Abstract
Cytokinesis is a dynamic and plastic process involving the co-ordinated regulation of many components. Accordingly, many proteins, including the putative scaffold protein anillin, localize to the cleavage furrow and are required for cytokinesis, but how they function together is poorly understood. Anillin can bind to numerous other furrow components, including F-actin, septins and myosin II, but its molecular functions are unclear. Recent data suggest that anillin participates in a previously unrecognized Rho-dependent pathway that can promote the association of anillin with the plasma membrane, septins, myosin II and microtubules. Studies using the inhibitor of F-actin assembly, Lat A (Latrunculin A), have revealed that these associations occur independently of F-actin; indeed they appear to be stabilized by the loss of F-actin. This pathway may explain previously described requirements for anillin in maintaining stable furrow positioning and for forming a stable midbody, and supports the notion that anillin is a central organizer at the hub of the cytokinetic machinery.
Keywords: contractile ring, cytokinesis, midbody ring, Rho, septin
Introduction
Cytokinesis involves sophisticated interplay between membranes, microtubules and the actin cytoskeleton [1–3]. It begins in anaphase, when the spindle specifies the cell equator, the location where an F-actin- and myosin II-dependent contractile ring assembles and pulls the plasma membrane into the cell interior. Elegant micromanipulation studies have helped set ground-rules for furrow positioning and have demonstrated how plastic and robust the process is [4]. Many genetic and molecular studies have helped identify important factors and the repertoire of essential cytokinesis genes is now largely known, with ~15–20 conserved proteins constituting the core cytokinetic machinery [1]. The challenge is now to understand how these proteins operate together in space and time to bring about cytokinesis. The anillin/Mid proteins belong to this core set of evolutionarily conserved proteins required for cytokinesis (see [5] for a review).
Anillin is a conserved cleavage furrow component that can interact with many other furrow components
First identified in Drosophila melanogaster, anillin localizes to the nucleus during interphase, to the cortex upon nuclear envelope breakdown, to the cleavage furrow in anaphase and to midbody rings during telophase and into the next cell cycle [6]. RNAi (RNA interference)-mediated depletion of anillin causes furrow instability in HeLa cells [7–9] and D. melanogaster S2 cells [10]. In these cells, furrows form normally at the cell equator but then oscillate back and forth across the equator, parallel to the spindle axis. Loss of anillin also promotes membrane blebbing and, in cases where a relatively stable furrow forms, a loss of stability of the midbody structure that forms after furrowing [11]. In the first division of Caenorhabditis elegans embryos, anillin depletion does not appear to prevent cytokinesis but causes a loss of furrow asymmetry [12].
Anillin was isolated as an F-actin binding and bundling protein from D. melanogaster embryo extracts [6]. Subsequent studies revealed interactions between human anillin and septins [13,14] and between Xenopus laevis anillin and the phosphorylated form of the MRLC (myosin II regulatory light chain) [7]. Anillin binds septins [13,14] and recruits them to the cleavage furrow [10,15,16].
In addition, anillin homologues have a C-terminal pleckstrin homology domain, consistent with a membrane-anchoring role. Many of the putative interactions of anillin appear to require distinct regions of the polypeptide that are generally evolutionarily conserved (see [5] for review). Thus anillin appears, at least in principle, capable of linking several key components of the cytokinetic apparatus. However, it has not been clear how anillin itself localizes to the cleavage furrow, or how significant its various interactions are to the success of cytokinesis.
Multiple inputs control the localization of anillin in anaphase
The assembly of the actomyosin contractile ring depends upon activation of Rho via its cytokinesis-specific guanine-nucleotide-exchange factor, Pebble (Drosophila)/ECT2 (mammals) [17,18]. In its active GTP-bound conformation, Rho binds to and activates formins such as Diaphanous [19], promoting F-actin assembly, and Rhokinase, activating myosin II [20–22].
Pebble also controls anillin accumulation at the equatorial cortex in anaphase (although not its cortical localization in metaphase), as evidenced by analysis of pebble mutant spermatocytes [23], embryos [24] and S2 cells depleted of Pebble by RNAi [10]. A priori, this could occur through indirect actions on F-actin and myosin II, but recent data indicate that multiple inputs are involved [10]. Using RNAi and/or the inhibitor of F-actin assembly in S2 cells expressing anillin-GFP, we found that anillin localization during anaphase occurs independent of myosin II and F-actin, albeit abnormally [10]. When myosin II function was perturbed, cortical anillin did not focus properly at the equator; and when F- actin was abolished with LatA (Latrunculin A), anillin changed localization from a diffuse cytoplasmic pattern to one where it localized near the equator in the form of linear, filamentous structures [10]. Importantly, these structures appeared at the normal time (during anaphase) and location (equatorial plasma membrane) of cytokinesis, indicating that anillin can respond to spatiotemporal signals even in the absence of F-actin. However, washout of the LatA after formation of the filamentous structures caused their coalescence into a focused equatorial band, indicating that an F-actin-dependent mechanism also contributes to equatorial anillin localization, perhaps via myosin II-driven cortical flow [10].
