Summary
-
1
Emerging wildlife diseases pose a significant threat to natural and human systems. Because of real or perceived risks of delayed actions, disease management strategies such as culling are often implemented before thorough scientific knowledge of disease dynamics is available. Adaptive management is a valuable approach in addressing the uncertainty and complexity associated with wildlife disease problems and can be facilitated by using a formal model.
-
2
We developed a multi‐state computer simulation model using age, sex, infection‐stage, and seasonality as a tool for scientific learning and managing chronic wasting disease (CWD) in white‐tailed deer Odocoileus virginianus. Our matrix model used disease transmission parameters based on data collected through disease management activities. We used this model to evaluate management issues on density‐ (DD) and frequency‐dependent (FD) transmission, time since disease introduction, and deer culling on the demographics, epizootiology, and management of CWD.
-
3
Both DD and FD models fit the Wisconsin data for a harvested white‐tailed deer population, but FD was slightly better. Time since disease introduction was estimated as 36 (95% CI, 24–50) and 188 (41–>200) years for DD and FD transmission, respectively. Deer harvest using intermediate to high non‐selective rates can be used to reduce uncertainty between DD and FD transmission and improve our prediction of long‐term epidemic patterns and host population impacts. A higher harvest rate allows earlier detection of these differences, but substantially reduces deer abundance.
-
4
Results showed that CWD has spread slowly within Wisconsin deer populations, and therefore, epidemics and disease management are expected to last for decades. Non‐hunted deer populations can develop and sustain a high level of infection, generating a substantial risk of disease spread. In contrast, CWD prevalence remains lower in hunted deer populations, but at a higher prevalence the disease competes with recreational hunting to reduce deer abundance.
-
5
Synthesis and applications. Uncertainty about density‐ or frequency‐dependent transmission hinders predictions about the long‐term impacts of chronic wasting disease on cervid populations and the development of appropriate management strategies. An adaptive management strategy using computer modelling coupled with experimental management and monitoring can be used to test model predictions, identify the likely mode of disease transmission, and evaluate the risks of alternative management responses.
Keywords: adaptive management, back‐calculation analysis, culling, density‐dependent transmission, disease control, frequency‐dependent transmission, multi‐state matrix, transmission mode, transmissible spongiform encephalopathy, Wisconsin
Introduction
Emerging wildlife diseases may have significant health, demographic, and ecosystem consequences for the natural host as well as incidental hosts such as humans or livestock (Daszak, Cunningham & Hyatt 2000). Regretfully, wildlife diseases are often managed in a post hoc or crisis fashion because limited scientific information on disease dynamics creates uncertainty about alternative management actions and disagreement among stakeholders (Peterson 1991). Success in managing most wildlife diseases depends on understanding host–pathogen dynamics and the way in which infectious contact‐rate is affected by host density (McCallum, Barlow & Hone 2001). Typically, infectious contacts are assumed to increase with host density (density‐dependent transmission, DD) or to be independent of host density (frequency‐dependent transmission, FD). When transmission is DD, host population reduction below a threshold density can eliminate the disease; however, for FD transmission depopulation of the host is required (McCallum, Barlow & Hone 2001; Lloyd‐Smith et al. 2005). For most wildlife diseases, estimating disease transmission can be challenging, and there is little scientific knowledge about whether transmission is DD or FD.
Adaptive management provides an important framework for managing natural resources under the constraints of limited knowledge (Walters & Holling 1990). This approach integrates policy, management, and monitoring activities through purposeful experiments which provide opportunities for scientific learning. An active adaptive management process involves generation of alternative hypotheses and specific management experiments to evaluate these hypotheses (Stankey, Clark & Bormann 2005). Due to the complexity of most ecological systems, hypotheses generation and experimental management can be facilitated by development of a formal model (Starfield 1997) which promotes problem and goal definition, identifies underlying assumptions, and allows integration of information from disparate sources. Most importantly, models can be used to evaluate alternative management strategies, test scientific hypotheses, and assess the sensitivity of model predictions to underlying ecological and epidemiological processes. Because wildlife disease outbreaks usually require immediate response in the face of limited scientific knowledge, we believe that control plans (e.g. culling, vaccination) should be designed as management experiments that provide disease control and enhance scientific understanding of the underlying epidemiologic processes.
Chronic wasting disease (CWD) is an emerging neurodegenerative prion disease belonging to a family of transmissible spongiform encephalopathies (TSE) and is the only ‘infectious’ TSE in free‐living animals (Williams & Miller 2002). First recognized in Colorado during the 1960s in captive mule deer Odocoileus hemionus Rafinesque, CWD subsequently was detected throughout much of Colorado and Wyoming as well as in Nebraska, South Dakota, New Mexico, Saskatchewan, Utah, Wisconsin, Illinois and was recently discovered in New York, Kansas, Alberta, and West Virginia (http://wildlifedisease.nbii.gov/cwdupdates.jsp). The disease infects mule deer, elk Cervus elaphus Baily, white‐tailed deer O. virginianus Zimmerman, and moose Alces alces Nelson. The potentially detrimental effects on cervid populations (Gross & Miller 2001), the human and domestic animal health concerns, and uncertain economic consequences have led management agencies to seek effective strategies to control CWD distribution and prevalence. In the absence of a treatment or vaccine for CWD, non‐selective culling to reduce deer populations or selective culling of infected individuals are the main tools available for disease control in free‐ranging populations (Williams & Miller 2002). In Wisconsin, CWD was discovered in three white‐tailed deer harvested in south‐central Wisconsin in 2001 (Joly et al. 2003). Faced with limited knowledge about the basic epizootiology, distribution, and length of time CWD had been present, the Wisconsin Department of Natural Resources initiated a management programme with the goals of eradicating CWD from affected areas using generalized culling, evaluating the state‐wide distribution of disease, and preventing disease spread. Wisconsin's CWD management plan was defined as an adaptive management programme with culling, as the first stage, providing data for detection and evaluation of epizootiological patterns (Bartelt, Pardee & Thiede 2003).
