SUMMARY
Endoplasmic reticulum (ER) stress is a hallmark of advanced atherosclerosis, but its causative role in plaque progression is unknown. In-vitro studies have implicated the ER stress effector CHOP in macrophage apoptosis, a process involved in plaque necrosis in advanced atheromata. To test the effect of CHOP deficiency in vivo, aortic root lesions of fat-fed Chop+/+;Apoe−/− and Chop−/−;Apoe−/− mice were analyzed for size and morphology. Despite similar plasma lipoproteins, lesion area was 35% smaller in Chop−/−;Apoe−/− mice. Most importantly, plaque necrosis was reduced by ~50% and lesional apoptosis by 35% in the CHOP-deficient mice. Similar results were found in fat-fed Chop−/−;Ldlr−/− vs. Chop+/+;Ldlr−/− mice. Thus, CHOP promotes plaque growth, apoptosis, and plaque necrosis in fat-fed Apoe−/− and Ldlr−/− mice. These data provide direct evidence for a causal link between the ER stress effector CHOP and plaque necrosis and suggest that interventions weakening this arm of the UPR may lessen plaque progression.
INTRODUCTION
Understanding how asymptomatic atherosclerotic lesions are converted into plaques that are at high risk to trigger acute lumenal thrombosis is a major goals of molecular cardiology. These so-called “vulnerable” plaques are characterized by large necrotic cores; focal thinning of the fibrous cap; a high level of inflammatory cytokines and matrix proteases; and apoptosis of intimal cells (Libby et al., 1996; Kolodgie et al., 2004; Clarke and Bennett, 2006). This latter process is critical, because advanced lesional macrophage apoptosis, coupled with defective phagocytic clearance, results in post-apoptotic necrosis (Tabas, 2005; Schrijvers et al., 2007). Likewise, smooth muscle cell can lead to fibrous cap thinning (Clarke and Bennett, 2006), and endothelial apoptosis may promote a pro-thrombotic state and vascular dysfunction (Choy et al., 2001).
Recent studies in vitro have implicated a signal transduction pathway called the Unfolded Protein Response (UPR) in the death of all three cell types. The UPR is normally a repair pathway that responds to a variety of perturbations that affect endoplasmic reticulum (ER) homeostasis (Kaufman, 2002; Ron, 2002; Ma and Hendershot, 2001). However, a particular branch of the UPR involving the transcription factor CHOP (GADD153) can trigger apoptosis in the setting of severe or prolonged ER stress (Zinszner et al., 1998; Feng et al., 2003a; Lin et al., 2007). Over the last several years, mechanistic studies with cultured cells and analyses of mRNA and protein expression in murine and human atheromata have suggested roles for the UPR in general, and CHOP in particular, in intimal cell apoptosis (Feng et al., 2003a; Feng et al., 2003b; Hossain et al., 2003; Zhou et al., 2005; Gargalovic et al., 2006; Myoishi et al., 2007; Pedruzzi et al., 2004; Sanson et al., 2009). For example, CHOP plays a role in macrophage apoptosis induced by combinations of ER stressors and pattern recognition receptor (PRR) ligand (Feng et al., 2003a; DeVries-Seimon et al., 2005). In these studies, deletion of CHOP blocked apoptosis without leading to “default” necrosis (Feng et al., 2003a). In vivo significance is suggested by studies showing elevated levels of mRNA and protein of UPR effectors, including CHOP, in advanced murine aortic root and human coronary artery plaques (Feng et al., 2003a; Zhou et al., 2005; Gargalovic et al., 2006; Myoishi et al., 2007; Sanson et al., 2009). For example, Myoishi et al. found a very strong correlation between expression of UPR markers, including CHOP, and plaque necrosis and rupture in human coronary artery lesions (Myoishi et al., 2007). In this regard, a large number of molecules or processes that are known to exist in advanced plaques are UPR inducers, including oxidized lipids, homocysteine, oxidative stress, and insulin resistance (Gargalovic et al., 2006; Hossain et al., 2003; Han et al., 2006).
Despite this large body of correlative data, there are no studies testing the causal relationship between CHOP expression and the development of atherosclerotic lesions in vivo. Here we report that advanced aortic root lesions of both Chop−/−;Apoe−/− mice and Chop−/−;Ldlr−/− fed an atherogenic diet are smaller in total area and, most importantly, have substantially diminished plaque necrosis and less intimal cell apoptosis than the lesions of these mice with normal CHOP expression.
