Abstract
Hydrotropic polymers (HPs) and their micelles have been recently developed as vehicles for delivery of poorly water-soluble drugs, such as paclitaxel (PTX), by oral administration. The release of PTX from HP micelles, however, was slow and it took more than a day for complete release of the loaded PTX. Since the gastrointestinal (GI) transit time is known to be only several hours, pH-sensitive HP micelles were prepared for fast release of the loaded PTX responding to pH changes along the GI tract. Acrylic acid (AA) was introduced, as a release modulator, into HPs by copolymerization with 4-(2-vinylbenzyloxy)-N,N-(diethylnicotinamide) (VBODENA). The AA content was varied from 0% to 50 % (in the molar ratio to VBODENA). HPs spontaneously produced micelles in water, and their critical micelle concentrations (CMCs) ranged from 31 μg/mL to 86 μg/mL. Fluorescence probe study using pyrene showed that blank HP micelles possessed a good pH-sensitivity, which was clearly observed at relatively high AA contents and pH > 6. The pH sensitivity also affected the PTX loading property. Above pH 5, the PTX loading content and loading efficiency in HP micelles were significantly reduced. Although this may be primarily due to the AA moieties, other factors may include PTX degradation and polymer aggregation. The PTX release from HP micelles with more than 20% (mol) AA contents was completed within 12 h in a simulated intestinal fluid (SIF, pH=6.5). The HP micelles without any AA moiety showed very slow release profiles. In the simulated gastric fluid (SGF, pH=1.6), severe degradation of the released PTX was observed. The pH-dependent release of PTX from HP micelles can be used to increase the bioavailability of PTX upon oral delivery.
Keywords: Hydrotropic polymer, Micelle, Paclitaxel, Acrylic acid, pH sensitive
1. Introduction
Polymer micelles have been widely used as a carrier system for poorly water-soluble drugs. However, traditional polymer micelles assembled by physical driving force have been limited in their application primarily due to the poor stability in aqueous environments and the low drug loading capacity [1, 2]. To alleviate these problems, hydrotropic polymer (HP) micelles have been developed in our previous studies. Similar to typical polymer micelles, HP micelles possess a hydrophilic corona and a hydrophobic core. A distinct difference between the conventional and HP micelles is the presence of a hydrotropic agent in the hydrophobic core of the HP micelles. It has been reported that hydrotropes, e.g., nicotinamide and p-toluenesulfonate, can solubilize poorly soluble drugs [3-5] by specific interactions such as π-π stacking [6]. Although the exact mechanism is still unclear, the overall driving force inducing drug solubilization is called the hydrotropic interaction. The HP micelles showed higher drug loading capacity and higher aqueous stability than the conventional micelles, due to the hydrotropic interaction in addition to hydrophobic interaction. The HP micelles also resolved a problem associated with using hydrotropes for oral administration. Because hydrotropes are usually low molecular weight compounds, they can be absorbed into the body after oral administration, and this may cause undesirable side effects. This particular problem can be removed by polymer micelles, as they are not expected to be absorbed after oral administration. In vitro cell culture tests showed that HP micelles were not cytotoxic [7].
HP micelles that have been studied to date contain a common hydrotropic monomer unit, N,N-diethylnicotinamide (DENA) [2, 7-9]. The DENA moiety was identified after an extensive screening of many hydrotrope candidates for solubilizing paclitaxel (PTX), a representative of poorly water soluble drugs [6]. PTX is an effective anti cancer drug for several types of cancers, but its poor water solubility is limiting its clinical application [10, 11]. The PTX solubility in 3.5 M aqueous DENA solution (39.07 mg/mL) was drastically increased from that in pure water (0.3 μg/mL). Based on the DENA structure, a new monomer of 4-(2-vinylbezyloxy)-DENA (VBODENA) was synthesized and polymerized. HP micelles containing DENA moieties showed high PTX loading capacity and excellent aqueous stability in vitro [2, 7]. The drug release study, however, showed that it took about 24 hours to complete PTX release from the HP micelle [2]. Since the retention time of an oral drug formulation in the gastrointestinal (GI) tract is known to be much less than 12 h [12], it is desirable to release all PTX from the HT micelles within 12 h to maximize the bioavailability. One way to accelerate the PTX release from HT micelles is to exploit the pH changes in the GI tract.
In the GI tract, the pH varies from 1.0-2.5 in the stomach to 5.1-7.8 in the small intestine [13, 14]. Such wide variation of pH along the GI tract has been utilized for controlled drug release from carriers [15]. Acrylic acid (AA) is a monomer that has been widely used in the development of pH-sensitive drug delivery systems. It becomes hydrophilic by deprotonation above pH 4.5 [16, 17]. Therefore, it is hypothesized that physical stability of HP micelles containing AA moieties decreases at the pH in the intestine, releasing the loaded PTX faster in the intestine than in the stomach. To examine this hypothesis, we prepared AA-containing HP micelles and tested PTX loading/release profiles by changing the medium pH. The primary goal is to evaluate a pH-sensitive HP micelle as a controlled PTX delivery system for oral application.
2. Materials and Methods
2.1. Materials
Methoxy poly(ethylene glycol) (MPEG, MW 5,000 g/mole), 2-hydroxynicotinic acid (HNA, 98%), 1,1’-carbonyldiimidazole (CDI), 4-vinylbenzyl chloride (VBC, 90%), N,N-diethylamine (DEA), potassium carbonate (K2CO3), tert-butyl acrylate (tBA), 2-bromopropionyl bromide (BPB, 97%), N,N,N’,N”,N”-pentamethyldiethylenetriamine (PMDETA), triethylamine (TEA, 99.99%), 2-(N-morpholino)-ethansulfonic acid (MES), tris-(hydroxymethyl) aminoethane (Tris), copper (I) bromide (Cu(I)Br), sodium dodecyl sulfate (SDS), sodium salicylate, sodium taurocholate, lecithin (L-α-phosphatidylcholine), silica gel (60 Å, 130-270 mesh), acetone, dichloromethane (DCM, anhydrous), and toluene (anhydrous) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Paclitaxel was provided by Samyang Genex Co. (Daejeon, Korea). The tBA was double distilled under vacuum condition and acetone was distilled with excess K2CO3 before use. All other chemicals were used as received.
2.2. Synthesis of hydrotropic polymers
The hydrotropic monomer, VBODENA, was synthesized by the same method as described previously [7, 8]. HNA (20 g, 140.8 mmol) was reacted with CDI (23 g, 142.0 mmol) in 900 mL THF at 70 °C. After 24 h, 400 mL THF was added into the crude solution and 30 mL DEA (299.0 mmol) was slowly dropped and further reaction was performed at 70 °C for 18 h. White crystal of 2-hydroxy-DENA (HDENA) was obtained by repeated recrystallization in 50 mL THF. The VBODENA was prepared by reaction between HDENA (10 g, mmol) and VBC (15 mL, mmol) in 900 mL anhydrous acetone containing K2CO3 (20 g) at 60 °C for a day. White VBODENA crystal was generated by silica gel column chromatography (THF:n-hexane = 7:1) and recrystallization in THF/n-hexane (1:1) (final yield 60 %). 1H-NMR assignment revealed that structure of the VBODENA was identical to previously reported results [7].
