Abstract
Neutralization of stratum corneum (SC) adversely impacts key epidermal functions, including permeability barrier homeostasis and SC integrity. Conversely, acidification of SC improves these functions in developmentally impaired (neonatal or aged) skin, and enhances function in normal skin. Hence, we hypothesized that acidification could alter the course of inflammatory dermatoses, which invariably exhibit an increased SC pH. Maintenance of a low pH by topical applications of the polyhydroxyl acid, lactobionic acid, during the repeated-challenge phase inhibited the development of oxazolone-induced atopic dermatitis (AD). Neither gross/histological dermatitis nor altered barrier function developed, and emergence of epidermal hyperplasia was prevented; however, cytokine generation decreased. Acidification also largely normalized the development of hapten-induced changes in eosinophil/mast cell densities, density of chemoattractant receptor-homologous molecule expressed on TH2-positive lymphocytes, and serum IgE levels. The pH-induced improvement in barrier function most likely accounts for the anti-inflammatory activity, which could be further attributed to normalization of both lamellar body secretion and lamellar bilayer formation. Acidification of SC alone substantially prevents development of barrier abnormalities and downstream immune abnormalities during the elicitation phase of murine AD. These results provide direct evidence for the “outside–inside” pathogenesis of AD and further suggest that maintenance of an acidic SC pH could prevent the emergence of AD in humans.
INTRODUCTION
The normally acidic pH of stratum corneum (SC) regulates several key protective functions of the skin, including permeability barrier homeostasis (Mauro et al., 1998; Fluhr et al., 2001; Hachem et al., 2003), SC integrity and cohesion (the converse of desquamation) (Fluhr et al., 2001; Hachem et al., 2003), antimicrobial defense (Schade, 1928; Korting et al., 1990; Elias, 2007), as well as primary cytokine activation (Hachem et al., 2002). Accordingly, both in developmentally impaired (neonatal (Behne et al., 2003; Fluhr et al., 2004), aged (Choi et al., 2007a)) and inflamed skin [reviewed by Fluhr and Elias, 2002), the pH of SC increases toward neutrality. The increased pH of SC alone inactivates lipid-processing enzymes that generate ceramides in SC, although simultaneously activating a family of serine proteases (SPs) (that is, kallikreins), which compromise SC structure and function by divergent downstream mechanisms (Hachem et al., 2003, 2005b).
As pH regulates so many key cutaneous functions, we recently explored the efficacy of therapeutic approaches that normalized (lowered) pH in developmentally impaired settings. In separate reports, we first showed that hyperacidifying the SC with the polyhydroxyl acids (PHAs) enhances the structure and function of normal mouse skin (Hachem et al., 2009). Moreover, topical PHAs normalized pH and barrier function in parallel both in neonatal (Fluhr et al., 2004) and in moderately aged (Choi et al., 2007b) human and rodent skin. In both age groups, lowering of pH in turn corrected the epidermal structural and functional abnormalities that distinguish these divergent age groups from young (normal) skin. In this study, we assessed hyperacidification as a preventive/therapeutic strategy in a recently described model of hapten-induced atopic dermatitis (AD) in hairless mice (Man et al., 2008). In this model, an initial sensitizing dose with the hapten, oxazolone (Ox), followed 1 week later by a single challenge dose, elicits acute allergic contact dermatitis (Sheu et al., 2002; Fowler et al., 2003). If sensitized mice then are challenged repeatedly (10 doses over 3 weeks), an AD-like dermatitis evolves (Man et al., 2008). This oxazalone-induced atopic-like dermatitis (Ox-AD) model recapitulates virtually every clinical, structural, lipid biochemical, and immunological feature of human AD, including: (i) a chronic, pruritic, inflammatory dermatosis, (ii) elevated transepidermal water loss (TEWL) levels; (iii) ultrastructural and lipid biochemical abnormalities typical of AD; (iv) tissue eosinophilia, elevated serum IgE levels, and a Th2-dominant immunophenotype; and (v) decreased expression of two key antimicrobial peptides (AMPs), that is, cathelicidin-related antimicrobial peptide (CRAMP), the mouse homolog of the human cathelicidin carboxy-terminal fragment, LL-37, and mBD3, the murine homolog of human β-defensin 2 (Man et al., 2008). Pertinent to this study, the mice also develop an elevated surface pH, as in human AD (cited in the review by Man et al., 2008). Therefore, we asked here whether SC acidification could either prevent emergence of or reverse Ox-induced acute allergic contact dermatitis or AD. Reduction of the pH of SC with the PHA, lactobionic acid (LBA), did not modify the clinical, structural, functional, or biochemical abnormalities either in hapten-induced acute allergic contact dermatitis or in established Ox-AD, whereas maintenance of an acidic SC pH during the 3-week challenge phase largely prevented emergence of key components of Ox-AD. We show here further that the responsible mechanisms are pH-dependent improvements in barrier function, rather than anti-inflammatory effects of the LBA, providing further support for the “outside–inside” concept of AD pathogenesis (Elias et al., 1999, 2008; Taieb, 1999).
RESULTS
SC acidification prevents emergence of the pH abnormality, but does not prevent the development of acute allergic contract dermatitis
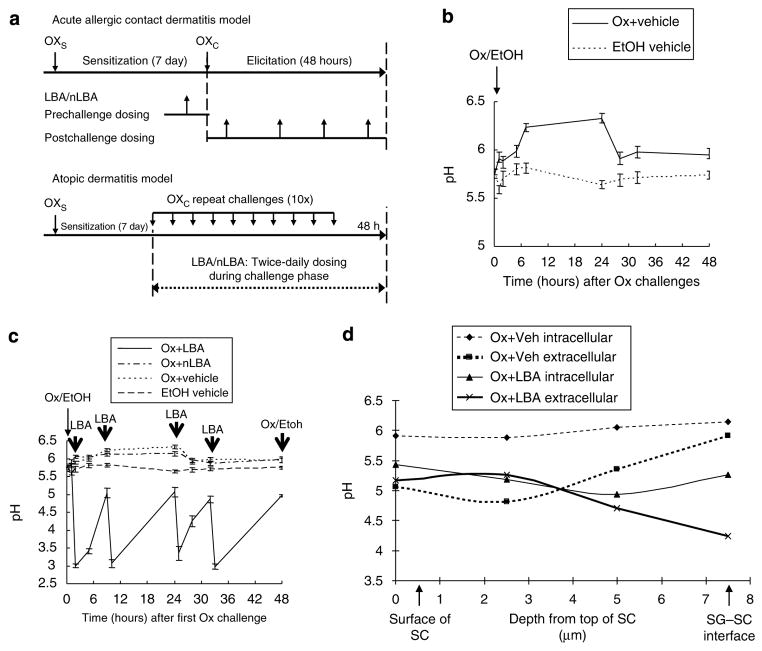
After the initial Ox challenge (Figure 1a), surface pH increases by 6 hours, well before the appearance of acute allergic contact dermatitis (Figure 1b), and surface pH remains increased during subsequent Ox challenges that eventually elicit an AD-like dermatosis (Man et al., 2008). Therefore, we first assessed whether the reduction of pH, achieved with applications of the PHA, LBA, immediately before, coincident with, or immediately after the first challenge dose, would prevent or delay the development of the pH abnormality in singly or repeatedly Ox-challenged mice (Figure 1a). In control animals, challenged one time with Ox alone, the mean surface pH increased from a mean of 5.7 to a mean of 6.3 by 8 hours, remaining increased for 24 hours before slowly normalizing over 48 hours (Figure 1b). By applying LBA immediately after the initial Ox challenge, and then twice daily thereafter, skin surface pH could be maintained at normal or subnormal levels (≤5) over the 48 hours post-challenge period (Figure 1c).
Figure 1. Topical PHA prevents the hapten-induced increase in stratum corneum pH.