RESULTS
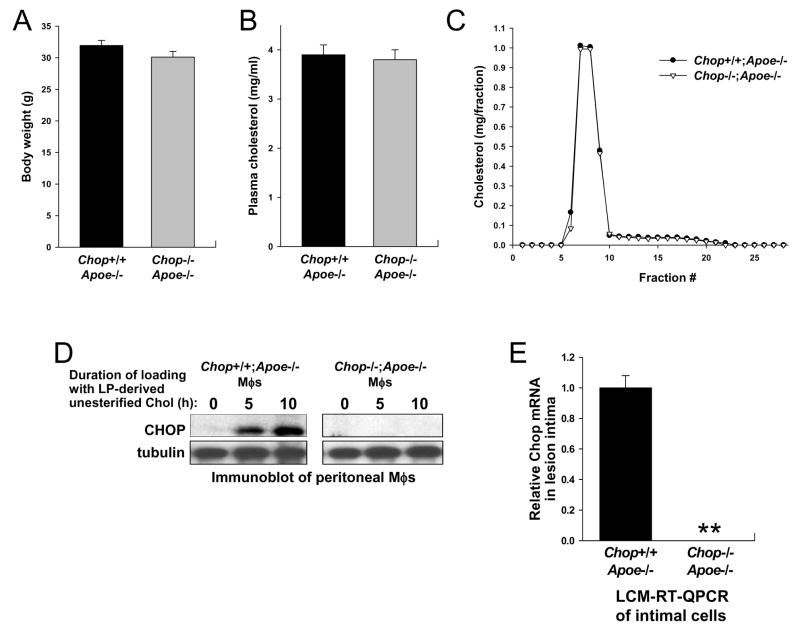
Chop+/+;Apoe−/− and Chop−/−;Apoe−/− mice on the C57BL6 background were placed on a Western-type diet at age 8 weeks and maintained on this diet for an additional 10 weeks. The mice were then sacrificed and analyzed for lipoprotein, metabolic, and plaque characteristics. As shown in Figure 1A–C, the two groups of mice did not differ significantly in body weight, total plasma cholesterol, or fasting lipoprotein profile. Moreover, plasma triglycerides, glucose, and insulin did not differ significantly between the two groups of mice (data not shown). As expected, peritoneal macrophages from the Chop+/+;Apoe−/− mice expressed CHOP when loaded with lipoprotein-derived unesterified cholesterol (Feng et al., 2003a), while macrophages from the Chop−/−;Apoe−/− did not (Figure 1D). To determine CHOP expression in plaques, we quantified Chop mRNA using RT-QPCR of samples obtained by laser capture microdissection (LCM) of non-endothelial intimal cells. On average, greater than 70% of the intimal cells were F4/80-positive macrophages. This technique was used because CHOP immunohistochemistry gives non-specific staining in atheromata (unpublished observations). As shown in Figure 1E, Chop mRNA was readily detected in the lesions of Chop+/+;Apoe−/− mice but not in the lesions of Chop−/−;Apoe−/− mice.
Figure 1. Total Body Weight, Plasma Lipoproteins, and CHOP Expression in Chop+/+;Apoe−/− and Chop−/−;Apoe−/− Mice.
(A–B) Body weight and total plasma cholesterol of Western diet-fed male Chop+/+;Apoe−/− and Chop−/−;Apoe−/− mice (n = 20 and 21, respectively).
(C) Pooled plasma samples were subjected to fast performance liquid chromatography gel-filtration, and the fractions were assayed for cholesterol concentration. None of the differences between the two groups of mice in A–C were statistically significant.
(D) Peritoneal macrophages from Chop+/+;Apoe−/− and Chop−/−;Apoe−/− mice were loaded with lipoprotein-derived unesterified cholesterol for the indicated times, and then whole-cell lysates were subjected to immunoblot analysis for CHOP and, as a loading control, tubulin.
(E) RNA from the intima of aortic root lesions of Chop+/+;Apoe−/− and Chop−/−;Apoe−/− mice (see Figure 2) was captured by LCM, and Chop and Cypa mRNA were quantified by RT-QPCR. Relative expression was normalized to Cypa. Results are displayed as the mean ± S.E.M. (n = 3 RT-PCR replicates). **, Chop mRNA not detected.
