Abstract
To assess the role of the Ogg1 DNA glycosylase in the transcription-coupled repair (TCR) of the mutagenic lesion, 7,8-dihydro-8oxoguanine (8-OxoG), we have investigated the removal of this lesion in wild-type and ogg1−/− null mouse embryo fibroblast (MEF) cell lines. We used nonreplicating plasmids containing a single 8-OxoG·C base pair in a different assay that allowed us to study the removal of 8-OxoG located in a transcribed sequence (TS) or in a nontranscribed sequence (NTS). The results show that the removal of 8-OxoG in a wild-type MEF cell line is faster in the TS than in the NTS, indicating TCR of 8-OxoG in murine cells. In the homozygous ogg1−/− MEF cell line, 8-OxoG was not removed from the NTS whereas there was still efficient 8-OxoG repair in the TS. Expression of the mouse Ogg1 protein in the homozygous ogg1−/− cell line restored the ability to remove 8-OxoG in the NTS. Therefore, we have demonstrated that Ogg1 is essential for the repair of 8-OxoG in the NTS but is not required in the TS. These results indicate the existence of an Ogg1-independent pathway for the TCR of 8-OxoG in vivo.
Reactive oxygen species, generated either endogenously by cellular metabolism or by exposure to environmental mutagens or ionizing radiation, induce oxidative damage to DNA, which has been implicated in human pathologies such as cancer, neurodegenerative diseases, and aging (1–3). Reactive oxygen species produce different types of lesions in DNA such as apurinic/apyrimidinic (AP) sites, DNA strand breaks, and oxidized bases (4, 5). In all organisms, oxidatively damaged bases in DNA are primarily corrected by the base excision repair process initiated by a specialized DNA glycosylase/AP lyase that recognizes and releases the altered base and incises DNA at the resulting AP site (6–9). An oxidatively damaged guanine, 7,8-dihydro-8-oxoguanine (8-OxoG), is an important premutagenic lesion due to its potential to mispair with adenine, thus generating G·C to T·A transversions (10). Its biological significance is revealed by the existence of a three-tiered defense system composed of MutT, Fpg, and MutY proteins in Escherichia coli: MutT hydrolyses 8-OxodGTP from the pool of DNA precursors, the Fpg DNA glycosylase/AP lyase releases 8-OxoG from damaged DNA, while the MutY DNA glycosylase excises adenine inserted opposite 8-OxoG by DNA polymerases (7, 11, 12). Inactivation of any of these three genes generates a mutator phenotype attributed to the persistence of 8-OxoG in DNA or in the pool of deoxynucleoside-triphosphates (11, 12). In Saccharomyces cerevisiae, the OGG1 gene encodes a DNA glycosylase/AP lyase that catalyses the removal of 8-OxoG and incises DNA at the resulting AP site (13, 14). Furthermore, Ogg1-deficient strains of S. cerevisiae exhibit a spontaneous G·C to T·A mutator phenotype (15). Homologs of MutY and MutT have not been identified in the S. cerevisiae genome. However, a recent study (16) shows that the mismatch repair proteins Msh2 and Msh6 are involved in the removal of adenine inserted opposite 8-OxoG in S. cerevisiae.
In human cells, MutT, MutY, and Ogg1 homologs have been identified (17–25). The human OGG1 gene encodes two isoforms, α-hOgg1 and β-hOgg1, resulting from alternative splicing of the transcript (reviewed in ref. 26). α-hOgg1 is a 37-kDa protein localized in the nucleus whereas β-Ogg1 is a 44-kDa protein targeted to the mitochondria (27, 28). Mouse Ogg1 has a predicted molecular mass of 37 kDa and is 84% identical to the human nuclear α-hOgg1 protein (29, 30). Both human and mouse Ogg1 are DNA glycosylases/AP lyases that excise 2,6-diamino-4-hydroxy-5-N-methylformamidopyrimidine and 8-OxoG and incise DNA at AP sites (reviewed in ref. 26). To investigate the biological role of the Ogg1 protein in mammalian cells, homozygous ogg1−/− null mice have been generated. Null animals are viable, do not develop malignancies, and show no marked pathological changes up to 12 months of age (31, 32). However, Ogg1-deficient mice show an abnormal accumulation of 8-OxoG in their genomes and exhibit a significantly higher spontaneous mutation rate in nonproliferative tissues compared to the isogenic wild-type (31, 32). Furthermore, analysis of a mouse embryo fibroblast (MEF) cell line indicated slow but substantial repair of Fpg-sensitive sites in ogg1−/− null cells after oxidative stress induced by a photosensitizer, suggesting the presence of an alternative pathway for repair of oxidative DNA damage in the absence of Ogg1 (31). Recently, a study of 8-OxoG repair in human cells has revealed the existence of a transcription-coupled repair (TCR) mechanism that requires proteins involved in nucleotide excision repair such as XPG, TFIIH, and CSB (33). Finally, global nucleotide excision repair has been suggested to contribute to the repair of 8-OxoG in eukaryotes in vitro and in vivo (34, 35).
To assess the contribution of the Ogg1 glycosylase in the TCR of 8-OxoG in mammalian cells, we have investigated the removal of 8-OxoG from shuttle vectors containing a single 8-OxoG paired with a C (8-OxoG.C) in a transcribed (TS) or in a nontranscribed (NTS) sequence in MEF cell lines derived from ogg1−/− and wild-type mice.
