size (bars) and zeta potential (points) of polyplexes formulated by condensing siRNA (A) or plasmid DNA (B) with PEI-peptide conjugates at a N/P ratios of 10 or 3, respectively.
3.4.2. Plasmid delivery to PC-12 cells
Transfection efficiency of PEI-peptide conjugates to 2-day differentiated PC-12 cells was evaluated using the luciferase reporter system. Transfection within 2-3 days of differentiation is necessary for evaluating plasmid delivery to PC-12 cells because, at this stage, the PC-12 cells have started to exhibit a neuron-like phenotype with sprouting neurites, but the cells are still slowly dividing, allowing for plasmid delivery to the nucleus. Because the transfection efficiency of PEI-based carriers is sensitive to N/P ratio especially at low charge ratios, the polymer to DNA ratios between the different formulations were confirmed before transfection by gel retardation assays using fine increments (0.5 N/P) of charge ratios. These assays demonstrated DNA condensation at the same N/P ratios for all formulations and were therefore consistent with calculated N/P ratios. Luciferase activity was assessed 48 hours after delivery (Figure 5). PEI modified with membrane-lytic peptides HGP and L240 mediated significantly increased transfection compared to PEI (120-fold (p<0.01) and 35-fold (p<0.05) increases, respectively). PEI modified with targeting peptide Tet1 also mediated significantly increased transfection over PEI (520-fold increase (p<0.001)). The highest transfection efficiency was achieved by the double conjugate, Tet1-PEI-HGP. This conjugate mediated a 9-fold increase in reporter gene expression compared to PEI-HGP (p<0.01), a 30-fold increase compared to PEI-L240 (p<0.001), and a 2-fold increase compared to Tet1-PEI (p<0.05).
Figure 5.
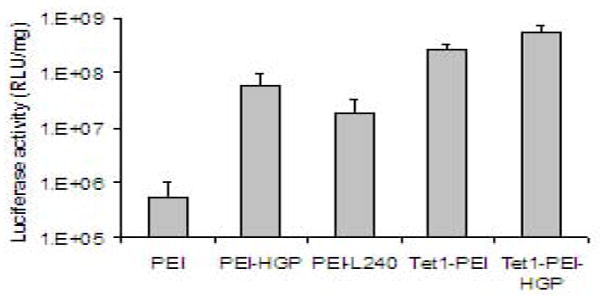
Transfection efficiency of PEI, PEI-HGP, PEI-L240, Tet1-PEI, and Tet1-PEI-HGP to 2-day differentiated PC-12 cells. Results are reported as the mean RLU/mg protein ± SD for samples in replicates of 6.
3.4.3. siRNA delivery to PC-12 cells
siRNA delivery by PEI-peptide conjugates to 6-day differentiated PC-12 cells was evaluated by quantifying the degree of specific knockdown of the endogenous gene GAPDH (Figure 6). siRNA transfection can be evaluated in fully differentiated PC-12 cells since, unlike plasmid DNA, siRNA is active in the cytosol. Peptides designed to enhance endosomal escape (HGP and L240) increased the percentage of GAPDH knockdown by ~3-fold compared to PEI (p<0.05). Still, siRNA knockdown efficiency is lower in PC-12 cells compared to HeLa cells [23]. The Tet1 targeting ligand did not enhance siRNA delivery to differentiated PC-12 cells either as a PEI-Tet1 or Tet1-PEI-HGP conjugate. Unlike plasmid DNA delivery, siRNA must be complexed at high N/P ratios for efficient knockdown activity. The lack of targeting by Tet1 is possibly due to high nonspecific binding to cells by the highly positive-charged particles formulated at N/P 10.
Figure 6.
