Abstract
Dietary conjugated linoleic acids (CLA), have been reported to have a number of isomer-dependent effects on lipid metabolism including reduction in adipose tissue deposition, changes in plasma lipoprotein concentrations and hepatic lipid accumulation. The aim of this study was to compare the effect of individual CLA isomers against lipogenic and high “Western” fat background diets. Golden Syrian hamsters were fed a high-carbohydrate rodent chow or chow supplemented with 17.25% fat formulated to represent the type and amount of fatty acids found in a typical “Western” diet (including 0.2% cholesterol). Diets were further supplemented with 0.25% (w/w) rapeseed oil, cis9, trans11 (c9,t11)-CLA or trans10, cis12 (t10,c12)-CLA. Neither isomer had a significant impact on plasma lipid or lipoprotein concentrations. The t10,c12-CLA isomer significantly reduced perirenal adipose tissue depot mass. While adipose tissue acetyl coenzyme A carboxylase and fatty acid synthase mRNA concentrations (as measured by quantitative PCR) were unaffected by CLA, lipoprotein lipase mRNA was specifically reduced by t10,c12-CLA, on both background diets (p<0.001). This was associated with a specific reduction of SREBP1c expression in perirenal adipose tissue (p=0.018). The isomers appear to have divergent effects on liver triacylglycerol content with c9,t11-CLA producing lower concentrations than t10,c12-CLA. We conclude that t10,c12-CLA modestly reduces adipose tissue deposition in the Golden Syrian hamster independently of background diet and this may possibly result from reduced uptake of lipoprotein fatty acids, as a consequence of reduced LPL gene expression.
Keywords: Conjugated linoleic acid, lipoprotein lipase, SREBP, hamster
Introduction
Conjugated Linoleic Acid (CLA) refers to a mix of geometric and positional isomers of linoleic acid where the two double bonds are conjugated. The last decade has seen a plethora of claims, mainly supported by work with animal models that dietary intake of CLA is associated with potential health benefits1,2. These include reduction in fat deposition, protection from atherosclerosis and cancer and enhanced immunity. Translation of these effects into benefits to human health has proven intractable with little evidence to support effects on cardiovascular disease, immunity or cancer2-4. Effects on fat deposition have also been variable, with a recent meta-analysis of 18 human trials concluding that at a dose of 3.2g/day, consumption of mixed CLA isomers (primarily c9,t11-CLA and t10, c12-CLA) produces modest body fat loss in humans5. It is of note that mixed isomers of CLA have recently been awarded Generally Regarded as Safe (GRAS) status by the Food and Drug Administration in the USA and can now be added to fluid and flavoured milks, yoghurts, milk-based meal replacements, meal replacement bars, soy milk, and fruit juice*.
The predominant CLA isomer in the diet is the c9,t11 isomer, which is primarily found in milk and meat of ruminant animals, and is produced from desaturation of rumenal trans-11 C18:1 through the action of stearoyl CoA desaturase (SCD) in the tissues of the animal6. Much smaller amounts of other isomers can also be found in ruminant products. In addition to naturally occurring CLA, dietary supplements are also widely available. Until recently these have tended to be isomeric mixtures (largely equal mixtures of c9,t11-CLA and t10,c12-CLA), however more recently pure c9,t11-CLA and t10,c12-CLA have become available.
Many of the health benefits attributed to CLA have been linked to specific isomers. The t10,c12-CLA isomer is most potent in reducing body fat deposition7. The c9,t11-CLA isomer has been suggested to have tumour suppressive actions8. Evidence for effects of individual isomers on cardiovascular disease and its associated risk factors has been less conclusive2,3.
Much of the data currently available on the effects of CLA isomers in vivo has been gathered from studies using mice. However, it has become increasingly clear that this species shows a much greater reduction in body fat in response to CLA than most other species9. In recent years a number of studies have reported the comparative effects of pure CLA isomers on lipid metabolism in the Golden Syrian hamster10-23. While most of these have reported some degree of reduction in adipose tissue deposition, effects on plasma lipids have been less conclusive. All of these studies have tended to use high doses (0.5% w/w or greater) of CLA against the background of a high fat diet and usually substituting linoleic acid-rich oils with CLA. Such diets have often been supplemented with highly saturated plant oils containing very low concentrations of unsaturated fatty acids. In the present study we feed 0.25% (w/w) pure c9,t11-CLA or t10,c12-CLA against a background diet of either low-fat chow or a high fat diet designed to mimic the fatty acid composition and quantity of a typical “Western” diet.
