Traumatic brain injury (TBI) activates several protein kinase signaling pathways in the hippocampus that are critical for hippocampal-dependent memory formation. In particular, extracellular signal-regulated kinase (ERK), a protein kinase activated during and necessary for hippocampal-dependent learning, is transiently activated after TBI. However, TBI patients experience hippocampal-dependent cognitive deficits that occur for several months to years after the initial injury. Although basal activation levels of ERK return to sham levels within hours after TBI, we hypothesized that activation of ERK may be impaired after TBI. Adult male Sprague-Dawley rats received either sham surgery or moderate parasagittal fluid-percussion brain injury. At 2, 8, or 12 weeks after surgery, the ipsilateral hippocampi of sham surgery and TBI animals were sectioned into transverse slices. After 2 h of recovery in oxygenated artificial cerebrospinal fluid, the hippocampal slices were stimulated with glutamate or KCl depolarization, then analyzed by western blotting for phosphorylated, activated ERK and one of its downstream effectors, the transcription factor cAMP response element-binding protein (CREB). We found that activation of ERK (p<0.05) and CREB (p<0.05) after 30 s of glutamate stimulation or KCl depolarization was decreased in hippocampal slices from animals at 2, 8, or 12 weeks after TBI as compared to sham animals. Basal levels of phosphorylated or total ERK were not significantly altered at 2, 8, or 12 weeks after TBI, although basal levels of phosphorylated CREB were decreased 12 weeks post-trauma. These results suggest that TBI results in chronic signaling deficits through the ERK-CREB pathway in the hippocampus.
There are an estimated 3.17 million people living with long-term disabilities due to traumatic brain injury (TBI) in the United States [51]. These neurological deficits result in the inability to perform daily living activities, return to work, and participate as productive members within their communities. Longitudinal studies assessing outcome after moderate to severe TBI have consistently found that cognitive performance is impaired for months to years after the initial trauma which significantly impedes rehabilitation [37]. Although some recovery is typically observed in the first year after injury, this either slows or reverses in the following years [23, 46]. In particular, memory problems are almost always observed in human TBI patients, due either to direct effects on memory encoding or secondary effects on concentration and attention [1, 30]. This is reflected in experimental models of TBI, which have reported enduring impairments in learning and memory as well [10, 24, 35].
Functional imaging studies in humans have found that underlying the neurological deficits in cognition is atrophy of the hippocampus and white matter tract damage [7, 41, 47]. Furthermore, there is neuronal loss throughout the CA1, CA3 and CA4 subregions of the hippocampus [31]. Besides these histopathological changes, whether the remaining hippocampal neurons exhibit persistent deficits in cell signaling pathways required for learning and memory after TBI remains relatively unexplored.
Using the parasagittal fluid-percussion brain injury (FPI) as a clinically relevant model of TBI, we and others have found that TBI activates a series of biochemical cascades in the hippocampus; several of these signaling pathways are critical for the formation of hippocampal-dependent memories. The protein kinases extracellular signal-regulated kinase (ERK), calcium/calmodulin-dependent protein kinase II (CaMKII), protein kinase C (PKC), and calcium/calmodulin-dependent protein kinase IV (CaMKIV) are all activated after TBI [2, 15, 19, 21, 25, 34, 36, 50]. However, these changes are rapid and transient, occurring on the timescale of minutes to hours and reflect the glutamate excitotoxicity and widespread potassium depolarization that initially occurs as a consequence of the trauma [26]. Over the course of hours to days, activation of these signaling molecules returns to noninjured levels. Although the acute activation of protein kinases resolves between hours to days after TBI, we hypothesized that activation of these signaling pathways is chronically impaired after TBI.
All procedures with animals were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the University of Miami Institutional Animal Care and Use Committee. Six experimental groups were used in the following experiments: sham surgery animals (2 week survival n=4, 8 week survival n=4, 12 week survival n=4) and TBI animals (2 week survival n=6, 8 week survival n=6, 12 week survival n=6). Male Sprague Dawley rats (270–320 gm) were anesthetized with 3% halothane, 70% N2O, and 30% O2 and received a 4.8 mm craniotomy (3.8 mm posterior to bregma, 2.5 mm lateral to the midline) to secure a plastic tube (3.5 mm inside diameter) over the right parietal cortex with cyanoacrylate and dental cement. Twenty-four h after the craniotomy, the animals were re-anesthetized, intubated and mechanically ventilated with 0.5–1% halothane, 70% N2O, and 30% O2. Pancuronium bromide (0.5 mg/kg, intravenously) was administered to immobilize the rats. Arterial blood pressure, blood gases, and blood pH were monitored for 30 min prior to and up to 30 min after TBI to maintain physiological ranges (blood pH: 7.35–7.45, pCO2: 35–40 mm Hg, pO2: 105–140 mm Hg). Brain and body temperature were monitored with thermistors placed in the left temporalis muscle and rectum and maintained at 36.5–37.0°C with self-adjusting warming lamps. The animals received a moderate (2.0 ± 0.2 atmospheres) fluid-percussion pulse or sham treatment.
