Abstract
AIM: To investigate endoscopic and histopathological findings in the duodenum of patients with Strongyloides stercoralis (S. stercoralis) hyperinfection.
METHODS: Over a period of 23 years (1984-2006), we investigated 25 patients with S. stercoralis hyperinfection who had had an esophagogastroduodenoscopy before undergoing treatment for strongyloidiasis. The clinical and endoscopic findings were analyzed retrospectively.
RESULTS: Twenty-four (96%) of the patients investigated were under immunocompromised condition which was mainly due to a human T lymphotropic virus type 1 (HTLV-1) infection. The abnormal endoscopic findings, mainly edematous mucosa, white villi and erythematous mucosa, were observed in 23 (92%) patients. The degree of duodenitis including villous atrophy/destruction and inflammatory cell infiltration corresponded to the severity of the endoscopic findings. The histopathologic yield for identifying larvae was 71.4% by duodenal biopsy. The endoscopic findings of duodenitis were more severe in patients whose biopsies were positive for larvae than those whose biopsies were negative (Endoscopic severity score: 4.86 ± 2.47 vs 2.71 ± 1.38, P < 0.05).
CONCLUSION: Our study clearly demonstrates that, in addition to stool analysis, endoscopic observation and biopsies are very important. We also emphasize that S. stercoralis and HTLV-1 infections should be ruled out before immunosuppressive therapy is administered in endemic regions.
Keywords: Strongyloides stercoralis, Strongyloidiasis, Hyperinfection, Endoscopy, Histopathology, Duodenum
INTRODUCTION
Strongyloides stercoralis (S. stercoralis) is an intestinal nematode which is a parasite of humans. About 100 million people are infected by this parasite in tropical and subtropical areas[1]. Infections are acquired when larvae penetrate the skin and migrate to the duodenum and upper jejunum to mature. An internal autoinfective cycle allows the parasite to reside within a human for years. Clinical syndromes of S. stercoralis vary widely. Chronic infection with S. stercoralis is most often asymptomatic. Hyperinfection describes a syndrome of accelerated autoinfection which results from immunosuppression. Detection of an increased number of larvae in stool, sputum and/or tissue is a hallmark of hyperinfection[2]. Gastrointestinal and pulmonary symptoms are common but non-specific, and include abdominal pain, diarrhea, vomiting, adynamic ileus, small bowel obstruction (SBO) and protein-losing enteropathy, as well as pneumonia. Disseminated infection is the migration of larvae to organs beyond the range of the autoinfective cycle (lungs and gastrointestinal tract) and is often complicated by Gram-negative sepsis. Such organs include the skin, liver, central nervous system as well as virtually every other organ. An immunocompromised condition consists of immunosuppressive drug therapy (e.g., corticosteroids, cyclosporine and anti-cancer drugs), hematologic malignancies, organ transplants, human T lymphotropic virus type 1 (HTLV-1) infection, and human immunodeficiency virus (HIV) infection[1,2]. As S. stercoralis colonizes in the duodenum where the larvae mature, endoscopic evaluation has been recognized as an important tool for diagnosing strongyloidiasis. Although there have been several reports demonstrating endoscopic findings of strongyloidiasis, most of these are case reports or small case series[3–19]. This study aims at investigating the relationship of endoscopic markers to clinical and histopathological findings of the duodenum in patients with S. stercoralis hyperinfection in an endemic region.
MATERIALS AND METHODS
Patients
Over a 23-year period from 1984 to 2006, we identified 25 patients (15 males and 10 females; mean age = 63.0 ± 14.1 years) with S. stercoralis hyperinfection who had had an esophagogastroduodenoscopy (EGD) before undergoing treatment for strongyloidiasis at Ryukyu University Hospital and other affiliated hospitals in Okinawa, Japan. The diagnosis of S. stercoralis hyperinfection was based on gastrointestinal, pulmonary and/or systemic symptoms along with the identification of an increased number of Strongyloides larvae or ova in the stool, sputum, gastroduodenal drainage and/or tissue. The clinical and endoscopic findings of the patients were investigated retrospectively. The study was conducted and carried out in accordance with the Helsinki Declaration.
Endoscopy and histopathology
EGD was performed with forward-viewing endoscopes (Olympus, Tokyo, Japan) at the second portion of the duodenum. Since the endoscopic severity scoring system of the duodenum has not been established, we created the scoring system, which was determined by the total number of points for duodenitis. One point was given for a mild form (edema, erythema and white villi), two points for a moderate form (erosion, fine granule and hemorrhage) and three points for a severe form (ulcer, dilatation, dilatation and pseudopolyps). At endoscopy, biopsy specimens were obtained from the duodenal mucosa in a routine fashion using standard forceps. The specimens were stained with hematoxylin and eosin for histopathological evaluation. Duodenal pathology was assessed as described elsewhere[20].