Materials and Methods
Cell Lines and Culture Conditions.
Spontaneously transformed MEF wild-type, heterozygous ogg1+/− and homozygous ogg1−/− clones were established by standard procedures from individual embryonic day 13.5 embryos derived from heterozygous matings (31). They were cultured in DMEM/Ham's F12 (3:1) supplemented with 10% (vol/vol) fetal calf serum, Fungizone and penicillin (100 units/ml), and streptomycin (100 μg/ml). To obtain ogg1−/− MEF cell lines corrected by expression of wild-type mouse Ogg1, ogg1−/− cells were transfected with a pB-MT-Epstein-Barr virus vector (36) or with the vector containing the cDNA encoding the wild-type mouse Ogg1 protein (27). Transfectants were selected by growth in medium containing hygromycin at increasing concentrations up to 375 μg/ml. Single clones were isolated after 10 days, propagated in 24-well plates, and analyzed for Ogg1 activity as described below.
Analysis of Transfectants Expressing Ogg1 Activity.
Cell-free protein extracts were prepared from clonal isolates to test 8-OxoG DNA glycosylase activity/AP lyase activity. A total of 5 × 106 cells were resuspended in 0.35 ml of lysis buffer (20 mM Tris⋅HCl, pH 8.0/1 mM Na2EDTA/250 mM NaCl/0.8 μg/ml antipain/ 0.8 μg/ml aprotinin/0.8 μg/ml leupeptin). The cell suspension was sonicated, the supernatant was recovered by centrifugation at 85,000 g at 4°C for 45 min, and its protein content determined according to Bradford. The 8-OxoG repair activity was measured by using a 34-mer oligodeoxyribonucleotide containing a single 8-OxoG: 5′-GGCTTCATCGTTGTC[8-OxoG]CAGACCTGGTGGATACCG-3′ (a kind gift of Jean Cadet, Commissariat à l'Energie Atomique-Grenoble). The 8-OxoG-containing strand was 32P-labeled at its 5′-end and hybridized with a complementary sequence carrying a cytosine (C) opposite the lesion yielding the [8-OxoG.C] duplex. Standard assay mixtures (20 μl of final volume) contained 25 mM Tris⋅HCl, pH 7.6, 2 mM Na2EDTA, 70 mM NaCl, 25 fmol of 32P-labeled [8-OxoG.C] duplex, and 10 μg of cell-free protein extract (37). The reactions were performed at 37°C for 30 min and the products separated by 20% denaturing PAGE as described (38). Selected independent clones expressing wild-type Ogg1 were maintained in culture medium supplemented with a hygromycin analog.
Preparation of Closed Circular Plasmids Carrying a Unique 8-OxoG·C Pair.
Plasmids used were derived from pS189, which was a generous gift of M. Seidman. Plasmid pSΔoriSV harbors a deletion of the simian virus (SV) 40 origin of replication, whereas plasmid pSΔ(ori-p)SV harbors a deletion at both the SV40 origin of replication and the promoter region. Plasmids pSΔoriSV-[8-OxoG.C] and pSΔ(ori-p)SV-[8-OxoG.C] containing a unique 8-OxoG·C bp at the same site were prepared as described (39, 33). The 19-mer oligodeoxyribonucleotide carrying a unique 8-OxoG: (5′-GATCGGCGCCG[8-OxoG]CGGTGTG-3′), was a kind gift of Jean Cadet (Commissariat à l'Energie Atomique-Grenoble).
Assay for Removal of 8-OxoG from Plasmid DNA in MEF Cells.
Eight hundred nanograms of closed circular double-stranded plasmids pSΔoriSV-[8-OxoG.C] or pSΔ(ori-p)SV-[8-OxoG.C] were transfected into MEF cell lines (semiconfluent cell culture in 10-cm2 Petri dishes) using the cationic liposome DOTAP procedure (Boehringer). Cells were then incubated for 2–12 h and harvested. Elimination of any contaminating extracellular input DNA was performed by treatment of cell cultures with DNase I before extraction. Extrachromosomal plasmid DNA was recovered by a small-scale alkaline lysis method (40). Recovered plasmid DNA was treated or not with 5 ng of homogeneous E. coli Fpg protein (41) and directly analyzed on a 0.8% agarose gel containing ethidium bromide to separate covalently closed (CC) and nicked (OC) plasmid molecules. Plasmid DNA was detected by Southern blotting using the bacterial Amp sequence present in all constructs, as a fluorescent-labeled probe (Amersham-Pharmacia). Recovery of plasmid DNA after transfection was ≈20% of the input DNA independently of the MEF cell line used. Visualization and quantification were done by using a PhosphorImager (Molecular Dynamics). The repair of 8-OxoG in MEFs was calculated as a ratio of covalently closed molecules to the total amount of recovered plasmid DNA.
Results
MEF Cell Lines.