At 2, 8, or 12 weeks of recovery after surgery, animals were anesthetized with 3% halothane, 70% N2O, and 30% O2 for 5 min, and then decapitated. The ipsilateral hippocampus was rapidly dissected and transversely sectioned into slices 400 μm thick at 4°C using a vibratome in artificial cerebrospinal fluid (aCSF, in mM: 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 10 D-glucose, 2 CaCl2, and 1 MgCl2, saturated with 95% O2/5% CO2). An average of 8–12 hippocampal slices were obtained from each sham and TBI animal. After 1 h of recovery at room temperature, slices were equilibrated in a submersion chamber at 31.5°C for 1 h. Hippocampal slices (n=2–4 for each treatment condition from each animal) were pharmacologically stimulated with glutamate (200 μM) or high KCl depolarization (90 mM) for 30 s in isotonic aCSF. After stimulation, the slices were transferred to a nylon screen and frozen on liquid nitrogen. Two hippocampal slices from each treatment condition (control, high KCl depolarization, or glutamate) were pooled and sonicated in 200 μl of fractionation homogenization buffer (15 mM Tris pH 7.6, 0.25 M sucrose, 1 mM MgCl2, 1 mM EGTA, 1 mM DTT, 1.25 μg/ml pepstatin A, 10 μg/ml leupeptin, 25 μg/ml aprotinin, 0.5 mM PMSF, 0.1 mM Na3VO4, 50 mM NaF, 2 mM Na4P2O7, and 1X phosphatase inhibitor cocktail set II, Calbiochem, San Diego, CA, USA). Samples were electrophoresed and western blotted for anti-phospho-ERK2 Thr202/Tyr204 (1:3000, Cell Signaling Technology, Danvers, MA, USA), anti-total ERK2 (1:5000, Cell Signaling Technology), anti-phospho-CREB Ser133 (1:1000, Cell Signaling Technology), anti-total CREB (1:1000, Cell Signaling Technology), and β-actin (1:10,000, Sigma-Aldrich, St. Louis, MO, USA). Epitopes were visualized with HRP-conjugated secondary antibodies (1:2000, Cell Signaling Technology) using enhanced chemiluminescence and developed on film (Phenix x-ray film BX; Phenix Research Products, Hayward, CA, USA). Films were developed to be in a linear range and densitized using ImageJ 1.38x (National Institutes of Health, USA). To analyze changes in phospho-ERK and phospho-CREB after glutamate or high KCl stimulation, levels of phospho-proteins from stimulated slices were normalized to levels phospho-proteins from non-stimulated slices within each animal. Thus, non-stimulated phosphorylation levels from both sham and TBI hippocampal slices were averaged to 1.0 to directly compare the changes in phosphorylation after high KCl depolarization or glutamate stimulation. To analyze changes in basal levels of phospho-ERK and phospho-CREB, levels of phospho-proteins from all TBI animals were normalized to levels of phospho-proteins from all sham surgery animals within each survival time point.
All of the data presented are mean ± SEM. Statistical analysis was performed with GraphPad Prism 5.02 (GraphPad Software, Inc., La Jolla, CA, USA). The differences among multiple groups were analyzed with a two-way analysis of variance (ANOVA) with significance set at p<0.05. Post-hoc comparisons were made using Tukey t-tests.
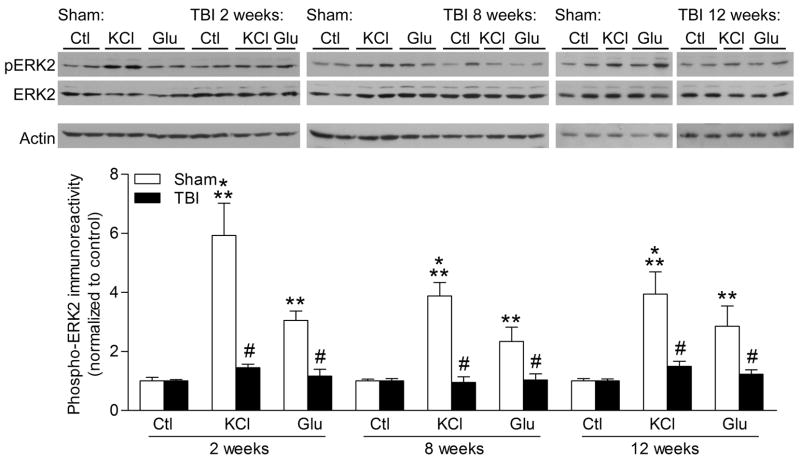
To investigate changes in protein kinase signaling after TBI, we dissected the ipsilateral, injured hippocampus from sham and TBI animals, and generated hippocampal slices. An average of 8–12 hippocampal slices were generated from each animal. After 2 h of recovery in oxygenated aCSF, we measured levels of phosphorylated, activated ERK in hippocampal slices after brief high KCl depolarization (90 mM) or glutamate stimulation (200 μM) for 30 s. These stimulation paradigms trigger a transient depolarization and brief calcium influx into hippocampal neurons mediated through voltage-gated calcium channels and glutamate receptors [5, 12, 13]. We found that there was a significant increase in ERK activation with KCl treatment or glutamate treatment in hippocampal slices from sham surgery animals, but not from TBI animals at 2 weeks after surgery (Fig. 1) [injury: F(1,46) = 33.87, p<0.0001, slice treatment F(2,46) = 18.51, p<0.0001]. This was seen as well at 8 weeks after surgery [injury: F(1,45) = 40.63, p<0.0001, slice treatment F(2,45) = 14.40, p<0.0001] and 12 weeks post-injury, although there was some variability in the response to stimulation in TBI hippocampal slices [injury: F(1,39) = 17.15, p=0.0002, slice treatment F(2,39) = 9.27, p=0.0005].