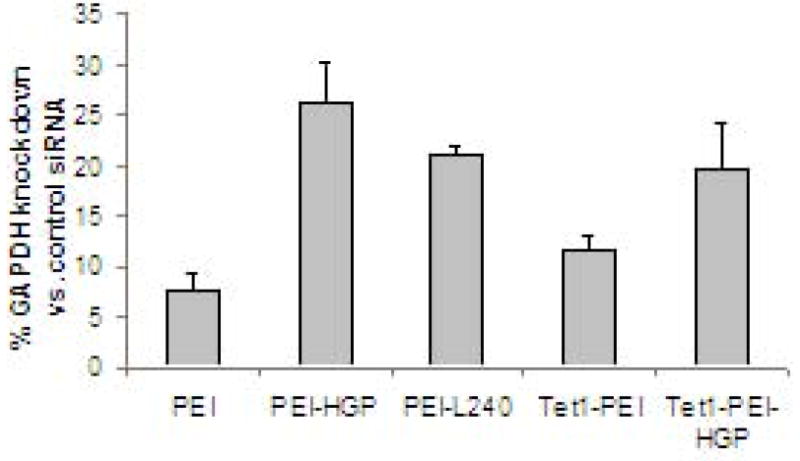
siRNA complexed with PEI, PEI-HGP, PEI-L240, Tet1-PEI and Tet1-PEI-HGP was delivered to 6-day differentiated PC-12 cells. The percentage of GAPDH expression compared to untreated control cells is reported as the mean ± SD for tripicate samples.
4. Conclusions and future directions
We have summarized our recent work identifying and applying bioactive peptides for enhancing non-viral gene delivery and have demonstrated the potential of multicomponent, peptide-modified vectors for neuron-targeted delivery. Synthetic peptides are promising materials because they can be economically produced [52] and modularly incorporated into existing vectors. In this work, we have demonstrated that multiple peptides can be combined to synergistically improve gene transfection efficiencies. We believe that the synergistic effect achieved by incorporating multiple peptides into a gene vector can be further improved by optimizing the relative peptide ratios and the method of display on the vector. Future work in our group will focus on the development of peptide-based vectors with controllable peptide ratios as well as the in vivo evaluation of these vectors for nucleic acid delivery to the nervous system.
Acknowledgments
This work was funded by the NIH/NINDS (R21NS052030) and an NSF CAREER Award (CBET 0448547). EJK and JMB acknowledge the University of Washington’s Engineered Biomaterials Training Program for graduate fellowship support (T32GM065098). JMB was supported by the Whitaker Foundation Graduate Fellowship. We are grateful to Patrick Stayton (UW Bioengineering) for the use of his ZetaPALS dynamic light scattering analyzer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dua T, Cumbrera MG, Mathers C, Saxena S. Neurological Disorders: public health challenges. World Health Organization; Geneva, Switzerland: 2006. pp. 27–39. [Google Scholar]
- 2.Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301(5634):839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 3.Ralph GS, Radcliffe PA, Day DM, Carthy JM, Leroux MA, Lee DC, Wong LF, Bilsland LG, Greensmith L, Kingsman SM, Mitrophanous KA, Mazarakis ND, Azzouz M. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat Med. 2005;11(4):429–433. doi: 10.1038/nm1205. [DOI] [PubMed] [Google Scholar]
- 4.Mandel RJ, Spratt SK, Snyder RO, Leff SE. Midbrain injection of recombinant adeno-associated virus encoding rat glial cell line-derived neurotrophic factor protects nigral neurons in a progressive 6-hydroxydopamine-induced degeneration model of Parkinson’s disease in rats. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(25):14083–14088. doi: 10.1073/pnas.94.25.14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harper SQ, Staber PD, He X, Eliason SL, Martins IH, Mao Q, Yang L, Kotin RM, Paulson HL, Davidson BL. RNA interference improves motor and neuropathological abnormalities in a Huntington’s disease mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(16):5820–5825. doi: 10.1073/pnas.0501507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia H, Mao Q, Eliason SL, Harper SQ, Martins IH, Orr HT, Paulson HL, Yang L, Kotin RM, Davidson BL. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med. 2004;10(8):816–820. doi: 10.1038/nm1076. [DOI] [PubMed] [Google Scholar]