Individual challenges with 0.1% oxazolone (Ox) were performed in hairless mice (h/kr) (n=8 in each group), sensitized 7 days previously with 1% Ox as described in Materials and Methods. Parallel groups of normal mice were treated with the ethanol vehicle (EtOH) alone. (a–c) LBA, a neutralized solution of LBA (nLBA), or vehicle were applied to Ox-sensitized mice at the time points indicated by the bold arrows (four times during the 48 hours between Ox challenges). (a) Experimental time line; (b) changes in surface pH over time after a single Ox challenge dose; (c) changes in surface pH in Ox-challenged mice, cotreated with LBA, nLBA, vehicle, or nonsensitized mice treated with vehicle alone (treatment time is shown by bold vertical arrows). (d) Changes in extracellular and intracellular pH within different SC microdomains after single Ox challenge plus co-applied LBA or vehicle, assessed by fluorescence life-time imaging. Ox challenge results in a significantly more neutral intracellular than extracellular pH in the mid- and lower SC, which was corrected by co-applications of LBA with Ox. Note: LBA acidifies both intracellular and extracellular domains of the SC.
Dual-photon, fluorescence lifetime imaging analysis allows localization of pH changes both at different levels of SC and in cellular (cytosolic) versus membrane (“extracellular”) domains (Behne et al., 2002). With fluorescence lifetime imaging, the LBA-induced reduction in pH could be seen to extend to all SC layers, with a further selective reduction of pH within extracellular (membrane) domains of the SC where SP and lipid-processing enzymes function, a difference that became more prominent deep in the SC (Figure 1d, representative experiment). Taken together, these results show that pH levels can be maintained at or below normal levels by the topical PHA, even in the face of single or repeated hapten challenges (cf., Figure 1c).
We next assessed whether maintenance of an acidic pH with topical LBA prevents development of hapten-induced acute allergic contact dermatitis (Figure 1a). LBA applications alone do not change ear thickness. Although both pre-applications and co-applications of LBA with Ox maintained an acidic SC pH (Figure 1) and normalized permeability barrier function (Figure S2a), Ox-challenged mice nevertheless developed levels of inflammation that were comparable with mice treated with either Ox plus neutralized LBA (nLBA) or Ox plus vehicle alone (Figure S1). These results were confirmed quantitatively both as an increase in ear thickness and as an increase in T-cell density (CD3-positive cells) occurred in singly challenged mice, regardless of the initial pH of the SC (Figures S1b and S2b). As neither topical pre-acidification nor co-acidification (Figures S1 and S2b) prevents development of hapten-induced acute allergic contact dermatitis, acidification with LBA most likely alters neither the antigenicity of the hapten nor hapten penetration. Taken together, these results show that LBA applications, despite maintaining an acidic SC pH and normal barrier function, do not prevent the emergence of Ox-induced acute allergic contact dermatitis.
SC acidification prevents the development of Ox-AD
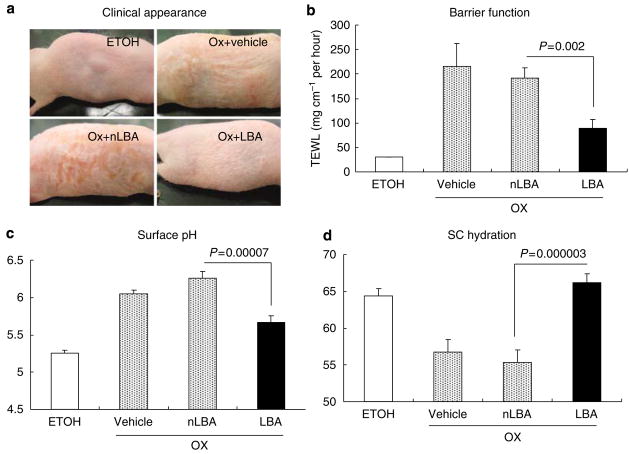
We next assessed whether normalization of SC pH would reverse preestablished Ox-AD. Reductions in SC pH alone altered neither gross nor histological evidence of inflammation or epidermal hyperplasia, nor did they normalize epidermal structure and function (data not shown). Therefore, we next asked whether maintenance of an acidic pH during the repeated-challenge phase would prevent emergence of Ox-AD (AD model; Figure 1a). In contrast to acute allergic contact dermatitis, maintenance of an acidic pH largely prevented emergence of Ox-AD (Figure 2a). Specifically, LBA-cotreated mice showed reduced erythema, scale, and almost no excoriations, indicative of pruritus, in comparison with Ox plus vehicle- or Ox plus nLBA-treated mice. As noted previously (Man et al., 2008), mice repeatedly challenged with either Ox plus vehicle alone or Ox plus nLBA develop abnormal permeability barrier function (TEWL levels increased six- to eightfold), reduced SC hydration, and notably a 1–1.5U increase in surface pH (Figure 2b–d). In contrast, these functional abnormalities did not emerge when the SC remained acidic with LBA applications during the hapten-challenge phase (Figure 2b–d; results for each parameter are statistically significant versus either Ox plus vehicle or Ox plus nLBA; P<0.01). Thus, maintenance of an acidic SC pH largely prevents the emergence of the macroscopic and functional abnormalities in Ox-AD.
Figure 2. Maintenance of normal pH prevents emergence of hapten-induced, murine atopic dermatitis.
(a) Macroscopic (clinical) appearance of repeated Ox-challenged mice, cotreated with topical LBA or controls, as above. (b–d) Transepidermal water loss (TEWL), pH and hydration (electrical capacitance) were measured after the tenth Ox challenge, as described in Materials and Methods. Normal control group indicates mice treated with the ethanol vehicle instead of Ox. Vehicle indicates control group in which water/ethanol (7:3) was applied twice daily instead of LBA or nLBA during repeated Ox challenges (cf., Figure 1a). LBA/nLBA: LBA/nLBA treatment group in which LBA/nLBA in water/ethanol (7:3) was applied twice daily, whereas Ox challenges were continued every other day. Each experiment was repeated twice and representative data are shown. For TEWL and SC hydration, n=24; for pH, n=16.
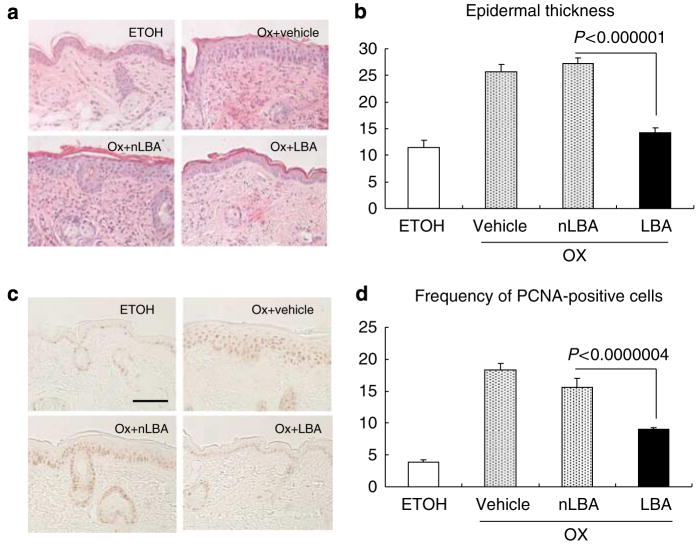
Histological studies further showed the ability of acidification to largely prevent the development of both dermal inflammation and epidermal hyperplasia in hematoxylin-and-eosin sections (Figure 3a). Both epidermal thickness and epidermal proliferation, assessed as the density of proliferating cell nuclear antigen (PCNA)-positive cells, also were largely normalized by hyperacidification (Figure 3b–d). Taken together, these studies show that hyperacidification prevents the emergence of Ox-AD, including the maintenance of normal barrier function, as well as a significant reduction in both epidermal hyperplasia and histological evidence of inflammation.
Figure 3. Maintenance of a normal pH largely prevents emergence of epidermal hyperplasia.