Fig. 1.
TBI results in deficits in ERK activation. Hippocampal slices at 2, 8 or 12 weeks after sham surgery (n=4 animals/time point) or TBI surgery (n=6 animals/time point) were stimulated with KCl (90 mM, n=6–10 slices/time point), glutamate (Glu, 200 μM, or vehicle (Ctl, n=6–8 slices/time point), then analyzed by western blotting for changes in phospho-ERK2 (pERK2). Phosphorylated ERK2 significantly increased in hippocampal slices stimulated with high KCl or glutamate from sham animals, but not in hippocampal slices from TBI animals. Values are mean ± SEM. **p<0.01, ***p<0.001, stimulated slices compared to non-stimulated slices, #p<0.05 stimulated slices from sham animals compared to stimulated slices from TBI animals
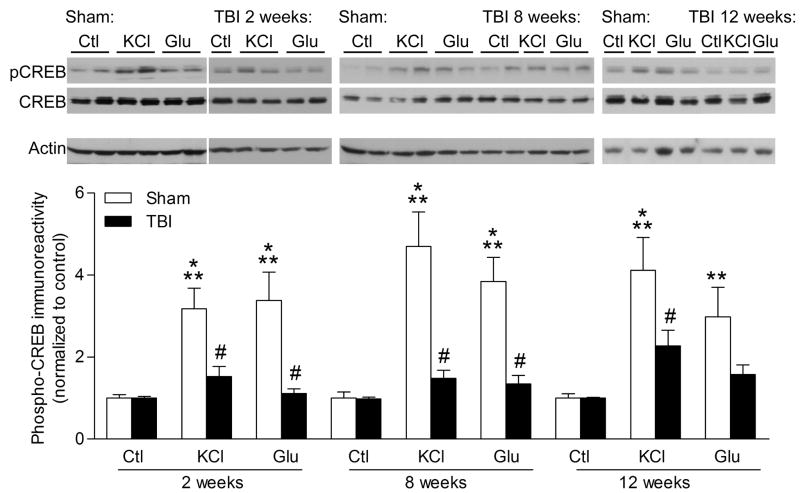
A downstream effector of ERK during hippocampal-dependent learning is the transcription factor CREB [42, 44, 48]. After stimulation with high KCl depolarization or glutamate (200 μM) for 30 s, hippocampal slices were analyzed by western blotting for changes in CREB phosphorylation (Fig. 2). We found that activation of CREB was reduced in hippocampal slices from TBI animals as compared to slices from sham animals at 2 weeks [injury: F(1,46) = 21.34, p<0.0001, slice treatment F(2,46) = 9.30, p=0.0004], 8 weeks [injury: F(1,46) = 34.66, p<0.0001, slice treatment F(2,46) = 15.90, p<0.0001], and 12 weeks post-injury [injury: F(1,38) = 9.67, p=0.0035, slice treatment F(2,38) = 13.47, p<0.0001].
Fig. 2.