To prepare a macroinitiator (MPEG-Br) for atom transfer radical polymerization (ATRP), MPEG (20 g) was dissolved in anhydrous DCM (30 mL) and precipitated twice against anhydrous diethyl ether (2 L), followed by vacuum drying with P2O5 for three days. And then, BPB (10 mL, mmol) wad dropped into MPEG (10 g, 2 mmol) solution in anhydrous DCM (100 mL) in the presence of TEA (1 mL) at 4 °C for a day. After treating with charcoal, the solution was concentrated and precipitated twice against excess diethyl ether. Yellowish powder was dissolved in 250 mL DCM and repeatedly extracted using deionized water. White powder of MPEG-Br was obtained by precipitation and vacuum drying with P2O5 (yield 93 %).
A series of HPs containing AA moieties were synthesized by ATRP method as shown in Fig. 1. Reagents used in ATRP and abbreviations for each polymer are listed in Table 1. To remove air, MPEG-Br, VBODENA, and Cu(I)Br were added into one-necked Schlenk flask, followed by three cycles of pumping and N2 gas purging for 30 min. Meanwhile, toluene and tBA were degassed by N2 gas bubbling. Degassed tBA and toluene (1 mL) was added into the flask, and finally PMDETA was added. Oxygen trace was further removed by repeated cycles of pumping and N2 gas purging. Reaction was performed at 80 °C under slightly reduced pressure. During polymerization, increased viscosity and color change from weak green to dark green were observed. In 6 h, polymer was retrieved by 10 mL DCM and copper catalyst was removed by silica gel column. Eluted solution was precipitated against diethyl ether and products of white powder, PEG-b-P(VBODENA-co-tBA)s, were dried under vacuum. Hydrolysis of tert-butyl group was accomplished by DCM/TFA (1:1) mixture (10 mL) for 2 h. After evaporating all liquids, polymers were dissolved in 5 mL DCM and then, repeatedly precipitated against diethyl ether. Final products, PEG-b-P(VBODENA-co-AA)s, were dried and stored under vacuum till further experiments.
Fig. 1.
Synthetic scheme of hydrotropic block copolymers by varying molar ratios of VBODENA to AA.
Table 1.
Recipe for synthesis of PEG-b-(VBODENA-co-AA)s.
| Polymers (VBODENA:AA molar ratio) | Abbrev. | MPEG-Br (mmol) | VBODENA (mmol) | tBA (mmol) | Cu(I)Br (mmol) | PMDETA (mmol) | Yield (%) |
|---|---|---|---|---|---|---|---|
| PEG-b-P(VBODENA) | HP0 | 0.1 | 1.608 | 0 | 0.4 | 0.4 | 73 |
| PEG-b-P(VBODENA-co-AA) (90:10) | HP1 | 0.1 | 1.567 | 0.1742 | 0.4 | 0.4 | 70 |
| PEG-b-P(VBODENA-co-AA) (80:20) | HP2 | 0.1 | 1.520 | 0.3799 | 0.4 | 0.4 | 65 |
| PEG-b-P(VBODENA-co-AA) (70:30) | HP3 | 0.1 | 1.463 | 0.627 | 0.4 | 0.4 | 79 |
| PEG-b-P(VBODENA-co-AA) (60:40) | HP4 | 0.1 | 1.392 | 0.928 | 0.4 | 0.4 | 80 |
| PEG-b-P(VBODENA-co-AA) (50:50) | HP5 | 0.1 | 1.305 | 1.305 | 0.4 | 0.4 | 80 |
2.3. Polymer characterization
Chemical structure of each polymer was confirmed by 1H-NMR (Bruker ARX300 spectrophotometer, 300 MHz, Madison, WI, USA). Since the amounts of tBA and AA were too small to be detected by 1H-NMR analysis, AA content was obtained by endpoint titration [18]. Titrant was prepared by dissolving KOH in water/methanol mixture (0.05 N). Polymer solution (50 mg) in 0.05 N HCl solution (10 mL, water:methanol mixture = 1:1) was titrated by adding the titrant. From acid-base titration curves using the same titrant and the same concentration of polymer solutions, pKa of each polymer was determined. Polymer molecular weight was calculated by GPC (Agilent 1100 Series with RI detector, Santa Clara, CA, USA) equipped with a set of three PLgel 5 μm MIXED–D and –E columns (Agilent). The flow rate was 0.5 mL/min and DMF was used as an eluent. Molecular weights were calculated from obtained chromatograms based on a series of PEG GPC standards (Polysciences Inc., Warrington, PA, USA).
2.4. Pyrene studies
Polymer micelles were prepared by dialysis against 4 L deionized water for a day. Dialysate of each polymer was filtered (0.45 μm), lyophilized and dried under vacuum condition. To determine the critical micelle concentration (CMC) of HP micelles, pyrene was dissolved in acetone and 2.4 μmol of pyrene was loaded in 4 mL vials. After evaporating acetone overnight in hood, 4 mL polymer solution in water of various concentrations were added to the vials (final pyrene conc. 6×10-7 M). All samples were incubated at 60 °C for an hour and further shaken at 37 °C for a day. Fluorescence spectra were obtained by Spex FluoroMax-2 spectrophotometer (Jobin Yvon Inc., Edison, NJ, USA) at room temperature. With a set of 0.5 nm excitation and 0.5 nm emission slits, fluorescence measurement was preformed as described elsewhere [7]. Intensity ratio (I339/I334) of excitation spectra (emission 390 nm) was calculated and plotted as a function of polymer concentration. CMC for each polymer was determined at an inflection point of the plot.
Pyrene study was also employed to examine the pH sensitivity of blank HP micelles. Polymer micelle was reconstituted by adding 4 mL of buffer solution (phosphate for pH 1, 2; acetate for pH 4; MES for pH 6; phosphate for pH 7; Tris for pH 8) to pre-micellized polymer powder (4 mg). Each buffer had 10 mM concentration and 154 mM ionic strength (physiological condition). Fluorescence intensity ratio of I339/I334 was obtained as described above.
2.5. Preparation of PTX-loaded HP micelles
PTX (17.5 mg) and each polymer (50 mg) were dissolved in 10 mL acetonitrile and stirred for 6 h at room temperature. Each solution was dialyzed (MWCO 3500) against 4 L of dialysis media, either deionized water (pH 6.2) or buffer solutions with different pHs described above. The dialysis media were refreshed every 8 h. After one day, each dialysate was filtered by a membrane filter (0.45 μm pore) to remove aggregated particles. The filtrates were lyophilized and powders were stored at -20 °C until further experiments.
PTX loading content and loading efficiency were determined by HPLC analysis. HPLC samples were prepared by dissolving PTX-containing micelles in the powder form in acetonitrile (1 mg/mL). After injecting a 100 μL sample solution, chromatograms were monitored at 227 nm (PTX) and 332 nm (λmax of HP polymers) by a diode array multi-wavelength detector (Agilent). To resolve PTX and polymer peaks, a binary gradient mode (water/acetonitrile) was employed at a constant flow rate of 1 mL/min using a reverse phase (RP) column (C18, 5 μm pore size, 3.9×150 mm, Waters Corp., Milford, MA, USA). After 5% acetonitrile flowing for 5 min, its content was gradually increased up to 95% for another 30 min. Based on standard curves, the amounts of PTX and polymers were calculated.
2.6. Polymer micelle size analysis
The sizes of blank HP micelles and PTX-loaded HP micelles were measured by PDLLS/Batch dynamic light scattering (DLS) connected to PD2000DLS detector (Precision Detectors, Bellingham, MA, USA) at room temperature. Freeze-dried powder of the micelles (1 mg/mL) was reconstituted in water, followed by filtering through a 0.45 μm syringe filter. Each sample was counted for 10 sec. Reported values correspond to the average from 10 replicate diameter measurements.