Statistical analysis
Mann-Whitney U-test was used, when appropriate, to compare any differences among the groups. Statistical comparisons were analyzed using SPSS for Windows version 15 (SPSS Inc., Japan). P values less than 0.05 were considered statistically significant.
RESULTS
Clinical features
The clinical features of the patients are summarized in Table 1. The main symptoms and/or illnesses complicated by the hyperinfection were as follows: vomiting in 13 (52.0%), abdominal pain in 10 (40.0%), diarrhea in 8 (32.0%), SBO in 8 (32.0%), weight loss in 7 (28.0%), bacterial meningitis in 3 (12.0%), sepsis in 3 (12.0%), pneumonia in 2 (8.0%), and gastrointestinal bleeding in 2 (8.0%) patients. Most patients had more than one clinical symptom or illness. Six patients had disseminated infection (bacterial meningitis and/or sepsis) and the outcome of 2 patients was fatal. Twenty-four (96%) patients were under immunocompromised conditions resulting from HTLV-1 infection, administration of corticosteroid, diabetes mellitus, alcoholism, liver cirrhosis and chronic renal failure. Of note, 18 (72.0%) patients were HTLV-1 carriers. The diagnostic samples used for identifying Strongyloides were as follows: stool samples from 17 (68.0%) patients, duodenal biopsy from 15 (60.0%) patients, gastroduodenal drainage from 9 (36.0%) patients and sputum from 3 (12.0%) patients. Most patients were treated with thiabendazole or ivermectin alone and 2 patients needed a combination of the two drugs.
Table 1.
Clinical characteristics of patients with S. stercoralis hyperinfection
| Case No. | Age/Gender | Presenting symptoms and/or illness | Immunosuppressive state | Diagnosis | Treatment | Outcome |
| 1 | 51/M | Meningitis, GI bleeding | Alcoholism | Duodenal biopsy, stool | TBZ | Cured |
| 2 | 72/M | Diarrhea | HTLV-1 | Stool | TBZ | Cured |
| 3 | 80/M | Sepsis, meningitis | HTLV-1, corticosteroids | Duodenal biopsy, stool, sputum, gastroduodenal drainage | TBZ | Cured |
| 4 | 38/M | Abdominal pain | HTLV-1 | Duodenal biopsy, stool | TBZ | Cured |
| 5 | 58/F | Sepsis, diarrhea, pneumonia | Corticosteroids | Sputum | IVM | Dead |
| 6 | 86/F | Vomiting, weight loss | HTLV-1, DM | Duodenal biopsy, stool, sputum | IVM | Cured |
| 7 | 31/F | SBO, abdominal pain, vomiting | HTLV-1, alcoholism | Duodenal biopsy, stool, gastroduodenal drainage | TBZ | Cured |
| 8 | 58/F | Vomiting, weight loss | HTLV-1 | Duodenal biopsy, gastroduodenal drainage | IVM | Cured |
| 9 | 62/M | Meningitis, diarrhea | HTLV-1, DM | Duodenal biopsy, gastroduodenal drainage | TBZ | Cured |
| 10 | 73/F | Abdominal pain | HTLV-1, liver cirrhosis | Stool | IVM | Cured |
| 11 | 58/M | SBO, abdominal pain, vomiting | HTLV-1, DM | Duodenal biopsy | IVM, TBZ | Cured |
| 12 | 52/M | SBO, abdominal pain, vomiting | HTLV-1 | Duodenal biopsy, stool, gastroduodenal drainage | TBZ | Cured |
| 13 | 74/M | Diarrhea, weight loss | Chronic renal failure | Stool | IVM | Cured |
| 14 | 66/F | Vomiting, diarrhea | HTLV-1 | Duodenal biopsy, stool | TBZ | Cured |
| 15 | 42/M | SBO, abdominal pain, GI bleeding | HTLV-1 | Duodenal biopsy, stool | IVM, TBZ | Cured |
| 16 | 56/F | Vomiting, abdominal pain | HTLV-1 | Duodenal biopsy, gastroduodenal drainage | TBZ | Cured |
| 17 | 62/M | SBO, abdominal pain, vomiting | None | Stool | TBZ | Cured |
| 18 | 80/M | SBO, abdominal pain, vomiting | Alcoholism | Stool | IVM | Cured |
| 19 | 82/M | SBO, meningitis, pneumonia | DM | Stool, sputum | IVM | Dead |
| 20 | 57/M | Diarrhea, weight loss | HTLV-1 | Stool | IVM | Cured |
| 21 | 76/F | Vomiting, weight loss | HTLV-1 | Duodenal biopsy, stool | TBZ | Cured |
| 22 | 50/F | SBO, abdominal pain, vomiting | HTLV-1, corticosteroids | Duodenal biopsy | IVM | Cured |
| 23 | 66/M | Vomiting, weight loss | HTLV-1 | Duodenal biopsy, gastroduodenal drainage | IVM | Cured |
| 24 | 71/M | Sepsis, diarrhea | Corticosteroids | Gastroduodenal drainage | IVM | Cured |
| 25 | 74/F | Vomiting, diarrhea, weight loss | HTLV-1 | Gastroduodenal drainage, stool | IVM | Cured |
GI: Gastrointestinal; SBO: Small bowel obstruction; DM: Diabetes mellitus; TBZ: Thiabendazole; IVM: Ivermectin.