Materials and Methods
Protocol of animal treatment
All procedures involving hamsters were subject to UK Home Office regulations and animals were housed as previously described14. Hamsters were anaesthetized using sodium pentabarbitone (Sagatal, 1 mL/kg) and 3-4 ml blood collected by cardiac puncture and placed into EDTA-tubes. While animals were not fasted, they were sacrificed during the light-phase (when food intake would be minimal), between the times of 09.00-12.00h. Plasma was isolated by centrifugation and stored at 4°C until lipoprotein separation which was commenced within 48h of collection. Livers and perirenal and epididymal fat pads were removed, weighed and snap-frozen in liquid nitrogen.
Experimental diets were fed for 6-wk. 8-12-wk old hamsters were randomly divided into 7 groups of 8 animals. Animals were fed either a chow diet (Rat and Mouse Diet 1, Special Diet Supplies) or the same chow supplemented with 172.5g/kg added fat and 0.2% cholesterol (High Fat/High Cholesterol (HF/HC):135g/kg beef tallow, 15g/kg tripalmitin and 22.5g/kg corn oil). Diets were prepared in 2kg batches and frozen at -20°C in 500g aliquots until use. Rapeseed oil/CLA were added directly to ground chow. The high fat diet was prepared by adding cholesterol to melted tallow+ tripalmitin and then mixing this with chow, corn oil and rapeseed oil/CLA. Animals were fed every 2-3 days with un-eaten food being completely removed and discarded. The total fat content of the chow diet was approximately 2.8% and that of the HF/HC was 19.4%. The major fatty acids in the chow diet were 4.38% C14:0, 24.4% C16:0, 10.5% C18:0, 27.4% C18:1 and 27.9% C18:2. Those in the HF/HC diet contained 3.21% C14:0, 31.4% C16:0, 16.7% C18:0, 28.6% C18:1 and 12.8% C18:2. Eight of the diets were further supplemented with 2.5g/kg of either high oleic rapeseed oil (human food grade), c9, t11 CLA (90% c9, t11-CLA, 3.8% t10,c12 -CLA, 3.3% c9-C18:1 and 2.5% other conjugated isomers) or t10, c12 –CLA (94.4% t10, c12-CLA, 2.0% c9, t11-CLA, 0.4% c9-C18:1 and 2.9% other conjugated isomers). The rapeseed supplemented chow and HF/HC diets both contained approximately 0.28% and 0.24% of c9, t11-CLA respectively and no detectable t10, c12-CLA. Supplementation with CLA increased the relative amount of the appropriate isomer to approximately 7% and 1% of the total fatty acids in the chow and HF/HC diets respectively. A further HF/HC diet was supplemented with 1% t10, c12-CLA. CLA isomers were provided by Larodan Fine Chemicals AB, Malmö, Sweden). Animals had free access to food and water, and food was replaced completely every 2-3d. Daily food intake was measured between days 21-27 of the trial.
Fatty acid analysis
The fatty acid composition of perirenal adipose tissue was determined by gas chromatography of fatty acid methyl esters as previously described17.
Lipoprotein separation and cholesterol and triacylglycerol (TAG) analysis
After removal of triacylglycerol-rich lipoproteins (d<1.02 g/ml), plasma LDL (d = 1.02-1.06g/ml) and HDL (d > 1.06g/ml) were isolated from hamster plasma as previously described24. Total plasma cholesterol and TAG concentrations were determined using diagnostic kits from Thermo-Trace (Infinity Cholesterol and Infinity TAG Enzymatic kits, Alpha Laboratories). Hepatic cholesterol ester and TAG concentrations were also determined using Thermo-Trace diagnostic kits.
Determination of mRNA levels
RNA was extracted from liver and perirenal adipose tissue using Trizol (Invitrogen) according to the manufacturer’s instructions. Genomic DNA was digested with DNase, RNA purity and yield determined and RT-PCR performed as described previously25. For the relative quantification of cDNA for ATP binding cassette transporter A1 (ABC-A1), acetyl CoA carboxylase (ACC), fatty acid synthase (FAS), lipoprotein lipase (LPL), low density lipoprotein receptor (LDLr), sterol regulatory element binding proteins 1a, 1c and 2 (SREBP1a, SREBP1c and SREBP2) quantitative, real-time PCR was performed using either a PRISM 7700 Sequence Detector (Applied Biosystems) or a Lightcycler 480 (Roche). Primer and probe sequences and real-time PCR methods were as previously described25. Standard curves were used to check assay linearity and to determine sample gene expression in RNA equivalents using the CT values. In addition to the genes of interest the mRNA concentrations for the housekeeping gene, β-actin, were measured and found not to differ between treatment groups. Relative expression of genes of interest were therefore normalized to β-actin and expressed as arbitrary units.
Statistical Analyses
Data from control animals and those fed either 0.25% c9,t11-CLA or t10,c12-CLA were analysed by two-way ANOVA, with diet as one factor (D), and conjugated linoleic acid (C) as another. Results are expressed as means ± Standard error of the difference (SED). Treatment effects and differences between means were considered significant when p<0.05. Where an effect of CLA was apparent, but no interaction with background diet, data were further analysed by post-hoc Bonferoni test to determined differential effects of isomers. Selected data for animals fed 0.25% and 1% t10, c12-CLA were analyzed by regression analysis to determine dose-dependent effects.