Transmission of CWD has been suspected to be FD based largely on the assumption of female site fidelity and matrilineal social structure which limits contact rate between female social groups (Gross & Miller 2001). However, the resulting FD prediction that CWD usually results in host extinction (Gross & Miller 2001) has been challenged (Schauber & Woolf 2003) based on insufficient empirical support and CWD epidemiology. For example, high CWD prevalence in captive deer herds (Williams & Miller 2002), positive correlation between prevalence and indices of deer abundance (Joly et al. 2006), and deer congregation on winter ranges (Van Deelen 1999), around bait‐piles, or at mineral licks suggest that DD transmission is also feasible. CWD management using non‐selective culling assumes DD transmission. Therefore, understanding the transmission mode is crucial for understanding CWD dynamics and for predicting management success (Schauber & Woolf 2003).
In this study, we consider adaptive management as a framework for CWD control. To facilitate this approach, we developed a mathematical model to evaluate the consequences of DD and FD transmission, host‐culling strategies, and their impacts on disease and deer populations. Specifically, we addressed the implications of DD and FD transmission, the length of time CWD has been present in Wisconsin, the potential impacts of CWD on white‐tailed deer populations, and CWD management using generalized deer culling. We incorporated prevalence data collected during a CWD control programme to improve model parameter estimates, reduce uncertainty, and improve model predictions. We show how culling can provide scientific insights on dynamics and control of CWD in white‐tailed deer.
Methods
Multi‐state matrix models have a well‐established theoretical basis, wide range of applications, and allow incorporation of considerable heterogeneity (e.g. demographic, epidemiologic, genetic, spatial, temporal) in a relatively simple manner. They have been used for human demographics (Land & Rogers 1982) as well as for natural populations (Caswell 2001). However, simple matrix and multi‐state matrix models are infrequently used for pathogen–host interactions (Yang et al. 1997). Such a model requires temporal resolution that corresponds to its constituent stages.
We developed a multi‐state non‐spatial deterministic matrix model (Caswell 2001), which accounts for age, sex, infection stage, and seasonal heterogeneity with respect to demographic and epidemiologic parameters, to simulate the effect of different culling strategies on disease and white‐tailed deer dynamics in the south‐central Wisconsin CWD core area (Joly et al. 2006). Our multi‐state model incorporates four infection stages for 20 age classes and two sex classes of deer, resulting in a transition matrix of 160 × 160 cells with eight sex and infection‐stage population sub‐vectors. We used semi‐annual time‐steps to accommodate parturition and fawn rearing during summer (April–September), hunting and increased natural mortality during winter (October–March), and the slow rate of CWD progression for infected deer. The model is non‐spatial and assumes a closed deer population with no immigration or emigration. We used maximum likelihood (ML) methods to estimate disease transmission probability (π), force of infection (λ), density‐dependent or density‐independent transmission coefficients (β or β′, respectively), and time since disease introduction (TDI) based on the age‐specific prevalence rates for deer harvested in the CWD affected area of south‐central Wisconsin during 2002–2005 (Table 1). A detailed description of the model parameters for deer demography, harvest, and disease stages is provided in Supporting Information Appendix S1.
Table 1.
Sample sizes (n), number of CWD positives (i), and percentage prevalence (p) by age class and year in the CWD core area of south‐central Wisconsin (Joly et al. 2003)
| Age | 2002 | 2003 | 2004 | 2005 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | i | p | n | i | p | n | i | p | n | i | p | ||
| Core area | F | 1605 | 5 | (0·3) | 329 | 0 | (0) | 445 | 3 | (0·7) | 310 | 2 | (0·6) |
| 1 | 965 | 23 | (2·4) | 669 | 18 | (2·7) | 589 | 9 | (1·5) | 492 | 11 | (2·2) | |
| 2 | 793 | 49 | (6·2) | 498 | 34 | (6·8) | 641 | 44 | (6·9) | 549 | 45 | (8·2) | |
| 3 | 468 | 43 | (9·2) | 264 | 16 | (6·1) | 391 | 25 | (6·4) | 341 | 30 | (8·8) | |
| 4+ | 257 | 13 | (5·1) | 112 | 7 | (6·3) | 204 | 11 | (5·4) | 143 | 13 | (9·1) | |
equilibrium, sensitivity, and elasticity
We assessed the effect of CWD transmission and stage transition probabilities on the deer finite population growth rate (Λ), equilibrium prevalence, and disease stage distribution by varying specific transition parameters and calculating the corresponding dominant eigenvalue and eigenvector. Sensitivity is expressed as the change in prevalence relative to the change in each transition parameter. We also conducted a lower‐level elasticity analysis (Caswell 2001) to assess the proportional effect of demographic and epidemiologic parameters on the finite population growth (Table 2). We conducted this analysis for female deer because only females affect Λ.
Table 2.