Skin samples were collected 48 hours after the tenth challenge with Ox, and (a) hematoxylin-and-eosin staining and (b) PCNA immunostaining were performed. All treatments were performed, as described in the legend to Figure 2. (a) Epidermal thickness and inflammation appear to be reduced in LBA-cotreated mice. (b–d) Epidermal thickness (mm) (n=30) and (c and d) PCNA immunostaining (n=14 & 28, respectively) were assessed and quantitated as described in Materials and Methods. Bar=50 μm.
SC acidification attenuates the development of Th2-dominant inflammation in Ox-AD mice
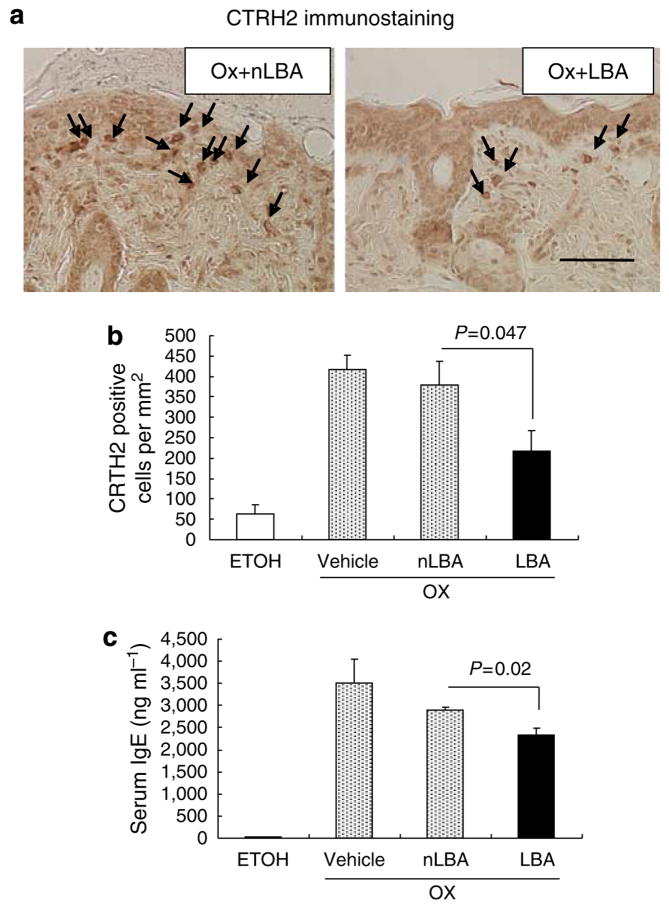
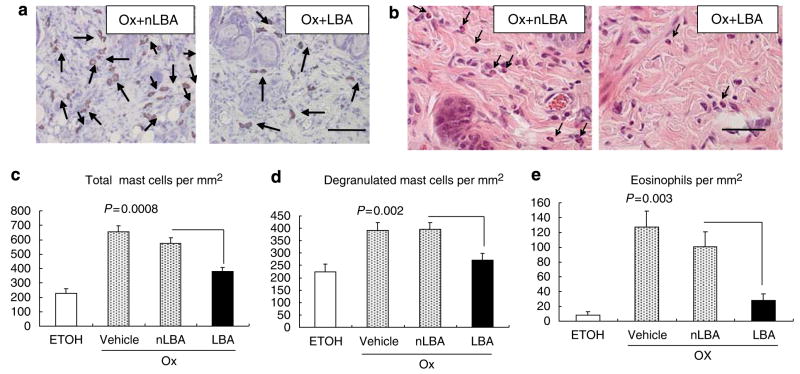
Although single Ox challenges provoke a Th1-dominant immunophenotype, with relatively low serum IgE levels, repeated Ox challenges that lead to Ox-AD are associated with both a Th2 immunophenotype and very high serum IgE levels (Man et al., 2008). We next assessed whether maintenance of an acidic pH with topical LBA prevents the emergence of the Th2 immunophenotype. Repeated Ox challenges, with or without co-applications of vehicle or nLBA (cf., Figure 1a), provoked a dense inflammatory infiltrate (Figure 3a), enriched in CTRH2-positive T cells (Figure 4a), an observation that was confirmed by quantitative studies (Figure 4b). In contrast, development of the chemoattractant receptor-homologous molecule expressed on TH2 (CRTH2)-positive cellular infiltrate was inhibited significantly when the SC remained acidified during Ox challenges (cf. Figure 4a versus b). Moreover, serum IgE levels decreased, a decrease that achieved statistical significance (Figure 4e). Yet, acidification did not reduce serum levels of thymus and activation-regulated cytokine (TARC), an epidermis-derived chemokine that signals Th2 cells, in acidified Ox-AD mice (Figure S3), reflecting the partial effect of SC acidification on inflammation induced by repetitive Ox challenges. Finally, both tissue mast cells (including both total and degranulated) and eosinophil densities were largely normalized in LBA-treated, Ox-AD mice (Figure 5a–e). Taken together, these results show that the maintenance of an acidic SC pH attenuates the development of a Th2-dominant infiltrate in Ox-AD mice.
Figure 4. Maintenance of a normal pH partially prevents emergence of TH2-type inflammation.
(a) Skin samples were collected 48 hours after the tenth challenge with Ox and immunostained for CRTH2-positive cells; (b) Quantitation of CRTH-positive cells (n=14 each on nLBA- and LBA-treated). (c) Serum IgE levels were measured by ELISA (see Materials and Methods; n=3 each). Bar=50 μm.
Figure 5. Maintenance of a normal SC pH prevents development of tissue eosinophilia and mast cell infiltration/degranulation.
(a) Toluidine blue-stained sections. Metachromasia (purple staining) of mast cells is indicated by arrows. (b) Tissue eosinophilia was assessed in hematoxylin-and-eosin-stained sections. (c–e) Quantitative data for eosinophils and mast cells (n=12–16 sections each). Bar=50 μm (a); 100 μm (b).
SC acidification normalizes antimicrobial peptide expression in Ox-AD mice
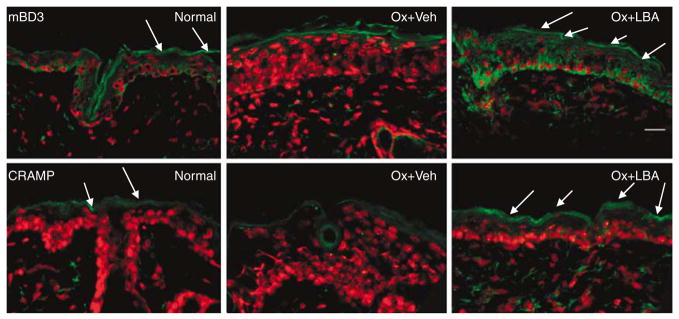
We next assessed whether co-acidification would normalize AMP expression in Ox-AD mice. Previous studies showed that levels of both CRAMP (murine homolog of LL-37) and mBD3 (murine homolog of human β-defensin 2) decrease in Ox-AD mice (Man et al., 2008). Co-acidification normalized or even supernormalized both CRAMP and mBD3 immunostaining in the outer epidermis in comparison with either Ox plus nLBA- or Ox plus Veh-treated skin (Figure 6; Ox plus nLBA not shown). These results suggest that the maintenance of an acidic pH prevents the hapten-induced decrease in AMP expression in Ox-AD skin.
Figure 6. Acidification normalizes or supernormalizes antimicrobial peptide expression in Ox-AD mice.
Frozen sections (6 μm) of Ox-AD skin cotreated with vehicle (Veh) or LBA, incubated with primary antibodies against either mBD3 (top three panels) or CRAMP (bottom three panels), with propidium iodide as the nuclear counterstain. Note decrease in Ox- plus Veh-treated skin, and increased (supernormal) immunostaining in LBA cotreated samples. Bar=20 μm.