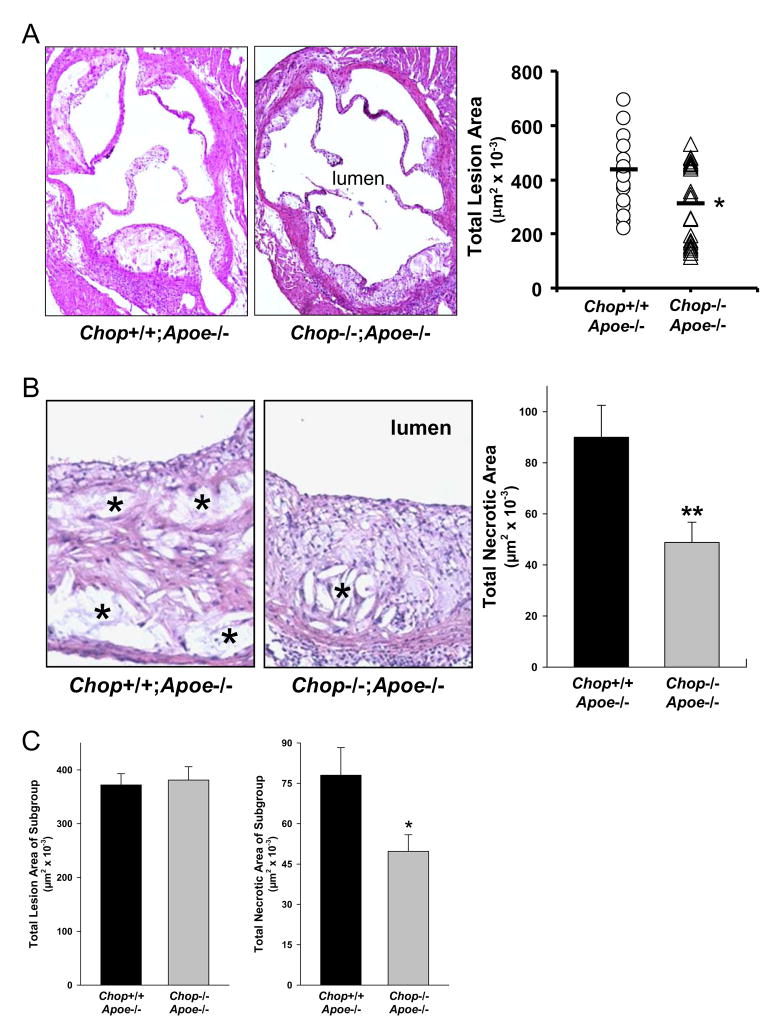
Atherosclerotic lesions were analyzed at the aortic root. As shown in the representative images and quantitative data in Figure 2A, lesion area was 35% less in Chop−/−;Apoe−/− mice compared with Chop+/+;Apoe−/− mice (P < 0.05). In vitro, macrophages from Chop+/− mice were equally susceptible to FC-induced apoptosis as those from Chop+/+ mice (unpublished observations). Consistent with these findings, aortic root lesion area of Apoe−/−;Chop+/+ and Apoe−/−;Chop+/− mice were statistically indistinguishable (data not shown).
Figure 2. Reduction in Aortic Root Lesion Area and Necrotic Area in Chop−/−;Apoe−/− Mice.
(A) The images show representative sections of aortic roots from each group of mice were stained with hematoxylin and eosin. Quantification was conducted on lesions from 20 Chop+/+;Apoe−/− mice and 21 Chop−/−;Apoe−/− mice. *, P = 0.03.
(B) Representative sections of hematoxylin and eosin-stained aortic root sections from Chop+/+;Apoe−/− and Chop−/−;Apoe−/− mice. (*= necrotic areas). The bar graph shows quantification of anuclear, afibrotic, and eosin-negative necrotic areas (n = 20 for both groups of mice). **, P < 0.01. (C) Quantification of necrotic area in a subgroup of 12 Chop+/+;Apoe−/− lesions and 10 Chop−/−;Apoe−/− lesions with statistically identical lesion area. *, P = 0.039.
In human atherosclerotic cardiovascular disease, plaque morphology is a more important predictor of plaque disruption and acute clinical events than plaque size (Brown et al., 1993). In particular, the size of the necrotic core of advanced plaques is a key determinant of plaque vulnerability (Brown et al., 1993; Kolodgie et al., 2004). Analysis of lesions for acellular non-fibrotic areas revealed a clear decrease in plaque necrosis in the Chop−/− lesions, and quantification of this parameter for the entire cohort of mice indicated a 46% decrease in total necrotic area in the lesions of Chop−/−;Apoe−/− mice (P < 0.01) (Figure 2B). To control for the potentially confounding influence of the decrease in lesion area, we carried out a subgroup analysis of 12 Chop+/+;Apoe−/− lesions and 10 Chop−/−;Apoe−/− lesions with statistically identical lesion area. In this subgroup, the Chop−/−;Apoe−/− lesions had a 36% decrease in necrosis (P = 0.039) (Figure 2C).
