Abstract
Heterotopic pancreas is a congenital anomaly characterized by ectopic pancreatic tissue. Treatment of heterotopic pancreas may include expectant observation, endoscopic resection or surgery. The aim of this report was to describe the technique of ligation-assisted endoscopic mucosal resection (EMR) for resection of heterotopic pancreas of the stomach. Two patients (both female, mean age 32 years) were referred for management of gastric subepithelial tumors. Endoscopic ultrasound in both disclosed small hypoechoic masses in the mucosa and submucosa. Band ligation-assisted EMR was performed in both cases without complications. Pathology from the resected tumors revealed heterotopic pancreas arising from the submucosa. Margins were free of pancreatic tissue. Ligation-assisted EMR is technically feasible and may be considered for the endoscopic management of heterotopic pancreas.
Keywords: Endoscopic mucosal resection, Endoscopic ultrasound, Heterotopic pancreas
INTRODUCTION
Heterotopic pancreas (HP), also known as pancreatic rest, was first described in 1727 when it was found in an ileal diverticulum[1]. It refers to an uncommon congenital anomaly characterized by the presence of ectopic pancreatic tissue far from the pancreas and without any anatomical or vascular communication with this organ. It occurs in 2% of the general population[2] and is more common in males than females[3]. HP may occur throughout the gastrointestinal tract but has a proclivity for involving the stomach and proximal small intestine. Most affected patients are asymptomatic although a minority may present with a variety of symptoms, most common being epigastric pain[2]. Options for treatment for heterotopic pancreas in the stomach include surgery[2–4], endoscopic resection[5–8] or conservative management. This report describes the first two cases of HP treated with band ligation-assisted endoscopic mucosal resection (EMR).
CASE REPORT
Case 1
A 49-year-old white female presented to her primary gastroenterologist with abdominal bloating and constipation. She was report anemic and found to have heme-occult positive stools. Work-up included a computer tomography (CT) scan of the abdomen and pelvis, colonoscopy and esophagogastroduodenoscopy (EGD). CT revealed small liver cysts but no other abnormalities. Colonoscopy demonstrated two small polyps that were removed. EGD showed a small, firm, umbilicated subepithelial antral mass. The mucosa overlying the mass was biopsied and showed chronic gastritis with no submucosal tissue present. The patient was referred to our endoscopy unit for upper endoscopic ultrasonography (EUS) of the antral lesion. She denied abdominal pain, nausea, vomiting, overt gastrointestinal bleeding or weight loss. Her physical examination revealed a healthy middle-aged white female in no apparent distress. Her abdominal examination was normal without tenderness. Prior to EUS, she was informed that the lesion most likely represented a pancreatic rest and that if suspected diagnosis was confirmed, then conservative management would be reasonable. Nevertheless, the patient expressed her preference for resection of the mass if possible. Informed consent was obtained and sedation was performed using a combination of intravenous midazolam and propofol. Initial EGD confirmed a single medium-sized subepithelial nodule along the greater curvature of the gastric antrum (Figure 1). The patient was then placed into the right lateral decubitus position and water was instilled into the distal stomach. Radial endosonography (GF-UE160-AL5; Olympus America Inc., Center Valley PA; USA) revealed a well-defined hypoechoic lesion from the deep mucosa/submucosa that measured 8 mm × 6 mm in maximal diameter (Figure 2A and B). There was no evidence of peritumoral adenopathy. Ligator-assisted EMR (Duette Multi-Band Mucosectomy; Cook Medical Inc., Winston-Salem, NC; USA) of the tumor was successfully performed using one band (Figure 3). The residual ulcer was closed using three endoclips (Resolution Clip; Boston Scientific Inc., Natick MA; USA). There were no complications. Surgical pathology from the resected specimen revealed heterotopic pancreatic tissue confined to the submucosa (Figure 4).
Figure 1.
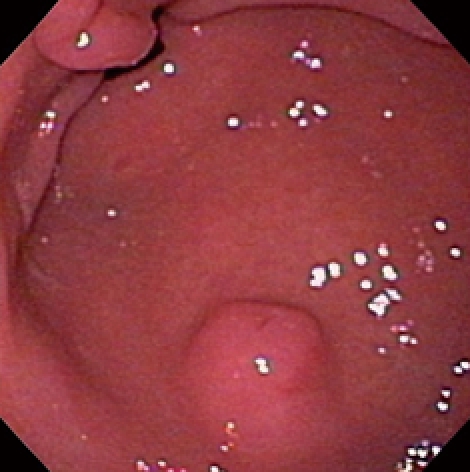
Endoscopic view of heterotopic pancreas of the stomach showing a medium-sized subepithelial nodule with central umbilication and normal overlying mucosa. The pylorus is visible distally.