Enzymatic and structural basis for the attenuation of Ox-AD by SC acidification
A sustained increase in the pH of the SC has deleterious consequences for normal mice, including provocation of permeability barrier dysfunction (Hachem et al., 2003, 2005b). These abnormalities could be further attributed to a parallel increase in SP activity, which, if sustained, degrades lipid-processing enzymes and blocks lamellar body (LB) secretion (Hachem et al., 2005b, 2006a). Likewise, a pH-induced increase in SP activity correlated with abnormal barrier function in Ox-AD mice (Man et al., 2008). Owing to the potential, central role of pH-induced increases in SP activity, we next assessed enzyme activity zymographically in frozen sections of Ox-AD mouse skin, with or without concurrent LBA cotreatment. Whereas control (nonsensitized, ethanol vehicle-treated) mice showed low levels of SP activity (Figure 7a), Ox-AD mice cotreated with either vehicle alone or nLBA (Ox plus Veh not shown) showed an intense band of SP activity at all levels of the SC, consistent with the Ox-induced elevation in pH (Figure 7a; cf. Figure 1b). In contrast, Ox-AD mice cotreated with LBA showed normal or even subnormal levels of SP activity. We next assessed whether SC acidification normalizes barrier function by “rescuing” the lipid-processing enzyme, β-glucocerebrosidase (β-GlcCer’ase) (Takagi et al., 1999). Again, by in situ zymography, cotreatment with LBA largely normalized the activity of this critical lipid-processing enzyme (Figure 7b).
Figure 7. Maintenance of normal SC pH normalizes both serine protease (SP) and lipid-processing enzyme activities.
(a and b) Skin samples were collected 48 hours after the tenth challenge with Ox for the assessment of both (a) SP and (b) β-glucocerebrosidase activities by in situ zymography (see Materials and Methods). Green stain in (a) represents SP activity; red counterstain in (a) represents nuclear staining with propidium iodide. Intense red staining in (b) represents β-glucocerebrosidase activity. Bar=20 μm.
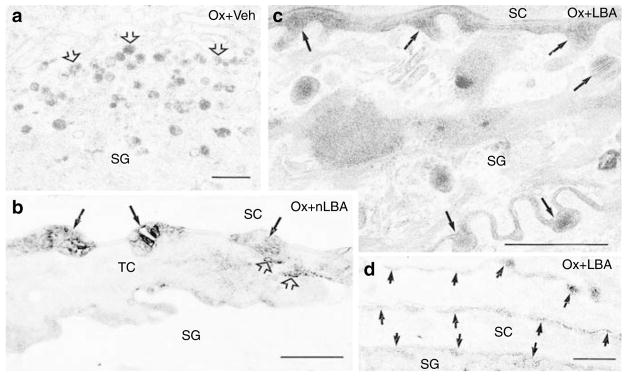
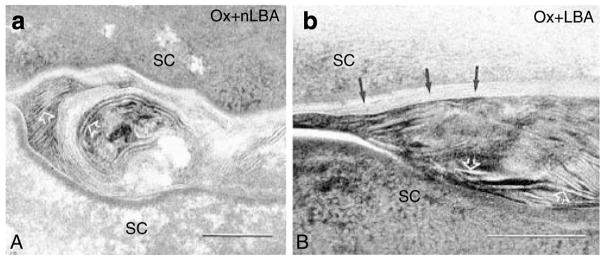
Human AD is characterized by decreased secretion of epidermal LBs (Fartasch, 1997), leading to decreased generation of extracellular lamellar bilayers (Chamlin et al., 2002). Hence, we next assessed the LB secretory system in Ox-AD mice treated with vehicle, nLBA, or LBA. Neither the density nor the internal contents of LB appeared altered in repeatedly Ox-challenged mouse epidermis (Figure 8a). Yet, there was a paucity of secreted LB contents at the stratum granulosum (SG)–SC interface, with accumulation of unsecreted LB in the apical cytosol of outermost SG cells. Thus, the apparent, partial failure of exocytosis is reflected by the entombment of substantial numbers of LB within nascent corneocytes (Figure 8a). In contrast, LB secretion appeared to be unimpaired in LBA-treated Ox-AD mice (Figure 8c).
Figure 8. Maintenance of an acidic pH normalizes lamellar body (LB) secretion.
(a) Ox-AD mice were cotreated twice daily with ethanol vehicle (Veh) above. Note the accumulation of LBs in apical cytosol of upper stratum granulosum (SG) (open arrows), consistent with partial blockade of secretion. (b) Using lipase cytochemistry, retention (entombment) of some LB contents (open arrows) can be seen within nascent corneocytes (TC=transitional cells). Solid arrows show normally secreted, extracellular contents. (c) Ox-AD mice cotreated with LBA display normal pattern of nearly complete secretion of LB contents at stratum granulosum (SG)–stratum corneum (SC) interface (solid arrows). (d) Efficiency of secretion in LBA-cotreated mice is shown independently with lipase ultrastructural cytochemistry (Note: all enzyme activity (short arrows) in SC is extracellular; cf. b). (a–d) Osmium tetroxide postfixation. Bar=0.5 μm.
To verify further that LB secretion is impaired in Ox-AD mice, we next assessed changes in the localization of the activity of an LB content marker, acidic lipase, by ultrastructural cytochemistry. In Ox-challenged mice, cotreated with either nLBA or vehicle, the partial blockade of LB secretion was confirmed by the visible entombment of acid lipase activity within nascent corneocytes (Figure 8b). Notably, in Ox-AD mice cotreated with LBA, all of the LB content marker appeared between corneocytes (Figure 8d), indicating unimpaired secretion, as in normal mice. Ox-AD mice cotreated with LBA also exhibited both fewer LB in the SG cell cytosol and evidence of abundant (complete) secretion at the SG–SC interface (cf. Figure 8a versusc). These results show that SC acidification normalizes LB secretion, even in the face of ongoing hapten challenges.
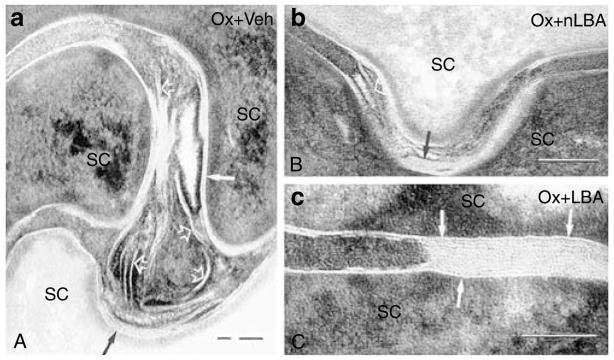
Finally, Ox-AD mice treated with either vehicle or nLBA show a delay in the extracellular processing of secreted LB contents into mature lamellar bilayers (Figures 9a, b, 10a and b). Whereas Ox-AD mice showed a paucity of extracellular bilayers in the SC, Ox-AD mice cotreated with LBA show a relative abundance of mature extracellular lamellar bilayers at comparable levels of the SC (Figure 10c). Taken together, these results show that the maintenance of an acidic pH prevents emergence of the enzymatic and structural abnormalities that underlie the barrier abnormality in Ox-AD mice.
Figure 9. Maintenance of an acidic pH normalizes lamellar bilayer structure.
(a) Ox-AD mice cotreated with either vehicle or nLBA show a paucity of lamellar bilayers (vehicle not shown) and failure of lamellar bilayer formation (also see Figure 10 below). (b) Ox-AD mice cotreated with LBA show rapid formation of mature lamellar bilayers in SC extracellular spaces (Figure 9b, arrows). (a and b) Ruthenium tetroxide postfixation. Bar=0.1 μm.
Figure 10. Maintenance of an acidic pH normalizes post-secretory lipid processing.
(a and b) Ox-AD mice cotreated with either nLBA or vehicle show secreted lamellar material that is incompletely processed into lamellar bilayers (arrows). (c) At a comparable level of stratum corneum (SC), Ox-AD mice cotreated with LBA show abundant, fully processed and mature lamellar bilayers (arrows). (a–c) Ruthenium tetroxide postfixation. Bar=0.2 μm.