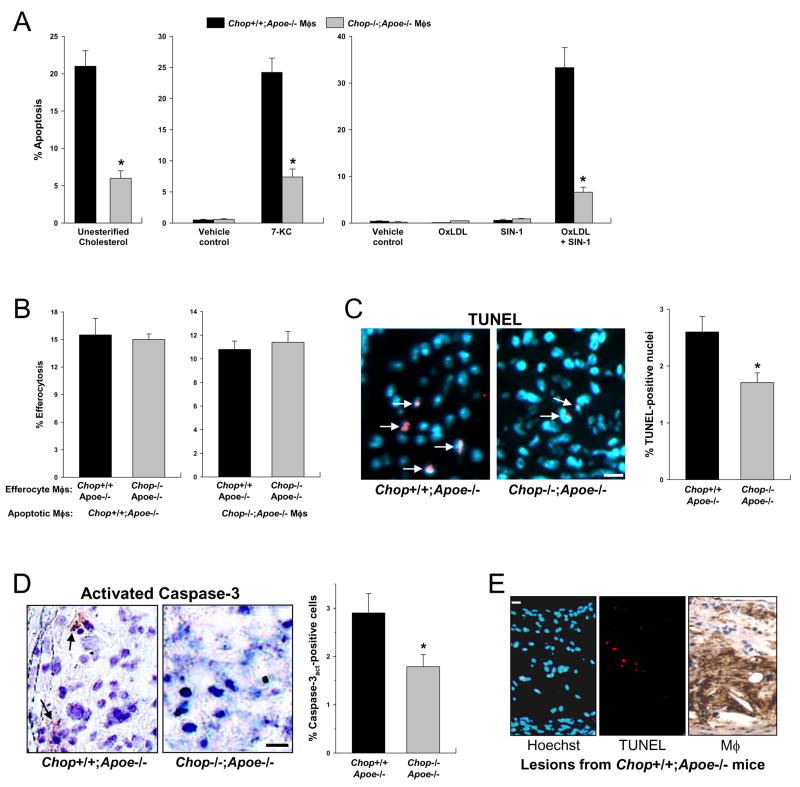
The decreased plaque necrosis in Chop−/−;Apoe−/− atheromata could reflect a primary decrease in macrophage apoptosis and/or increased efficiency of phagocytic clearance of the apoptotic cells (efferocytosis) in Chop−/−;Apoe−/− lesions (Tabas, 2005; Schrijvers et al., 2007). Consistent with our previous study (Feng et al., 2003a), macrophages from Chop−/−;Apoe−/− mice showed a 71% protection from apoptosis induced by loading the macrophages with lipoprotein-derived unesterified cholesterol (p < 0.01) (Figure 3A, left graph). Similar results were found with 7-ketocholesterol (Figure 3A, middle graph), an oxysterol and ER stressor present in advanced atheromata (Myoishi et al., 2007; Sanson et al., 2009). To test an ER stress-PRR combination known to be present in atheromata, we incubated macrophages with SIN-1, which generates peroxynitrite and induces ER stress in cells (Kawahara et al., 2001; Dickhout et al., 2005), and low dose oxidized LDL, which is a PRR ligand (Miller et al., 2003). At the doses used, neither SIN-1 nor oxidized LDL alone triggered apoptosis in macrophages (Figure 3A, right graph). However, the combination was a potent inducer of apoptosis, and apoptosis was markedly reduced in macrophages from CHOP-deficient mice. Regarding efferocytosis, there was no statistically significant difference in the ability of Chop+/+;Apoe−/− vs. Chop−/−;Apoe−/− macrophages to engulf apoptotic macrophages, regardless of whether the apoptotic cells were from either type of mouse (Figure 3B). Pending assessment of efferocytosis in vivo, these combined data are consistent with the interpretation that decreased susceptibility to apoptosis is a cause of decreased plaque necrosis in Chop−/−;Apoe−/− lesions.
Figure 3. Reduction in Peritoneal Macrophage and Aortic Root Lesional Macrophage Apoptosis in Apoe−/−;Chop−/− Mice.
(A) Left graph, Macrophages from Chop+/+;Apoe−/− or Chop−/−;Apoe−/− mice were loaded with lipoprotein-derived unesterified cholesterol and then assayed for apoptosis. *, P < 0.01. Middle graph, Macrophages were incubated for 18 h with vehicle control (0.2% ethanol) or 50 μM 7-ketocholesterol and then assayed for apoptosis. *, P<0.01 compared with Chop+/+;Apoe−/− macrophages. Right graph, Macrophages were incubated for 15 h with vehicle control, 50 μg/ml oxidized LDL (OxLDL), 1 mM SIN-1, or both compounds. The cells were then assayed for apoptosis. Asterisk = P<0.01 compared with Chop+/+;Apoe−/− macrophages. In the case of cholesterol loading, ~5% of apoptotic cells were PI-positive. With 7-ketocholesterol and OxLDL + SIN-1, the percentage of PI-positive cells was >50%, indicating advanced apoptosis, and both annexin-positive and PI-positive cells were decreased in the CHOP–deficient macrophages.
(B) Efferocytosis assays were conducted using various combinations of peritoneal macrophages from Chop+/+;Apoe−/− and Chop−/−;Apoe−/− mice, where the macrophages served as the source of either the apoptotic cells or the efferocytes. None of the differences shown are statistically significant.
(C) Representative micrographs show less TUNEL-positive signal (red; arrows) in nuclei (blue) of aortic root lesions from Chop−/−;Apoe−/− lesions. Bar, 10 μm. Quantification of TUNEL-positive nuclei was conducted on lesions from 20 Chop+/+;Apoe−/− mice and 21 Chop−/−;Apoe−/− mice. *, P<0.01.
(D) Representative micrographs show less activated caspase-3 (red; arrows) in aortic root lesions from Chop−/−;Apoe−/− vs. Chop−/−;Apoe−/− lesions. Bar, 10 μm. Quantification of TUNEL-positive cells was conducted on lesions from 7 Chop+/+;Apoe−/− mice and 7 Chop−/−;Apoe−/− mice. *, P<0.05.
(E) Representative sequential sections of a Chop+/+;Apoe−/− lesion stained for nuclei (Hoechst), TUNEL, and macrophages. Bar, 10 μm.