Lower‐level elasticity analysis: The proportional effect of model parameters on finite population growth (Λ) based on lower‐level elasticity analysis for the summer (Es, with reproduction) and winter (Ew, without reproduction) matrices for a non‐hunted white‐tailed deer population infected with CWD. Low transmission probability (π = 0·024) produces the current apparent prevalence and high transmission probability (π = 0·34) reduces the finite population growth rate to unity (Λ = 1). Demographic and epidemiologic parameter values are as shown in Supporting Information Appendix S1
| Symbol | Model parameter | Low π | High π | ||
|---|---|---|---|---|---|
| Es | Ew | Es | Ew | ||
| Fy | Yearling fecundity | 0·032 | 0·000 | 0·055 | 0·000 |
| F2 | 2 year old fecundity | 0·045 | 0·000 | 0·072 | 0·000 |
| Fa | Adult fecundity | 0·066 | 0·000 | 0·051 | 0·000 |
| Sf1 | Fawn survival (0–6 months) | 0·143 | 0·000 | 0·177 | 0·000 |
| Sf2 | Fawn survival (6–12 months) | 0·143 | 0·000 | 0·177 | 0·000 |
| Sa | Adult survival | 0·572 | 1·000 | 0·469 | 1·000 |
| π | Transmission probability | –0·024 | –0·040 | 0·098 | –0·074 |
| γ | I‐to‐O transition probability | 0·004 | 0·002 | 0·028 | –0·033 |
| φ | O‐to‐C transition probability | 0·006 | 0·000 | 0·069 | –0·000 |
| α | Mortality probability for clinically affected deer (C) | 0·004 | 0·000 | 0·039 | –0·408 |
model behaviour and predictions
We used our ML estimates of the DD and FD transmission coefficients (β and β′, respectively) (Table 3) to evaluate predicted disease prevalence and deer density under three deer management strategies: no‐hunting, harvest, and culling. Deer hunting is the principal population and disease control measure used to manage deer abundance (Anonymous 2001; Bartelt et al. 2003). Throughout this study, we generally use ‘harvest strategy’ to indicate recreational killing of deer for wildlife population management and ‘culling strategy’ as additional population reduction for disease management. However, both of these actions were generally achieved by changes in the recreational harvest rates of deer. For the no‐hunting strategy, we simulated exponential as well as logistic deer population growth based on density‐dependent fecundity (Keyser, Guynn & Hoke 2005). For logistic growth, we estimated a carrying capacity (K) of 200 deer mi−2[R. Rolley, Wisconsin Department of Natural Resources (WDNR), personal communication] and fecundity as f = f max – (N(t)/K) × f max, where f max is the original fecundity sub‐matrix and N(t) is the pre‐birth population size at time t. The average annual harvest rate prior to the detection of CWD (harvest strategy) was estimated as 48% and 26% for antlered and antlerless deer, respectively. These rates produced an approximately stable deer population (Λ ≈ 1·0) from 1987 to 2001 (Anonymous 2001). Following the detection of CWD in 2001, liberal regulations that extended the hunting period and encouraged killing of antlerless deer (culling strategy), were implemented to reduce CWD transmission via population reduction (Bartelt, Pardee & Thiede 2003). We simulated this culling strategy using harvest rates of 28% and 39% for antlered and antlerless deer, respectively. In the culling strategy, deer are harvested using the harvest strategy until CWD prevalence reaches the 2002 rate (CWD detection year), when the culling strategy is used. To assess the sensitivity of our model predictions to the fixed‐harvest rate we also simulated the harvest strategy with a density‐dependent harvest rate which decreased linearly with deer density.
Table 3.
Transmission coefficient, time‐to‐disease‐introduction (TDI), 95% confidence intervals (CI), and model fit (AIC) to observed CWD prevalence data based on likelihood profile analysis for density‐ and frequency‐dependent transmission
| Parameter | Density‐dependent transmission | Frequency‐dependent transmission | ||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
| Transmission coefficient | 1·67 × 10−4 | 1·61 × 10−4–1·82 × 10−4 | 0·82 | 0·82–0·94 |
| TDI | 36 | 24–50 | 188 | 41– > 200 |
| AIC | 189·92 | 186·76 | ||
Results
equilibrium, sensitivity, and elasticity
The finite population growth rate (Λ) of a non‐hunted, uninfected population without resource limitation is 1·35. The equilibrium proportion of female deer is 0·5, and the proportion of fawns and yearlings are 0·32 and 0·22, respectively. The finite population growth rate is negatively correlated to the disease transmission probability (π) and equilibrium prevalence. The 2005 prevalence in the CWD core area (5·4%) corresponds to a transmission probability of 0·024 and reduces the finite population growth rate by 0·03 (Λ = 1·32). Τhis results in a slight reduction in the proportion of fawns (0·31) and yearlings (0·21). When transmission probability increases to 0·34, corresponding to 62% equilibrium prevalence, population growth rate equals unity, and the proportion of fawns and yearlings decreases substantially (0·22, 0·09, respectively). For low‐ and high‐transmission probabilities equilibrium prevalence is negatively correlated and relatively insensitive to the rate of disease progression (γ, Φ, and α: see Supporting Information Appendix S1) although sensitivity increases threefold when transmission probability is high: γ (–0·011 vs. –0·034), Φ (–0·027 vs. –0·092), and α (–0·011 vs. 0·034).
When transmission probability is low, population growth is most sensitive to female adult survival, followed by female fawn survival, and fecundity, and least sensitive to epidemiologic transition probabilities (Table 2). However, when transmission probability is high, population growth becomes substantially more sensitive to the epidemiologic parameters in the summer, while in the winter, disease mortality (α) becomes the second most important parameter after adult survival (Table 2). The relative sensitivity of adult survival is higher in winter when reproduction does not occur.
The expected proportion of deer in the susceptible (0·961) and infectious (0·012) stages fit within the 95% confidence intervals of the observed estimates (0·952–0·962 and 0·007–0·012, respectively) and the expected proportion of deer in the obex‐clinical stages (0·027) fall slightly below the observed range (0·028–0·038).
transmission mode, disease introduction and transmission coefficient
Frequency‐dependent transmission has better support than DD transmission (ΔAIC = 3·16) (Table 3). However, both models fit observed CWD prevalence for 2002–2005 (χ2, 0·001 and 0·004, respectively, P > 0·999, d.f. = 3) and closely predict (4·21% and 4·31%, respectively) observed prevalence in 2006 (4·96% and 95%, CI = 3·93–6·17%).