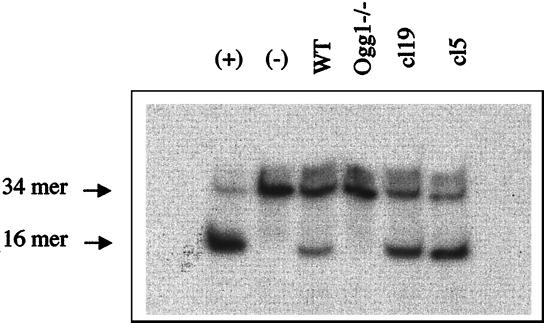
To investigate the biological role of the Ogg1 protein in mammalian cells, ogg1−/− null mice have been generated by targeted disruption of the OGG1 locus, and homozygous ogg1−/− null, heterozygous ogg1+/−, and wild-type MEF cell lines have been established (30). Moreover, we have constructed MEF cell lines, cl5 and cl19, that overexpress the wild-type mouse Ogg1 protein in the homozygous ogg1−/− background. Ogg1 enzyme activity was assayed in cell-free protein extracts from these various cell lines using a 34-mer oligodeoxyribonucleotide substrate containing a single 8-OxoG·C base pair. Fig. 1 shows that a wild-type MEF cell-free extract cleaves the [8-OxoG·C] duplex, whereas an ogg1−/− null cell-free extract has no detectable cleavage activity. This result confirms that Ogg1 is the major, if not the only, DNA glycosylase/AP lyase that recognizes 8-OxoG·C in murine cells (31). Fig. 1 also shows that cl5 and cl19 cell-free extracts contain a 2- to 3-fold higher [8-OxoG.C] cleavage activity compared to extracts from wild-type cells.
Figure 1.
Cleavage of a 34-bp [8-OxoG·C] duplex by crude cell-free protein extracts of MEF cell lines. A 34-mer oligodeoxyribonucleotide containing a single 8-OxoG was 5′ 32P-labeled and hybridized with a complementary strand yielding a duplex containing a cytosine opposite 8-OxoG [8-OxoG·C]. The [8-OxoG·C] duplex was incubated with 10 μg of crude cell-free protein extracts from wild-type (WT), ogg1−/− null, or two clones (cl5 and cl19) corrected after transfection of ogg1−/− cells by the mouse Ogg1 cDNA. The assay was performed at 37°C for 15 min and the products of the reaction were analyzed by denaturing 20% PAGE (38). Control: [8-OxoG·C] duplex incubated with (+) or without (−) 10 ng of purified yeast Ogg1 protein.
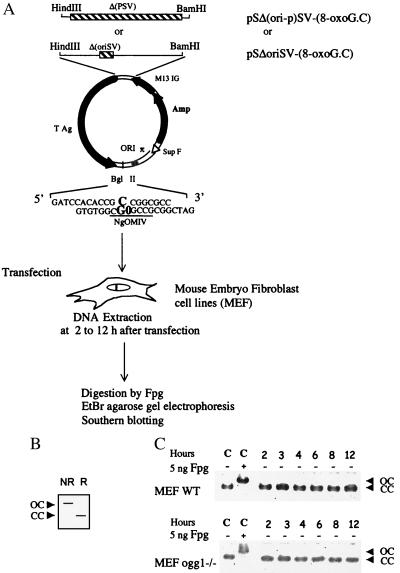
An Assay to Measure Removal of 8-OxoG from Mono-Modified Plasmids.
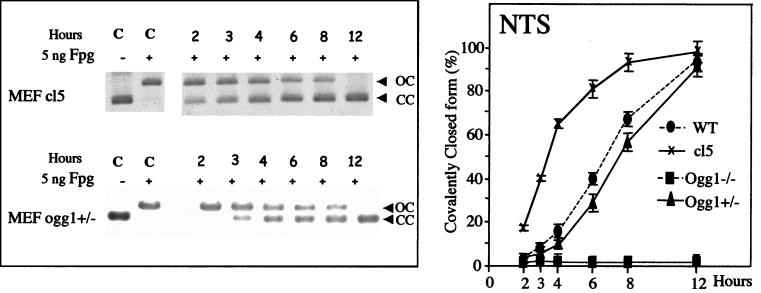
The repair of 8-OxoG in the MEF cell lines was monitored by using two nonreplicative plasmids that contain a unique 8-OxoG·C base pair (33). The sequence containing the unique 8-OxoG·C base pair was inserted into the 3′-untranslated region of the SV40 TAg cassette of the strand TS from the early promoter of SV40 in pSΔoriSV-[8-OxoG.C] or NTS when the promoter region has been deleted in pSΔ(ori-p)SV-[8-OxoG.C] (Fig. 2A). Therefore, these two constructs allow analysis of the repair of an 8-OxoG lesion in the same sequence context but different transcription status. The transcription of 8-OxoG in pSΔoriSV-[8-OxoG.C] was confirmed in the wild-type MEFs by reverse transcription-PCR analysis using two sets of primers to amplify an internal TAg sequence and the 8-OxoG containing sequence (ref. 33 and data not shown). To measure the removal of 8-OxoG from these mono-modified plasmids, we developed an assay that uses the capacity of the E. coli Fpg protein to specifically nick DNA at the 8-OxoG·C base pair (41). This assay allows the direct analysis of DNA molecules after their recovery from mammalian cells (Fig. 2A). Plasmids remaining CC after treatment with Fpg correspond to molecules in which the 8-OxoG lesion was replaced by a G in the cell. On the other hand, relaxed plasmids (OC) correspond to molecules that retained the 8-OxoG lesion and consequently are sensitive to Fpg. Therefore, removal of 8-OxoG is assessed by the increasing fraction of covalently closed molecules in Fpg-treated plasmid DNA recovered from transfected cells. Control experiments show that the plasmid preparations used migrate as covalently closed molecules, which are fully relaxed after treatment with 5 ng of Fpg protein (Fig. 2B). This assay requires that the integrity of covalently closed plasmid DNA molecules is preserved during their extraction from MEF cells. Therefore, wild-type and ogg1−/− MEF cells were transfected with pSΔoriSV-[8-OxoG.C], plasmid DNA was extracted after 2–12 h and analyzed as described. Fig. 2B shows that all of the plasmid DNA recovered from MEF cells 2 to 12 h after transfection migrates as covalently closed molecules, indicating an absence of detectable DNA degradation during the course of the experiments. Thus, conversion to relaxed molecules depends on Fpg treatment.