To directly assess apoptosis in situ, lesions from the two groups of mice were stained by the TUNEL method to detect apoptotic cells (Kockx, 1998). The representative images in Figure 3C show occasional TUNEL-positive nuclei, at the expected low frequency, in Chop+/+;Apoe−/− lesions (Kockx, 1998; Feng et al., 2003b). Most importantly, lesions from Chop−/−;Apoe−/− mice had 35% less TUNEL-positive nuclei than lesions from Chop+/+;Apoe−/− mice (P < 0.01) (graph in Figure 3C). Similar data were obtained using activated caspase-3 as an assay for lesional apoptosis (Figure 3D). The less robust effect of CHOP deficiency shown here compared with the cell culture studies above likely reflects the difficultly of assessing cumulative apoptotic events in vivo (see Discussion). Cellular immunostaining showed that many of the TUNEL-positive nuclei were in macrophage-rich regions (Figure 3E), and quantification revealed that 78% of these apoptotic cells co-localized with macrophages in Chop+/+;Apoe−/− lesions and 72% in Chop−/−;Apoe−/− lesions (P>0.05). These combined mechanistic and lesional data are consistent with the hypothesis that decreased macrophage apoptosis conferred by CHOP deficiency contributes, at least in part, to a reduction in advanced lesional plaque necrosis.
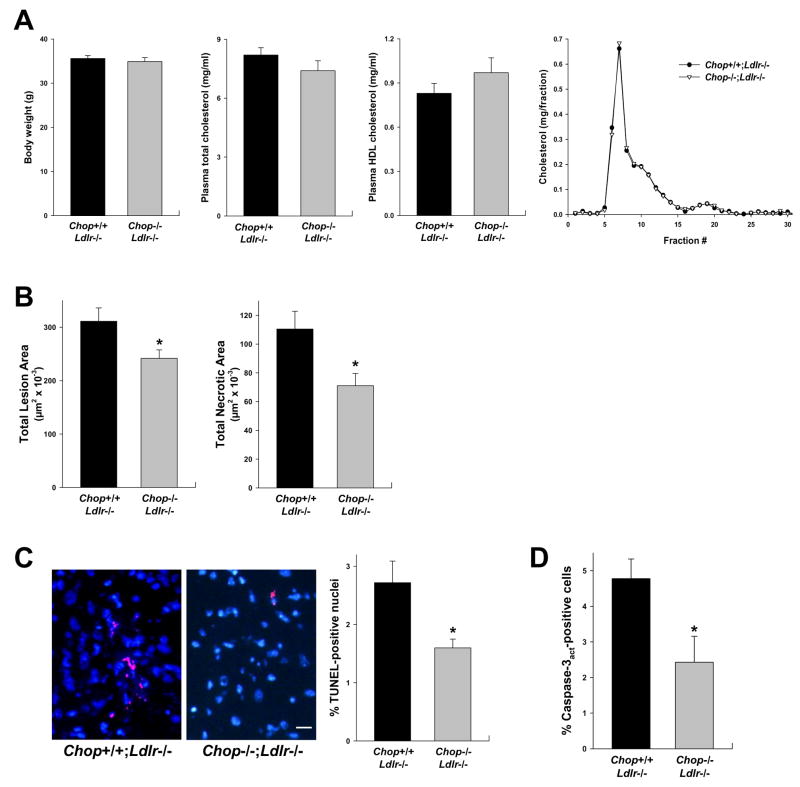
To test the effect of CHOP deficiency in a different model of advanced atherosclerosis, Chop+/+;Ldlr−/− and Chop−/−;Ldlr−/− mice were fed the Western diet for 12 weeks, and the aortic root was analyzed for overall lesion size, plaque necrosis, and TUNEL-positive cells. Fortunately, body weight, lipoprotein levels, and lipoprotein distribution did not differ between the Chop+/+ and Chop−/− Ldlr−/− mice (Figure 4A). Overall lesion area was modestly lower in the CHOP-deficient mice, but necrotic area was decreased more substantially (Figure 4B). TUNEL-positive nuclei and activated caspase-3-positive cells were significantly less abundant in the macrophage-rich intimal of the lesions of the CHOP-deficient mice (Figure 4C–D). Thus, CHOP deficiency in the two most widely studied models of murine atherosclerosis show strikingly similar effects: modest reduction in lesion area with more substantial reduction in both plaque necrosis and intimal apoptosis.
Figure 4. Reduction in Aortic Root Lesion Area, Necrotic Area, and Apoptosis in Ldlr−/−;Chop−/− Mice.
(A) Body weight, plasma total cholesterol, plasma HDL cholesterol, and FPLC plasma cholesterol profile of 12-wk Western diet-fed male Chop+/+;Ldlr−/− and Chop−/−;Ldlr−/− mice (n = 12 and 17, respectively). None of the differences between the two groups of mice in these parameters were statistically significant.
(B), Quantification of total aortic root lesion area and necrotic area of the mice described in A. *, P<0.05.
(C) Representative micrographs and quantification of TUNEL-positive signal (red) in nuclei (blue) of aortic root lesions from Chop−/−;Ldlr−/− lesions. Bar, 10 μm. *, P<0.05.