The likelihood profiles of both transmission modes demonstrated a negative relationship between the transmission coefficient and TDI which was discontinuous for FD transmission (Supporting Information Appendix S2). For DD, the confidence‐bounds for the transmission coefficient and TDI are clearly defined. For FD, the upper confidence‐bound for TDI could not be determined (Table 3) and the lower confidence‐bound for the transmission coefficient equals the ML estimate. The ML estimate of TDI is considerably longer for FD transmission than DD transmission (188 vs. 36 years) and has a larger 95% CI (41 to > 200 years) which slightly overlaps with DD transmission (24–50 years). TDI is slightly sensitive to the gender of the index case due to lower male survival. Initiating disease with a single 2‐year‐old male increases TDI from 36 to 37 years (2·8%) for DD transmission and 188 to 192 years (2·1%) for FD transmission. TDI is moderately sensitive to the initial number of infected individuals: with two infected 2‐year‐old females, TDI decreases by 11–12% for DD and FD transmission, respectively. Similarly, with five or 10 infected individuals, TDI is reduced by 28% and 41%, respectively.
Estimated transmission coefficients are 1·67 × 10−4 (per capita infections per 6 months) and 0·82 (infections per 6 months) for DD and FD transmission, respectively (Table 3). Based on ML estimates of the transmission coefficient and TDI, simulated number of infected deer at the time of CWD detection, and our harvest strategy, the annual infection growth rate (R) was 1·03 (CI, 1·03–1·13) and 1·15 (CI, 1·13–1·23) for FD and DD transmission, respectively.
deer harvest, disease prevalence, and host dynamics
No‐hunting
For exponential (unrestricted) and logistic (resource‐limited) population growth under DD transmission, prevalence and host density show damped oscillations which reach stability after 60–80 years (Fig. 1) with lower disease prevalence and deer density predicted for the resource‐limited population. Prevalence peaks earlier for the unrestricted population (11–12 years) than for the resource‐limited population (16–18 years). After a series of damped oscillations, disease reaches a stable prevalence which controls the host population near the initial population density (Fig. 1). Equilibrium prevalence and host density are determined by the balance between transmission, mortality and fecundity rates.
Figure 1.
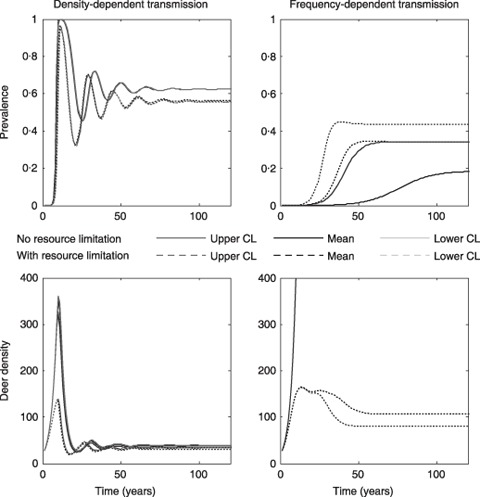
Prevalence and deer density dynamics (deer mi−2) for a non‐hunted resource‐limited and resource‐unlimited deer population under density‐ and frequency‐dependent transmission. Lines show the predicted trajectories for the mean, upper, and lower confidence limits for the estimated transmission coefficients. Note the substantial overlap of these lines for the prevalence in the density‐dependent transmission model (upper left panel) and for deer‐density in the resource‐unlimited population under frequency‐dependent transmission (lower right panel). A colour version of this figure is provided in Supporting Information Fig. S2.
For unrestricted and resource‐limited populations under FD transmission, prevalence shows a sigmoidal pattern over time with higher equilibrium prevalence for a resource‐limited population (Fig. 1) because density‐dependent birth rates reduce the recruitment of susceptible fawns into the population. The unrestricted population grows exponentially and was not limited by disease prevalence. The resource‐limited deer population initially increased (160 deer mi−2), but was reduced (80–107 deer mi−2) as disease prevalence and mortality increased (Fig. 1). For non‐hunted deer populations, FD transmission predicts that CWD should become enzootic.
Harvest strategy
Under the harvest strategy, DD transmission results in a single epizootic wave ranging from 70–100 years with peak prevalence ranging from 3–9% for the lower and upper confidence limits of β, respectively (Fig. 2). Higher transmission rates (β) produce shorter epizootic waves with higher peak prevalence and lower equilibrium deer densities ranging from 28 to 15–19 deer mi−2 before the disease is eradicated (Fig. 2).
Figure 2.
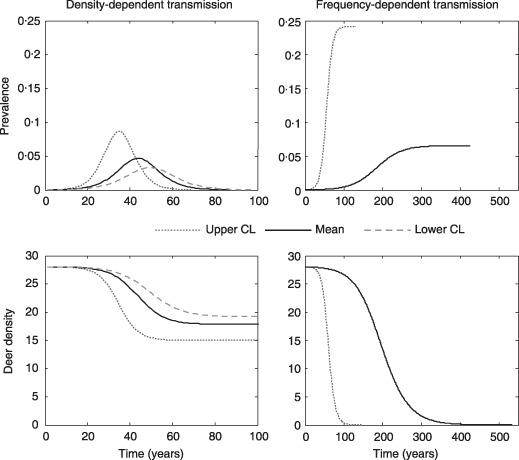
Prevalence and deer density dynamics (deer mi−2) for a deer population managed using a harvest strategy under density‐ and frequency‐dependent transmission. Lines show the predicted trajectories for the mean, upper, and lower confidence intervals for the estimated transmission coefficients. Note different time‐scales for density‐ and frequency‐dependent transmission models.
For FD transmission, a harvest strategy reduces equilibrium prevalence compared to no‐hunting, but does not alter the sigmoidal pattern with time (Fig. 2). However, harvest in combination with disease mortality produces a long‐term decline in host density with eventual extinction (Fig. 2) at a rate correlated to β′. Disease and host population went extinct after 131–425 and 145–535 years, for the lower and upper confidence limits of β′, respectively (Fig. 2).