Maintenance of an acidic pH inhibits cytokine generation in Ox-AD mice
We next assessed mechanisms whereby SC acidification could prevent the development of inflammation in Ox-AD mice. Barrier abnormalities result in a pH-induced increase in SP activity (Hachem et al., 2003, 2005b), which, in turn, has been shown to release IL-1β from the SC (Nylander-Lundqvist and Egelrud, 1997). Immunostaining for both IL-1α and tumor necrosis factor-α (TNFα) increased in Ox-AD mice treated with either vehicle or nLBA (Figure 11; TNF not shown). In contrast, maintenance of an acidic pH largely prevented the expected increase in IL-1α and TNFα immunostaining (Figure 11). Notably, the extent of the decrease was greater than in clobetasol-treated Ox-AD, which also partially attenuated cytokine immunostaining. These results show that maintenance of an acidic pH prevents generation of epidermal primary cytokines in Ox-AD mice.
Figure 11. Maintenance of an acidic pH decreases epidermal IL-1α production.
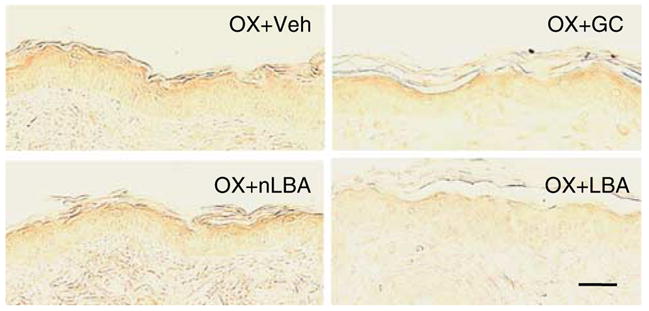
Ox-AD mice cotreated with either nLBA or vehicle (not shown) exhibit increased immunostaining for IL-1α (see Materials and Methods). The expected increase is blocked by cotreatment with LBA, but the glucocorticoid (clobetasol) has little effect. Paraffin-embedded sections: secondary antibody detected by the ABC-peroxidase method. Bar=20 μm.
DISCUSSION
The pH of the SC influences at least four key epidermal functions (permeability barrier homeostasis, integrity/cohesion (desquamation), initiation of inflammation, and antimicrobial defense) (Elias, 2005). Hence, the maintenance of low acidity provides a mechanism that coordinately integrates these key functions (Elias, 2005). The importance of pH in vivo was first shown in experiments where permeability barrier function is acutely abrogated, producing a parallel elevation in pH (Mauro et al., 1998). Under these conditions, it was pH itself, rather than barrier requirements, that upregulated endogenous acidification mechanisms, that is, sodium/hydrogen exchanger 1 (NHE1) antiporter expression— barrier restoration alone did not influence the rate of reacidification (Hachem et al., 2005a). As inflammatory dermatoses often show parallel alterations in all four of the above key functions, we asked here first whether maintenance of a normal or hyperacidic pH could reverse or prevent either hapten-induced acute allergic contact dermatitis or AD. Although exogenous SC acidification reversed neither hapten-induced acute allergic contact dermatitis nor preexistent Ox-AD, exogenous co-acidification alone restricted development of hapten-induced AD. Indeed, acidification of SC not only normalized epidermal structure and function in the face of ongoing hapten challenges, but also significantly attenuated most components of the inflammatory response, including Th2 markers, epidermal hyperplasia, tissue eosinophil and mast cell densities, and Th2 cell counts within the residual infiltrate. Even serum IgE levels decreased significantly with topical co-acidification. As neither pre- nor co-acidification prevented the development of inflammation during the AACD elicitation phase, despite normalizing epidermal function, it seems safe to assume that our results reflect neither a physical–chemical effect of acidification nor interference with hapten absorption. Instead, our results appear to show that it is pH-induced maintenance of normal epidermal structure and function that most likely accounts for the efficacy of this strategy in Ox-AD.
Normalization of barrier function most likely decreases inflammation by two unrelated mechanisms. First, it most likely restricts antigen access during repeated-challenge phase, but this supposition must still be verified. These studies suggest that an additional anti-inflammatory mechanism is operative, that is, reduced evidence for cytokine activation. Previous studies have shown that barrier disruption stimulates epidermal cytokine generation (Wood et al., 1992; Nickoloff and Naidu, 1994); and conversely, normalization of barrier function by occlusion decreases epidermal primary cytokine generation in models of chronic inflammation (Wood et al., 1994a, b). The acidification-induced reduction in cytokine generation most likely reflects the observed reduction in SP activity (Nylander-Lundqvist and Egelrud, 1997). In summary, it is most likely that both mechanisms are operative (that is, decreased hapten ingress and decreased cytokine activation), together accounting for the ability of SC acidification to largely protect against the development of inflammation in this mouse model of AD.
Our study provided additional mechanistic insights about how maintenance of an acidic pH most likely prevents emergence of the barrier abnormality in hapten-induced AD. A characteristic structural feature of human AD is a reduction in extracellular lamellar bilayers (Chamlin et al., 2002), as also shown here for Ox-AD, most likely rendering the SC more porous to transcutaneous water loss (and simultaneously more susceptible to pro-inflammatory ingress of haptens). We confirmed here that the basis for this abnormality in the Ox-AD model could be a pH-induced increase in SP activity in the outer epidermis (Man et al., 2008), with several potentially adverse consequences. The increased pH of Ox-AD epidermis downregulates the activities of the key ceramide-generating enzyme, β-glucocerebrosidase, which shows an acidic pH optimum (reviewed by Holleran et al., 2006). A sustained increase in SP activity not only inactivates lipid-processing enzymes, but also degrades the lipid-processing enzymes that generate the lamellar bilayers that provide for barrier function (Hachem et al., 2005b). However, whether lipid-processing enzyme protein content decreases in AD or in Ox-AD mice is not known. SP-mediated inhibition of LB secretion also occurs in Ox-AD, which most likely results from increased SP signaling of the PAR2 receptor (Hachem et al., 2006a). It should be noted that such a failure in both lipid secretion and ceramide generation correlates with the reported reduction in total SC lipids and further decrease in ceramide content that occurs in human AD (Melnik et al., 1988; Imokawa et al., 1991; Di Nardo et al., 1998). Finally, and in parallel, abnormal/heightened SP-PAR2 binding could accelerate terminal differentiation (physiological apoptosis or programmed cell death), a process that traps unsecreted LB within the cytosol of nascent corneocytes (Demerjian et al., 2008). Taken together, these mechanisms most likely account for the observed abnormalities in lamellar bilayer structure, and in an overall reduction in the quantities of extracellular lamellae in Ox-AD, as well as the abnormal desquamation (poor integrity) of the SC in AD (Cork et al., 2006). Conversely, we show here that the maintenance of a reduced pH improves permeability barrier function by preventing emergence of many of the above-described pathogenic mechanisms. Reduction in pH would not only allow optimal lipid-processing enzyme activity, but also block SP-mediated degradation of these enzymes (previously shown to be a pH-dependent process) (Hachem et al., 2005b, 2006b). In support of these proposed mechanisms, we showed here β-glucocerebrosidase activity increases in acidified, hapten-challenged skin, as well as ultrastructural evidence of accelerated lamellar membrane “maturation” in such acidified sites, consistent with normalization of this enzyme activity in the SC. Thus, the maintenance of an acidic pH could normalize barrier function in murine AD by several distinct, yet interdependent, mechanisms.
This work has implications for the primary or ancillary prevention of AD, as topical PHAs, such as LBA, are “GRAS” ingredients, that is, “Generally Regarded As Safe.” Applications of PHA have been shown to improve barrier function in both neonatal and aged rodent skin (Fluhr et al., 2004; Choi et al., 2007a), and even to “super-normalize” barrier function in normal mice (Hachem et al., 2009), and in humans (Gunathilake, 2009). Current forms of therapy for AD, regardless of type, could readily exploit these findings simply by ensuring that the vehicle used for topical applications is buffered to achieve a sustained reduction in the pH of diseased SC. Alternatively, SC pH could also be manipulated indirectly by stimulating activity or expression of endogenous epidermal acidifying mechanisms, such as the NHE1 antiporter and/or secretory phospholipase A2 (Elias, 2005). Both topical peroxisome proliferator activator receptor-α and liver-X receptor activators have already been deployed successfully to normalize SC acidity (and epidermal structure and function) in neonatal murine skin (Fluhr et al., 2005, 2008), and our recent results suggest their use in attenuating AD in a murine model (Hatano et al., in preparation). Nevertheless, the use of all of these approaches for human AD remains to be assessed.