Figure 2.
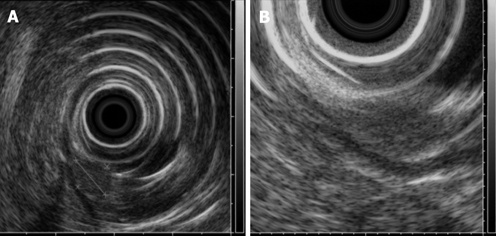
Radial EUS images. A pancreatic rest showing an 8 mm × 6 mm hypoechoic subepithelial mass appearing to involve the mucosa (A) and submucosa (B). The tumor was confirmed as submucosal in origin after resection without involvement of the mucosa.
Figure 3.
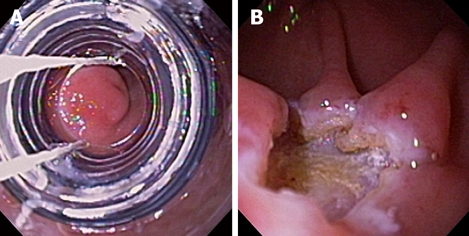
Ligation-assisted EMR of pancreatic rest. The banding device was positioned over the target lesion (A), suction was applied and a band was deployed. The lesion was then resected using electrocautery snare. Residual ulcer is shown (B).
Figure 4.
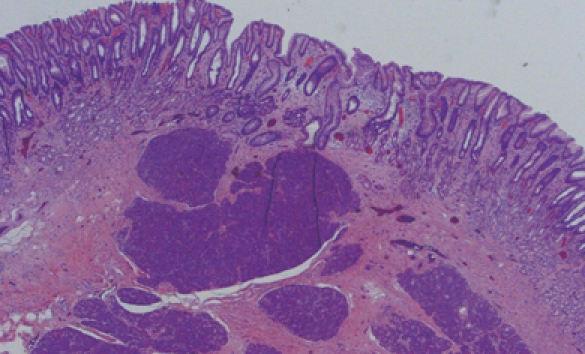
Photomicrograph of the resection specimen showing pancreatic tissue within the gastric submucosa (HE, × 10).
Case 2
A 15-year-old white female presented to her primary gastroenterologist with left upper quadrant abdominal pain. EGD showed a small subepithelial antral mass. The mucosa overlying the tumor was normal and biopsies showed only mild gastritis. The patient was then referred for an upper EUS. She denied nausea, vomiting, overt gastrointestinal bleeding or weight loss. Physical examination including abdominal examination was normal. Prior to EUS, review of endoscopic pictures from outside EGD suggested a pancreatic rest. Information was provided to the patient and parents that if suspected diagnosis was confirmed, then conservative management would be reasonable. Nevertheless, the patient and parents both expressed preference for tumor resection, if possible. Initial EGD confirmed a single medium-sized subepithelial nodule along the greater curvature of the prepyloric stomach (Figure 5). The patient was then placed into the right lateral decubitus position and water was instilled into the distal stomach. Radial endosonography revealed a well-defined 9 mm × 7 mm hypoechoic lesion from the deep mucosa and submucosa (Figure 6). The outer endosonographic borders were well defined. Ligator-assisted EMR of the tumor was successfully performed using one band (Figure 7). There were no complications. The resected specimen was submitted for pathologic examination, which revealed the presence of HP tissue confined to the submucosa with negative margins.
Figure 5.
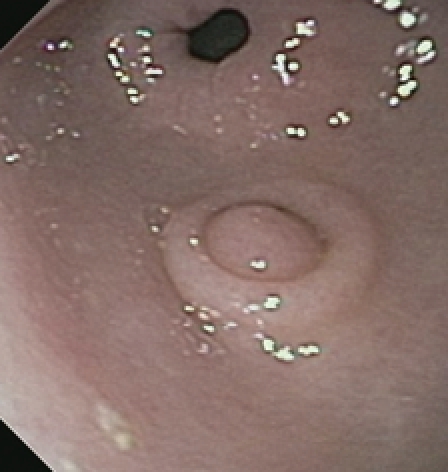
Endoscopic view of heterotopic pancreas of the stomach showing a medium-sized subepithelial nodule with central umbilication and normal overlying mucosa. The pylorus is visible distally.