Culling strategy
For DD transmission, the culling strategy produces moderate reductions in peak prevalence compared to the harvest strategy. The primary impacts of culling are to substantially shorten the epizootic wave by approximately 30–40 years and reduce host density below 5 deer mi−2 (Fig. 3). The DD model also predicts a delay of 4–7 years following initiation of culling before prevalence begins to decline (Fig. 3).
Figure 3.
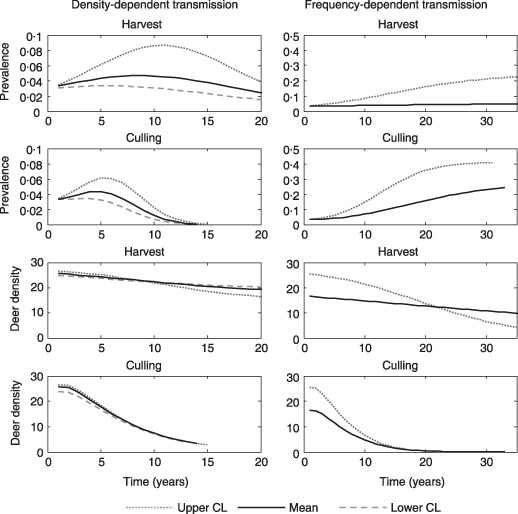
Prevalence and deer density dynamics (deer mi−2) after CWD detection in south‐central Wisconsin under harvest and culling strategies. Lines show the predicted trajectories for the mean, upper, and lower confidence intervals for the estimated transmission coefficients. Note different time‐scales for density‐ and frequency‐dependent transmission models.
For FD transmission, the culling strategy produces both higher equilibrium prevalence and disease growth rate, compared with the harvest strategy (Fig. 3). Equilibrium prevalence is higher because the population has a lower proportion of females (0·41 vs. 0·63), fawns (0·26 vs. 0·39), and yearlings (0·19 vs. 0·23), which are characterized by lower prevalence (Supporting Information Appendix S1) (Grear et al. 2006). Harvest and culling strategies result in both disease and host extinction; however, culling shortens time to extinction by at least 60–70 years.
Density‐dependent harvest
When harvest rate is density‐dependent, prevalence increases and progresses to asmyptote for both transmission modes (Fig. 4). Because harvest rate decreases with lower host population density, an increase in disease prevalence and associated mortality is offset by a decline in the harvest rate (Fig. 4). For DD transmission, the disease prevalence pattern is qualitatively different from the single epidemic wave and lower host density predicted for a fixed‐harvest rate. The reduction in harvest rate with lower host density keeps the population above the threshold density for disease extinction (Fig. 4). For FD transmission, equilibrium prevalence is slightly higher under density‐dependent harvest and the host population does not go extinct because harvest rate is reduced (Fig. 4). In general, the two main effects of density‐dependent harvest are that CWD becomes enzootic and that the dynamics of DD and FD transmission are less distinguishable.
Figure 4.
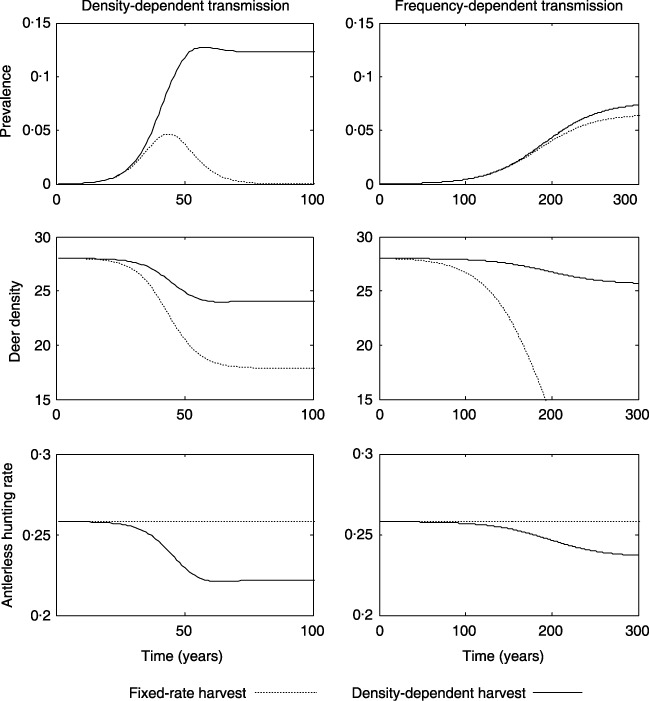
Prevalence, deer density (deer mi−2), and antlerless harvest‐rate for a deer population managed via fixed and density‐dependent harvest (maximum harvest rate equals 48% and 26% for antlered and antlerless deer, respectively) under density‐ and frequency‐dependent transmission models. Note different time‐scales for density‐ and frequency‐dependent transmission models.
Discussion
density‐ vs. frequency‐dependent transmission
Our model predicts qualitative and quantitative differences in disease dynamics between density‐ and frequency‐dependent transmission. Under intermediate (harvest strategy) or high (culling strategy) harvest rates, DD transmission predicts the typical epizootic curve of prevalence with time, whereas the FD transmission predicts increased prevalence to an asymptote (2, 3). Gross & Miller (2001) made a similar prediction for FD transmission for mule deer in Colorado. Although FD transmission fits better (ΔAIC = 3·16) than DD transmission, both models provide nearly identical predictions to our observed prevalence data. Distinguishing between DD and FD transmission was difficult given the slow growth of CWD and only 4 years of observed prevalence data, and because DD and FD transmission have similar patterns during the growth phase of the epizootic. Quantitatively, prevalence increases faster under DD transmission (R = 1·15) than under FD transmission (R = 1·03) (2, 3). The time required to achieve a detectable difference in prevalence patterns between DD and FD transmission depends strongly on a sustained harvest programme with higher harvest rates that facilitate deer population reduction and earlier assessment (Fig. 3).