Figure 2.
Removal of 8-OxoG in MEF cell lines. Experimental scheme: Plasmid pSΔoriSV-[8-OxoG.C] has a deletion of the SV40 origin of replication (hatched) whereas pS(ori-p)SV-[8-OxoG.C] has a deletion of both the SV40 origin of replication and the SV40 promoter from −10 to 273 that eliminates transcription of TAg and of the 8-OxoG (hatched). After transfection in MEF cell lines, plasmid DNA was recovered and incubated with 5 ng of Fpg protein or left untreated before ethidium bromide-agarose gel electrophoresis and Southern blot analysis. (A) In this assay, removal of 8-OxoG is identified by the presence of CC plasmid DNA after incubation with Fpg (R; repaired). Unrepaired 8-OxoG causes conversion of the (CC) plasmid to an open circle (OC) when treated with Fpg (NR). (B) Control lanes (C) represent plasmid DNA (pSΔoriSV-[8-OxoG.C]) with (+) or without (−) Fpg treatment. Plasmid DNA transfected into wild-type or ogg1−/− MEF cell lines was incubated 2–12 h, as indicated and analyzed without Fpg treatment. The same result was obtained with pS(ori-p)SV-[8-OxoG.C].
Finally, removal of 8-OxoG in MEF cell lines also has been assayed by NgoMIV restriction analysis of individual plasmid molecules recovered from mammalian cells and amplified in E. coli (fpg mutY) (33, 39). The results obtained for the repair of 8-OxoG·C in wild-type and ogg1−/− MEF cell lines were identical to those reported in the present study using Southern blotting analysis (data not shown).
TCR of 8-OxoG in a Wild-Type MEF Cell Line.
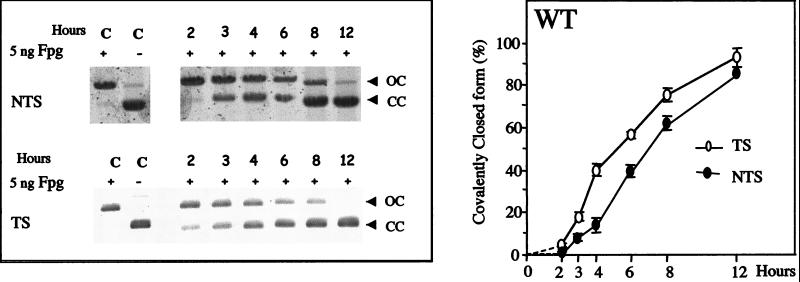
To study the repair of 8-OxoG in vivo, nonreplicative plasmids, pSΔoriSV-[8-OxoG.C] and pSΔ(ori-p)SV-[8-OxoG.C], were transfected into the wild-type OGG1+/+ MEF cells and incubated for 2–12 h before recovery. Fig. 3 (Left) illustrates repair kinetics of 8-OxoG in wild-type MEF cells either in the NTS or in the TS. Southern blotting analysis for both TS and NTS shows that, 2 h after transfection, recovered plasmid DNA molecules essentially migrate as open circles after treatment with Fpg, indicating that 8-OxoG had not been repaired. This delay is probably caused by the fact that DNA has to reach the nucleus to be repaired. Between 3 and 12 h after transfection, an increasing fraction of the TS or NTS plasmid molecules migrates as covalently closed molecules after Fpg treatment, indicating removal of 8-OxoG (Fig. 3). Quantitative analysis of the Southern blots (Fig. 3 Right), reveals that in wild-type MEFs, 8-OxoG is efficiently removed from both the TS and NTS within 12 h after transfection. However, 8-OxoG removal occurs at a faster rate when the lesion is located in the TS compared to the NTS, suggesting the existence of a TCR pathway in the wild-type MEF cell line (Fig. 3 Right).
Figure 3.
Kinetics of 8-OxoG removal in the wild-type MEF cell line. The nonreplicating shuttle vectors, pSΔoriSV-[8-OxoG.C] and pSΔ(ori-p)SV-[8-OxoG.C], were transfected into wild-type MEF cells, recovered after 2- to 12-h incubations, and analyzed for repair of 8-OxoG. Control lanes (C) show mono-modified constructs incubated with (+) or without (−) Fpg. (Left) Southern blots after transfection of plasmids in the wild-type MEF cell line and Fpg-cleavage. (Right) Quantitative analysis of Southern blots. Experimental values are the average of six blots resulting from three transfections with two independent preparations of mono-modified plasmid DNA. Error bars are shown.
Requirement of Ogg1 for the Repair of 8-OxoG in a NTS but Not in a TS.