MATERIALS AND METHODS
Materials
Female hairless mice (h/h), aged 6–8 weeks old, were purchased from Charles River Laboratories (Wilmington, MA) and fed on a mouse diet (Ralston-Purina Co., St Louis, MO) and water ad libitum. Ethanol was purchased from Fisher Scientific (Fairlane, NJ). Ox and LBA were purchased from Sigma Chemical Co. (St Louis, MO). Rabbit anti-mouse antibody against the prostaglandin D receptor, CRTH2/DP2, was from Cayman Chemical (Ann Arbor, MI). Goat anti-mouse antibodies against mBD3, IL-1α, and TNFα were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-human antibody against CD3 was purchased from Dako (Glostrup, Denmark). Biotinylated goat anti-rabbit IgG antibody and biotinylated horse, anti-goat antibody were purchased from Vector Laboratories (Burlingame, CA). Biotinylated mAb against PCNA was purchased from CalTag Laboratories (Burlingame, CA). Primary rabbit anti-mouse mBD3 antibody was purchased from Alpha Diagnostics (San Antonio, TX) and the primary rabbit anti-CRAMP antibody was a gift from Dr Gallo (UCSD). FITC-conjugated goat anti-rabbit secondary antibody was purchased from Alpha Diagnostics.
Experimental protocols
All animal procedures were approved by the Animal Studies Subcommittee of the San Francisco Veterans Administration Medical Center and performed in accordance with their guidelines. Animals were sensitized with a single topical application of 1% Ox (50 μl) to the flank. To assess the effects of acidification in acute allergic contact dermatitis, Ox (0.1%) or vehicle was then applied 1 week later to the ears, as described previously (Sheu et al., 2002; Fowler et al., 2003). We generated mice with an AD-like dermatosis, using the protocol described by Man et al. (2008). One week after Ox sensitization, hairless mice were treated topically with 60 μl of 0.1% Ox on both flanks once every other day for an additional 3 weeks (totally, 10 challenges over 20–21 days (Man et al., 2008)). Atopic mice were divided into three groups at the beginning of challenges, depending on the kind of the additional treatment, that is, applications of 60 μl of 10% LBA, 10% neutralized LBA (nLBA), or the ethanol-containing vehicle (water/ethanol 7:3 vol/vol) twice daily on both flanks. nLBA was produced by neutralization of LBA with NaOH for a control. An additional control group comprised animals that were treated with the ethanol vehicle alone (no hapten treatments). LBA, nLBA, or vehicle were applied twice daily at an interval of 6 hours at least 1 hour after previous hapten treatment. In the acute allergic contact dermatitis mice, LBA, nLBA, and vehicle were applied either 2 hours before Ox challenge or immediately before Ox challenge.
Functional studies
Basal TEWL was measured with an electrolytic water analyzer (Meeco, Warrington, PA), and SC hydration, assessed as electrical capacitance, was measured with a Corneometer CM820 (Courage & Khazaka, Cologne, Germany) (Choi et al., 2005), 48 hours after the final Ox challenge. SC surface pH was measured concurrently with a flat glass, surface electrode (Mettler-Toledo, Giessen, Germany), attached to a pH meter (PH900; Courage & Khazaka) as described previously (Hachem et al., 2005a, b). pH at different levels of SC and within SC microdomains was assessed by dual-photon, fluorescence life-time imaging (FLIM), using 2,7-bis-(2-carboxyEthyl)-5-(and-6-) carboxyfluorescein (BCECF) as the fluorophore (Behne et al., 2002). To evaluate the effect of pre- or co-acidification on the intensity of inflammation elicited by single Ox challenge, alterations in ear thickness were assessed histologically and with a dial thickness gauge (Ozaki Seisakusho Co., Tokyo, Japan), 3, 6, 24, and 48 hours after the Ox challenge.
Immunohistochemistry and immunofluorescence
Immunohistochemical staining for CRTH2, IL-1α, TNFα, and CD3 was performed as described previously (Demerjian et al., 2006; Man et al., 2008). Briefly, 5-μm paraffin-embedded sections were incubated with the primary antibodies overnight at 4 °C. After washing × 3, sections were incubated with the secondary antibody for 30 minutes. Staining was detected with the ABC-peroxidase kit (Vector Labs). The density of CRTH2-positive cells, eosinophils assessed in hematoxylin-and-eosin-stained sections, mast cells detected by toluidine blue stain, and the CD3-positive cells in the area of 220 μm × 170 μm were counted in more than 15 fields in the dermis of each sample.
CRAMP and mBD3 were detected in 5-μm frozen sections, using rabbit polyclonal antibodies against the mouse proteins (CRAMP antibody was a gift from Dr Gallo). After incubation with the secondary anti-rabbit and anti-goat antibodies, sections were visualized and photographed in a confocal microscope (Leica TCS-SP).
To assess changes in the epidermal proliferation, changes in the overall morphology were visualized after hematoxylin-and-eosin staining of 5-μm paraffin-embedded sections, and proliferating cells were detected and quantitated by PCNA staining. Briefly, 5-μm paraffin sections were incubated with biotinylated mAb against PCNA (Ki67 antigen) overnight at 4 °C, and positive staining was detected by the ABC-peroxidase method, as described above. Sections were examined with a Zeiss light microscope (Jena, Germany), and digital images were captured with AxioVision software (Carl Zeiss Vision, Munich, Germany). Epidermal thickness in hematoxylin-and-eosin sections was measured with AxioVision software. Measurements were performed in more than 15 fields of intervals of 100 μm in each sample. The number of PCNA-positive cells observed within 50 μm length of epidermis was counted at more than 10 points for each sample.
For immunofluorescence detention of mBD3 and CRAMP, freshly obtained biopsies were snap-frozen in liquid nitrogen. Five-micrometer frozen sections were soaked in acetone for 10 minutes, washed in phosphate-buffered saline, and blocked with 4% BSA and 0.05% cold fish gelatin in phosphate-buffered saline for 30 minutes. Slides were incubated overnight at 4 °C with either the CRAMP or mBD3 primary antibodies, followed by incubation with the secondary antibody for 40 minutes at room temperature. Some slides were counterstained with propidium iodide and examined in a Leica TCS-SP confocal microscope.
Zymographic assessment of enzyme activity
Serine protease activity was assessed in freshly obtained skin samples by in situ zymography, as described previously (Hachem et al., 2003, 2005b). Five-micrometer frozen sections were incubated with BODIPY-FI0-casein for 2 hours at 37 °C. β-glucocerebrosidase was detected using 4-methylumbelliferyl-β-D-glucoside as the substrate, as described previously (Takagi et al., 1999). After 3 × washing with 1% Tween-20, sections were counter-stained with propidium iodide and examined with the confocal microscope, as described above.
Serum IgE and thymus and activation-regulated cytokine measurements
Blood samples were collected from mice after 10 Ox- or vehicle-challenges, and serum IgE levels were determined with a mouse IgE ELISA quantitation kits from Bethyl Laboratories (Montgomery, TX), following the instructions provided by the manufacturer. Serum thymus and activation-regulated cytokine (TARC) levels were measured by ELISA, using a quantitation kit (Quantikine) from R&D Systems (Minneapolis, MN).
Electron microscopy
Skin biopsies from both vehicle and 10 × Ox-treated mice were fixed in Karnovsky’s fixative overnight, and postfixed with either 0.25% ruthenium tetroxide or 1% aqueous osmium tetroxide, containing 1.5% potassium ferrocyanide, as described previously (Hou et al., 1991). Ultrathin sections were examined using an electron microscope (Zeiss 10A, Carl Zeiss, Thornwood, NY), operated at 60 kV.