2.7. PTX release studies
Before conducting release experiments, optical transmittance of each PTX-loading micelle was observed by varying pH values. Initially, HP micelles containing PTX were reconstituted in 10 mM phosphate buffer (pH 1). By adding 1 N NaOH solution, pH was elevated from 1 to 8. The optical transmittance at 500 nm was measured by Cary 50Bio UV-VIS spectrophotometer (Varian Inc., Palo Alto, CA, USA).
For in vitro PTX release test, HP micelles containing 100 μg PTX were suspended in 1 mL distilled water and placed in a dialysis bag (MWCO 3500). To examine the release profile of free PTX, 100 μg PTX in 50 μL acetonitrile was added in 1 mL distilled water, and then, transferred to the dialysis bag. PTX in the bag was immediately precipitated. The whole bag containing each micelle or PTX particle was placed into 40 mL release media and shaken (120 rpm) at 37 °C. Simulated gastric fluid without pepsin (SGF, pH 1.6; 0.01-0.05 N HCl, 2.5 g SDS, 2 g NaCl, 1 L water) and simulated intestinal fluid without pancreatin (SIF, pH 6.5; 3 mM sodium taurocholate, 0.75 mM lecithin, 3.0 g K2HPO4, 7.7 g KCl, 1 L water) were used as release media [19-21]. For release test in SIF, after one hour incubation of HP micelles in 40 mL SGF at 37 °C, sample bags were transferred into 40 mL SIF and further incubated at 37 °C. At a given time, 0.5 mL samples were taken and the same volume of fresh solution was added to tubes. Into each sample, 0.5 mL acetonitrile was added and stored till RP-HPLC analysis. As release media, either sodium salicylate solution (0.8 M, pH 6.5) or 10 mM MES buffer (pH 6.0, μ = 154 mM) containing 1 % (v/v) Tween 80 was used in another release experiments.
3. Results and Discussion
3.1. Polymer synthesis
HPs used in this study were polymerized by ATRP method as shown in Fig. 1. ATRP is known to be an excellent polymerization method providing well-defined structure and good controllability of the molecular weight [22, 23]. As reported previously, VBODENA is a good monomer for ATRP [2, 7, 8]. It is noted that the AA monomer was not polymerized by the ATRP method, as it forms a metal carboxylate by rapidly reacting with the metal catalysts [23]. Because of this reason, the AA moieties were introduced by hydrolysis of tBA, a good monomer for ATRP [22, 23]. In the 1H-NMR analysis, tBA peaks were shown around 1.0-1.1 ppm, and disappeared after the hydrolysis process. Since it was difficult to integrate tBA peaks due to their overlapping with diethyl peaks of VBODENA, the AA content of each HP was determined by endpoint titration. As listed in Table 2, the determined AA contents were in agreement with the initial feed ratios.
Table 2.
Characterization of hydrotropic block copolymers and their micelles.
| Polymer | Mn (GPC) | CMC (μg/mL) | pKa | Target AA content (%, mol) | Calculated AA content (%, mol) | LE (wt%) | LC (wt%) | Micelle Size (nm) | |
|---|---|---|---|---|---|---|---|---|---|
| w/o PTX | w/PTX | ||||||||
| HP0 | 6900 | 31.0 | 7.51 | 0 | 0 | 88.7 | 31.0 | 87.4±5.6 | 94.4±9.9 |
| HP1 | 7100 | 84.7 | 4.53 | 10 | 10.2 | 87.8 | 30.8 | 90.2±3.8 | 92.5±4.9 |
| HP2 | 7100 | 86.6 | 4.50 | 20 | 16.8 | 87.5 | 30.6 | 87.4±7.9 | 87.4±7.3 |
| HP3 | 7520 | 83.4 | 4.58 | 30 | 26.4 | 85.2 | 29.8 | 84.7±4.0 | 89.7±2.3 |
| HP4 | 9100 | 83.6 | 4.57 | 40 | 38.6 | 87.4 | 30.6 | 87.1±5.6 | 90.2±3.8 |
| HP5 | 9620 | 75.3 | 4.50 | 50 | 49.1 | 87.4 | 30.6 | 86.5±4.2 | 87.4±7.9 |
Mn: Calculated based on PEG standards.
CMC: Determined by fluorescence spectrometry using pyrene (6 × 10-7 M).
pKa: Determined by the acid-base titration curve (titrant 0.05 N KOH in water/methanol (1:1)).
Calculated AA content: Determined by titration(titrant 0.05 N KOH). AA content (mole %) + VBODENA content (mole %) = 100 %.
Loading efficiency (LE) = (the amount of paclitaxel in micelle (μg)/the initially fed amount of paclitaxel (μg))×100 % (w/w).
Loading content (LC) = (the amount paclitaxel in micelle (μg)/total weight of paclitaxel-loading micelle (μg))×100 % (w/w).
Micelle size with or without PTX (nm) = mean ± S.D. (n = 3)
3.2. The pH sensitivity of HP micelles
To examine the pH-sensitivity of HPs, acid-base titration curves were constructed. The pKa of each polymer was also listed in Table 2, which was similar to pKa values of poly(acrylic acid) [24]. As shown in Fig. 2a, HP1-5 possessed a broad range of buffering zone from pH 4.5 (pKa of AA) to 7.5, while HP0 without AA did not show any buffering effect. The buffering effect of HPs was enhanced by increasing the AA content. Even a small molar fraction (10 mole%) of AA effectively showed the pH sensitivity.
Fig. 2.
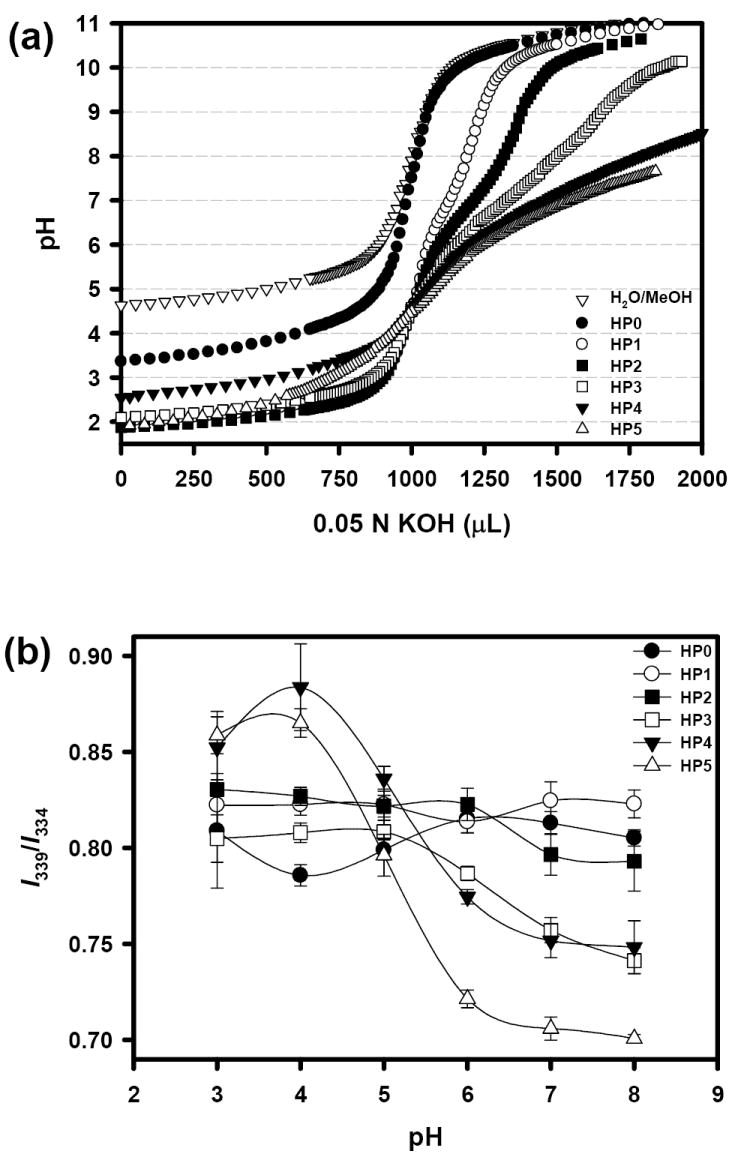
Acid-base titration curves for blank (methanol:water=1:1) and six hydrotropic polymers (HPs) (a), and the pyrene study monitoring the pH-sensitivity of HP micelles (b). The low value of I339/I334 represents the micelle destabilization. (n = 3).