Figure 6.
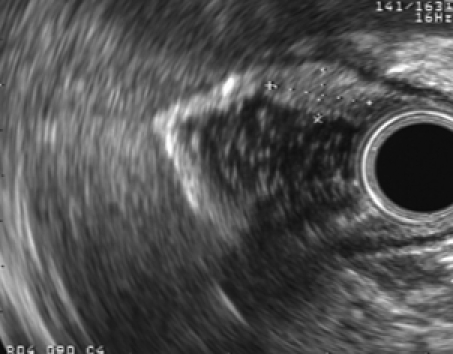
Radial EUS images of a pancreatic rest showing a 9 mm × 7 mm hypoechoic subepithelial mass appearing to involve the deep mucosa and submucosa. The tumor was confirmed as submucosal in origin after resection without involvement of the mucosa.
Figure 7.
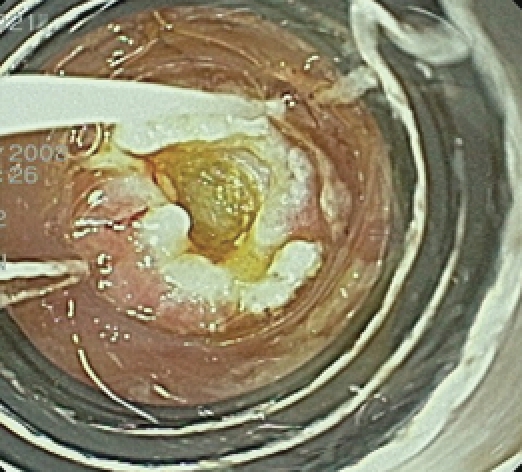
Ligation-assisted EMR of pancreatic rest.
DISCUSSION
Heterotopic pancreas (HP), also known as pancreatic rest, is most often detected as an incidental finding during routine upper endoscopy. The typical endoscopic appearance in the stomach is as a firm round or oval umbilicated subepithelial nodule along the greater curvature situated several centimeters proximal to the pylorus. Most patients are asymptomatic, but symptoms may rarely occur due to the irritative effect of the hormones and enzymes secreted by the HP at a particular site[3]. Histologically, pancreatic rests vary from resembling the normal pancreas (acini, ducts and islets of Langerhans) to widely separated ducts within a muscular stroma[4,9]. Hence, complications in HP resemble those seen with the pancreas itself including acute pancreatitis[10,11], pancreatic cancer[12], cystic degeneration[13] and islet cell tumors[14]. Other rare reported complications include gastrointestinal bleeding[15], gastric outlet obstruction[16], jaundice[13] and abscess formation[10]. Asymptomatic HP can generally be followed expectantly with treatment reserved for patients who are symptomatic, have enlarging lesions or to ensure diagnostic certainty. Both pancreatic rests in these two patients were incidentally discovered during EGD for investigation of anemia and left upper quadrant pain, respectively. Nevertheless, removal was requested by both patients to ensure the suspected pre-procedural diagnosis.
The diagnosis of HP is not straightforward. Although umbilication is characteristic, the specificity of this endoscopic finding for HP remains unknown. The typical EUS image of a pancreatic rest in the stomach is a hypoechoic, heterogeneous submucosal mass, although the muscularis propria and mucosa may be occasionally involved. A ductal structure may also be discernible within the lesion. Although these endosonographic findings are suggestive of HP, the accuracy of EUS for the diagnosis of subepithelial tumors (other than gastrointestinal stromal tumors such as HP) is limited[7,17]. This fact is illustrated in our cases as the mucosa was incorrectly predicted to be involved in both patients.
Since HP is usually a submucosal tumor, pinch mucosal biopsies are invariably non-diagnostic. Nevertheless, Shalaby et al[18] reported a case of esophageal HP diagnosed with biopsies obtained with a jumbo forceps. Teixeira[19] has described the use of ethanol injection to create an artificial ulcer that then facilitated the removal of sufficient submucosal tissue to establish the diagnosis of HP. Goto et al[20] reported a case of esophageal HP diagnosed by EUS-guided FNA. Historically, the diagnosis of HP was often made by histological examination of surgical specimens. Some authors believe that it is difficult to obtain a definitive diagnosis of HP preoperatively[21] and therefore advocate surgical resection[3].