Ultrastructural lipase detection
Ultrastructural cytochemistry of various lipases serve as content markers for epidermal LBs, allowing assessment of the efficacy of organelle secretion (Menon et al., 1992), as modified from Nagata (Nagata and Murata, 1972). Briefly, samples were incubated in 5% Tween 85 (1 ml) in 0.2M HEPES buffer (2.5 ml), 2.5% sodium taurocholate (2 ml), and 10% calcium chloride (1 ml) in 25 ml distilled water (pH 7.4). Microwave incubations were performed, as described previously (Rassner et al., 1997). Microwave incubations were performed twice for 30 seconds at 2,450MHz and the water bath was changed between the pulses, to maintain temperatures below 40 °C. The samples then were further incubated in the same medium for an additional 30 minutes at 37 °C. After incubations, all samples were processed for electron microscopy (see above). As controls, duplicate samples were preincubated in 0.1 M cacodylate buffer containing 0.2% Tween 85 with or without 200 μm of the lipase inhibitor, tetrahydrolipstatin (Hochuli et al., 1987), and incubated as above.
Statistical analyses
Data are expressed as means±SEM. A two-tailed Student’s t-test was used to determine significant differences between two groups. An ANOVA analysis was performed when three or more groups were compared.
Supplementary Material
Figure S1. Pre-acidification does not prevent Ox-induced acute allergic contact dermatitis.
Figure S2. Co-acidification prevents development of barrier abnormality, but not inflammation in Ox-induced acute allergic dermatitis.
Figure S3. No change in serum thymus and activation-regulated cytokine levels after co-acidification of Ox-AD mice.
Acknowledgments
We gratefully acknowledge the superb editorial assistance of Ms Joan Wakefield. This work was supported by NIH grants AI 59311 [WMH] and AR 19098 [PME], DOD grant W81XWH-05-2-0094, and the Medical Research Service, Department of Veterans Affairs. No company provided support for this article.
Abbreviations
- AD
atopic dermatitis
- AMP
antimicrobial peptide
- BD
β-defensin
- CRTH
chemoattractant receptor-homologous molecule expressed on TH2
- LB
lamellar body
- LBA
lactobionic acid
- Ox
oxazolone
- Ox-AD
oxazalone-induced atopic-like dermatitis
- PCNA
proliferating cell nuclear antigen
- PHA
polyhydroxyl acid
- SC
stratum corneum
- SG
stratum granulosum
- SP
serine protease
- TEWL
transepidermal water loss
- TNFα
tumor necrosis factor-α
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Behne MJ, Barry NP, Hanson KM, Aronchik I, Clegg RW, Gratton E, et al. Neonatal development of the stratum corneum pH gradient: localization and mechanisms leading to emergence of optimal barrier function. J Invest Dermatol. 2003;120:998–1006. doi: 10.1046/j.1523-1747.2003.12262.x. [DOI] [PubMed] [Google Scholar]
- Behne MJ, Meyer JW, Hanson KM, Barry NP, Murata S, Crumrine D, et al. NHE1 regulates the stratum corneum permeability barrier homeostasis. Microenvironment acidification assessed with fluorescence lifetime imaging. J Biol Chem. 2002;277:47399–406. doi: 10.1074/jbc.M204759200. [DOI] [PubMed] [Google Scholar]
- Chamlin SL, Kao J, Frieden IJ, Sheu MY, Fowler AJ, Fluhr JW, et al. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: changes in barrier function provide a sensitive indicator of disease activity. J Am Acad Dermatol. 2002;47:198–208. doi: 10.1067/mjd.2002.124617. [DOI] [PubMed] [Google Scholar]
- Choi EH, Man MQ, Wang F, Zhang X, Brown BE, Feingold KR, et al. Is endogenous glycerol a determinant of stratum corneum hydration in humans? J Invest Dermatol. 2005;125:288–93. doi: 10.1111/j.0022-202X.2005.23799.x. [DOI] [PubMed] [Google Scholar]
- Choi EH, Man MQ, Xu P, Xin S, Liu Z, Crumrine DA, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007a;127:2847–56. doi: 10.1038/sj.jid.5700913. [DOI] [PubMed] [Google Scholar]
- Choi EH, Man MQ, Xu P, Xin S, Liu Z, Crumrine DA, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007b;127:2847–56. doi: 10.1038/sj.jid.5700913. [DOI] [PubMed] [Google Scholar]
- Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, et al. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. J Allergy Clin Immunol. 2006;118:3–21. doi: 10.1016/j.jaci.2006.04.042. quiz 22–3. [DOI] [PubMed] [Google Scholar]
- Demerjian M, Hachem JP, Tschachler E, Denecker G, Declercq W, Vandenabeele P, et al. Acute modulations in permeability barrier function regulate epidermal cornification: role of caspase 14 and the protease-activated receptor type 2. Am J Path. 2008;172:86–97. doi: 10.2353/ajpath.2008.070161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerjian M, Man MQ, Choi EH, Brown BE, Crumrine D, Chang S, et al. Topical treatment with thiazolidinediones, activators of peroxisome proliferator-activated receptor-gamma, normalizes epidermal homeostasis in a murine hyperproliferative disease model. Exp Dermatol. 2006;15:154–60. doi: 10.1111/j.1600-0625.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- Di Nardo A, Wertz P, Giannetti A, Seidenari S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol. 1998;78:27–30. doi: 10.1080/00015559850135788. [DOI] [PubMed] [Google Scholar]
- Elias P, Hatano Y, Williams M. Basis for the barrier abnormality in atopic dermatitis: “outside–inside–outside” pathogenic mechanisms. J Allergy Clin Immunol. 2008;121:1337–43. doi: 10.1016/j.jaci.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- Elias PM. The skin barrier as an innate immune element. Sem Immunopath. 2007;29:3–14. doi: 10.1007/s00281-007-0060-9. [DOI] [PubMed] [Google Scholar]
- Elias PM, Wood LC, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermat. 1999;10:119–26. [PubMed] [Google Scholar]
- Fartasch M. Epidermal barrier in disorders of the skin. Microsc Res Tech. 1997;38:361–72. doi: 10.1002/(SICI)1097-0029(19970815)38:4<361::AID-JEMT4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Fluhr JW, Crumrine D, Mao-Qiang M, Moskowitz DG, Elias PM, Feingold KR. Topical liver X receptor activators accelerate postnatal acidification of stratum corneum and improve function in the neonate. J Invest Dermatol. 2005;125:1206–14. doi: 10.1111/j.0022-202X.2005.23964.x. [DOI] [PubMed] [Google Scholar]
- Fluhr JW, Elias PM. Stratum corneum pH: formation and function of the “acid mantle”. Exog Dermatol. 2002;1:163–75. [Google Scholar]
- Fluhr JW, Kao J, Jain M, Ahn SK, Feingold KR, Elias PM. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol. 2001;117:44–51. doi: 10.1046/j.0022-202x.2001.01399.x. [DOI] [PubMed] [Google Scholar]
- Fluhr JW, Mao-Qiang M, Brown BE, Hachem JP, Moskowitz DG, Demerjian M, et al. Functional consequences of a neutral pH in neonatal rat stratum corneum. J Invest Dermatol. 2004;123:140–51. doi: 10.1111/j.0022-202X.2004.22726.x. [DOI] [PubMed] [Google Scholar]