HP micelles were prepared by dialysis against water. Their CMC values obtained from the pyrene study and the micelle size (85–90 nm) are listed in Table 2. Introduction of the AA moiety resulted in substantial increase in the CMC values of HP1-5 to 75-87 μg/mL, as compared with 31 μg/mL for HP0. Increased hydrophilicity of the hydrophobic block is attributed to the increased CMC values. The content of the AA moiety in HPs, however, did not affect the CMC values significantly. It is possible that AAs may not fully overcome the hydrotropic and hydrophobic interaction between VBODENA (310 g/mol) because of its smaller size (72 g/mol).
The pH sensitivity of blank HP micelles was monitored by the fluorescence spectrometry (Fig. 2b). Excitation or emission spectra of pyrene have been a useful indicator for determining the CMC and stability of micelles [25-29]. The I339/I334 ratio decreases when micelles are destabilized. Micelles prepared from HP0 and HP1 were not influenced by the environmental pH change whereas micelles of HP3-5 showed good sensitivity, especially at pH > 6. At pH 4, the HP micelles containing more than 40 mol% of AA moieties showed the highest I339/I334 ratio. This means that the micelle core becomes more hydrophobic. Because the pKa value of AA is about 4.0-4.5, protonated AAs would increase the I339/I334 ratio by stacking more pyrene molecules. While only 10 mole% of free AAs could generate buffering zone in the titration experiment, the blank HP micelles required more than 30 mole% of AA. Since the micelle core consists of two monomers, VBODENA and AA, disassembly of the HP micelles may require more protonation of AAs in the core [27]. It is similar to pH-sensitive changes of poly(L-lactide)-b-PEG-b-poly(L-histidine) micelles, in which the hydrophobic poly(L-lactide) block is a key unit maintaining the micelle structure after ionization of the histidine moieties [26].
3.3. Loading PTX in HP micelles
Based on the pH sensitivity of blank HP micelles, we hypothesized that PTX load in the micelle could depend on the pH of the medium. When distilled water was used as a dialysis medium, the PTX loading efficiency reached almost 90% and the loading content was also up to 30%, regardless of the AA content in HP micelles (Table 2). After loading PTX, the micelle size was slightly increased in general (Table 2). However, it was observed that the loading content of PTX in HP micelles highly depended on the pH of loading media (Fig. 3a). Low pH (≤ 4) provided approximately 20% (w/w) loading content. It is reasonable that, because pKa values of HPs except HP0 are around 4.5, the amount of PTX loaded in HP micelles can be significantly lowered at higher pH (> 4) by the AA deprotonation. Similarly, increase in the AA content significantly deteriorated the PTX loading content. The effect of AA and pH on the PTX loading content was also supported by the negligible change of the PTX loading content in HP0 micelles. Fig. 3b shows that the PTX loading efficiency of HP micelles was governed by the pH of the loading media. The maximum loading efficiency did not exceed 60%. Interestingly, the maximum loading efficiency was obtained at pH 4 regardless of AA contents in HP micelles. Rapid decrease in the loading efficiency was observed by increasing pH (> 4), especially in the HP micelles with higher AA contents. For HP2-5 micelles, the loading efficiency was relatively high at lower pH (< 4) than at higher pH (< 4). However, it was difficult to find a relationship between the AA content and loading efficiency.
Fig. 3.
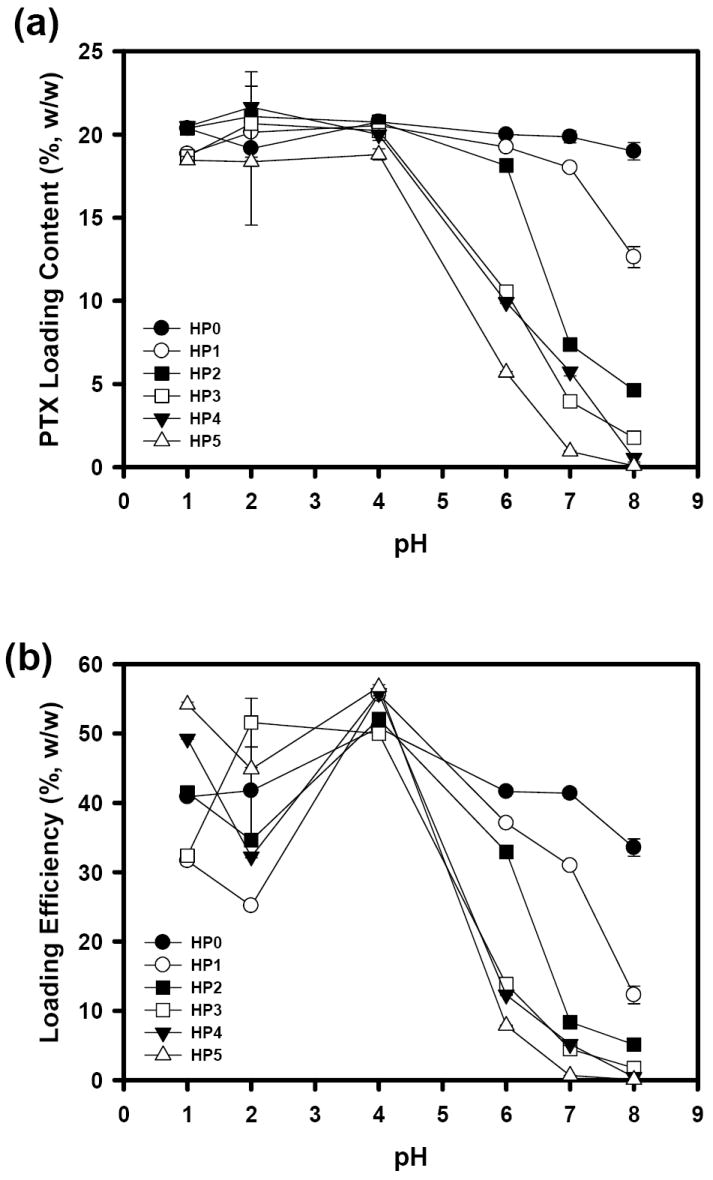
PTX loading content (a) and loading efficiency (b) as a function of pH. The PTX loading content (%, w/w) was calculated by [PTX weight/ (PTX + polymer) weight)] × 100. The PTX loading efficiency (%, w/w) was determined by [(measured PTX weight)/(initially fed PTX weight)] × 100. (n=3 for both experiments).