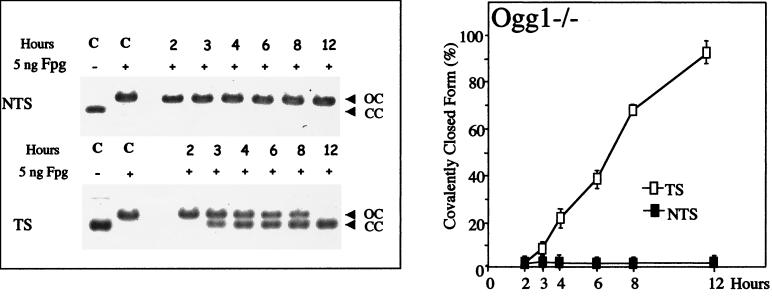
To investigate the role of the Ogg1 DNA glycosylase/AP lyase in the repair of 8-OxoG in mammalian cells, we have transfected MEF ogg1−/− null cell lines with pSΔoriSV-[8-OxoG.C] and pSΔ(ori-p)SV-[8-OxoG.C]. In ogg1−/− cells, we do not observe any removal of 8-OxoG located in the NTS, even 12 h after transfection (Fig. 4). The absence of repair is demonstrated by the presence of open molecules after treatment with Fpg protein, indicating the persistence of the lesion in the plasmid (Fig. 4). Therefore, Ogg1 is absolutely required for removal of 8-OxoG in the NTS in nonreplicating DNA. In contrast, 8-OxoG located in the TS is efficiently removed by the ogg1−/− null MEF cell line (Fig. 4). To demonstrate that the lack of repair of 8-OxoG in the NTS is caused by the inactivation of Ogg1, we have analyzed removal of this lesion in ogg1−/− MEFs expressing the murine Ogg1 cDNA. The results show that 8-OxoG in the NTS is very efficiently repaired in complemented clone (cl5 cells), whereas there is no removal in cells transfected with the vector alone (Fig. 5 and data not shown). This demonstrates that the absence of 8-oxoG repair in ogg1−/− cells is caused by the inactivation of Ogg1 and not to another genetic alteration. Fig. 5 also shows that the removal of 8-OxoG in the NTS in a heterozygous ogg1+/− cell line occurs at a slower rate compared to the wild-type. However, the rate of removal of 8-OxoG in heterozygous ogg1+/− cells is only moderately affected, which seems to indicate that, in our assay conditions, Ogg1 is not limiting in wild-type cells (Fig. 5). In conclusion, our results show that the Ogg1 DNA glycosylase is required for the repair of 8-OxoG in the NTS and indicate the occurrence of an alternative repair mechanism independent of Ogg1 to remove 8-OxoG in the TS.
Figure 4.
Kinetics of 8-OxoG removal in the ogg1−/− MEF cell line. The nonreplicating shuttle vectors, pSΔoriSV-[8-OxoG.C] and pSΔ(ori-p)SV-[8-OxoG.C], were transfected into the ogg1−/− MEF cells, recovered after 2- to 12-h incubations, and analyzed for repair of 8-OxoG. Control lanes (C) show mono-modified constructs incubated with (+) or without (−) Fpg. (Left) Southern blots after transfection of plasmids in the ogg1−/− MEF cell line and Fpg-cleavage. (Right) Quantitative analysis of Southern blots. Experimental values are calculated as described in the legend of Fig. 3.
Figure 5.
Kinetics of 8-OxoG removal in heterozygous ogg1+/− cells and homozygous ogg1−/− cells complemented with murine Ogg1. The nonreplicating shuttle vector, pSΔ(ori-p)SV-[8-OxoG.C], was transfected into cl5 an ogg1−/− MEF cell line complemented for murine Ogg1 or heterozygous ogg1−/+ MEFs, recovered after 2- to 12-h incubations, and analyzed for repair of 8-OxoG. Control lanes (C) show mono-modified constructs incubated with (+) or without (−) Fpg. (Left) Southern blots after transfection of plasmids in the MEF cell lines and Fpg-cleavage. (Right) Quantitative analysis of Southern blots. Experimental values are calculated as described in the legend of Fig. 3.
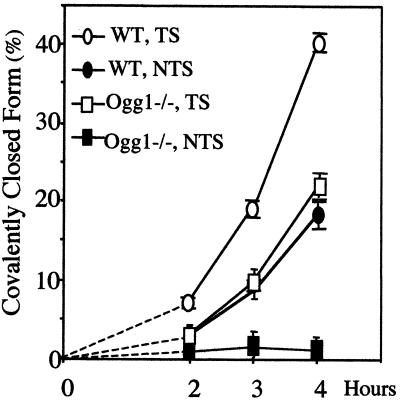
Although not essential for TCR, Ogg1 may still act on 8-OxoG repair in the TS. This hypothesis is suggested by the fact that removal of 8-OxoG from the TS in wild-type cells is faster than that in ogg1−/− null cells, particularly at early time points after transfection (Fig. 6).
Figure 6.
Early kinetics of 8-OxoG removal in the NTS and TS in wild-type and ogg1−/− MEF cell lines. The values are those reported in Figs. 3 and 4.