(D) Quantification of activated caspase-3 in aortic root lesions from Chop+/+;Ldlr−/− and Chop−/−;Ldlr−/ lesions. *, P<0.05.
DISCUSSION
In humans, advanced necrotic atheromata are responsible for acute atherothrombotic clinical events. While the mouse is not a good model for plaque disruption and acute atherothrombosis (Rosenfeld et al., 2002), it is a reasonable model for plaque necrosis (Tabas, 2008). Therefore, understanding processes that promote plaque necrosis in mice may give insight into plaque disruption and atherothrombosis in humans. The data herein with two separate models of advanced murine atherosclerosis, together with correlative findings with advanced human coronary atheromata (Gargalovic et al., 2006; Myoishi et al., 2007), suggest that the CHOP branch of the UPR contributes significantly to advanced atherosclerotic plaque progression. While it is theoretically possible that CHOP affects macrophage apoptosis plus a non-apoptotic process involved in plaque necrosis per se, we hypothesize that the decrease in plaque necrosis is a direct consequence of the decrease in apoptosis in the CHOP-deficient mice. This hypothesis is based upon the concept that in advanced atheromata, where efferocytosis appears to be defective, apoptosis becomes rate-limiting for post-apoptotic necrosis (Schrijvers et al., 2005; Tabas, 2005; Schrijvers et al., 2007).
The concept that CHOP plays an important role in macrophage apoptosis in advanced atherosclerotic lesions is now supported by several experimental findings: (a) the role of CHOP in apoptosis of ER-stressed cultured macrophages (Feng et al., 2003a) (Figure 3A); (b) the fact that CHOP is expressed in lesional macrophages (Feng et al., 2003a; Zhou et al., 2005; Myoishi et al., 2007); and, most importantly, (c) our data here showing decreased apoptosis in macrophage-rich lesional regions in both models of CHOP-deficient atherosclerosis-prone mice. Although the presence of ER stress in advanced atheromata is likely the major force behind CHOP expression, it is theoretically possible that non-ER stress activators of the phospho-eIF2α-ATF4-CHOP pathway, like amino acid starvation in deep regions of the intima, may contribute as well (Bruhat et al., 1997). Furthermore, while one might expect a somewhat greater decrease in lesional apoptotic cells based on our cell culture data, in situ measurements of apoptosis reflect the net effect of apoptosis and efferocytosis rather than cumulative apoptotic events over a long period of time, and so one can only obtain an estimate of trends in one direction or another from these measurements. Finally, studies to address the role of CHOP specifically in macrophages using bone marrow transplantation is not possible due to the fact that lethal irradiation fundamentally alters the relationship between CHOP and atherosclerosis (unpublished data). Thus, creation of Cre-Lox models will be necessary to address this important issue.
While CHOP expression is pronounced in advanced atherosclerotic lesions, it is also expressed in early lesions, albeit in lesser amounts (Zhou et al., 2005; Myoishi et al., 2007). Using LCM-RT-QPCR, we found that Chop mRNA was 2.9-fold less in lesions from Apoe−/− mice fed the Western diet for 4 wks vs. 10 wks (n = 3; P < 0.05). In the 4-wk-diet study, the Chop+/+;Apoe−/− and Chop−/−;Apoe−/− lesion areas were 162,961 ± 34,288 and 159,841 ± 11,861 μm2, respectively (n = 6 per group; P>0.05). These early lesions had only very small pockets of plaque necrosis, and the areas were not statistically different between the two groups (data not shown). Thus, CHOP does not appear to play a significant role in early atherogenesis. However, the finding that CHOP deficiency did have a modest effect on lesion area in the 10-wk-diet lesions (Figures 2A and 4B) raises the possibility that CHOP plays a role in one or more processes that promote the expansion of lesions after they have reached the relatively early foam cell stage.
The results of this study, together with recent findings showing expression of CHOP in advanced human atherosclerotic plaques (Gargalovic et al., 2006; Myoishi et al., 2007), suggest that the CHOP pathway may be a potential therapeutic target related to the formation of dangerous atheromata. In particular, it will be interesting to determine whether so-called chemical chaperones, which have be successfully used in other animal models of UPR-associated diseases (Ozcan et al., 2006), have a beneficial effect on advanced atherosclerotic lesion progression.
EXPERIMENTAL PROCEDURES
Materials and additional methods are available in Supplemental Experimental Procedures.
Mice and Diets
Chop−/− mice (Zinszner et al., 1998) on the C57BL6/J were bred onto the Apoe−/− and Ldlr−/− C57BL6/J backgrounds to generate Chop+/−;Apoe−/− and Chop+/−;Ldlr−/− breeding pairs. According to genotyping with a fixed whole genome panel of 768 murine single-nucleotide polymorphisms, Chop−/−;Apoe−/− mice were 97.3% C57BL6/J and Chop−/−;Ldlr−/− mice 99.1% C57BL6/J. Starting at 8 wks of age, male progeny from these breeding pairs were fed a high-fat (21.2%), high-cholesterol (0.2%) Western-type diet (Catalog # TD88137) from Harlan Teklad (Madison, WI) for 10 wks (Apoe−/− study) or 12 wks (Ldlr−/− study).