The bell-shape plots in the PTX loading efficiency are very similar to the degradation profile of PTX responding to pH change. The PTX can be degraded by base-catalyzed hydrolysis [30]. The degradation rate is the slowest in aqueous buffer of pH 4, and becomes accelerated by either decreasing or increasing pH [31]. Thus, it seems that, in addition to the pH sensitivity of HP micelles based on AA moieties, secondary determinant controlling the PTX loading in HP micelles is the pH-dependent PTX degradation rate.
Even though PTX is stable at pH 4, the loading efficiency of less than 60% is still too low. Another possible explanation is the salting-in effect to disturb the stability of PTX. Due to three hydroxyl groups, PTX forms a dimer structure. Hydrogen bonding between the hydroxyl groups of PTX is important to generate the PTX crystal [32]. On the other hand, it was reported that the best stability of non-covalent PTX dimer was achieved in acetonitrile/acetic acid mixture rather than methanol/acetic acid mixture [33]. In that experiment, the acetic acid (pKa=4.7) was used to provide an appropriate pH to prevent PTX degradation. Acetonitrile is preferred to methanol for PTX dimer stabilization, because acetonitrile has neither hydrogen-bonding donor nor acceptor group. As a result, the PTX stabilization and crystallization highly depend on the hydrogen bonding between PTX molecules. In the present study, PTX was loaded in micelles after dissolving in acetonitrile and dialysis against water or buffer. Therefore, the existence of salts probably affected the loading efficiency of PTX, because ion species might interfere with the hydrogen bonding between PTX molecules. Moreover, HP polymers also possess multiple sites for hydrogen bonding (e.g., ether of PEG, pyridine ring of VBODENA, and AA). By the same reason, ionic species in buffer solution might also disturb the interaction between HP polymers or between PTX and polymer.
It was observed that PEG-b-P(VBODENA-co-AA) itself was not stable in buffer solution. The AA moiety at pH lower than its pKa is known to form a complex with ether backbone of the PEG via hydrogen bonding. There is a critical pH to facilitate the complexation (pHcrit), and below the pHcrit, precipitation occurs. The pHcrit depends on ionic species of additive salts and usually, it becomes higher as the salt concentration increases [34]. Due to this reason, it is possible that the HPs containing the AA moiety are aggregated at low pH (< 4). In fact, severe aggregation was observed during the PTX loading process at low pH, and this could be originated from polymer-polymer interactions.
The above observations indicate that the PTX loading efficiency in buffer solutions can be explained by multiple factors, such as PTX degradation/destabilization and inter-polymer aggregation. It is also possible that due to protonation of nitrogen atom in the pyridine ring, the VBODENA loses its hydrotropic effect at pH below 3.35 (pKa of nicotinamide) [35]. Moreover, the ion species possibly interfere with the stacking ability of the hydrotropic monomer. By the same reason, the difference between the PTX loading contents in Table 2 and Fig. 3a can be explained. Since the best result of PTX loading in HP micelles were obtained from dialysis against water, those HP micelles loading PTX were used for further experiments.
3.4. PTX release studies in simulated body fluids
When the PTX-loaded HP micelles were immediately dispersed in an acidic buffer (pH 1), neither precipitation nor aggregation was observed (Fig. 4 left insert). It means that after PTX loading, the HP micelles can to be stable under the stomach condition, at least for a short period. By increasing pH, the micelles showed rapid change in the optical transmittance (turbidity). This change reflected the pH-sensitivity of PTX-loading HP micelles [26]. Fig. 4 displays the change in the optical transparency of HP micelle dispersion. Higher pH (> 6) and more AAs led to more opaque solution. The right insert of Fig. 4 shows the difference in optical transparency at pH 8. This result indicated that PTX release from the HP micelles could be modulated by changing the medium pH.
Fig. 4.
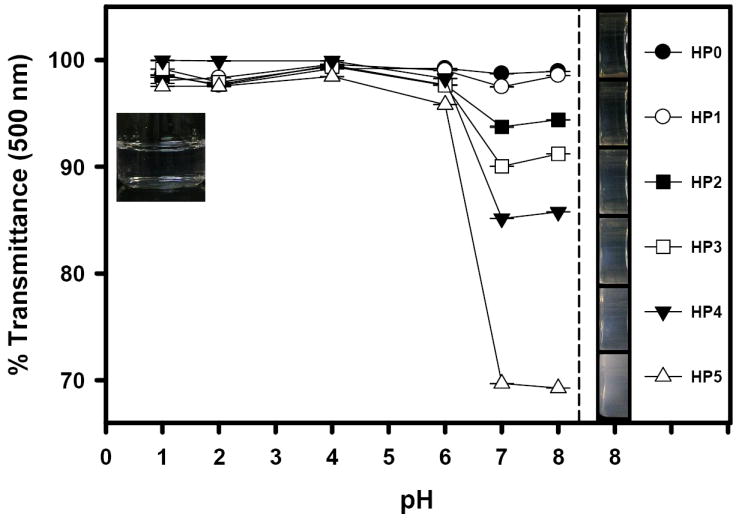
Optical transmittance at 500 nm as a function of pH. The optical transmittance was changed by adding 1 N NaOH solution into each HP micelle dispersion (10 mM phosphate buffer, pH 1.0, μ = 154 mM). Left insert shows the HP micelles in the acidic buffer (pH 1.0) and right insert presents their optical transparency at pH 8.0.
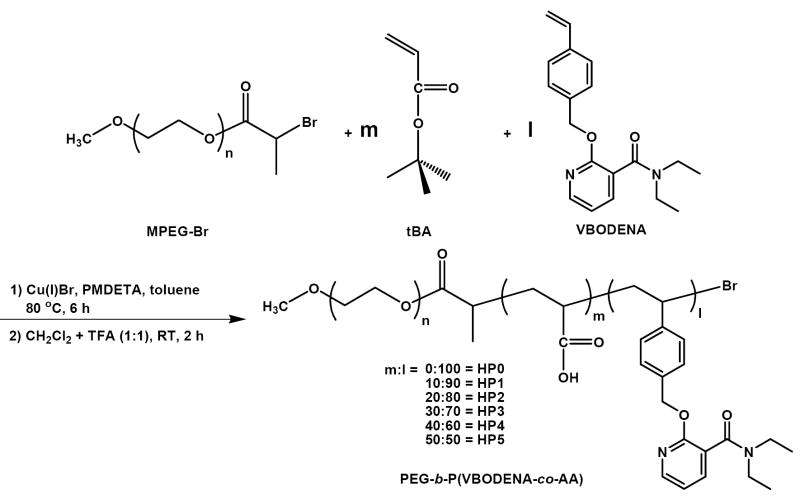
In vitro release test was performed using either simulated intestinal fluid (SIF, Fig. 5a) or simulated gastric fluid (SGF, Fig. 5b), in order to mimic the body conditions. For Fig. 5a, the whole dialysis bag containing the PTX-loading HP micelles was placed in 40 mL SGF for an hour, which was transferred to 40 mL SIF for further the experiment (3 days). Although both simulated fluids are standard solutions for the drug dissolution test [19, 20], there exist few reports on the drug release tests conducted by SGF and SIF [36, 37]. It was reported that solubility of PTX in lecithin/sodium deoxycholate system was 71 μg/mL [38]. Because the initial PTX amount was 100 μg and the volume of SIF was 40 mL in this study, it was considered that the SIF could provide a good sink condition for PTX release test. In SGF, PTX was severely degraded within a few hours so that it was difficult to define the solubility.