Impairments in CREB activation after TBI. Hippocampal slices at 2, 8 or 12 weeks after sham surgery (n=4 animals/time point) or TBI (n=6 animals/time point) were stimulated with high KCl (90 mM, 30 s, n=6–10 slices/time point), glutamate (Glu, 200 μM, 30 s, n=7–9 slices/time point), or vehicle (Ctl, n=6–8 slices/time point), then analyzed by western blotting for changes in phospho-CREB (pCREB). Levels of phospho-CREB were significantly increased in stimulated hippocampal slices from sham animals, but not from TBI animals. Values are mean ± SEM. **p<0.01, ***p<0.001, non-stimulated slices compared to stimulated slices; #p<0.05 stimulated slices from sham animals compared to stimulated slices from TBI animals
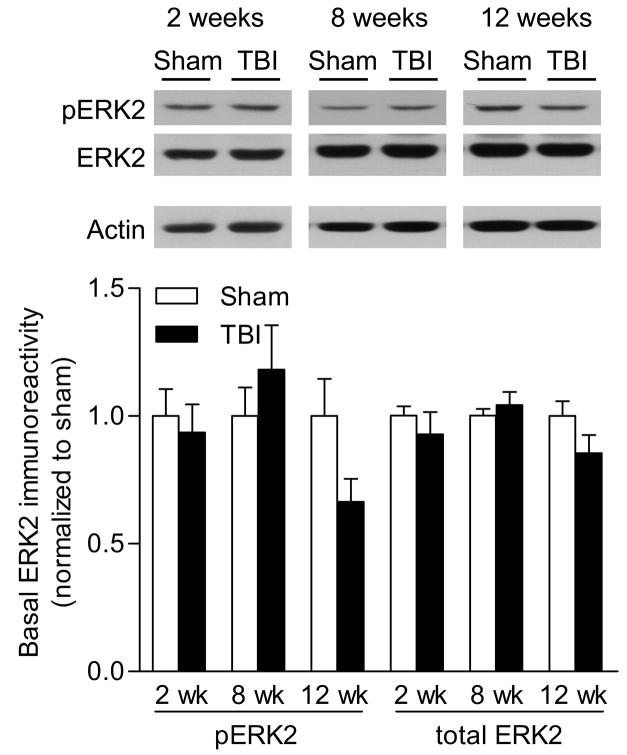
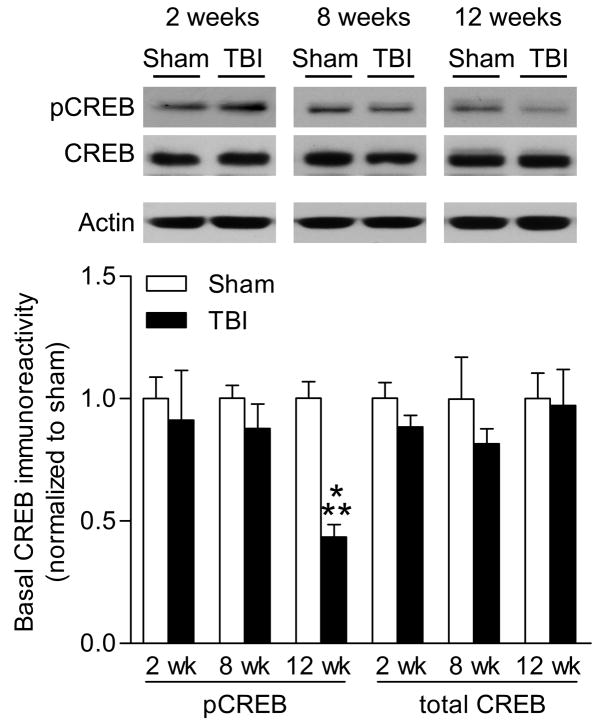
To evaluate whether basal levels of ERK or CREB could account for the deficits in activation, we directly compared basal levels of phosphorylated and total ERK and CREB from non-stimulated hippocampal slices of sham surgery or TBI animals. To control for changes in total protein levels, we normalized the changes in phospho- and total ERK and CREB levels to β-actin levels within each sample. Basal levels of ERK activation or total levels of ERK in hippocampal slices were not significantly different between sham and TBI animals at 2 weeks or 8 weeks post-TBI (Fig. 3), although a nonsignificant trend towards a decrease in ERK phosphorylation was observed at 12 weeks post-TBI [injury: F(1,33) = 0.41, p=0.5256, recovery time F(2,33) = 1.84, p=0.1740]. Basal levels of CREB activation or total CREB levels were not significantly decreased at 2 or 8 weeks post-injury (Fig. 4). At 12 weeks after injury, there was a significant decrease in basal phospho-CREB levels in hippocampal slices from TBI animals as compared to sham animals [injury: F(1,31) = 11.48, p=0.0019, recovery time F(2,31) = 4.16, p=0.0250, interaction: : F(2,31) = 4.18, p=0.0246].
Fig. 3.
Basal levels of phosphorylated ERK at chronic time points after TBI. Basal levels of phospho-ERK2 and total ERK2 levels were analyzed in non-stimulated hippocampal slices by western blotting at 2 weeks (n=8 sham slices, n=4 TBI slices), 8 weeks (n=7 sham slices, n=7 TBI slices) and 12 weeks (n=8 sham slices, n=5 TBI slices) after surgery. Basal levels of phospho- and total ERK2 were not significantly different between TBI and sham surgery animals. Values are mean ± SEM.
Fig. 4.