- 7.Bar-Or A, Vollmer T, Antel J, Arnold DL, Bodner CA, Campagnolo D, Gianettoni J, Jalili F, Kachuck N, Lapierre Y, Niino M, Oger J, Price M, Rhodes S, Robinson WH, Shi FD, Utz PJ, Valone F, Weiner L, Steinman L, Garren H. Induction of antigen-specific tolerance in multiple sclerosis after immunization with DNA encoding myelin basic protein in a randomized, placebo-controlled phase 1/2 trial. Archives of Neurology. 2007;64(10):1407–1415. doi: 10.1001/archneur.64.10.nct70002. [DOI] [PubMed] [Google Scholar]
- 8.Schubert M, Breakefield X, Federoff H, Frederickson R, Lowenstein P. Gene Delivery to the Nervous System. Molecular Therapy. 2008;16(4):640–646. doi: 10.1038/mt.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergen JM, Park IK, Horner PJ, Pun SH. Nonviral approaches for neuronal delivery of nucleic acids. Pharmaceutical Research. 2008;25(5):983–998. doi: 10.1007/s11095-007-9439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suk JS, Suh J, Choy K, Lai SK, Fu J, Hanes J. Gene delivery to differentiated neurotypic cells with RGD and HIV Tat peptide functionalized polymeric nanoparticles. Biomaterials. 2006;27(29):5143–5150. doi: 10.1016/j.biomaterials.2006.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergen JM, Pun SH. Analysis of the intraceltular barriers encountered by nonviral gene carriers in a model of spatially controlled delivery to neurons. Journal of Gene Medicine. 2008;10(2):187–197. doi: 10.1002/jgm.1137. [DOI] [PubMed] [Google Scholar]
- 12.Suk JS, Suh J, Lai SK, Hanes J. Quantifying the intracellular transport of viral and nonviral gene vectors in primary neurons. Exp Biol Med (Maywood) 2007;232(3):461–469. [PubMed] [Google Scholar]
- 13.Papadopoulos S, Jurgens KD, Gros G. Protein diffusion in living skeletal muscle fibers: Dependence on protein size, fiber type, and contraction. Biophysical Journal. 2000;79(4):2084–2094. doi: 10.1016/S0006-3495(00)76456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verkman AS. Solute and macromolecule diffusion in cellular aqueous compartments. Trends in Biochemical Sciences. 2002;27(1):27–33. doi: 10.1016/s0968-0004(01)02003-5. [DOI] [PubMed] [Google Scholar]
- 15.Lakadamyali M, Rust MJ, Babcock HP, Zhuang XW. Visualizing infection of individual influenza viruses. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(16):9280–9285. doi: 10.1073/pnas.0832269100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald D, Vodicka MA, Lucero G, Svitkina TM, Borisy GG, Emerman M, Hope TJ. Visualization of the intracellular behavior of HIV in living cells. The Journal of cell biology. 2002;159(3):441–452. doi: 10.1083/jcb.200203150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leopold PL, Kreitzer G, Miyazawa N, Rempel S, Pfister KK, Rodriguez-Boulan E, Crystal RG. Dynein- and microtubule-mediated translocation of adenovirus serotype 5 occurs after endosomal lysis. Hum Gene Ther. 2000;11(1):151–165. doi: 10.1089/10430340050016238. [DOI] [PubMed] [Google Scholar]
- 18.Dohner K, Wolfstein A, Prank U, Echeverri C, Dujardin D, Vallee R, Sodeik B. Function of dynein and dynactin in herpes simplex virus capsid transport. Molecular Biology of the Cell. 2002;13(8):2795–2809. doi: 10.1091/mbc.01-07-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith GA, Enquist LW. Break ins and break outs: viral interactions with the cytoskeleton of Mammalian cells. Annu Rev Cell Dev Biol. 2002;18:135–161. doi: 10.1146/annurev.cellbio.18.012502.105920. [DOI] [PubMed] [Google Scholar]
- 20.Martin ME, Rice KG. Peptide-guided gene delivery. Aaps Journal. 2007;9(1):E18–E29. doi: 10.1208/aapsj0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergen JM, Pun SH. Peptide-enhanced nucleic acid delivery. Mrs Bulletin. 2005;30(9):663–667. [Google Scholar]