Endoscopic and histopathological findings
The endoscopic findings of the patients are summarized in Table 2. Gross abnormal findings were observed in 23 (92.0%) patients and normal findings in 2 (8.0%) patients. A broad range of endoscopic findings included edematous mucosa in 16 (69.5%) patients, white villi in 13 (56.5%), erythematous mucosa in 9 (39.1%), erosion in 6 (26.0%), stenosis in 4 (17.3%), fine granule in 4 (17.3%), hemorrhage in 3 (13.0%), dilatation in 3 (13.0%), and ulcer in 2 (8.6%) patients (Figure 1). Most patients had more than one finding. Strongyloidiasis was diagnosed histopathologically in 71.4% (15/21) of patients who had duodenal biopsies. According to the endoscopic severity score of the duodenum, the endoscopic findings of duodenitis were more severe in patients whose biopsies were positive for larvae than in those with negative biopsies (4.86 ± 2.47 vs 2.71 ± 1.38, P < 0.05, Figure 2). Representative endoscopic images of white villi and edematous mucosa (Figure 3), white villi and stenosis (Figure 4), and ulcer and pseudopolyps (Figure 5) are shown along with the histopathological findings. As shown together with the endoscopic and histopathological images, the degree of duodenitis, including villous atrophy/destruction and inflammatory cell infiltration, was associated with the severity of the endoscopic findings. In 6 cases whose stool was not obtained due to SBO or larvae were not identified in the stool, strongyloidiasis was diagnosed only by duodenal biopsies.
Table 2.
Endoscopic and histopathological findings of the duodenum
| Case No. | Endoscopic findings of the duodenum | Histopathologic detection of S. stercoralis in the duodenum |
| 1 | Edema, erythema, erosion, hemorrhage | Larvae |
| 2 | Erythema | Negative |
| 3 | Edema, white villi, erosion | Larvae |
| 4 | Edema, white villi, erythema, dilatation | Larvae |
| 5 | White villi | ND |
| 6 | Edema, fine granule | Larvae |
| 7 | Erythema | Larvae |
| 8 | Edema, erosion, stenosis | Larvae |
| 9 | Edema, white villi | Larvae |
| 10 | Normal | ND |
| 11 | Edema, white villi, dilatation | Larvae |
| 12 | Edema, white villi | Larvae |
| 13 | White villi, erythema | Negative |
| 14 | Edema, white villi, fine granule | Larvae |
| 15 | Edema, erythema, ulcer, hemorrhage, pseudopolyps | Larvae |
| 16 | Edema, white villi, stenosis | Larvae |
| 17 | Edema, stenosis | Negative |
| 18 | White villi, erythema, erosion | Negative |
| 19 | Edema, ulcer | Negative |
| 20 | Normal | ND |
| 21 | Edema, white villi, erosion, hemorrhage | Larvae |
| 22 | Edema, white villi, erosion, fine granule, stenosis | Larvae |
| 23 | Fine granule, dilatation | Larvae |
| 24 | Edema, white villi, erythema | Negative |
| 25 | Erythema | Negative |
Edema: Edematous mucosa; Erythema: Erythematous mucosa; ND: Biopsies were not done.
Figure 1.