Fig. 5.
Cumulative amount of the released PTX as a function of time in SIF (a) and in SGF (b). (n = 3). The amounts of PTX release in SGF (0-1 h) and in SIF (1-2 h) are compared to determine the difference of release rate in both solutions (c). The asterisk indicates that there exists statistical significance (one-way ANOVA P < 0.05, c)
The release profile of PTX in SIF (after the first one hour in SGF) for three days is shown in Fig. 5a. Free PTX particle produced by precipitation showed very slow release rate, and its total cumulative amount was no more than 10 μg during three days of experiment. The HP2-5 micelles released almost half of the initially loaded PTX within 2–3.5 h, and the plateau was accomplished within 8-12 h. After 12 h, the release profiles showed negative slope, which meant that PTX was slowly degraded (GIF = pH 6.5). In contrast, the HP0 micelle showed relatively slow release rate, and the total amount of released PTX was limited to less than 50 μg. However, negative slope was not observed. Possible explanation is that the amount of released PTX was either larger than or compatible to the amount of degraded PTX. The total amounts of released PTX from the HP2–5 micelles were about 81–86%, and this may be also due to the degradation of PTX. The release rate of HP1 micelle (10% AAs) was slower than HP2-5 micelles , but faster than HP0 micelle. Its plateau was achieved in one day, from which point slow degradation of PTX was also observed.
The data in Fig. 5a shows that the AA moieties in HP micelles with more than 20 mole% efficiently increased the PTX release rate in SIF. Fig. 5c shows that the amounts of released PTX from each HP micelle for the first hour at pH 1.6 and for another hour at pH 6.5 (0–2 h of Fig. 5a, not cumulative amount). The One-way ANOVA result suggests that there exists a statistically significant difference (*P < 0.05) between the amounts of released PTX in SGF and in SIF. The difference was obtained from HP2-5. This means that the HP micelles have the pH sensitivity and that the release rate of PTX depends on the environmental pH.
Administration of Technetium (99mTc)-labeled resin particle revealed that the GI transit time is about 12 h in human [12]. Although GI transit time can be varied by multiple factors [14], it is desirable that drug release from any oral drug formulation is completed within 12 h to avoid the drug loss and to maximize the bioavailability. The present study was also initiated from a hypothesis that the HP0 micelle possibly could not liberate all PTX within GI transit time (<12 h) due to the strong hydrotropic interaction. Based on the result shown in Fig. 5a, it was confirmed that the HP0 micelle held significant portion of the initially loaded PTX over 12 h. The HP1-5 micelles showed promising results for oral delivery of PTX, as the drug release can be completed during the GI transit time.
Fig. 5b shows the PTX release profile in SGF, which was generally lower than that in SIF. After reaching the maximum in 2-4 h, the released PTX was rapidly degraded and finally, no PTX was detectable in a day. There was a small difference in the release profiles between HP1-5 micelles up to 12 h, but it was not significant as observed from the SIF. HP0 micelles seemed to strongly hold PTX even at low pH because the maximal amount of released PTX was about half of that from other micelles. This result suggested that, at least in the PTX release test, hydrophobicity increased by protonation of AAs (HP1-5) was not stronger than hydrotropic effect (HP0). As shown in Fig. 5c, however, the release rate of PTX in the SGF was significantly slower than that in the SIF. Therefore, loss of the PTX during the stomach transit time (~1 h) is not expected to be significant.
3.5. PTX release test in aqueous Tween 80 and sodium salicylate
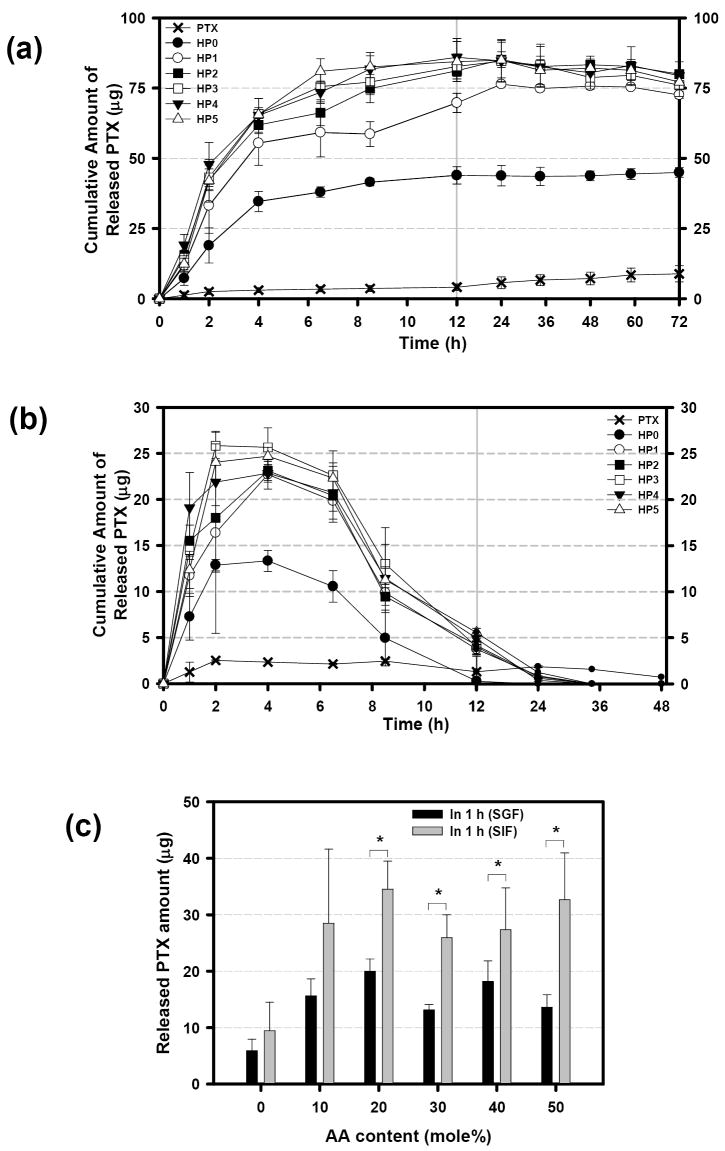
In our previous study, 0.8 M sodium salicylate solution was used as a release medium to generate a sink condition [2, 7, 8]. As a control experiment, the release test was performed using 0.8 M sodium salicylate solution (pH 6.5). Fig. 6a, shows that the PTX release from various micelles was completed within 8 h, and relatively fast degradation of the released PTX was observed. In another experiment, 1%(v/v) Tween 80 in MES buffer (pH 6.5) was used for the PTX release test (HP0-2 micelles only, Fig 6b). The Tween 80 has been used as a sink-inducing agent for release tests of hydrophobic drugs [39, 40]. In Tween 80-containing buffer, the PTX release rate was very slow and the plateau was achieved in 48 h. Although the AA effect on release profile was abolished in this buffer, it was observed that the HP0 micelles could not completely release PTX. The negative slope shown in Fig 6a (0.8 M sodium salicyate) and Fig. 5a (SIF) is due to the PTX degradation. The order of the PTX release rate was sodium salicylate > SIF > Tween 80-containing buffer. It is noticeable that the order of the slope steepness was the same. This means that the PTX is liable to be degraded only after release from micelles. Therefore, the plateau does not mean that there is no degradation, but that the amount of released PTX is compatible to that of degraded PTX.