- Fluhr JW, Man MQ, Hachem JP, Crumrine D, Mauro TM, Elias PM, et al. Topical peroxisome proliferator activated receptor activators accelerate postnatal stratum corneum acidification. J Invest Dermatol. 2008;179:365–74. doi: 10.1038/jid.2008.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler AJ, Sheu MY, Schmuth M, Kao J, Fluhr JW, Rhein L, et al. Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine production. J Invest Dermatol. 2003;120:246–55. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- Gunathilake R, Schurer N, Shoo B, Celli A, Hachem JP, Crumrine D, et al. pH-regulated mechanisms account for pigment type differences in epidermal barrier function. J Invest Dermatol. 2009 doi: 10.1038/jid.2008.442. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem JP, Behne M, Aronchik I, Demerjian M, Feingold KR, Elias PM, et al. Extracellular pH Controls NHE1 expression in epidermis and keratinocytes: implications for barrier repair. J Invest Dermatol. 2005a;125:790–7. doi: 10.1111/j.0022-202X.2005.23836.x. [DOI] [PubMed] [Google Scholar]
- Hachem JP, Crumrine D, Fluhr J, Brown BE, Feingold KR, Elias PM. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 2003;121:345–53. doi: 10.1046/j.1523-1747.2003.12365.x. [DOI] [PubMed] [Google Scholar]
- Hachem JP, Fowler A, Behne M, Fluhr J, Feingold K, Elias P. Increased stratum corneum pH promotes activation and release of primary cytokines from the stratum corneum attributable to activation of serine proteases. J Invest Dermatol. 2002;119:258. (abstract) [Google Scholar]
- Hachem JP, Houben E, Crumrine D, Man MQ, Schurer N, Roelandt T, et al. Serine protease signaling of epidermal permeability barrier homeostasis. J Invest Dermatol. 2006a;126:2074–86. doi: 10.1038/sj.jid.5700351. [DOI] [PubMed] [Google Scholar]
- Hachem JP, Man MQ, Crumrine D, Uchida Y, Brown BE, Rogiers V, et al. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J Invest Dermatol. 2005b;125:510–20. doi: 10.1111/j.0022-202X.2005.23838.x. [DOI] [PubMed] [Google Scholar]
- Hachem J-P, Roelandt T, Schurer N, Pu X, Fluhr J, Guiddelo C, et al. Acute acidification of stratum corneum membrane domains using polyhydroxyl acids improves lipid processing and inhibits degradation of corneodesmosomes. J Invest Dermatol. 2009 doi: 10.1038/jid.2009.249. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem JP, Wagberg F, Schmuth M, Crumrine D, Lissens W, Jayakumar A, et al. Serine protease activity and residual LEKTI expression determine phenotype in Netherton syndrome. J Invest Dermatol. 2006b;126:1609–21. doi: 10.1038/sj.jid.5700288. [DOI] [PubMed] [Google Scholar]
- Hochuli E, Kupfer E, Maurer R, Meister W, Mercadal Y, Schmidt K. Lipstatin, an inhibitor of pancreatic lipase, produced by Streptomyces toxytricini. II. Chemistry and structure elucidation. J Antibiot (Tokyo) 1987;40:1086–91. doi: 10.7164/antibiotics.40.1086. [DOI] [PubMed] [Google Scholar]
- Holleran WM, Takagi Y, Uchida Y. Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett. 2006;580:5456–66. doi: 10.1016/j.febslet.2006.08.039. [DOI] [PubMed] [Google Scholar]
- Hou SY, Mitra AK, White SH, Menon GK, Ghadially R, Elias PM. Membrane structures in normal and essential fatty acid-deficient stratum corneum: characterization by ruthenium tetroxide staining and X-ray diffraction. J Invest Dermatol. 1991;96:215–23. doi: 10.1111/1523-1747.ep12461361. [DOI] [PubMed] [Google Scholar]
- Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. 1991;96:523–6. doi: 10.1111/1523-1747.ep12470233. [DOI] [PubMed] [Google Scholar]
- Korting HC, Hubner K, Greiner K, Hamm G, Braun-Falco O. Differences in the skin surface pH and bacterial microflora due to the long-term application of synthetic detergent preparations of pH 5.5 and pH 7.0. Results of a crossover trial in healthy volunteers. Acta Derm Venereol. 1990;70:429–31. [PubMed] [Google Scholar]
- Man MQ, Hatano Y, Lee SH, Man M, Chang S, Feingold KR, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008;128:79–86. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro T, Holleran WM, Grayson S, Gao WN, Man MQ, Kriehuber E, et al. Barrier recovery is impeded at neutral pH, independent of ionic effects: implications for extracellular lipid processing. Arch Dermatol Res. 1998;290:215–22. doi: 10.1007/s004030050293. [DOI] [PubMed] [Google Scholar]
- Melnik B, Hollmann J, Plewig G. Decreased stratum corneum ceramides in atopic individuals—a pathobiochemical factor in xerosis? Br J Dermatol. 1988;119:547–9. doi: 10.1111/j.1365-2133.1988.tb03262.x. [DOI] [PubMed] [Google Scholar]
- Menon GK, Ghadially R, Williams ML, Elias PM. Lamellar bodies as delivery systems of hydrolytic enzymes: implications for normal and abnormal desquamation. Br J Dermatol. 1992;126:337–45. doi: 10.1111/j.1365-2133.1992.tb00675.x. [DOI] [PubMed] [Google Scholar]
- Nagata T, Murata F. Supplemental studies on the method for electron microscopic demonstration of lipase in the pancreatic acinar cells of mice and rats. Histochemie. 1972;29:8–15. doi: 10.1007/BF00305696. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Naidu Y. Perturbation of epidermal barrier function correlates with initiation of cytokine cascade in human skin. J Am Acad Dermatol. 1994;30:535–46. doi: 10.1016/s0190-9622(94)70059-1. [DOI] [PubMed] [Google Scholar]
- Nylander-Lundqvist E, Egelrud T. Formation of active IL-1 beta from pro-IL-1 beta catalyzed by stratum corneum chymotryptic enzyme in vitro. Acta Derm Venereol. 1997;77:203–6. doi: 10.2340/0001555577203206. [DOI] [PubMed] [Google Scholar]
- Rassner UA, Crumrine DA, Nau P, Elias PM. Microwave incubation improves lipolytic enzyme preservation for ultrastructural cytochemistry. Histochem J. 1997;29:387–92. doi: 10.1023/a:1026438917856. [DOI] [PubMed] [Google Scholar]
- Schade H. Zur physikalischen chemie der hautoberflache. Arch Dermatol Syphilis. 1928;154:690–716. [Google Scholar]
- Sheu MY, Fowler AJ, Kao J, Schmuth M, Schoonjans K, Auwerx J, et al. Topical peroxisome proliferator activated receptor-alpha activators reduce inflammation in irritant and allergic contact dermatitis models. J Invest Dermatol. 2002;118:94–101. doi: 10.1046/j.0022-202x.2001.01626.x. [DOI] [PubMed] [Google Scholar]
- Taieb A. Hypothesis: from epidermal barrier dysfunction to atopic disorders. Contact Dermatitis. 1999;41:177–80. doi: 10.1111/j.1600-0536.1999.tb06125.x. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Kriehuber E, Imokawa G, Elias PM, Holleran WM. Beta-glucocerebrosidase activity in mammalian stratum corneum. J Lipid Res. 1999;40:861–9. [PubMed] [Google Scholar]
- Wood LC, Elias PM, Sequeira-Martin SM, Grunfeld C, Feingold KR. Occlusion lowers cytokine mRNA levels in essential fatty acid-deficient and normal mouse epidermis, but not after acute barrier disruption. J Invest Dermatol. 1994a;103:834–8. doi: 10.1111/1523-1747.ep12413597. [DOI] [PubMed] [Google Scholar]
- Wood LC, Feingold KR, Sequeira-Martin SM, Elias PM, Grunfeld C. Barrier function coordinately regulates epidermal IL-1 and IL-1 receptor antagonist mRNA levels. Exp Dermatol. 1994b;3:56–60. doi: 10.1111/j.1600-0625.1994.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Wood LC, Jackson SM, Elias PM, Grunfeld C, Feingold KR. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992;90:482–7. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Pre-acidification does not prevent Ox-induced acute allergic contact dermatitis.
Figure S2. Co-acidification prevents development of barrier abnormality, but not inflammation in Ox-induced acute allergic dermatitis.
Figure S3. No change in serum thymus and activation-regulated cytokine levels after co-acidification of Ox-AD mice.