Discussion
The evidence for TCR of oxidative DNA damage such as thymine glycol and 8-OxoG was provided by the finding of preferential removal of these lesions from transcribed sequences both on γ-irrradiated genomic DNA and mono-modified plasmids, in human cell lines (33, 42, 43). TCR of thymine glycol was also identified in mouse embryonic stem cells (44). Furthermore, TCR of oxidative DNA base damage is defective in CSB, XPB/CS, XPD/CS, XPG/CS patients (33, 43). These results strongly suggest that Cockayne syndrome (CS) could result from defects in TCR of oxidative DNA lesions (33, 43). In addition, other proteins such as MSH2 and BRCA1 also are involved in the TCR of oxidative DNA damage (44, 45). Studies using shuttle vectors containing a single 8-OxoG·C base pair strongly suggest that 8-OxoG blocks transcription mediated by RNA polymerase II, which in turn could explain the lack of repair of 8-OxoG in the TS in CS cells (33). However, the specific roles of these different proteins in the repair of 8-OxoG in the TS is not clear. Some of them such as MSH2 may be responsible for transcription arrest, whereas others such as CSB are probably involved in the release of the RNA polymerase (33, 44, 45). The role of XPG may more directly be related to the repair of the lesion because this protein is required for the repair of 8-OxoG in the TS but also stimulates its repair in the NTS (33). Biochemical studies also show that purified XPG stimulates cleavage and binding of oxidized pyrimidine-containing DNA duplexes by the human Nth1 DNA glycosylase (46). The role of the Ogg1 DNA glycosylase in the repair of oxidative DNA damage has been demonstrated in Ogg1-deficient mice, which accumulate 8-OxoG in the genome (31, 32). However, an ogg1−/− MEF cell line still releases Fpg-sensitive oxidative DNA damage, albeit at a slower rate compared to a wild-type control, suggesting an additional repair pathway in proliferating cells (31).
Because there is no known way to induce only 8-OxoG in treated cells and because there is still no specific assay to follow only the 8-OxoG repair in cultured cells, we had to choose the shuttle vector technology containing a unique 8-OxoG residue for carrying out these experiments. Shuttle vectors have been used in the last decade to analyze repair and mutagenesis in human cells (47) and mutagenic potency of unique lesions (48). In every cases, in which the same mutagen was used with shuttle vectors and for studying endogenous gene mutagenesis, results have been similar. Finally, the comparison of TCR deficiency between thymine glycol on genomic DNA and 8-OxoG repair on the shuttle vectors used in this study has shown the two techniques analyzed the same pathway (33). Therefore, we believed that shuttle vectors with unique 8-OxoG represent, now, the best and unique model to study the fate of 8-OxoG in mammalian cells.
In the present study, we have demonstrated TCR of 8-OxoG in wild-type and ogg1−/− MEF cell lines. The TCR of 8-OxoG in an ogg1−/− cell line suggests the existence of an alternative repair pathway independent of the Ogg1 DNA glycosylase. In contrast, we show that the Ogg1 protein is required for the repair of 8-OxoG in the NTS; this is the first report on the selective absence of repair of oxidative DNA damage in the NTS. This result suggests that Ogg1 is the only enzyme that recognizes the 8-OxoG base by itself in the DNA in the cell. However, additional factors such as XPG can enhance the efficiency of Ogg1 (33). This result raises the possibility that XPG could act stimulating Ogg1 activity. Such a stimulatory function of XPG has been demonstrated on the hNth1 activity (46). The inability of the Ogg1-independent pathway to repair 8-OxoG in the NTS suggests that this pathway does not recognize the lesion by itself but rather the lesion associated with other proteins required for 8-OxoG repair in the TS such as XPG, TFIIH, MSH2, or BRCA1. However, some action of Ogg1 on 8-OxoG in the TS is suggested by the fact that 8-OxoG removal from the TS is faster in wild-type as compared to Ogg1-defective cells. To reconcile these observations, we propose that in the wild-type context, Ogg1 acts on 8-OxoG in the NTS and both Ogg1 and the alternative pathway contribute to the TCR of 8-OxoG.
The nature of the repair pathway acting on 8-OxoG in the TS in the ogg1−/− context remains unidentified. However, an obvious candidate is nucleotide excision repair that could recognize the stalled RNA polymerase at 8-OxoG (33). This proposal is not in conflict with the fact that repair of 8-OxoG in the TS occurs in cells strictly deficient in nucleotide excision repair (33), if we accept that in wild-type cells Ogg1 can act on both the TS and NTS. On the other hand, another DNA glycosylase such as Ogg2 that recognizes 8-OxoG paired with a purine, also may play a role in vivo (49). One approach to identify the repair pathway acting on 8-OxoG in the TS is to construct MEF cell lines defective in both Ogg1 and other repair gene such as CSB or XPA.
The alternative repair pathway reported here could explain the absence of evident pathogenesis in the homozygous ogg1−/− null mice (31, 32). In ogg1−/− cells, transcription can proceed through oxidative DNA base damage whereas mutagenesis induced by lesions in the NTS may be limited by the action of a MutY homolog and possibly mismatch repair. Therefore, the expected consequence of the inactivation of Ogg1 is a relatively modest mutator phenotype as observed in nonproliferating tissues of ogg1−/− null mice (31, 32) and Ogg1-deficient yeast strains (15). However, accumulation of 8-OxoG caused by the inactivation of Ogg1 may be deleterious in the long term and may be responsible for pathologies upon aging or exposure to environmental oxidative stress.
Acknowledgments
We thank Dr. Jean Cadet (Commissariat à l'Energie Atomique-Grenoble) for the kind gift of the 8-OxoG containing oligodeoxyribonucleotide used in this study. We also thank Drs. Tomas Lindahl (London) and Erling Seeberg (Oslo) for their constant interest and critical reading of the manuscript. This work was supported by the Centre National de la Recherche Scientifique and the Commissariat à l'Energie Atomique. This work also was supported by the “Action Spécifique: Radiobiologie 98” from the Ministère de l'Education Nationale et de la Recherche et de la Technologie to S.B. and A.S. A.K. acknowledges the support from the Research Council of Norway and the Norwegian Cancer Society.