Fig. 6.
Cumulative amount of released PTX as a function of time in 0.8 M sodium salicylate aqueous solution at pH 6.5 (a) and in 10 mM MES buffer containing 1 % (v/v) Tween 80 at pH 6 and ionic strength of 154 mM (b). (n=3 for a, and n=4 for b).
The difference in release profiles of Fig. 6a and 6b can be explained by the different nature of sink-inducing agents. Tween 80 is a surfactant forming micelle structure in water [41], while sodium salicylate is a hydrotrope for PTX [6]. The release rate of PTX into buffer solutions containing Tween 80 is known to be very slow, usually showing a linear release profile during weeks of release. Therefore, Tween 80 is not a good material for creating a sink condition for PTX. Also, it may be hard to disrupt the HP micelles by Tween 80 through a dialysis membrane due to its micelle structure in water. In contrast, sodium salicylate is a powerful solubilizing agent for the PTX. The solubility of PTX in 0.8 M sodium salicylate is 5.54 mg/mL [6], which produced a good sink condition. It was found, however, that sodium salicylate solution at 0.8 M significantly affected the stability of PTX-loaded HP0 micelle as functions of time and concentration. Moreover, the 0.8 M concentration (equivalent to 12. 8%(w/v)) is very high in comparison to the Tween 80-containing buffer. Therefore, it is highly possible that sodium salicylate significantly diffuses through the dialysis membrane, and directly interacts with HP micelles to disrupt them.
The lack of the pH sensitivity in Fig. 6 can be explained by the low solubilizing power of Tween 80 as well as by the polymer micelle destabilization by sodium salicylate. For this reason, SIF was relatively good to monitor the AA effect on PTX release. SIF could successfully produce a sink condition (relatively high solubility of PTX in the aqueous solution of lecithin/deoxycholate), but appeared to hardly interfere with the HP micelle structure. However, the physiological condition cannot be perfectly mimicked by any simulated fluid. It is unknown whether the orally administered micelles can be significantly affected by constituents of the fluid in the GI tract. Thus, the bioavailability of PTX formulated in the HP micelles should be further examined by in vivo experiments. The promising in vitro results of PTX release from HP micelles sensitive to environmental pH changes provide useful information for the future in vivo studies.
4. Conclusion
The HP micelles that have been developed in our laboratory have high PTX loading capacity and aqueous stability in comparison with conventional micelles. The AA moiety was introduced into the HP micelles to achieve fast release of the loaded PTX in SIF. By varying VBODENA/tBA molar ratios, a series of HPs were prepared by ATRP method, which provided good controllability of the AA content. The HP showed a pH buffering zone ranging from pH 4.5-7.5, and could spontaneously produce micelles in water. The micelle structure was dynamically changed depending on the medium pH and AA content. The loading content of PTX in each HP micelle also dependent on the pH of loading media. And, the loading content was significantly lowered above pH 4. This was especially prominent with the HP micelles containing more than 30 mole% of AA. The loading efficiency of PTX seemed to be primarily dependent on the AA protonation, but the PTX degradation and the PTX dimer destabilization at high pH (> 4) also affected the loading efficiency. In addition, polymer aggregation resulting from the hydrogen bonding between PEG and AA moiety was also a factor leading to the low loading efficiency.
The in vitro release test in SIF showed a promising result. HP micelles containing more than 20 mole% AA completed the PTX release within 12 h, which was desirable for oral delivery. The HP0 micelle showed a very slow release rate due to strong hydrotropic interaction. Failure of the Tween 80-containing buffer to provide a sink condition and destabilization of the micelle structure by 0.8 M sodium salicylate eliminated the pH-dependency of PTX release. It is expected that continuous investigation on the HP micelles with various structures leads to an optimal formulation for oral PTX delivery.
Acknowledgments
This work was supported in part by NIH through GM065284.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaucher G, Dufresne MH, Sant VP, Kang N, Maysinger D, Leroux JC. Block copolymer micelles: preparation, characterization and application in drug delivery. J Control Release. 2005;109(13):169–188. doi: 10.1016/j.jconrel.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 2.Huh KM, Lee SC, Cho YW, Lee J, Jeong JH, Park K. Hydrotropic polymer micelle system for delivery of paclitaxel. J Control Release. 2005;101(13):59–68. doi: 10.1016/j.jconrel.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Coffman RE, Kildsig DO. Hydrotropic solubilization - Mechanistic studies. Pharm Res. 1996;13(10):1460–1463. doi: 10.1023/a:1016011125302. [DOI] [PubMed] [Google Scholar]
- 4.Holmberg K, Shah DO, Schwuger MJ Knovel (Firm) Handbook of applied surface and colloid chemistry. Wiley; Chichester, England ; New York: 2002. [Google Scholar]
- 5.Mansur CRE, Spinelli LS, Lucas EF, Gonzalez G. The influence of a hydrotropic agent in the properties of aqueous solutions containing poly(ethylene oxide)-poly (propylene oxide) surfactants. Colloid Surface A. 1999;149(13):291–300. [Google Scholar]
- 6.Lee J, Lee SC, Acharya G, Chang CJ, Park K. Hydrotropic solubilization of paclitaxel: analysis of chemical structures for hydrotropic property. Pharm Res. 2003;20(7):1022–1030. doi: 10.1023/a:1024458206032. [DOI] [PubMed] [Google Scholar]
- 7.Lee SC, Huh KM, Lee J, Cho YW, Galinsky RE, Park K. Hydrotropic polymeric micelles for enhanced paclitaxel solubility: in vitro and in vivo characterization. Biomacromolecules. 2007;8(1):202–208. doi: 10.1021/bm060307b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huh KM, Min HS, Lee SC, Lee HJ, Kim S, Park K. A new hydrotropic block copolymer micelle system for aqueous solubilization of paclitaxel. J Control Release. 2008;126(2):122–129. doi: 10.1016/j.jconrel.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SC, Acharya G, Lee J, Park K. Hydrotropic polymers: Synthesis and characterization of polymers containing picolylnicotinamide moieties. Macromolecules. 2003;36(7):2248–2255. [Google Scholar]
- 10.Liggins RT, Burt HM. Polyether-polyester diblock copolymers for the preparation of paclitaxel loaded polymeric micelle formulations. Adv Drug Deliv Rev. 2002;54(2):191–202. doi: 10.1016/s0169-409x(02)00016-9. [DOI] [PubMed] [Google Scholar]
- 11.Singla AK, Garg A, Aggarwal D. Paclitaxel and its formulations. Int J Pharm. 2002;235(12):179–192. doi: 10.1016/s0378-5173(01)00986-3. [DOI] [PubMed] [Google Scholar]