Reduced levels of phospho-CREB after TBI. Basal levels of phospho-CREB and total CREB were analyzed by western blotting at 2 weeks (n=8 sham slices, n=4 TBI slices), 8 weeks (n=7 sham slices, n=5 TBI slices) and 12 weeks (n=8 sham slices, n=5 TBI slices) after surgery. Basal phospho-CREB levels significantly decreased at 12 weeks after TBI in non-stimulated hippocampal slices as compared to non-stimulated levels in sham hippocampal slices. Values are mean ± SEM. **p<0.01, sham animals compared to TBI animals
After TBI, there are both acute and chronic behavioral deficits [23, 30, 46]. With the fluid-percussion brain injury model, we can reliably observe deficits in hippocampal-dependent learning that persist from days to months after trauma [10, 11, 22, 29, 35]. There are a number of mechanisms that may contribute to hippocampal-dependent learning deficits. Accordingly, there is hippocampal atrophy, neuronal and synaptic loss in select regions of the hippocampus, atrophy of white matter tracts, as well as changes in inputs from other brain regions into the hippocampus [8, 9, 17, 20, 38, 49]. Our results suggest that another mechanism that may underlie chronic deficits in learning after TBI is the inability to activate the ERK-CREB signaling pathway in remaining hippocampal neurons.
Although we normalized the changes in phospho-ERK and phospho-CREB to total protein levels, and total ERK, total CREB or β-actin levels were not significantly different between sham and TBI injured animals, the number of synapses and neurons activated in a hippocampal slice from sham animals is likely to be much greater than from TBI animals [8, 9, 20, 39, 49]. Thus, the deficits in ERK and CREB activation may be due to neuronal and synaptic loss which results in atrophy of the hippocampus in the weeks following injury. Whether deficits in this signaling pathway are seen in mild fluid-percussion brain injury where there is no significant cell loss or atrophy of the hippocampus remains to be determined [21, 29]. Evaluation of injury severity may differentiate the contribution of neuronal loss to the misregulation of the ERK-CREB signaling pathway after TBI and is an important area of future investigation. Furthermore, there is select neuronal loss in the CA3 region of the hippocampus and dentate hilar region as compared to other subregions of the hippocampus with the parasagittal fluid-percussion brain injury model [9, 20, 49]. Whether there are greater deficits in ERK and CREB activation in these areas or whether this occurs throughout all subregions of the hippocampus remains to be explored [19].
TBI results in significant impairments in the ability to maintain calcium homeostasis [33]. Accordingly, although glutamate elicits similar levels of peak calcium entry into CA3 hippocampal neurons at 4 weeks after TBI, the restoration of basal calcium levels in CA3 neurons from TBI animals is impaired [16, 45]. This suggests that the molecular mechanisms that maintain homeostatic calcium levels are chronically altered after TBI. However, since the peak calcium influx was comparable in cultured neurons from sham and TBI animals and we assessed ERK and CREB activation during the peak calcium influx, it is unlikely that the deficits we observed are due to changes in total calcium levels during the glutamate pulse or potassium depolarization.
Given that we observed deficits in ERK activation with glutamate or potassium depolarization, the synaptic mechanisms that may underlie the deficits in ERK activation may involve changes in glutamate receptors and/or voltage-gated calcium channels. There are transient, biphasic changes in NMDA receptor levels after TBI, but by 14 days post-injury, these changes have returned to sham levels [6, 27, 32]. Levels of the GluR1 subunit of the AMPA receptor are increased 3 days post-TBI [2]; however whether these changes are persistent or if there are any differences in voltage-gated calcium channel expression at chronic time points after brain trauma remains unknown. Underlying these biochemical changes are deficits in hippocampal synaptic plasticity. Previous studies have found that there are significant impairments in long-term potentiation (LTP) after TBI, a physiological correlate of learning and memory formation [14, 38, 40, 43]. Inhibition of ERK activation blocks maintenance of hippocampal LTP [4, 18], which mimics the deficits in hippocampal LTP seen after TBI. Thus, one potential mechanism for the deficit in hippocampal LTP after TBI may be the inability of the glutamatergic synapse to activate ERK and trigger CREB-mediated gene expression.
ERK is activated by a canonical pathway that involves B-Raf activation of mitogen-activated protein kinase kinase (MEK) which then phosphorylates and activates ERK. Another possible mechanism for the impairments in ERK activation is the inability to stimulate one or several upstream activators of ERK. However, a causal link between the deficit in ERK and CREB activation after TBI remains to be established. CREB is phosphorylated by a number of protein kinases downstream of ERK, such as p90 ribosomal S6 kinase, mitogen- and stress-activated kinase 1/2, but is also directly phosphorylated by CaMKIV and protein kinase A (PKA) [42]. We have previously demonstrated that cAMP levels and PKA activation decrease from 15 min to 24 h after TBI in the injured hippocampus [3]. Although cAMP levels return to non-injured levels at 48 h after TBI, PKA activation is still depressed at 48 h post-TBI. Furthermore, whether there is a decrease in cAMP levels or PKA activation in the weeks after brain injury is unknown. A decrease in either cAMP levels or PKA activation could underlie the deficits in ERK and/or CREB activation. Thus, the deficits in CREB activation may have occurred through chronic alterations in one or several protein kinase pathways. An alternative mechanism that could account for the deficits in ERK and CREB activation is increased phosphatase activity. Although persistent alterations in phosphatases that dephosphorylate ERK have not yet been examined after TBI, calcineurin activity is increased 2–3 weeks after TBI [28].