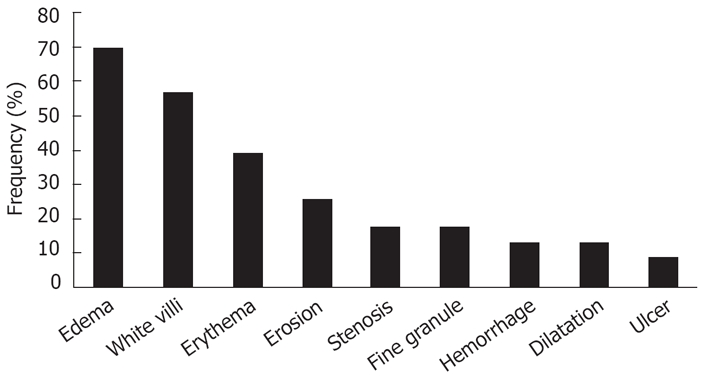
Frequency of abnormal endoscopic findings in the duodenum
Figure 2.
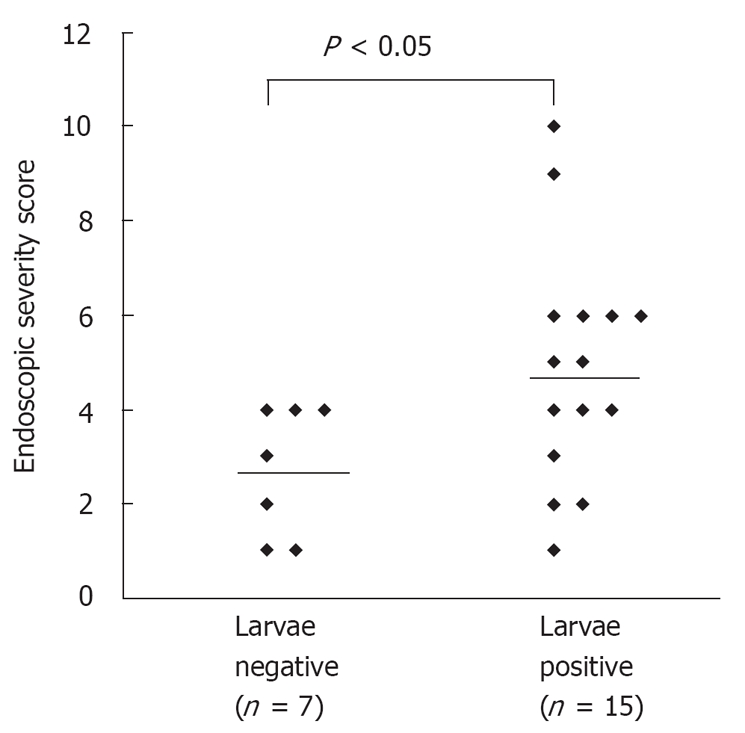
Comparison of endoscopic severity of the duodenum between the patients with larvae present and larvae absent in the duodenal biopsy (P < 0.05, Mann-Whitney U-test).
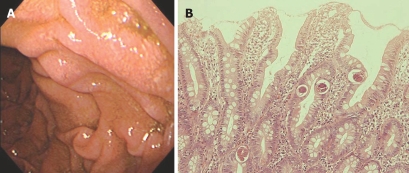
Figure 3.
Representative endoscopic image and HE staining of duodenal biopsy. A: EGD showing white villi and edematous mucosa in the second part of duodenal (Case 11); B: Biopsy specimen from the mucosa showing numerous larvae with villous atrophy and mild inflammatory cell infiltration (HE, × 200).
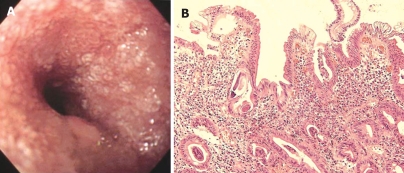
Figure 4.
Endoscopic image and HE staining of duodenal biopsy. A: EGD showing white villi and stenosis in the second part of duodenal (Case 16); B: Biopsy specimen from the mucosa showing numerous larvae with severe villous atrophy and moderate inflammatory cell infiltration (HE, × 200).
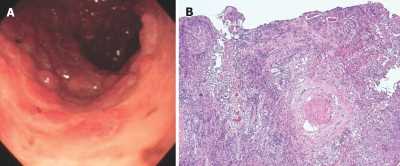
Figure 5.
Endoscopic findings and HE staining of duodenal biopsy. A: EGD showing large ulcers and pseudopolyps in the second part of duodenal (Case 15); B: Biopsy specimen from the margin of the ulcer showing formation of granulation tissue and complete destruction of the villi. Numerous larvae are observed within the granulation and lymph vessels (HE, × 100).