- 12.McDowell A, Nicoll JJ, McLeod BJ, Tucker IG, Davies NM. Gastrointestinal transit in the common brushtail possum measured by gamma scintigraphy. Int J Pharm. 2005;302(12):125–132. doi: 10.1016/j.ijpharm.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 13.Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29(8):1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDowell A, McLeod BJ. Physiology and pharmacology of the brushtail possum gastrointestinal tract: relationship to the human gastrointestinal tract. Adv Drug Deliv Rev. 2007;59(11):1121–1132. doi: 10.1016/j.addr.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Bromberg L. Polymeric micelles in oral chemotherapy. J Control Release. 2008 doi: 10.1016/j.jconrel.2008.1001.1018. [DOI] [PubMed] [Google Scholar]
- 16.Foss AC, Goto T, Morishita M, Peppas NA. Development of acrylic-based copolymers for oral insulin delivery. Eur J Pharm Biopharm. 2004;57(2):163–169. doi: 10.1016/S0939-6411(03)00145-0. [DOI] [PubMed] [Google Scholar]
- 17.Tian Y, Bromberg L, Lin SN, Hatton TA, Tam KC. Complexation and release of doxorubicin from its complexes with pluronic P85-b-poly(acrylic acid) block copolymers. J Control Release. 2007;121(3):137–145. doi: 10.1016/j.jconrel.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Park KH, Kim SW, Bae YH. Interaction of sulfonylurea-conjugated polymer with insulinoma cell line of MIN6 and its effect on insulin secretion. J Biomed Mater Res. 2001;55(1):72–78. doi: 10.1002/1097-4636(200104)55:1<72::aid-jbm100>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 19.Dressman JB, Amidon GL, Reppas C, Shah VP. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm Res. 1998;15(1):11–22. doi: 10.1023/a:1011984216775. [DOI] [PubMed] [Google Scholar]
- 20.Galia E, Nicolaides E, Horter D, Lobenberg R, Reppas C, Dressman JB. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm Res. 1998;15(5):698–705. doi: 10.1023/a:1011910801212. [DOI] [PubMed] [Google Scholar]
- 21.Ingels F, Deferme S, Destexhe E, Oth M, Van den Mooter G, Augustijns P. Simulated intestinal fluid as transport medium in the Caco-2 cell culture model. Int J Pharm. 2002;232(12):183–192. doi: 10.1016/s0378-5173(01)00897-3. [DOI] [PubMed] [Google Scholar]
- 22.Coessens V, Pintauer T, Matyjaszewski K. Functional polymers by atom transfer radical polymerization. Prog Polym Sci. 2001;26(3):337–377. [Google Scholar]
- 23.Patten TE, Matyjaszewski K. Atom transfer radical polymerization and the synthesis of polymeric materials. Adv Mater. 1998;10(12):901–915. [Google Scholar]
- 24.Fujiwara M, Grubbs RH, Baldeschwieler JD. Characterization of pH-Dependent Poly(acrylic Acid) Complexation with Phospholipid Vesicles. J Colloid Interface Sci. 1997;185(1):210–216. doi: 10.1006/jcis.1996.4608. [DOI] [PubMed] [Google Scholar]
- 25.Jeong JH, Park TG. Novel polymer-DNA hybrid polymeric micelles composed of hydrophobic poly(D,L-lactic-co-glycolic acid) and hydrophilic oligonucleotides. Bioconjug Chem. 2001;12(6):917–923. doi: 10.1021/bc010052t. [DOI] [PubMed] [Google Scholar]
- 26.Lee ES, Oh KT, Kim D, Youn YS, Bae YH. Tumor pH-responsive flower-like micelles of poly(L-lactic acid)-b-poly(ethylene glycol)-b-poly(L-histidine) J Control Release. 2007;123(1):19–26. doi: 10.1016/j.jconrel.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang CH, Wang CH, Hsiue GH. Polymeric micelles with a pH-responsive structure as intracellular drug carriers. J Control Release. 2005;108(1):140–149. doi: 10.1016/j.jconrel.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Opanasopit P, Ngawhirunpat T, Chaidedgumjorn A, Rojanarata T, Apirakaramwong A, Phongying S, Choochottiros C, Chirachanchai S. Incorporation of camptothecin into N-phthaloyl chitosan-g-mPEG self-assembly micellar system. Eur J Pharm Biopharm. 2006;64(3):269–276. doi: 10.1016/j.ejpb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Shim WS, Kim SW, Choi EK, Park HJ, Kim JS, Lee DS. Novel pH sensitive block copolymer micelles for solvent free drug loading. Macromol Biosci. 2006;6(2):179–186. doi: 10.1002/mabi.200500182. [DOI] [PubMed] [Google Scholar]
- 30.Tian J, Stella VJ. Degradation of paclitaxel and related compounds in aqueous solutions II: Nonepimerization degradation under neutral to basic pH conditions. J Pharm Sci. 2007 doi: 10.1002/jps.21214. [DOI] [PubMed] [Google Scholar]
- 31.Dordunoo SK, Burt HM. Solubility and stability of taxol: Effects of buffers and cyclodextrins. Int J Pharm. 1996;133(12):191–201. [Google Scholar]
- 32.Mastropaolo D, Camerman A, Luo Y, Brayer GD, Camerman N. Crystal and molecular structure of paclitaxel (taxol) Proc Natl Acad Sci U S A. 1995;92(15):6920–6924. doi: 10.1073/pnas.92.15.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorenz SA, Bigwarfe PM, Jr, Balasubramanian SV, Fetterly GJ, Straubinger RM, Wood TD. Noncovalent dimerization of paclitaxel in solution: evidence from electrospray ionization mass spectrometry. J Pharm Sci. 2002;91(9):2057–2066. doi: 10.1002/jps.10194. [DOI] [PubMed] [Google Scholar]
- 34.Khutoryanskiy VV, Mun GA, Nurkeeva ZS, Dubolazov AV. pH and salt effects on interpolymer complexation via hydrogen bonding in aqueous solutions. Polym Int. 2004;53(9):1382–1387. [Google Scholar]
- 35.Schiewe J, Mrestani Y, Neubert R. Application and Optimization of Capillary Zone Electrophoresis in Vitamin Analysis. J Chromatogr A. 1995;717(12):255–259. [Google Scholar]
- 36.Pierri E, Avgoustakis K. Poly(lactide)-poly(ethylene glycol) micelles as a carrier for griseofulvin. J Biomed Mater Res A. 2005;75(3):639–647. doi: 10.1002/jbm.a.30490. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z, Feng SS. Self-assembled nanoparticles of poly(lactide)--Vitamin E TPGS copolymers for oral chemotherapy. Int J Pharm. 2006;324(2):191–198. doi: 10.1016/j.ijpharm.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Sznitowska M, Klunder M, Placzek M. Paclitaxel solubility in aqueous dispersions and mixed micellar solutions of lecithin. Chem Pharm Bull (Tokyo) 2008;56(1):70–74. doi: 10.1248/cpb.56.70. [DOI] [PubMed] [Google Scholar]
- 39.Potineni A, Lynn DM, Langer R, Amiji MM. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive biodegradable system for paclitaxel delivery. J Control Release. 2003;86(23):223–234. doi: 10.1016/s0168-3659(02)00374-7. [DOI] [PubMed] [Google Scholar]
- 40.Oh KS, Lee KE, Han SS, Cho SH, Kim D, Yuk SH. Formation of core/shell nanoparticles with a lipid core and their application as a drug delivery system. Biomacromolecules. 2005;6(2):1062–1067. doi: 10.1021/bm049234r. [DOI] [PubMed] [Google Scholar]
- 41.Varade D, Ushiyama K, Shrestha LK, Aramaki K. Wormlike micelles in Tween-80/CmEO3 mixed nonionic surfactant systems in aqueous media. J Colloid Interface Sci. 2007;312(2):489–497. doi: 10.1016/j.jcis.2007.02.090. [DOI] [PubMed] [Google Scholar]