Macrophage Apoptosis and Efferocytosis Assays
Macrophage apoptosis was induced by one of three methods: (a) loading with lipoprotein-derived unesterified cholesterol by incubation with 100 μg/ml of acetyl-LDL plus 10 μg/ml of the ACAT inhibitor 58-035 (Yao and Tabas, 2000; Feng et al., 2003a); (b) incubation with 50 μM 7-ketocholesterol; or (c) incubation with the combination of 50 μg/ml oxidized LDL and 1 mM SIN-1. Apoptosis was measured by annexin V and propidium iodide staining as previously described (Yao and Tabas, 2000; Feng et al., 2003a). Efferocytosis was assessed as previously described (Li et al., 2006; Thorp et al., 2008). Briefly, macrophages were labeled for 1 h with the fluorescent green vital stain calcein AM and then rendered apoptotic by loading with lipoprotein-derived unesterified cholesterol (above). Based upon quantification of annexin V-positivity in parallel plates, the apoptotic macrophages were added to monolayers of unloaded macrophages (“efferocytes”) at a ratio of 1:1. After a 45 min of co-culture, the non-ingested apoptotic cells were removed and the ingested apoptotic cells quantified as described (Li et al., 2006).
Atherosclerotic Lesion Analysis
Mouse hearts were perfused in-situ with saline and removed. Aortic roots were fixed in 10% formalin, placed in biopsy cassettes, and processed in a Leica tissue-processing machine followed by embedment in paraffin blocks. Paraffin-embedded sections were cut serially at 6-μm intervals from the aortic sinus and mounted on slides. Prior to section staining, sections were deparaffinized in xylene and rehydrated in graded series of ethanol. For area measurements and morphometric analysis, the sections were stained with Harris’ hematoxylin and eosin. Total intimal lesional area was quantified by averaging six sections that were spaced 30 μm apart, starting from the base of the aortic root. Images were viewed and captured with a Nikon Labophot 2 microscope and analyzed using Image Pro Plus software. Apoptotic cells in lesions were detected by TUNEL (Tdt-mediated dUTP nick end labeling) after proteinase K treatment, using the in-situ cell death detection kit, TMR red (Roche). Nuclei were counterstained with Hoechst for 5 minutes, and the slides were viewed and imaged by fluorescent microscopy. Apoptotic cells in lesions were also detected by immunostaining for activated caspase-3 using rabbit anti-cleaved caspase-3 antibody #9661 from Cell Signaling Technology. The sections were then incubated for 30 min with horseradish peroxidase-avidin conjugate using ABC kit (Vector Laboratories), developed with substrate diaminobenzidine, and then counterstained with hematoxylin. Plaque necrosis was quantified by measuring the area of hematoxylin and eosin-negative acellular areas in the intima, as described previously (Feng et al., 2003b). For macrophage staining, the sections were heated in an EDTA solution and then endogenous peroxidase was blocked using hydrogen peroxide and methanol. Antigen blocking was performed using immunoglobulin from the species of the secondary antibody. The sections were then incubated with rabbit anti-macrophage antibody (AIA31240) from Accurate Chemical and Scientific Corporation, followed by incubation with biotinylated secondary antibody and streptavidin-horseradish peroxidase (HRP) and diaminobenzidine. Images were viewed and captured using a Nikon Labophot 2 microscope equipped with a Sony CCD-Iris/RGB color video camera attached to a computerized imaging system with Image Pro Plus 3.0 software.