- 22.Park IK, Lasiene J, Chou SH, Horner PJ, Pun SH. Neuron-specific delivery of nucleic acids mediated by Tet1-modified poly(ethylenimine) The journal of gene medicine. 2007;9(8):691–702. doi: 10.1002/jgm.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon EJ, Bergen JM, Pun SH. Application of an HIV gp41-Derived Peptide for Enhanced Intracellular Trafficking of Synthetic Gene and siRNA Delivery Vehicles. Bioconjugate chemistry. 2008;19(4):920–927. doi: 10.1021/bc700448h. [DOI] [PubMed] [Google Scholar]
- 24.Bergen JM, Wei K, Pun SH. An HPV-derived peptide with dynein-binding and membranolytic properties enhances nonviral gene delivery through multiple mechanisms. 2008 submitted. [Google Scholar]
- 25.von Harpe A, Petersen H, Li Y, Kissel T. Characterization of commercially available and synthesized polyethylenimines for gene delivery. J Control Release. 2000;69(2):309–322. doi: 10.1016/s0168-3659(00)00317-5. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif) 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Halpern JL, Neale EA. Neurospecific binding, internalization, and retrograde axonal transport. Current topics in microbiology and immunology. 1995;195:221–241. doi: 10.1007/978-3-642-85173-5_10. [DOI] [PubMed] [Google Scholar]
- 28.Knight A, Carvajal J, Schneider H, Coutelle C, Chamberlain S, Fairweather N. Non-viral neuronal gene delivery mediated by the HC fragment of tetanus toxin. European journal of biochemistry / FEBS. 1999;259(3):762–769. doi: 10.1046/j.1432-1327.1999.00108.x. [DOI] [PubMed] [Google Scholar]
- 29.Fishman PS, Carrigan DR. Retrograde Trans-Neuronal Transfer of the C-Fragment of Tetanus Toxin. Brain Research. 1987;406(12):275–279. doi: 10.1016/0006-8993(87)90792-x. [DOI] [PubMed] [Google Scholar]
- 30.Liu JK, Teng Q, Garrity-Moses M, Federici T, Tanase D, Imperiale MJ, Boulis NM. A novel peptide defined through phage display for therapeutic protein and vector neuronal targeting. Neurobiology of disease. 2005;19(3):407–418. doi: 10.1016/j.nbd.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 31.Federici T, Liu JK, Teng Q, Yang J, Boulis NM. A means for targeting therapeutics to peripheral nervous system neurons with axonal damage. Neurosurgery. 2007;60(5):911–918. doi: 10.1227/01.NEU.0000255444.44365.B9. [DOI] [PubMed] [Google Scholar]
- 32.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonawane ND, Szoka FC, Jr, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. The Journal of biological chemistry. 2003;278(45):44826–44831. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 34.Martin ME, Rice KG. Peptide-guided gene delivery. The AAPS journal. 2007;9(1):E18–29. doi: 10.1208/aapsj0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrer-Miralles N, Vazquez E, Villaverde A. Membrane-active peptides for non-viral gene therapy: making the safest easier. Trends in biotechnology. 2008;26(5):267–275. doi: 10.1016/j.tibtech.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Wiethoff CM, Wodrich H, Gerace L, Nemerow GR. Adenovirus protein VI mediates membrane disruption following capsid disassembly. Journal of virology. 2005;79(4):1992–2000. doi: 10.1128/JVI.79.4.1992-2000.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreno MR, Giudici M, Villalain J. The membranotropic regions of the endo and ecto domains of HIV gp41 envelope glycoprotein. Biochimica et biophysica acta. 2006;1758(1):111–123. doi: 10.1016/j.bbamem.