DISCUSSION
This study was conducted in the Okinawa islands, a subtropical region of Japan, where both S. stercoralis and HTLV-1 are endemic and epidemiological studies have been thoroughly conducted[21–24]. There is an increasing body of evidence regarding the strong association between S. stercoralis and HTLV-1 co-infection and hyperinfection syndrome[21–26], which has been further strengthened by our striking result that a majority (72%) of the patients with hyperinfection were co-infected with HTLV-1. We also confirmed the well-described role of corticosteroids in triggering hyperinfection regardless of the presence of HTLV-1 co-infection. Corticosteroids not only have a well-known effect impairing human immunity but also directly affect the female larvae to increase output of infective larvae with a structural similarity to larval ecdysteroids[1,27].
Enteritis by S. stercoralis has been studied since the early 1960’s before the endoscopic era. de Paola et al[28] classified the histopathological changes in fatal cases into three forms: catarrhal enteritis, edematous enteritis and ulcerative enteritis. Catarrhal enteritis is a minor form characterized by mild mucosal congestion with larvae restricted to the mucous membrane. Edematous enteritis is a moderately serious form characterized by edematous thickening of the wall, swelling folds and villous atrophy. Larvae occupy lymph-vessel spaces. They also observed that mucosal edema was not only a result of inflammation and protein deficiency but also an effect of larval invasion in the lymph vessels and lymphangiectasia. Ulcerative enteritis is a serious form characterized by ulcers and fibrosis. Larvae are encountered in the entire wall. In the endoscopic era, there have been several case reports describing endoscopic findings of the duodenum in strongyloidiasis, including normal mucosa, edema, erythema, erosion, swollen folds, fine granule, tiny ulcer, polyps, hemorrhage, megaduodenum, deformity, and stenosis (Table 3). To our knowledge, the present retrospective study represents the largest endoscopic experience with S. stercoralis hyperinfection. In the histopathological studies, Coutinho et al[29] reported that duodenal villous atrophy and crypt hyperplasia were proportional to the degree of clinical severity of strongyloidiasis. Suarez and Sanchez[19] confirmed that plasma cell infiltration, villous atrophy and severe duodenitis were the characteristics of severe strongyloidiasis. A recent study clearly demonstrated that strongyloidiasis disrupted epithelial kinetics in the human small intestine by the induction of apoptosis and inhibition of cell proliferation, thereby resulting in villous atrophy and impaired barrier function[17]. Our observations disclosed that endoscopic findings of duodenitis were more severe in the patients whose biopsies were positive for larvae than in those with negative biopsies. Considering the fact that increased numbers of larvae are strongly associated with the progression to hyperinfection, our result supports their findings.
Table 3.
Reported endoscopic findings of the duodenum with strongyloidiasis in literature
| Authors | Year | No. of cases | Immunosuppressive state | Endoscopic findings of the duodenum |
| Brasitus et al[3] | 1980 | 1 | None | Brisk bleeding, deformed bulb |
| Milder et al[4] | 1981 | 2 | ND | Enlarged folds |
| Bone et al[5] | 1982 | 1 | ND | Deformed cap, obliterated second part |
| Bhatt et al[6] | 1990 | 1 | None | Mild erythema |
| Chen et al[7] | 1994 | 1 | Corticosteroids | Flattened folds, swelling mucosa, tiny ulcer |
| Choudhry et al[8] | 1995 | 3 | DM, none | Multiple serpiginous lesions, duodenal nodule |
| Hizawa et al[9] | 1996 | 1 | HTLV-1 and corticosteroids | Edema, tiny white spots |
| Friedenberg et al[10] | 1999 | 1 | HTLV-1 | Severe stenosis |
| Overstreet et al[11] | 2003 | 1 | HIV | White punctate dotting mucosa |
| Asano et al[12] | 2004 | 1 | HTLV-1 | Fine granule, coarse mucosa, disappeared folds |
| Thompson et al[13] | 2004 | 6 | Corticosteroids, DM, HIV, none | Edema, brown discoloration, erythematous spots, |
| subepithelial hemorrhage, megaduodenum | ||||
| Seet et al[14] | 2005 | 1 | Anti-myeloma drugs | Erythematous and granular mucosa |
| Karmo et al[15] | 2006 | 1 | Corticosteroids | Normal |
| Ghoshal et al[16] | 2006 | 1 | Corticosteroids | Multiple nodules |
| Werneck-Silva et al[17] | 2006 | 4 | ND | Erythema, erosion |
| Csermely et al[18] | 2006 | 1 | Anti-myeloma drugs | Necrotic ulcerations |
| Suarez and Sanchez[19] | 2006 | 11 | ND | Swollen folds of nodular aspect |
ND: Not determined.