Supplementary Material
Acknowledgments
This work was supported by NIH grants HL75662 and HL57560 to I.T.; DK47119 and ES08681 to D.R.; and an American Heart Association, Heritage Affiliate, post-doctoral fellowship grant to E.T.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brown BG, Zhao XQ, Sacco DE, Albers JJ. Atherosclerosis regression, plaque disruption, and cardiovascular events: a rationale for lipid lowering in coronary artery disease. Annu Rev Med. 1993;44:365–376. doi: 10.1146/annurev.me.44.020193.002053. [DOI] [PubMed] [Google Scholar]
- Bruhat A, Jousse C, Wang XZ, Ron D, Ferrara M, Fafournoux P. Amino acid limitation induces expression of CHOP, a CCAAT/enhancer binding protein-related gene, at both transcriptional and post-transcriptional levels. J Biol Chem. 1997;272:17588–17593. doi: 10.1074/jbc.272.28.17588. [DOI] [PubMed] [Google Scholar]
- Choy JC, Granville DJ, Hunt DW, McManus BM. Endothelial cell apoptosis: biochemical characteristics and potential implications for atherosclerosis. J Mol Cell Cardiol. 2001;33:1673–1690. doi: 10.1006/jmcc.2001.1419. [DOI] [PubMed] [Google Scholar]
- Clarke M, Bennett M. The emerging role of vascular smooth muscle cell apoptosis in atherosclerosis and plaque stability. Am J Nephrol. 2006;26:531–535. doi: 10.1159/000097815. [DOI] [PubMed] [Google Scholar]
- DeVries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, Flavell R, Tabas I. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickhout JG, Hossain GS, Pozza LM, Zhou J, Lhotak S, Austin RC. Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: implications in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:2623–2629. doi: 10.1161/01.ATV.0000189159.96900.d9. [DOI] [PubMed] [Google Scholar]
- Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003a;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- Feng B, Zhang D, Kuriakose G, Devlin CM, Kockx M, Tabas I. Niemann-Pick C heterozygosity confers resistance to lesional necrosis and macrophage apoptosis in murine atherosclerosis. Proc Natl Acad Sci U S A. 2003b;100:10423–10428. doi: 10.1073/pnas.1732494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Baruch-Oren T, Berliner JA, Kirchgessner TG, Lusis AJ. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:2490–2496. doi: 10.1161/01.ATV.0000242903.41158.a1. [DOI] [PubMed] [Google Scholar]
- Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Hossain GS, van Thienen JV, Werstuck GH, Zhou J, Sood SK, Dickhout JG, de Koning AB, Tang D, Wu D, Falk E, Poddar R, Jacobsen DW, Zhang K, Kaufman RJ, Austin RC. TDAG51 is induced by homocysteine, promotes detachment-mediated programmed cell death, and contributes to the cevelopment of atherosclerosis in hyperhomocysteinemia. J Biol Chem. 2003;278:30317–30327. doi: 10.1074/jbc.M212897200. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara K, Oyadomari S, Gotoh T, Kohsaka S, Nakayama H, Mori M. Induction of CHOP and apoptosis by nitric oxide in p53-deficient microglial cells. FEBS Lett. 2001;506:135–139. doi: 10.1016/s0014-5793(01)02898-8. [DOI] [PubMed] [Google Scholar]
- Kockx MM. Apoptosis in the atherosclerotic plaque: quantitative and qualitative aspects. Arterioscler Thromb Vasc Biol. 1998;18:1519–1522. doi: 10.1161/01.atv.18.10.1519. [DOI] [PubMed] [Google Scholar]
- Kolodgie FD, Virmani R, Burke AP, Farb A, Weber DK, Kutys R, Finn AV, Gold HK. Pathologic assessment of the vulnerable human coronary plaque. Heart. 2004;90:1385–1391. doi: 10.1136/hrt.2004.041798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gerbod-Giannone MC, Seitz H, Cui D, Thorp E, Tall AR, Matsushima GK, Tabas I. Cholesterol-induced apoptotic macrophages elicit an inflammatory response in phagocytes, which is partially attenuated by the Mer receptor. J Biol Chem. 2006;281:6707–6717. doi: 10.1074/jbc.M510579200. [DOI] [PubMed] [Google Scholar]
- Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, Sukhova GK, Lee RT. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol. 1996;7:330–335. doi: 10.1097/00041433-199610000-00012. [DOI] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM. The unfolding tale of the unfolded protein response. Cell. 2001;107:827–830. doi: 10.1016/s0092-8674(01)00623-7. [DOI] [PubMed] [Google Scholar]
- Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem. 2003;278:1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G, Bochaton-Piallat ML, Mochizuki N, Kitakaze M. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim C, O’dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, Ogier-Denis E. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002;110:1383–1388. doi: 10.1172/JCI16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld ME, Carson KG, Johnson JL, Williams H, Jackson CL, Schwartz SM. Animal models of spontaneous plaque rupture: the holy grail of experimental atherosclerosis research. Curr Atheroscler Rep. 2002;4:238–242. doi: 10.1007/s11883-002-0025-3. [DOI] [PubMed] [Google Scholar]
- Sanson M, Auge N, Vindis C, Muller C, Bando Y, Thiers JC, Marachet MA, Zarkovic K, Sawa Y, Salvayre R, Negre-Salvayre A. Oxidized low-density lipoproteins trigger endoplasmic reticulum stress in vascular cells: prevention by oxygen-regulated protein 150 expression. Circ Res. 2009;104:328–336. doi: 10.1161/CIRCRESAHA.108.183749. [DOI] [PubMed] [Google Scholar]
- Schrijvers DM, De Meyer GR, Herman AG, Martinet W. Phagocytosis in atherosclerosis: Molecular mechanisms and implications for plaque progression and stability. Cardiovasc Res. 2007;73:470–480. doi: 10.1016/j.cardiores.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- Tabas I. Mouse models of apoptosis and efferocytosis. Curr Drug Targets. 2008;8:1288–1296. doi: 10.2174/138945007783220623. [DOI] [PubMed] [Google Scholar]
- Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:1421–1428. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao PM, Tabas I. Free cholesterol loading of macrophages induces apoptosis involving the fas pathway. J Biol Chem. 2000;275:23807–23813. doi: 10.1074/jbc.M002087200. [DOI] [PubMed] [Google Scholar]
- Zhou J, Lhotak S, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2005;111:1814–1821. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.