Abbreviations
- 8-OxoG
7,8-dihydro-8-oxoguanine
- TCR
transcription-coupled repair
- MEF
mouse embryo fibroblast
- TS
transcribed sequence
- NTS
nontranscribed sequence
- AP
apurinic/apyrimidinic
- SV
simian virus
- CC
covalently closed
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.140137297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.140137297
References
- 1.Feig D I, Reid T M, Loeb L A. Cancer Res Suppl. 1994;54:1890–1894. [PubMed] [Google Scholar]
- 2.Wiseman H, Kaur H, Halliwell B. Cancer Lett. 1995;93:113–120. doi: 10.1016/0304-3835(95)03792-U. [DOI] [PubMed] [Google Scholar]
- 3.Beckman K B, Ames B N. J Biol Chem. 1997;272:19633–19636. doi: 10.1074/jbc.272.32.19633. [DOI] [PubMed] [Google Scholar]
- 4.Dizdaroglu M. Free Radic Biol Med. 1991;10:225–242. doi: 10.1016/0891-5849(91)90080-m. [DOI] [PubMed] [Google Scholar]
- 5.Cadet J, Berger M, Douki T, Ravanat J L. Rev Physiol Biochem Pharmacol. 1997;131:1–87. doi: 10.1007/3-540-61992-5_5. [DOI] [PubMed] [Google Scholar]
- 6.Friedberg E C, Walker G C, Siede W. DNA Repair and Mutagenesis. Washington, DC: Am. Soc. Microbiol.; 1995. [Google Scholar]
- 7.Boiteux S, Laval J. In: Repair of Oxidized Purines in DNA in Base Excision Repair of DNA Damage. Hickson I D, editor. Austin, TX: Landes-Springer; 1997. pp. 31–44. [Google Scholar]
- 8.Krokan H E, Standal R, Slupphaug G. Biochem J. 1997;325:1–16. doi: 10.1042/bj3250001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindahl T, Wood R D. Science. 1999;286:1897–1905. doi: 10.1126/science.286.5446.1897. [DOI] [PubMed] [Google Scholar]
- 10.Grollman A P, Moriya M. Trends Genet. 1993;9:246–249. doi: 10.1016/0168-9525(93)90089-z. [DOI] [PubMed] [Google Scholar]
- 11.Michaels M L, Miller J H. J Bacteriol. 1992;174:6321–6325. doi: 10.1128/jb.174.20.6321-6325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tajiri T, Maki H, Sekiguchi M. Mutat Res. 1995;336:257–267. doi: 10.1016/0921-8777(94)00062-b. [DOI] [PubMed] [Google Scholar]
- 13.Auffret van der Kemp P, Thomas D, Barbey R, De Oliveira R, Boiteux S. Proc Natl Acad Sci USA. 1996;93:5197–5202. doi: 10.1073/pnas.93.11.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nash H M, Bruner S D, Scharer O D, Kawate T, Addona T A, Spooner E, Lane W S, Verdine G L. Curr Biol. 1996;6:968–980. doi: 10.1016/s0960-9822(02)00641-3. [DOI] [PubMed] [Google Scholar]
- 15.Thomas D, Scott A D, Barbey R, Padula M, Boiteux S. Mol Gen Genet. 1997;254:171–178. doi: 10.1007/s004380050405. [DOI] [PubMed] [Google Scholar]
- 16.Ni T T, Marsichky G T, Kolodner R D. Mol Cell. 1999;4:439–444. doi: 10.1016/s1097-2765(00)80346-9. [DOI] [PubMed] [Google Scholar]
- 17.Sakumi K, Furuichi M, Tsuzuki T, Kakuma T, Kawabata S, Maki H, Sekiguchi M. J Biol Chem. 1993;268:23524–23530. [PubMed] [Google Scholar]
- 18.Slupska M M, Baikalov C, Luther W M, Chiang J H, Wei W-F, Miller J H. J Bacteriol. 1996;178:3885–3892. doi: 10.1128/jb.178.13.3885-3892.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radicella J P, Dhérin C, Desmaze C, Fox M S, Boiteux S. Proc Natl Acad Sci USA. 1997;94:8010–8015. doi: 10.1073/pnas.94.15.8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roldan-Arjona T, Wei Y-F, Carter K C, Klungland A, Anselmino C, Wang R P, Augustus M, Lindahl T. Proc Natl Acad Sci USA. 1997;94:8016–8020. doi: 10.1073/pnas.94.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjoras M, Luna L, Johnsen B, Hoff B, Haug T, Rognes T, Seeberg E. EMBO J. 1997;16:6314–6322. doi: 10.1093/emboj/16.20.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arai K, Morishita K, Shinmura K, Kohno T, Kim S R, Nohmi T, Taniwaki M, Ohwada S, Yokota J. Oncogene. 1997;14:2857–2861. doi: 10.1038/sj.onc.1201139. [DOI] [PubMed] [Google Scholar]
- 23.Aburatani H, Hippo Y, Ishida T, Takashima R, Matsuba C, Kodama T, Takao M, Yasui A, Yamamoto K, Asano M. Cancer Res. 1997;57:2151–2156. [PubMed] [Google Scholar]
- 24.Lu R, Nash H M, Verdine G L. Curr Biol. 1997;7:397–407. doi: 10.1016/s0960-9822(06)00187-4. [DOI] [PubMed] [Google Scholar]