Anillin participates in a novel Rho-dependent pathway during cytokinesis
Further analysis of anillin behaviour in LatA-treated cells led to additional interesting observations [10]. In particular, Rho1 and the septin Peanut (septinPnut) co-localized with anillin in the filamentous structures that formed during anaphase. Testing the interdependencies of these proteins revealed that Rho1 was required for anillin recruitment and that anillin was required for septinPnut recruitment. SeptinPnut was itself required for the filamentous nature of the structures, but anillin was able to localize to the equatorial membrane independently of septinPnut. These data argue that a Rho1 → anillin → septin pathway exists and this can explain the actomyosin-independent targeting of anillin to the equatorial membrane observed in LatA-treated cells [10]. These results are also consistent with a direct interaction between human anillin and RhoA [25], recently reported to occur via the anillin homology domain of anillin [9]. Interestingly, anillin, septins and F-actin have previously been shown to assemble into multimeric structures independently of membranes and Rho [14], while our data suggest that anillin and septins can also form a different complex that is dependent upon Rho and the plasma membrane, but independent of F-actin.
Like anillin, myosin II is recruited to the equator in the presence of LatA [22], where it also forms filamentous structures that partially colocalize with anillin [10]. This filamentous appearance of myosin II (but not its localization) depends on anillin [10]. Thus, anillin contributes to myosin II behaviour in the absence of F-actin, while myosin II contributes to cortical anillin focusing when the F-actin cortex is intact.
Anillin as a linker between the contractile ring and spindle microtubules?
Another striking feature of the Rho1, anillin and septin-containing filamentous structures that form in LatA-treated cells is the fact that they form stable associations with the plus ends of equatorially positioned MTs (microtubules) [10]. Using cells with a compromised spindle assembly checkpoint, MT depolymerization revealed that MTs were dispensable for assembly of the structures. However, in such cells the filamentous structures appeared uniformly at the membrane rather than restricted to the equator. Thus MTs direct where the filamentous structures formed, presumably via equatorial stimulation spatially controlling Rho activation [26]. Once formed, the structures in turn associated with the MT plus ends. This association between anillin and spindle MTs could explain why loss of anillin leads to lateral instability of the furrow [7–10]: through a loss of anillin-dependent anchoring of the contractile apparatus to MTs at the spindle midzone. This putative anchoring role could occur via the RhoGAP, RacGAP50C, which was recently found to bind anillin and is a known component of the spindle midzone required for cytokinesis [27]. Collectively, these data also support the initial finding that anillin and septins were recovered from Drosophila embryo extracts by tubulin affinity chromatography [28].
Do events in LatA-treated cells reveal F-actin-dependent control of anillin and provide a snapshot of normally dynamic events?
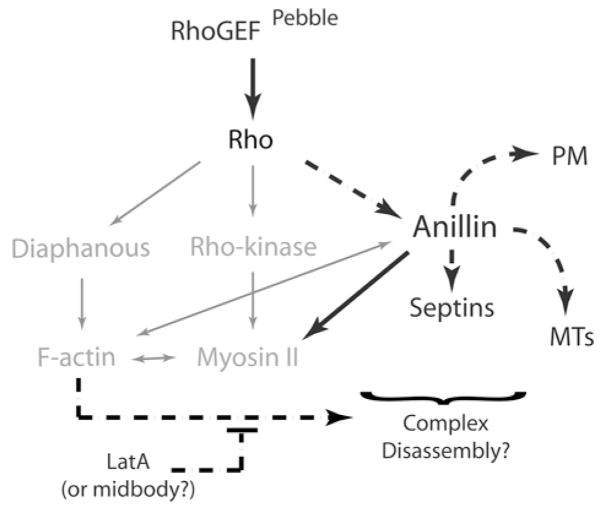
Cleavage furrows are highly dynamic and plastic, but the filamentous structures formed in LatA-treated cells were intrinsically stable. Although not apparent in unperturbed furrowing cells, they probably represent an intermediate step or an alternative outcome of normal events. For example, Rho, anillin and septins may form complexes that undergo dynamic cycles of assembly and disassembly, and LatA, in blocking F-actin polymerization, may prevent disassembly while allowing continued assembly (Figure 1). The cycling of events that this predicts would promote transient associations between furrow components (Rho, septins, phosphorylated MRLC), MT plus-ends and the plasma membrane.