Understanding the molecular mechanisms that underlie enduring deficits in learning and memory after TBI may open new therapeutic avenues to pursue to improve cognition after TBI. In the current study, we have identified deficits in the ability of the hippocampus to activate signaling through ERK and CREB after brain trauma. Development of pharmacological strategies to stimulate signaling through ERK and CREB may thus be a therapeutically feasible strategy in the rehabilitation repertoire for TBI patients.
Acknowledgments
This work was supported by USAMRMC PR054538 and NIH grant NS056072.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ashman TA, Cantor JB, Gordon WA, Sacks A, Spielman L, Egan M, Hibbard MR. A comparison of cognitive functioning in older adults with and without traumatic brain injury. J Head Trauma Rehabil. 2008;23:139–148. doi: 10.1097/01.HTR.0000319930.69343.64. [DOI] [PubMed] [Google Scholar]
- 2.Atkins CM, Chen S, Alonso OF, Dietrich WD, Hu BR. Activation of calcium/calmodulin-dependent protein kinases after traumatic brain injury. J Cereb Blood Flow Metab. 2006;26:1507–1518. doi: 10.1038/sj.jcbfm.9600301. [DOI] [PubMed] [Google Scholar]
- 3.Atkins CM, Oliva AA, Jr, Alonso OF, Pearse DD, Bramlett HM, Dietrich WD. Modulation of the cAMP signaling pathway after traumatic brain injury. Exp Neurol. 2007;208:145–158. doi: 10.1016/j.expneurol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- 5.Baron C, Benes C, Van Tan H, Fagard R, Roisin MP. Potassium chloride pulse enhances mitogen-activated protein kinase activity in rat hippocampal slices. J Neurochem. 1996;66:1005–1010. doi: 10.1046/j.1471-4159.1996.66031005.x. [DOI] [PubMed] [Google Scholar]
- 6.Biegon A, Fry PA, Paden CM, Alexandrovich A, Tsenter J, Shohami E. Dynamic changes in N-methyl-D-aspartate receptors after closed head injury in mice: Implications for treatment of neurological and cognitive deficits. Proc Natl Acad Sci USA. 2004;101:5117–5122. doi: 10.1073/pnas.0305741101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bigler ED, Anderson CV, Blatter DD. Temporal lobe morphology in normal aging and traumatic brain injury. Am J Neuroradiol. 2002;23:255–266. [PMC free article] [PubMed] [Google Scholar]
- 8.Bramlett HM, Dietrich WD. Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol (Berl) 2002;103:607–614. doi: 10.1007/s00401-001-0510-8. [DOI] [PubMed] [Google Scholar]
- 9.Bramlett HM, Dietrich WD, Green EJ, Busto R. Chronic histopathological consequences of fluid-percussion brain injury in rats: Effects of post-traumatic hypothermia. Acta Neuropathol (Berl) 1997;93:190–199. doi: 10.1007/s004010050602. [DOI] [PubMed] [Google Scholar]
- 10.Bramlett HM, Green EJ, Dietrich WD. Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Res. 1997;762:195–202. doi: 10.1016/s0006-8993(97)00387-9. [DOI] [PubMed] [Google Scholar]
- 11.Carbonell WS, Maris DO, McCall T, Grady MS. Adaptation of the fluid percussion injury model to the mouse. J Neurotrauma. 1998;15:217–229. doi: 10.1089/neu.1998.15.217. [DOI] [PubMed] [Google Scholar]
- 12.Cole AE, ffench-Mullen JM, Fisher RS. Fade of the response to prolonged glutamate application in the rat hippocampal slice. Synapse. 1989;4:11–18. doi: 10.1002/syn.890040103. [DOI] [PubMed] [Google Scholar]
- 13.Collins DR, Davies SN. Potentiation of synaptic transmission in the rat hippocampal slice by exogenous L-glutamate and selective L-glutamate receptor subtype agonists. Neuropharm. 1994;33:1055–1063. doi: 10.1016/0028-3908(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 14.D’Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Selective loss of hippocampal long-term potentiation, but not depression, following fluid percussion injury. Brain Res. 1998;786:64–79. doi: 10.1016/s0006-8993(97)01412-1. [DOI] [PubMed] [Google Scholar]
- 15.Dash PK, Mach SA, Moore AN. The role of extracellular signal-regulated kinase in cognitive and motor deficits following experimental traumatic brain injury. Neuroscience. 2002;114:755–767. doi: 10.1016/s0306-4522(02)00277-4. [DOI] [PubMed] [Google Scholar]
- 16.Deshpande LS, Sun DA, Sombati S, Baranova A, Wilson MS, Attkisson E, Hamm RJ, DeLorenzo RJ. Alterations in neuronal calcium levels are associated with cognitive deficits after traumatic brain injury. Neurosci Lett. 2008;441:115–119. doi: 10.1016/j.neulet.2008.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon CE, Bao J, Johnson KM, Yang K, Whitson J, Clifton GL, Hayes RL. Basal and scopolamine-evoked release of hippocampal acetylcholine following traumatic brain injury in rats. Neurosci Lett. 1995;198:111–114. doi: 10.1016/0304-3940(95)11979-7. [DOI] [PubMed] [Google Scholar]