It is important to note that predicted disease patterns are sensitive to the assumption of density‐independent harvest (Fig. 4). Furthermore, we used simple linear DD or FD transmission which represent opposite relationships between infectious contact rate and host density. Uncertainties about the relative importance of direct and indirect routes of CWD transmission (Miller et al. 2004) or the role of social structure, age, gender, or spatial heterogeneity and scale in contact among deer (Miller & Conner 2005; Grear 2006; Grear et al. 2006) suggests that transmission dynamics are likely between these extremes. Future empirical research and model development should address some of these issues by using non‐linear contact functions, evaluating sex‐specific transmission rates, incorporating spatial structure, and considering environmental sources of infection. Preliminary analyses (unpublished results) indicate that when the decay rate of infectious prions in the environment is low (Georgsson, Sigurarson & Brown 2006) and infectious contact rate is sufficiently high, irrespective of transmission mode, prevalence will continuously increase to nearly 100% and cause host extinction. Observations from high‐density pen studies corroborate this prediction (Miller et al. 2004; Miller, Hobbs & Tavener 2006), and emphasize the importance of understanding environmental reservoirs of CWD in free‐ranging deer populations.
cwd introduction in southern wisconsin
DD and FD transmission suggest different CWD histories in Wisconsin. DD transmission suggests CWD was introduced to southern Wisconsin during 1950–1980, which overlaps initial detection of CWD in Colorado and Wyoming in the 1960s (Williams & Miller 2002). In contrast, FD transmission suggests that disease introduction is equally likely to have occurred between the early 1960s and sometime in the 19th century. White‐tailed deer were scarce in southern Wisconsin during the late 1800s and early 1900s (Bersing 1966); therefore, the recent appearance of CWD makes more sense. A larger initial number of infected deer or smaller deer population would shorten our TDI estimates, especially for the DD model.
Back‐calculation analyses have been used to reconstruct epidemics and forecast short‐term future dynamics for chronic diseases with long and variable incubation time such as Creutzfeldt‐Jakob disease (vCJD) (Ghani et al. 2003). However, these models were criticized for being static and neglecting disease transmission dynamics (Donnelly et al. 2003). Although we explicitly addressed this issue, we were unable to resolve much of the uncertainty regarding the history of CWD in Wisconsin. Our work is a first attempt to reconstruct the history of CWD introduction into Wisconsin (or any area) and, in contrast to previous opinions, clearly indicates that CWD introduction in Wisconsin is unlikely to have been a recent event. This general knowledge about the length of time that the disease has been present in Wisconsin can be used to approximate the historical rate of disease spread, assess risk to uninfected areas, evaluate alternative surveillance strategies, and assess the need for rapid management actions.
implications for deer populations and management
Our analysis indicates that irrespective of DD or FD transmission, a non‐hunted deer population can coexist with the disease, but with high levels of infection (Fig. 1). In a hunted population, as predicted also by Gross & Miller (2001), where CWD prevalence is allowed to grow unchecked, deer harvest (particularly of females) will eventually become unsustainable. For DD transmission, irrespective of resource limitation, CWD will generate damped oscillations in the host population before equilibrium is reached (Fig. 1). In contrast, for FD transmission under the realistic assumption of resource limitation, disease increases until it regulates the deer population considerably below the carrying capacity.
In most parts of North America, white‐tailed deer (and other cervids) are harvested to maintain a sustainable population. In other areas (e.g. National Parks, state parks, urban areas, deer refugia), hunting is not permitted or is severely reduced. Our results suggest that CWD infection can rapidly increase in non‐hunted areas and reach high prevalence levels (30 to 60%). These areas are likely to function as a source for disease spread when infected animals disperse to surrounding areas. The results of the present study indicate that non‐selective culling strategies can be potentially effective in reducing CWD infection and spread. Alternatively, selective culling of infected deer might be a suitable strategy in local areas where generalized culling is not feasible (Wolfe, Miller & Williams 2004).
One of our main questions was whether non‐selective culling can be used to control CWD. Our simulation results indicate that for FD transmission, eradication of CWD necessitates eradication of the host population. Gross & Miller (2001), who implicitly assumed FD transmission, predicted a similar effect. In contrast, CWD could be eradicated while maintaining a viable deer population only if transmission is DD. However, achieving disease eradication is contingent upon maintaining a high and sustained harvest rate. If harvest rate is density‐dependent and therefore not sustained, then CWD cannot be eradicated, deer density and disease prevalence stabilize at higher levels (Fig. 4), and the functional form of harvest (Van Deelen & Etter 2003) affects the predicted levels of prevalence and deer density (results not shown).
Our model also provides important insights regarding the time‐scale associated with disease epidemics and management. Under DD transmission and a harvest strategy, CWD can last for 60–80 years or become permanently endemic under FD transmission. Similarly, the time required to achieve disease management can be considerable. For DD transmission, time to disease eradication can be reduced to 13–15 years by aggressive culling, but the result is a lower deer density (5 vs. 15–19 deer mi−2). For FD transmission, time to eradication could range from 90 to 385 years (2, 3) and aggressive culling could substantially decrease disease eradication time to between 31 and 33 years, although host eradication is a requirement for disease eradication (Fig. 3). On shorter time‐scales, DD transmission predicts a 5‐ to 11‐year lag before CWD prevalence begins to decline (Fig. 3) for the harvest strategy, whereas a 4‐ to 5‐ year lag is predicted for the culling strategy (Fig. 3). These results demonstrate that CWD prevalence may temporarily increase even when management culling strategies are successfully controlling the disease, and emphasizes the need for both aggressive and sustained culling to successfully reduce or eradicate chronic diseases such as CWD. These results may help explain the lack of prevalence reduction in Colorado mule deer following 3 years of intensive culling (Conner et al. 2007) or 6–7 years of low to intermediate culling (Miller & Conner 2005). Under FD transmission, prevalence never declines, suggesting that alternative metrics such as the density of infected animals should be considered for monitoring and surveillance.