Although there are some reports of surgical resection of HP[2–4], EMR may be an attractive, less invasive option for resection of accessible lesions. However, there have only been a few reports[5–8] that describe the use EMR for HP resection (Table 1). Two groups have described the use of cap-assisted EMR[5,6]. The “inject, lift and cut”, or “strip biopsy” EMR technique[7] was reported for the resection of 6 HP submucosal masses. Finally, Sun et al[8] studied the use of EUS-guided injection and the “inject and cut” EMR technique for the resection of 16 upper gastrointestinal submucosal tumors, two of which were gastric HP tumors. Ligation-assisted EMR may be more operator-friendly than the other EMR techniques. It requires neither saline injection nor snare prepositioning, and the concept of tissue capture is similar to the familiar variceal ligation technique. The current series, to our knowledge, is the first to describe the use of ligation-assisted EMR for gastric HP. This technique permitted a histologic confirmation of the suspected diagnosis and in both cases achieved a margin-negative resection without complication.
Table 1.
Literature summary of HP cases treated with EMR
| Author | Yr | Number of tumors | Mean tumor size (mm) | Tumor location | EMR technique | Follow-up1 (mo) |
| Lee et al[5] | 1999 | 1 | Small | Stomach | CAP-assisted | NR |
| Faigel et al[6] | 2001 | 1 | 10 | Stomach | CAP-assisted | 6 |
| Kojima et al[7] | 1999 | 6 | 13.5 | Stomach | Strip biopsy | 15 |
| Sun et al[8] | 2002 | 2 | NR | Stomach | Inject and cut | 12-17 |
| Khashab et al (current study) | 2008 | 2 | 8.5 | Stomach | Ligation-assisted | NR |
EMR: Endoscopic mucosal resection; NR: Not reported.
All cases with reported follow-up were without any recurrence.
Footnotes
Peer reviewers: Atsushi Nakajima, Professor, Division of Gastroenterology, Yokohama City University Graduate School of Medicine, 3-9 Fuku-ura, Kanazawa-ku, Yokohama 236-0004, Japan; Dr. Mitsuhiro Fujishiro, Department of Gastroenterology, Faculty of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, Japan; Dr. Peter Draganov, University of Florida, 1600 SW Archer Rd., Gainesville, FL 32610, United States
S- Editor Tian L L- Editor Logan S E- Editor Yin DH
References
- 1.Elfving G, Hästbacka J. Pancreatic heterotopia and its clinical importance. Acta Chir Scand. 1965;130:593–602. [PubMed] [Google Scholar]
- 2.Lai EC, Tompkins RK. Heterotopic pancreas. Review of a 26 year experience. Am J Surg. 1986;151:697–700. doi: 10.1016/0002-9610(86)90045-0. [DOI] [PubMed] [Google Scholar]
- 3.Ormarsson OT, Gudmundsdottir I, Mårvik R. Diagnosis and treatment of gastric heterotopic pancreas. World J Surg. 2006;30:1682–1689. doi: 10.1007/s00268-005-0669-6. [DOI] [PubMed] [Google Scholar]
- 4.Dolan RV, ReMine WH, Dockerty MB. The fate of heterotopic pancreatic tissue. A study of 212 cases. Arch Surg. 1974;109:762–765. doi: 10.1001/archsurg.1974.01360060032010. [DOI] [PubMed] [Google Scholar]
- 5.Lee TH, Wang HP, Huang SF, Wang TH, Lin JT. Endoscopic mucosal resection for treatment of heterotopic pancreas in the stomach. J Formos Med Assoc. 1999;98:643–645. [PubMed] [Google Scholar]
- 6.Faigel DO, Gopal D, Weeks DA, Corless C. Cap-assisted endoscopic submucosal resection of a pancreatic rest. Gastrointest Endosc. 2001;54:782–784. doi: 10.1067/mge.2001.116620. [DOI] [PubMed] [Google Scholar]