Figure 1. Rho-dependent control of anillin.
In addition to well-characterized inputs into the contractile ring formation (grey), Rho controls anillin during cytokinesis via a novel pathway (black) that can promote the association of anillin with the plasma membrane (PM), MTs, septins and myosin II, independently of F-actin. LatA may block an F-actin dependent disassembly pathway and stabilize events in a manner mimicking midbody ring formation.
It is noteworthy that anillin-containing structures, remarkably similar to those formed in LatA-treated cells, appeared in the cleavage furrows of HeLa cells arrested with the myosin II inhibitor, blebbistatin [7]. Since blebbistatin also inhibits the turnover of F-actin [29,30], blebbistatin and LatA may affect anillin behaviour in similar ways, and may reflect both F-actin and myosin II-dependent control of anillin.
Events in LatA-treated cells may be physiologically relevant to another important aspect of cytokinesis. At the close of furrowing, the F-actin ring disassembles [31] and a stable midbody ring containing anillin and septins forms. The integrity of the resulting intercellular bridge becomes resistant to LatA treatment [11]. Thus LatA may trigger/allow events normally restricted to midbody biogenesis to occur more globally.
In summary, anillin appears to act as a pivotal organizer of the cytokinetic machinery, capable of linking F-actin, myosin II, Rho, septins, the plasma membrane and MTs. A true understanding of the mechanics of cytokinesis will require further dissection of this network of interactions.
Acknowledgments
This work was supported by postdoctoral fellowships from Susan G. Komen for the Cure, the Leukemia and Lymphoma Society and by NIH (National Institutes of Health) grant GM37193.
Abbreviations used
- LatA
Latrunculin A
- MRLC
myosin regulatory light chain
- MT
microtubule
- RNAi
RNA interference
References
- 1.Eggert US, Mitchison TJ, Field CM. Animal cytokinesis: from parts list to mechanisms. Annu Rev Biochem. 2006;75:543–566. doi: 10.1146/annurev.biochem.74.082803.133425. [DOI] [PubMed] [Google Scholar]
- 2.Barr FA, Gruneberg U. Cytokinesis: placing and making the final cut. Cell. 2007;131:847–860. doi: 10.1016/j.cell.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- 4.Rappaport R. Cytokinesis in Animal Cells. Cambridge University Press; Cambridge: 1996. [Google Scholar]
- 5.Paoletti A, Chang F. Anillins and mid proteins: organizers of the contractile ring during cytokinesis. In: Miki T, editor. Signal Transduction of Cell Division . Research Signpost; Kerala, India: 2005. pp. 39–51. [Google Scholar]
- 6.Field CM, Alberts BM. Anillin, a contractile ring protein that cycles from the nucleus to the cell cortex. J Cell Biol. 1995;131:165–178. doi: 10.1083/jcb.131.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Straight AF, Field CM, Mitchison TJ. Anillin binds nonmuscle myosin II and regulates the contractile ring. Mol Biol Cell. 2005;16:193–201. doi: 10.1091/mbc.E04-08-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao WM, Fang G. Anillin is a substrate of anaphase-promoting complex/cyclosome (APC/C) that controls spatial contractility of myosin during late cytokinesis. J Biol Chem. 2005;280:33516–33524. doi: 10.1074/jbc.M504657200. [DOI] [PubMed] [Google Scholar]
- 9.Piekny AJ, Glotzer M. Anillin is a scaffold protein that links RhoA, actin and myosin during cytokinesis. Curr Biol. 2008;18:30–36. doi: 10.1016/j.cub.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 10.Hickson GR, O’Farrell PH. Rho-dependent control of anillin behavior during cytokinesis. J Cell Biol. 2008;180:285–294. doi: 10.1083/jcb.200709005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Echard A, Hickson GR, Foley E, O’Farrell PH. Terminal cytokinesis events uncovered after an RNAi screen. Curr Biol. 2004;14:1685–1693. doi: 10.1016/j.cub.2004.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maddox AS, Lewellyn L, Desai A, Oegema K. Anillin and the septins promote asymmetric ingression of the cytokinetic furrow. Dev Cell. 2007;12:827–835. doi: 10.1016/j.devcel.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Oegema K, Savoian MS, Mitchison TJ, Field CM. Functional analysis of a human homologue of the Drosophila actin binding protein anillin suggests a role in cytokinesis. J Cell Biol. 2000;150:539–552. doi: 10.1083/jcb.150.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ. Self- and actin-templated assembly of mammalian septins. Dev Cell. 2002;3:791–802. doi: 10.1016/s1534-5807(02)00366-0. [DOI] [PubMed] [Google Scholar]