Conclusion
Our results indicate that CWD is a slowly spreading disease in wild white‐tailed deer and, therefore disease management will probably require decades. There is considerable controversy about the appropriate management actions to control CWD, primarily because FD vs. DD transmission and the long‐term impacts on white‐tailed deer populations are unknown. If CWD transmission is FD, then disease prevalence may reach high levels and disease eradication can only be achieved by eradication of deer, action that might be best justified for protecting a larger uninfected population. If CWD transmission is DD, then disease prevalence could remain at lower levels with minimal impact on deer populations, and disease eradication might be achieved by a dramatic reduction in deer abundance. In either situation, deer culling appears to be an important tool for limiting the rate of disease growth by removing infected deer from the population, altering host density, and affecting the rate of disease prevalence and spread. Higher harvest rates can also be used to learn about managing CWD by producing prevalence patterns that distinguish DD transmission (a single epidemic wave) from FD transmission (asymptotic growth). High harvest rates allow earlier detection of these differences, but culling strategies must be sustained over a sufficiently long period. The limited scientific knowledge about environmental transmission, density‐dependent harvest, and other epizootiological conditions presents additional challenges that require further research to effectively manage CWD.
Adaptive management is an iterative process in which we are ‘... learning to manage by managing to learn’ (Stankey, Clark & Bormann 2005) by using modelling to integrate existing interdisciplinary experience and scientific information for predicting impacts of alternative policies (Walters & Holling 1990). Our modelling approach uses prior information on deer population dynamics and harvest rates combined with monitoring data from ongoing CWD management (Joly et al. 2003, 2006; Grear 2006; Grear et al. 2006) to improve our knowledge about disease dynamics, reduce uncertainty in model predictions, and evaluate how FD and DD transmission affect disease management programmes such as generalized culling. We believe an adaptive management approach which closely integrates management programmes within a predictive modelling framework (Nishi, Shury & Elkin 2006) should be used to conduct future experiments that perturb deer densities and evaluate DD and FD transmission. Alternatively, if dramatic deer reduction is not an acceptable control strategy due to social, political, and financial limitations, modelling can be used to evaluate alternative management programmes (e.g. maintenance of low level endemic disease and/or spatial containment). In addition, our modelling approach can be used to evaluate new epidemiological strategies (e.g. vaccination) or expanded to incorporate future research findings (e.g. role for environmental transmission) on disease dynamics, host demographics, and management actions.
Supporting information
Appendix S1. Multi‐state model parameters and structure
Fig. S1. Beta‐TDI likelihood profile.
Fig. S2. A colour version of Fig. 1.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Acknowledgements
We thank the more than one thousand volunteers and WDNR staff who worked tirelessly to collect tissue samples; the Wisconsin Interagency Chronic Wasting Disease Task Force; the hunters who provided samples and participated in CWD management. We thank M. Verdon, WDNR, for organizing and verification of the database. We thank P. Cross and E. Cooch for reviewing an earlier version of this manuscript. G. Wasserberg's research fellowship was supported by funding from the US Geological Survey – National Wildlife Health Center. The Max McGraw Wildlife Foundation provided support for publication.
Re‐use of this article is permitted in accordance with the Creative Commons Deed, Attribution 2·5, which does not permit commercial exploitation.
References
- Anonymous (2001) Management Workbook for White‐tailed Deer, 2nd edn. Bureaus of Wildlife Management and Integrated Science Services, Wisconsin Department of Natural Resources, Madison, WI, USA. [Google Scholar]
- Bartelt, G. , Pardee, J. & Thiede, K. (2003) Environmental Impact Statement: On Rules to Eradicate Chronic Wasting Disease from Wisconsin's Free‐Ranging White‐Tailed Deer Herd. Wisconsin Department of Natural Resources, Madison, WI, USA. [Google Scholar]
- Bersing, O.S. (1966) A Century of Wisconsin Deer, 2nd edn. Wisconsin Conservation Department, Madison, WI, USA. [Google Scholar]
- Caswell, H. (2001) Matrix Population Models, 2nd edn. Sinauer Associates, Sunderland, MA, USA. [Google Scholar]
- Conner, M.M. , Miller, M.W. , Ebinger, M.R. & Burnham, K.P. (2007) A meta‐BACI approach for evaluating management intervention on chronic wasting disease in free‐ranging mule deer. Ecological Applications, 17, 140–153. [DOI] [PubMed] [Google Scholar]
- Daszak, P. , Cunningham, A.A. & Hyatt, A.D. (2000) Wildlife ecology – Emerging infectious diseases of wildlife – Threats to biodiversity and human health. Science, 287, 443–449. [DOI] [PubMed] [Google Scholar]
- Donnelly, C.A. , Ferguson, N.M. , Ghani, A.C. & Anderson, R.M. (2003) Extending backcalculation to analyse BSE data. Statistical Methods in Medical Research, 12, 177–190. [DOI] [PubMed] [Google Scholar]