- 18.English JD, Sweatt JD. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J Biol Chem. 1997;272:19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- 19.Folkerts MM, Parks EA, Dedman JR, Kaetzel MA, Lyeth BG, Berman RF. Phosphorylation of calcium calmodulin-dependent protein kinase II following lateral fluid percussion brain injury in rats. J Neurotrauma. 2007;24:638–650. doi: 10.1089/neu.2006.0188. [DOI] [PubMed] [Google Scholar]
- 20.Grady MS, Charleston JS, Maris D, Witgen BM, Lifshitz J. Neuronal and glial cell number in the hippocampus after experimental traumatic brain injury: Analysis by stereological estimation. J Neurotrauma. 2003;20:929–941. doi: 10.1089/089771503770195786. [DOI] [PubMed] [Google Scholar]
- 21.Griesbach GS, Gomez-Pinilla F, Hovda DA. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J Neurotrauma. 2007;24:1161–1171. doi: 10.1089/neu.2006.0255. [DOI] [PubMed] [Google Scholar]
- 22.Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: Brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125:129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 23.Himanen L, Portin R, Isoniemi H, Helenius H, Kurki T, Tenovuo O. Longitudinal cognitive changes in traumatic brain injury: a 30-year follow-up study. Neurology. 2006;66:187–192. doi: 10.1212/01.wnl.0000194264.60150.d3. [DOI] [PubMed] [Google Scholar]
- 24.Hoskison MM, Moore AN, Hu B, Orsi S, Kobori N, Dash PK. Persistent working memory dysfunction following traumatic brain injury: Evidence for a time-dependent mechanism. Neuroscience. 2009;159:483–491. doi: 10.1016/j.neuroscience.2008.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu B, Liu C, Bramlett H, Sick TJ, Alonso OF, Chen S, Dietrich WD. Changes in trkB-ERK1/2-CREB/Elk-1 pathways in hippocampal mossy fiber organization after traumatic brain injury. J Cereb Blood Flow Metab. 2004;24:934–943. doi: 10.1097/01.WCB.0000125888.56462.A1. [DOI] [PubMed] [Google Scholar]
- 26.Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- 27.Kumar A, Zou L, Yuan X, Long Y, Yang K. N-methyl-D-aspartate receptors: transient loss of NR1/NR2A/NR2B subunits after traumatic brain injury in a rodent model. J Neurosci Res. 2002;67:781–786. doi: 10.1002/jnr.10181. [DOI] [PubMed] [Google Scholar]
- 28.Kurz JE, Parsons JT, Rana A, Gibson CJ, Hamm RJ, Churn SB. A significant increase in both basal and maximal calcineurin activity following fluid percussion injury in the rat. J Neurotrauma. 2005;22:476–490. doi: 10.1089/neu.2005.22.476. [DOI] [PubMed] [Google Scholar]
- 29.Lyeth BG, Jenkins LW, Hamm RJ, Dixon CE, Phillips LL, Clifton GL, Young HF, Hayes RL. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 1990;526:249–258. doi: 10.1016/0006-8993(90)91229-a. [DOI] [PubMed] [Google Scholar]
- 30.Mathias JL, Mansfield KM. Prospective and declarative memory problems following moderate and severe traumatic brain injury. Brain Inj. 2005;19:271–282. doi: 10.1080/02699050400005028. [DOI] [PubMed] [Google Scholar]
- 31.Maxwell WL, Dhillon K, Harper L, Espin J, MacIntosh TK, Smith DH, Graham DI. There is differential loss of pyramidal cells from the human hippocampus with survival after blunt head injury. J Neuropathol Exp Neurol. 2003;62:272–279. doi: 10.1093/jnen/62.3.272. [DOI] [PubMed] [Google Scholar]
- 32.Osteen CL, Giza CC, Hovda DA. Injury-induced alterations in N-methyl-D-aspartate receptor subunit composition contribute to prolonged 45calcium accumulation following lateral fluid percussion. Neuroscience. 2004;128:305–322. doi: 10.1016/j.neuroscience.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 33.Osteen CL, Moore AH, Prins ML, Hovda DA. Age-dependency of 45calcium accumulation following lateral fluid percussion: acute and delayed patterns. J Neurotrauma. 2001;18:141–162. doi: 10.1089/08977150150502587. [DOI] [PubMed] [Google Scholar]
- 34.Otani N, Nawashiro H, Fukui S, Nomura N, Yano A, Miyazawa T, Shima K. Differential activation of mitogen-activated protein kinase pathways after traumatic brain injury in the rat hippocampus. J Cereb Blood Flow Metab. 2002;22:327–334. doi: 10.1097/00004647-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Pierce JE, Smith DH, Trojanowski JQ, McIntosh TK. Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience. 1998;87:359–369. doi: 10.1016/s0306-4522(98)00142-0. [DOI] [PubMed] [Google Scholar]