- 7.Kojima T, Takahashi H, Parra-Blanco A, Kohsen K, Fujita R. Diagnosis of submucosal tumor of the upper GI tract by endoscopic resection. Gastrointest Endosc. 1999;50:516–522. doi: 10.1016/s0016-5107(99)70075-1. [DOI] [PubMed] [Google Scholar]
- 8.Sun S, Wang M, Sun S. Use of endoscopic ultrasound-guided injection in endoscopic resection of solid submucosal tumors. Endoscopy. 2002;34:82–85. doi: 10.1055/s-2002-19386. [DOI] [PubMed] [Google Scholar]
- 9.DeBord JR, Majarakis JD, Nyhus LM. An unusual case of heterotopic pancreas of the stomach. Am J Surg. 1981;141:269–273. doi: 10.1016/0002-9610(81)90172-0. [DOI] [PubMed] [Google Scholar]
- 10.Kaneda M, Yano T, Yamamoto T, Suzuki T, Fujimori K, Itoh H, Mizumoto R. Ectopic pancreas in the stomach presenting as an inflammatory abdominal mass. Am J Gastroenterol. 1989;84:663–666. [PubMed] [Google Scholar]
- 11.Matsushita M, Hajiro K, Takakuwa H. Acute pancreatitis occurring in gastric aberrant pancreas accompanied by paralytic ileus. Am J Gastroenterol. 1997;92:2121–2122. [PubMed] [Google Scholar]
- 12.Jeng KS, Yang KC, Kuo SH. Malignant degeneration of heterotopic pancreas. Gastrointest Endosc. 1991;37:196–198. doi: 10.1016/s0016-5107(91)70687-1. [DOI] [PubMed] [Google Scholar]
- 13.Fléjou JF, Potet F, Molas G, Bernades P, Amouyal P, Fékété F. Cystic dystrophy of the gastric and duodenal wall developing in heterotopic pancreas: an unrecognised entity. Gut. 1993;34:343–347. doi: 10.1136/gut.34.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose C, Kessaram RA, Lind JF. Ectopic gastric pancreas: a review and report of 4 cases. Diagn Imaging. 1980;49:214–218. [PubMed] [Google Scholar]
- 15.Endo Y, Yazumi S, Kimura Y, Uza N, Matsuura M, Kodama Y, Nakase H, Chiba T. A case of ileal heterotopic pancreas with repeated melena. Gastrointest Endosc. 2007;65:156–157; discussion 157. doi: 10.1016/j.gie.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Shaib YH, Rabaa E, Feddersen RM, Jamal MM, Qaseem T. Gastric outlet obstruction secondary to heterotopic pancreas in the antrum: case report and review. Gastrointest Endosc. 2001;54:527–530. doi: 10.1067/mge.2001.116461. [DOI] [PubMed] [Google Scholar]
- 17.Brand B, Oesterhelweg L, Binmoeller KF, Sriram PV, Bohnacker S, Seewald S, De Weerth A, Soehendra N. Impact of endoscopic ultrasound for evaluation of submucosal lesions in gastrointestinal tract. Dig Liver Dis. 2002;34:290–297. doi: 10.1016/s1590-8658(02)80150-5. [DOI] [PubMed] [Google Scholar]
- 18.Shalaby M, Kochman ML, Lichtenstein GR. Heterotopic pancreas presenting as dysphagia. Am J Gastroenterol. 2002;97:1046–1049. doi: 10.1111/j.1572-0241.2002.05627.x. [DOI] [PubMed] [Google Scholar]
- 19.Teixeira CR, Haruma K, Shimamoto T, Tsuda T, Okamoto S, Sumii K, Kajiyama G. Heterotopic pancreas diagnosed by endoscopic ultrasonography and endoscopic injection of ethanol to make a histologic diagnosis. J Clin Gastroenterol. 1992;15:52–54. doi: 10.1097/00004836-199207000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Goto J, Ohashi S, Okamura S, Urano F, Hosoi T, Ishikawa H, Segawa K, Hirooka Y, Ohmiya N, Itoh A, et al. Heterotopic pancreas in the esophagus diagnosed by EUS-guided FNA. Gastrointest Endosc. 2005;62:812–814. doi: 10.1016/j.gie.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 21.Hsia CY, Wu CW, Lui WY. Heterotopic pancreas: a difficult diagnosis. J Clin Gastroenterol. 1999;28:144–147. doi: 10.1097/00004836-199903000-00012. [DOI] [PubMed] [Google Scholar]