Prior reports have indicated that findings frequently include edematous mucosa, swollen folds and erythematous mucosa. However, pathognomonic findings are apparently not evident[3–19]. Our present study confirmed the aforementioned frequent findings. In addition, we noticed that an endoscopic feature of duodenal white villi seemed to be a frequent finding. The findings of tiny white spots[9] and white punctuate dotting mucosa[11] appear to be similar in appearance. The endoscopic finding of white villi is well-known in intestinal lymphangiectasia with protein-losing enteropathy. It represented markedly dilated lymphatics in the stroma of the villi and fats, including fat droplets in the absorptive cells from the impaired transport of fats from intestinal epithelial cells to intestinal lymphatics[30,31]. Considering the fact that larvae invade the lymph vessels and that there is subsequent lymphangiectasia in edematous enteritis as reported by de Paola et al[28], the appearance of white villi may reflect villous atrophy/destruction and mucosal edema similar to intestinal lymphangiectasia. We, therefore, emphasize that white villi can be a good endoscopic marker for strongyloidiasis in endemic regions.
There have been very few reports regarding the histopathologic yield by endoscopy for strongyloidiasis. In a study by Thompson et al[13], a minimum of six biopsies were obtained from each lesion, resulting in a 100% histopathologic yield from the 6 patients. The reason for our low yield (71.4%) may be due to the fact that our study is a retrospective study conducted at multiple hospitals and only one to three biopsies were taken from each lesion. Obtaining multiple biopsy specimens might increase the histopathologic yield. However, looking at our study from another point of view, only duodenal biopsies were able to establish a diagnosis when the stool analysis was negative in 6 (24%) patients, which lead to the avoidance of a fatal outcome.
In conclusion, S. stercoralis hyperinfection can rapidly become fatal, so early diagnosis and treatment is very important. Although diagnosis is usually made by stool analysis, our results clearly demonstrate that endoscopic observation and biopsies, in addition to gastroduodenal drainage analysis, are important tools for diagnosing strongyloidiasis. We also emphasize that infection with S. stercoralis and HTLV-1 should be ruled out before immunosuppressive therapy is administered for patients living in or coming from endemic regions.
COMMENTS
Background
Strongyloides stercoralis (S. stercoralis) is an intestinal nematode that infects about 100 million people worldwide. As S. stercoralis colonizes in the duodenum, endoscopic evaluation has been recognized as an important tool for diagnosing strongyloidiasis. Although there have been several case reports demonstrating endoscopic findings of strongyloidiasis, the relationship of endoscopic markers to clinical and histopathological findings have not been intensively studied.
Research frontiers
There is an increasing body of epidemiological evidence regarding the association between S. stercoralis infection and HTLV-1 infection. Studies of molecular mechanism of this association have become one of the hot spots at present. For the diagnostic purpose, detection of S. stercoralis in clinical samples has been improved by the agar plate culture method which was invented recently.
Related publications
Nakada et al[21] clearly disclosed the first evidence of the association between S. stercoralis infection and HTLV-1infection. Hirata et al[24] conducted a large scale epidemiological study which strengthened this association and showed the impairment of host immune response against S. stercoralis by HTLV-1 infection.
Innovations and breakthroughs
This study clarified the strong association between S. stercoralis hyperinfection and HTLV-1 infection. Moreover, presence of white villi can be a good endoscopic marker for the duodenal strongyloidiasis. Endoscopic biopsy helped early diagnosis of strongyloidiasis.
Applications
Early endoscopic diagnosis of strongyloidiasis can have a marked impact on disease outcome. Co-infection with S. stercoralis and HTLV-1 should be ruled out to prevent hyperinfection before immunosuppressive therapy (e.g., corticosteroids) is administered for patients living in or coming from endemic regions.
Terminology
Hyperinfection is a syndrome of accelerated larval autoinfection which results from immunosuppression. Disseminated strongyloidiasis is the migration of larvae to organs beyond the range of the autoinfective cycle and is often complicated by Gram-negative sepsis which results in high mortality rates.
Peer review
This is a well conducted study clarifying the strong association between S. stercoralis hyperinfection and HTLV-1 infection.
Acknowledgments
We thank Drs. Osamu Zaha, Ryoji Matayoshi and Nobufumi Uchima for their support.