- 25.Rosenquist T A, Zharkov D O, Grollman A P. Proc Natl Acad Sci USA. 1997;94:7429–7434. doi: 10.1073/pnas.94.14.7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boiteux S, Radicella J P. Biochimie. 1999;81:59–67. doi: 10.1016/s0300-9084(99)80039-x. [DOI] [PubMed] [Google Scholar]
- 27.Boiteux S, Dhérin C, Reille F, Apiou F, Dutrillaux B, Radicella J P. Free Radical Res. 1998;29:487–497. doi: 10.1080/10715769800300541. [DOI] [PubMed] [Google Scholar]
- 28.Nishioka K, Ohtsubo T, Oda H, Fujiwara T, Kang D, Sugimachi K, Nakabeppu Y. Mol Biol Cell. 1999;10:1637–1652. doi: 10.1091/mbc.10.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takao M, Aburatani H, Kobayashi K, Yasui A. Nucleic Acids Res. 1998;26:2917–2922. doi: 10.1093/nar/26.12.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tani M, Shinmura K, Kohno T, Shiroishi T, Wakana S, Kim S R, Nohmi T, Kasai H, Takenoshita S, Nagamuchi Y, Yokota J. Mamm Genome. 1998;9:32–37. doi: 10.1007/s003359900675. [DOI] [PubMed] [Google Scholar]
- 31.Klungland A, Rosewell I, Hollenbach S, Larsen E, Daly G, Epe B, Seeberg E, Lindahl T, Barnes D E. Proc Natl Acad Sci USA. 1999b;96:13300–13305. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minowa O, Arai T, Hirano M, Monden Y, Nakai S, Fukuda M, Itoh M, Takano M, Hippou Y, Aburatani H, et al. Proc Natl Acad Sci USA. 2000;97:4156–4161. doi: 10.1073/pnas.050404497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Page F, Kwoh E E, Avrutskaya A, Gentil A, Leadon S A, Sarasin A, Cooper P K. Cell. 2000;101:159–171. doi: 10.1016/s0092-8674(00)80827-2. [DOI] [PubMed] [Google Scholar]
- 34.Reardon J T, Bessho T, Kung H C, Bolton P H, Sancar A. Proc Natl Acad Sci USA. 1997;94:9463–9468. doi: 10.1073/pnas.94.17.9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott A D, Neishabury M, Jones D H, Reed S H, Boiteux S, Waters R. Yeast. 1999;15:205–218. doi: 10.1002/(SICI)1097-0061(199902)15:3<205::AID-YEA361>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 36.Lafarge-Frayssinet C, Duc H T, Frayssinet C, Sarasin A, Anthony D, Guo Y, Trojan J. Cancer Gene Ther. 1997;4:276–285. [PubMed] [Google Scholar]
- 37.Hollenbach S, Dhénaut A, Eckert I, Radicella J P, Epe B. Carcinogenesis. 1999;20:1863–1868. doi: 10.1093/carcin/20.9.1863. [DOI] [PubMed] [Google Scholar]
- 38.Girard P M, Guibourt N, Boiteux S. Nucleic Acids Res. 1997;25:3404–3411. doi: 10.1093/nar/25.16.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Page F, Guy A, Cadet J, Sarasin A, Gentil A. Nucleic Acids Res. 1998;26:1276–1281. doi: 10.1093/nar/26.5.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stary A, Menck C F, Sarasin A. Mutat Res. 1992;272:101–110. doi: 10.1016/0165-1161(92)90038-n. [DOI] [PubMed] [Google Scholar]
- 41.Boiteux S, O'Connor T R, Lederer F, Gouyette A, Laval J. J Biol Chem. 1990;265:3916–3922. [PubMed] [Google Scholar]
- 42.Leadon S A, Cooper P K. Proc Natl Acad Sci USA. 1993;90:10499–10503. doi: 10.1073/pnas.90.22.10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cooper P K, Nouspikel T, Clarkson S G, Leadon S A. Science. 1997;275:990–993. doi: 10.1126/science.275.5302.990. [DOI] [PubMed] [Google Scholar]
- 44.Leadon S A, Avrutskaya A V. Cancer Res. 1997;57:3784–3791. [PubMed] [Google Scholar]
- 45.Gowen L C, Avrustskaya A V, Latour A M, Koller B H, Leadon S A. Science. 1998;5379:1009–1012. doi: 10.1126/science.281.5379.1009. [DOI] [PubMed] [Google Scholar]
- 46.Klungland A, Höss M, Gunz D, Constantinou A, Clarkson S G, Doetsch P W, Bolton P H, Wood R D, Lindahl T. Mol Cell. 1999;3:33–42. doi: 10.1016/s1097-2765(00)80172-0. [DOI] [PubMed] [Google Scholar]
- 47.Menck C F M, Madzak C, Renault G, Margot A, Sarasin A. Mutat Res. 1989;220:101–106. doi: 10.1016/0165-1110(89)90015-8. [DOI] [PubMed] [Google Scholar]
- 48.Gentil A, Le Page F, Sarasin A. Biol Chem. 1997;378:1287–1292. doi: 10.1515/bchm.1997.378.11.1287. [DOI] [PubMed] [Google Scholar]
- 49.Hazra T K, Izumi T, Maidt L, Floyd R A, Mitra S. Nucleic Acids Res. 1998;26:5116–5122. doi: 10.1093/nar/26.22.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]