- Georgsson, G. , Sigurarson, S. & Brown, P. (2006) Infectious agents of sheep scrapie may persist in the environment for at last 16 years. Journal of General Virology, 87, 3737–3740. [DOI] [PubMed] [Google Scholar]
- Ghani, A.C. , Ferguson, N.M. , Donnelly, C.A. & Anderson, R.M. (2003) Factors determining the pattern of the variant Creutzfeldt‐Jakob disease (vCJD) epidemic in the UK. Proceedings of the Royal Society, Series B, 270, 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grear, D.A. (2006) Chronic Wasting Disease Infection Patterns in Female White‐tailed Deer Related to Demographics, Genetic Relationships, and Spatial Proximity of Infected Deer in Southern Wisconsin. M.Sc. University of Wisconsin, Madison, WI, USA. [Google Scholar]
- Grear, D.A. , Samuel, M.D. , Langenberg, J.A. & Keane, D. (2006) Demographic patterns and harvest vulnerability of chronic wasting disease infected white‐tailed deer in Wisconsin. Journal of Wildlife Management, 70, 546–553. [Google Scholar]
- Gross, J.E. & Miller, M.W. (2001) Chronic wasting disease in mule deer: disease dynamics and control. Journal of Wildlife Management, 65, 205–215. [Google Scholar]
- Joly, D.O. , Ribic, C.A. , Langenberg, J.A. , Beheler, K. , Batha, C.A. , Dhuey, B.J. , Rolley, R.E. , Bartelt, G. , Van Deelen, T.R. & Samuel, M.D. (2003) Chronic wasting disease in free‐ranging Wisconsin white‐tailed deer. Emerging Infectious Diseases, 9, 599–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly, D.O. , Samuel, M.D. , Langenberg, J.A. , Blanchong, J.A. , Batha, C.A. , Rolley, R.E. , Keane, D. & Ribic, C.A. (2006) Spatial epidemiology of chronic wasting disease in Wisconsin white‐tailed deer. Journal of Wildlife Diseases, 42, 578–588. [DOI] [PubMed] [Google Scholar]
- Keyser, P.D. , Guynn, D.C. & Hoke, S.H. (2005) Density dependent recruitment patterns in white‐tailed deer. Wildlife Society Bulletin, 33, 222–232. [Google Scholar]
- Land, K.C. & Rogers, A. (1982) Multidimensional Mathematical Demography. Academic Press, New York. [Google Scholar]
- Lloyd‐Smith, J.O. , Cross, P.C. , Briggs, C.J. , Daugherty, M. , Getz, W.M. , Latto, J. , Sanchez, M.S. , Smith, A.B. & Swei, A. (2005) Should we expect population thresholds for wildlife disease? Trends in Ecology & Evolution, 20, 511–519. [DOI] [PubMed] [Google Scholar]
- McCallum, H. , Barlow, N. & Hone, J. (2001) How should pathogen transmission be modelled? Trends in Ecology & Evolution, 16, 295–300. [DOI] [PubMed] [Google Scholar]
- Miller, M.W. & Conner, M.M. (2005) Epidemiology of chronic wasting disease in free‐ranging mule deer: spatial, temporal, and demographic influences on observed prevalence patterns. Journal of Wildlife Diseases, 41, 275–290. [DOI] [PubMed] [Google Scholar]
- Miller, M.W. , Hobbs, N.T. & Tavener, S.J. (2006) Dynamics of prion disease transmission in mule deer. Ecological Applications, 16, 2208–2214. [DOI] [PubMed] [Google Scholar]
- Miller, M.W. , Williams, E.S. , Hobbs, N.T. & Wolfe, L.L. (2004) Environmental sources of prion transmission in mule deer. Emerging Infectious Diseases, 10, 1003–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi, J.S. , Shury, T. & Elkin, B.T. (2006) Wildlife reservoirs for bovine tuberculosis (Mycobacterium bovis) in Canada: strategies for management and research. Veterinary Microbiology, 112, 325–338. [DOI] [PubMed] [Google Scholar]
- Peterson, M.J. (1991) Wildlife parasitism, science, and management policy. Journal of Wildlife Management, 55, 782–789. [Google Scholar]
- Schauber, E.M. & Woolf, A. (2003) Chronic wasting disease in deer and elk: a critique of current models and their application. Wildlife Society Bulletin, 31, 610–616. [Google Scholar]
- Stankey, G.H. , Clark, R.N. & Bormann, B.T. (2005) Adaptive Management of Natural Resources: Theory, Concepts, and Management Institutions. Gen. Tech. Rep. PNW‐GTR‐654. U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station, Portland, OR. 73p. [Google Scholar]
- Starfield, A.M. (1997) A pragmatic approach to modeling for wildlife management. Journal of Wildlife Management, 61, 261–270. [Google Scholar]
- Van Deelen, T.R. (1999) Deer‐cedar interactions during a period of mild winters: implications for conservation of conifer swamp deeryards in the Great Lakes Region. Natural Areas Journal, 19, 263–274. [Google Scholar]
- Van Deelen, T.R. & Etter, D.R. (2003) Effort and the functional response of deer hunters. Human Dimensions of Wildlife, 8, 97–108. [Google Scholar]
- Walters, C.J. & Holling, C.S. (1990) Large‐scale management experiments and learning by doing. Ecology, 71, 2060–2068. [Google Scholar]
- Williams, E.S. & Miller, M.W. (2002) Chronic wasting disease in deer and elk in North America. Revue Scientifique et Technique de l Office International des Epizooties, 21, 305–316. [DOI] [PubMed] [Google Scholar]
- Wolfe, L.L. , Miller, M.W. & Williams, E.S. (2004) Feasibility of ‘test‐and‐cull’ for managing chronic wasting disease in urban mule deer. Wildlife Society Bulletin, 32, 500–505. [Google Scholar]
- Yang, Y. , Allen, J.C. , Knapp, J.L. & Stansly, P.A. (1997) An age structured population model of citrus rust mite: a fruit‐mite‐fungal pathogen system. Ecological Modelling, 104, 71–85. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Multi‐state model parameters and structure
Fig. S1. Beta‐TDI likelihood profile.
Fig. S2. A colour version of Fig. 1.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item