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Kamper N, Day PM, Nowak T, Selinka HC, Florin L, Bolscher J, Hilbig L, Schiller JT, Sapp M. A membrane-destabilizing peptide in capsid protein L2 is required for egress of papillomavirus genomes from endosomes. Journal of virology. 2006;80(2):759–768. doi: 10.1128/JVI.80.2.759-768.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popov S, Poo M. Diffusional transport of macromolecules in developing nerve processes. J Neuroscience. 1992;12:77–85. doi: 10.1523/JNEUROSCI.12-01-00077.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seksek O, Biwersi J, Verkman AS. Translational diffusion of macromolecule-sized solutes in cytoplasm and nucleus. Journal of Cell Biology. 1997;138(1):131–142. doi: 10.1083/jcb.138.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klingen Y, Conzelmann KK, Finke S. Double-labeled rabies virus: Live tracking of enveloped virus transport. Journal of virology. 2008;82(1):237–245. doi: 10.1128/JVI.01342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelkmans L, Kartenbeck J, Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nature Cell Biology. 2001;3(5):473–483. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- 43.Florin L, Becker KA, Lambert C, Nowak T, Sapp C, Strand D, Streeck RE, Sapp M. Identification of a dynein interacting domain in the papillomavirus minor capsid protein L2. Journal of virology. 2006;80(13):6691–6696. doi: 10.1128/JVI.00057-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mastrobattista E, van der Aa M, Hennink WE, Crommelin DJA. Artificial viruses: a nanotechnological approach to gene delivery. Nature Reviews Drug Discovery. 2006;5(2):115–121. doi: 10.1038/nrd1960. [DOI] [PubMed] [Google Scholar]
- 45.Bergen JM, Pun SH. Evaluation of an LC8-binding peptide for the attachment of artificial cargo to dynein. Molecular Pharmaceutics. 2007;4(1):119–128. doi: 10.1021/mp060086o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.RN C, Rashkin M, Wen X, Szoka F. Molecular motors as drug delivery vehicles. Drug Discovery Today. 2005;2:111–118. doi: 10.1016/j.ddtec.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Vale RD. The molecular motor toolbox for intracellular transport. Cell. 2003;112(4):467–480. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- 48.Vallee RB, Williams JC, Varma D, Barnhart LE. Dynein: An ancient motor protein involved in multiple modes of transport. Journal of Neurobiology. 2004;58(2):189–200. doi: 10.1002/neu.10314. [DOI] [PubMed] [Google Scholar]
- 49.Benison G, Karplus PA, Barbar E. Structure and dynamics of LC8 complexes with KXTOT-motif peptides: Swallow and dynein intermediate chain compete for a common site. Journal of Molecular Biology. 2007;371(2):457–468. doi: 10.1016/j.jmb.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 50.Wu HW, Maciejewski MW, Takebe S, King SM. Solution structure of the Tctex1 dimer reveals a mechanism for dynein-cargo interactions. Structure. 2005;13(2):213–223. doi: 10.1016/j.str.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 51.Kamper N, Day PM, Nowak T, Selinka HC, Florin L, Bolscher J, Hilbig L, Schiller JT, Sapp M. A membrane-destabilizing peptide in capsid protein L2 is required for egress of papillomavirus genomes from endosomes. Journal of virology. 2006;80(2):759–768. doi: 10.1128/JVI.80.2.759-768.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bray BL. Large-scale manufacture of peptide therapeutics by chemical synthesis. Nature Reviews Drug Discovery. 2003;2(7):587–593. doi: 10.1038/nrd1133. [DOI] [PubMed] [Google Scholar]