Peer reviewer: James M Scheiman, Professor, Division of Gastroenterology, University of Michigan Medical Center, 3912 Taubman Center, Box 0362, Ann Arbor, Michigan 48109-0362, United States
S- Editor Decorti G L- Editor Kumar M E- Editor Lu W
References
- 1.Concha R, Harrington W Jr, Rogers AI. Intestinal strongyloidiasis: recognition, management, and determinants of outcome. J Clin Gastroenterol. 2005;39:203–211. doi: 10.1097/01.mcg.0000152779.68900.33. [DOI] [PubMed] [Google Scholar]
- 2.Keiser PB, Nutman TB. Strongyloides stercoralis in the Immunocompromised Population. Clin Microbiol Rev. 2004;17:208–217. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brasitus TA, Gold RP, Kay RH, Magun AM, Lee WM. Intestinal strongyloidiasis. A case report and review of the literature. Am J Gastroenterol. 1980;73:65–69. [PubMed] [Google Scholar]
- 4.Milder JE, Walzer PD, Kilgore G, Rutherford I, Klein M. Clinical features of Strongyloides stercoralis infection in an endemic area of the United States. Gastroenterology. 1981;80:1481–1488. [PubMed] [Google Scholar]
- 5.Bone MF, Chesner IM, Oliver R, Asquith P. Endoscopic appearances of duodenitis due to strongyloidiasis. Gastrointest Endosc. 1982;28:190–191. doi: 10.1016/s0016-5107(82)73054-8. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt BD, Cappell MS, Smilow PC, Das KM. Recurrent massive upper gastrointestinal hemorrhage due to Strongyloides stercoralis infection. Am J Gastroenterol. 1990;85:1034–1036. [PubMed] [Google Scholar]
- 7.Chen JJ, Lee CM, Changchan CS. Duodenal strongyloides stercoralis infection. Endoscopy. 1994;26:272. doi: 10.1055/s-2007-1008965. [DOI] [PubMed] [Google Scholar]
- 8.Choudhry U, Choudhry R, Romeo DP, Cammerer RC, Gopalswamy N. Strongyloidiasis: new endoscopic findings. Gastrointest Endosc. 1995;42:170–173. doi: 10.1016/s0016-5107(95)70077-3. [DOI] [PubMed] [Google Scholar]
- 9.Hizawa K, Iida M, Aoyagi K, Kimura Y, Eguchi K, Fujishima M. Early detection of strongyloidiasis using endoscopic duodenal biopsy: report of a case. J Clin Gastroenterol. 1996;22:157–159. doi: 10.1097/00004836-199603000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Friedenberg F, Wongpraparut N, Fischer RA, Gubernick J, Zaeri N, Eiger G, Ozden Z. Duodenal obstruction caused by Strongyloides stercoralis enteritis in an HTLV-1-infected host. Dig Dis Sci. 1999;44:1184–1188. doi: 10.1023/a:1026636509713. [DOI] [PubMed] [Google Scholar]
- 11.Overstreet K, Chen J, Rodriguez JW, Wiener G. Endoscopic and histopathologic findings of Strongyloides stercoralis infection in a patient with AIDS. Gastrointest Endosc. 2003;58:928–931. doi: 10.1016/s0016-5107(03)02280-6. [DOI] [PubMed] [Google Scholar]
- 12.Asano K, Tada S, Kamio T, Matsumoto T, Suko H, Iida M. Strongyloidiasis. Gastrointest Endosc. 2004;60:606–607. doi: 10.1016/s0016-5107(04)01861-9. [DOI] [PubMed] [Google Scholar]
- 13.Thompson BF, Fry LC, Wells CD, Olmos M, Lee DH, Lazenby AJ, Monkemuller KE. The spectrum of GI strongyloidiasis: an endoscopic-pathologic study. Gastrointest Endosc. 2004;59:906–910. doi: 10.1016/s0016-5107(04)00337-2. [DOI] [PubMed] [Google Scholar]
- 14.Seet RC, Gong LL, Tambyath PA. Image of the month. Strongyloides stercoralis hyperinfection and syndrome of inappropriate secretion of antidiuretic hormone. Gastroenterology. 2005;128:8, 252. doi: 10.1053/j.gastro.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 15.Karmo M, Goh J, Boulton R, Sanders DS. An unusual cause of diarrhoea in a patient with colitis. Gut. 2005;54:77, 96. doi: 10.1136/gut.2004.043240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghoshal UC, Alexender G, Ghoshal U, Tripathi S, Krishnani N. Strongyloides stercoralis infestation in a patient with severe ulcerative colitis. Indian J Med Sci. 2006;60:106–110. [PubMed] [Google Scholar]