- 15.Maddox AS, Habermann B, Desai A, Oegema K. Distinct roles for two C. elegans anillins in the gonad and early embryo. Development. 2005;132:2837–2848. doi: 10.1242/dev.01828. [DOI] [PubMed] [Google Scholar]
- 16.Field CM, Coughlin M, Doberstein S, Marty T, Sullivan W. Characterization of anillin mutants reveals essential roles in septin localization and plasma membrane integrity. Development. 2005;132:2849–2860. doi: 10.1242/dev.01843. [DOI] [PubMed] [Google Scholar]
- 17.Hime G, Saint R. Zygotic expression of the pebble locus is required for cytokinesis during the postblastoderm mitoses of Drosophila. Development. 1992;114:165–171. doi: 10.1242/dev.114.1.165. [DOI] [PubMed] [Google Scholar]
- 18.Lehner CF. The pebble gene is required for cytokinesis in Drosophila. J Cell Sci. 1992;103:1021–1030. doi: 10.1242/jcs.103.4.1021. [DOI] [PubMed] [Google Scholar]
- 19.Castrillon DH, Wasserman SA. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development. 1994;120:3367–3377. doi: 10.1242/dev.120.12.3367. [DOI] [PubMed] [Google Scholar]
- 20.Matsumura F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 2005;15:371–377. doi: 10.1016/j.tcb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Hickson GR, Echard A, O’Farrell PH. Rho-kinase controls cell shape changes during cytokinesis. Curr Biol. 2006;16:359–370. doi: 10.1016/j.cub.2005.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dean SO, Rogers SL, Stuurman N, Vale RD, Spudich JA. Distinct pathways control recruitment and maintenance of myosin II at the cleavage furrow during cytokinesis. Proc Natl Acad Sci USA. 2005;102:13473–13478. doi: 10.1073/pnas.0506810102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giansanti MG, Bonaccorsi S, Gatti M. The role of anillin in meiotic cytokinesis of Drosophila males. J Cell Sci. 1999;112:2323–2334. doi: 10.1242/jcs.112.14.2323. [DOI] [PubMed] [Google Scholar]
- 24.Prokopenko SN, Brumby A, O’Keefe L, Prior L, He Y, Saint R, Bellen HJ. A putative exchange factor for Rho1 GTPase is required for initiation of cytokinesis in Drosophila. Genes Dev. 1999;13:2301–2314. doi: 10.1101/gad.13.17.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki C, Daigo Y, Ishikawa N, Kato T, Hayama S, Ito T, Tsuchiya E, Nakamura Y. ANLN plays a critical role in human lung carcinogenesis through the activation of RHOA and by involvement in the phosphoinositide 3-kinase/AKT pathway. Cancer Res. 2005;65:11314–11325. doi: 10.1158/0008-5472.CAN-05-1507. [DOI] [PubMed] [Google Scholar]
- 26.Bement WM, Benink HA, von Dassow G. A microtubule-dependent zone of active RhoA during cleavage plane specification. J Cell Biol. 2005;170:91–101. doi: 10.1083/jcb.200501131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregory SL, Ebrahimi S, Milverton J, Jones WH, Bejsovec A, Saint R. Cell division requires a direct link between microtubule-bound RacGAP and Anillin in the contractile ring. Curr Biol. 2008;18:25–29. doi: 10.1016/j.cub.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 28.Sisson JC, Field C, Ventura R, Royou A, Sullivan W. Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization. J Cell Biol. 2000;151:905–918. doi: 10.1083/jcb.151.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murthy K, Wadsworth P. Myosin II-dependent localization and dynamics of F-actin during cytokinesis. Curr Biol. 2005;15:724–731. doi: 10.1016/j.cub.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 30.Guha M, Zhou M, Wang YL. Cortical actin turnover during cytokinesis requires myosin II. Curr Biol. 2005;15:732–736. doi: 10.1016/j.cub.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 31.Schroeder TE. The contractile ring. II Determining its brief existence, volumetric changes, and vital role in cleaving Arbacia eggs. J Cell Biol. 1972;53:419–434. doi: 10.1083/jcb.53.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]