- 36.Raghupathi R, Muir JK, Fulp CT, Pittman RN, McIntosh TK. Acute activation of mitogen-activated protein kinases following traumatic brain injury in the rat: Implications for posttraumatic cell death. Exp Neurol. 2003;183:438–448. doi: 10.1016/s0014-4886(03)00166-3. [DOI] [PubMed] [Google Scholar]
- 37.Ruttan L, Martin K, Liu A, Colella B, Green RE. Long-term cognitive outcome in moderate to severe traumatic brain injury: a meta-analysis examining timed and untimed tests at 1 and 4.5 or more years after injury. Arch Phys Med Rehabil. 2008;89:S69–76. doi: 10.1016/j.apmr.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Sanders MJ, Sick TJ, Perez-Pinzon MA, Dietrich WD, Green EJ. Chronic failure in the maintenance of long-term potentiation following fluid percussion injury in the rat. Brain Res. 2000;861:69–76. doi: 10.1016/s0006-8993(00)01986-7. [DOI] [PubMed] [Google Scholar]
- 39.Scheff SW, Price DA, Hicks RR, Baldwin SA, Robinson S, Brackney C. Synaptogenesis in the hippocampal CA1 field following traumatic brain injury. J Neurotrauma. 2005;22:719–732. doi: 10.1089/neu.2005.22.719. [DOI] [PubMed] [Google Scholar]
- 40.Schwarzbach E, Bonislawski DP, Xiong G, Cohen AS. Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. Hippocampus. 2006 doi: 10.1002/hipo.20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serra-Grabulosa JM, Junque C, Verger K, Salgado-Pineda P, Maneru C, Mercader JM. Cerebral correlates of declarative memory dysfunctions in early traumatic brain injury. J Neurol Neurosurg Psychiatry. 2005;76:129–131. doi: 10.1136/jnnp.2004.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaywitz AJ, Greenberg ME. CREB: A stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 43.Sick TJ, Perez-Pinzon MA, Feng ZZ. Impaired expression of long-term potentiation in hippocampal slices 4 and 48 h following mild fluid-percussion brain injury in vivo. Brain Res. 1998;785:287–292. doi: 10.1016/s0006-8993(97)01418-2. [DOI] [PubMed] [Google Scholar]
- 44.Sindreu CB, Scheiner ZS, Storm DR. Ca2+-stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron. 2007;53:79–89. doi: 10.1016/j.neuron.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun DA, Deshpande LS, Sombati S, Baranova A, Wilson MS, Hamm RJ, DeLorenzo RJ. Traumatic brain injury causes a long-lasting calcium (Ca2+)-plateau of elevated intracellular Ca levels and altered Ca2+ homeostatic mechanisms in hippocampal neurons surviving brain injury. Eur J Neurosci. 2008;27:1659–1672. doi: 10.1111/j.1460-9568.2008.06156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Till C, Colella B, Verwegen J, Green RE. Postrecovery cognitive decline in adults with traumatic brain injury. Arch Phys Med Rehabil. 2008;89:S25–34. doi: 10.1016/j.apmr.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Tomaiuolo F, Carlesimo GA, Di Paola M, Petrides M, Fera F, Bonanni R, Formisano R, Pasqualetti P, Caltagirone C. Gross morphology and morphometric sequelae in the hippocampus, fornix, and corpus callosum of patients with severe non-missile traumatic brain injury without macroscopically detectable lesions: a T1 weighted MRI study. J Neurol Neurosurg Psychiatry. 2004;75:1314–1322. doi: 10.1136/jnnp.2003.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trifilieff P, Herry C, Vanhoutte P, Caboche J, Desmedt A, Riedel G, Mons N, Micheau J. Foreground contextual fear memory consolidation requires two independent phases of hippocampal ERK/CREB activation. Learn Mem. 2006;13:349–358. doi: 10.1101/lm.80206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Witgen BM, Lifshitz J, Smith ML, Schwarzbach E, Liang SL, Grady MS, Cohen AS. Regional hippocampal alteration associated with cognitive deficit following experimental brain injury: A systems, network and cellular evaluation. Neuroscience. 2005;133:1–15. doi: 10.1016/j.neuroscience.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 50.Yang K, Taft WC, Dixon CE, Todaro CA, Yu RK, Hayes RL. Alterations of protein kinase C in rat hippocampus following traumatic brain injury. J Neurotrauma. 1993;10:287–295. doi: 10.1089/neu.1993.10.287. [DOI] [PubMed] [Google Scholar]
- 51.Zaloshnja E, Miller T, Langlois JA, Selassie AW. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Trauma Rehabil. 2008;23:394–400. doi: 10.1097/01.HTR.0000341435.52004.ac. [DOI] [PubMed] [Google Scholar]