- 17.Werneck-Silva AL, Alvares EP, Gama P, Damiao AO, Osaki LH, Ogias D, Sipahi AM. Intestinal damage in strongyloidiasis: the imbalance between cell death and proliferation. Dig Dis Sci. 2006;51:1063–1069. doi: 10.1007/s10620-006-8010-2. [DOI] [PubMed] [Google Scholar]
- 18.Csermely L, Jaafar H, Kristensen J, Castella A, Gorka W, Chebli AA, Trab F, Alizadeh H, Hunyady B. Strongyloides hyper-infection causing life-threatening gastrointestinal bleeding. World J Gastroenterol. 2006;12:6401–6404. doi: 10.3748/wjg.v12.i39.6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suarez A, Sanchez C. Strongyloides stercoralis: histopathological findings of duodenal mucosa (1999-2005) Rev Gastroenterol Peru. 2006;26:44–48. [PubMed] [Google Scholar]
- 20.Jenkins D, Goodall A, Gillet FR, Scott BB. Defining duodenitis: quantitative histological study of mucosal responses and their correlations. J Clin Pathol. 1985;38:1119–1126. doi: 10.1136/jcp.38.10.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakada K, Kohakura M, Komoda H, Hinuma Y. High incidence of HTLV antibody in carriers of Strongyloides stercoralis. Lancet. 1984;1:633. doi: 10.1016/s0140-6736(84)91030-4. [DOI] [PubMed] [Google Scholar]
- 22.Satoh M, Kiyuna S, Shiroma Y, Toma H, Kokaze A, Sato Y. Predictive markers for development of strongyloidiasis in patients infected with both Strongyloides stercoralis and HTLV-1. Clin Exp Immunol. 2003;133:391–396. doi: 10.1046/j.1365-2249.2003.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaha O, Hirata T, Uchima N, Kinjo F, Saito A. Comparison of anthelmintic effects of two doses of ivermectin on intestinal strongyloidiasis in patients negative or positive for anti-HTLV-1 antibody. J Infect Chemother. 2004;10:348–351. doi: 10.1007/s10156-004-0345-z. [DOI] [PubMed] [Google Scholar]
- 24.Hirata T, Uchima N, Kishimoto K, Zaha O, Kinjo N, Hokama A, Sakugawa H, Kinjo F, Fujita J. Impairment of host immune response against strongyloides stercoralis by human T cell lymphotropic virus type 1 infection. Am J Trop Med Hyg. 2006;74:246–249. [PubMed] [Google Scholar]
- 25.Gotuzzo E, Terashima A, Alvarez H, Tello R, Infante R, Watts DM, Freedman DO. Strongyloides stercoralis hyperinfection associated with human T cell lymphotropic virus type-1 infection in Peru. Am J Trop Med Hyg. 1999;60:146–149. doi: 10.4269/ajtmh.1999.60.146. [DOI] [PubMed] [Google Scholar]
- 26.Carvalho EM, Da Fonseca Porto A. Epidemiological and clinical interaction between HTLV-1 and Strongyloides stercoralis. Parasite Immunol. 2004;26:487–497. doi: 10.1111/j.0141-9838.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- 27.Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clin Microbiol Rev. 1992;5:345–355. doi: 10.1128/cmr.5.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Paola, Dias LB, da Silva J. Enteritis due to Strongyloides stercoralis. A report of 5 fatal cases. Am J Dig Dis. 1962;7:1086–1098. doi: 10.1007/BF02232985. [DOI] [PubMed] [Google Scholar]
- 29.Coutinho HB, Robalinho TI, Coutinho VB, Almeida JR, Filho JT, King G, Jenkins D, Mahida Y, Sewell HF, Wakelin D. Immunocytochemistry of mucosal changes in patients infected with the intestinal nematode Strongyloides stercoralis. J Clin Pathol. 1996;49:717–720. doi: 10.1136/jcp.49.9.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asakura H, Miura S, Morishita T, Aiso S, Tanaka T, Kitahora T, Tsuchiya M, Enomoto Y, Watanabe Y. Endoscopic and histopathological study on primary and secondary intestinal lymphangiectasia. Dig Dis Sci. 1981;26:312–320. doi: 10.1007/BF01308371. [DOI] [PubMed] [Google Scholar]
- 31.Aoyagi K, Iida M, Yao T, Matsui T, Okada M, Oh K, Fujishima M. Characteristic endoscopic features of intestinal lymphangiectasia: correlation with histological findings. Hepatogastroenterology. 1997;44:133–138. [PubMed] [Google Scholar]