Results
Effect on body composition
The average initial starting weight of all animals was 93.0 ± 8.3g (mean ± SD) with no significant differences between groups of animals. Over the course of the experiment animals gained an average of 26.2 ± 9g. As can be seen in Table 1, no significant difference in weight gain (expressed as a percentage of initial weight) was seen between the groups. While there was no effect of either CLA isomer on food intake, animals adapted to the higher energy density of the HF/HC diet by reducing their food intake.
Table 1. Changes in body composition of hamsters fed a chow or high fat, high cholesterol diet supplemented with 0.25% (wt/wt) CLA isomers (n= 8 animals per group).
| CLA | ANOVA1 | |||||||
|---|---|---|---|---|---|---|---|---|
| None | c9,t11 | t10,c12 | mean | SED | P value | |||
| Initial body weight (g) |
Chow HF/HC mean |
95.1 89.2 92.1 |
90.2 90.6 90.4 |
97.2 96.0 96.6 |
94.1 91.9 93.0 |
D C D×C |
2.34 2.87 4.06 |
0.355 0.096 0.531 |
| Final body weight (g) |
Chow HF/HC mean |
124.9 114.1 119.5 |
110.9 118.7 114.8 |
120.2 127.2 123.7 |
118.7 120.0 119.3 |
D C D×C |
3.72 3.03 5.26 |
0.071 0.662 0.025 |
| Change in body weight (g)) |
Chow HF/HC mean |
29.8 24.9 27.3 |
20.7 28.1 24.4 |
23.0 31.1 27.1 |
24.5 28.0 26.3 |
D C D×C |
3.07 2.51 4.35 |
0.580 0.167 0.067 |
| Daily feed intake (g) |
Chow HF/HC mean |
7.70 5.75 6.72 |
6.51 5.65 6.08 |
6.70 6.02 6.36 |
6.97 5.80 6.39 |
D C D×C |
0.38 0.31 0.53 |
<0.001 0.248 0.192 |
| PR adipose weight (g) |
Chow HF/HC mean |
2.05 1.71 1.88 |
1.50 1.70 1.60 |
1.49 1.79 1.64 |
1.68 1.73 1.71 |
D C D×C |
0.16 0.13 0.22 |
0.160 0.700 0.106 |
| PR adipose weight (% final BW) 2 |
Chow HF/HC mean |
1.61 1.49 1.55a |
1.34 1.40 1.37a,b |
1.24 1.40 1.32b |
1.40 1.43 1.41 |
D C D×C |
0.08 0.09 0.13 |
0.712 0.049 0.334 |
| Epi adipose weight (g) |
Chow HF/HC mean |
2.35 2.12 2.24 |
2.01 2.38 2.19 |
2.37 2.43 2.40 |
2.24 2.31 2.28 |
D C D×C |
0.17 0.14 0.24 |
0.441 0.628 0.231 |
| Epi adipose weight (% final BW) 2 |
Chow HF/HC mean |
1.86 1.84 1.85 |
1.80 1.99 1.90 |
1.97 1.90 1.93 |
1.88 1.91 1.89 |
D C D×C |
0.10 0.09 0.15 |
0.718 0.705 0.434 |
| Liver weight (g) |
Chow HF/HC mean |
5.25 5.67 5.46a |
4.45 5.98 5.21a |
5.99 6.65 6.32b |
5.23 6.10 5.66 |
D C D×C |
0.26 0.22 0.37 |
<0.001 <0.001 0.098 |
| Liver weight (% final BW) 2 |
Chow HF/HC mean |
4.19 4.99 4.59 |
4.03 4.94 4.49 |
4.96 5.21 5.08 |
4.39 5.04 4.72 |
D C D×C |
0.11 0.14 0.19 |
<0.001 <0.001 0.041 |
| Liver TAG (μmole/liver) |
Chow HF/HC mean |
4.63 5.05 4.84a |
2.30 3.93 3.12b |
4.11 6.52 5.31a |
3.68 5.17 4.42 |
D C D×C |
0.50 0.61 0.86 |
0.005 0.002 0.275 |
| Liver Total Cholesterol (μmole/liver) |
Chow HF/HC mean |
1.43 14.35 7.99 |
1.87 12.27 7.21 |
0.45 13.87 7.16 |
1.34 13.57 7.45 |
D C D×C |
0.20 0.25 0.35 |
<0.001 0.240 0.738 |
Statistical values were obtained by two-way ANOVA with background diet (D, chow or HF/HC) as one factor and CLA (C, none, c9,t11 or t10,c12) as a second factor. SED: standard error of the difference
Tissue weight expressed as percent of final body weight (BW)
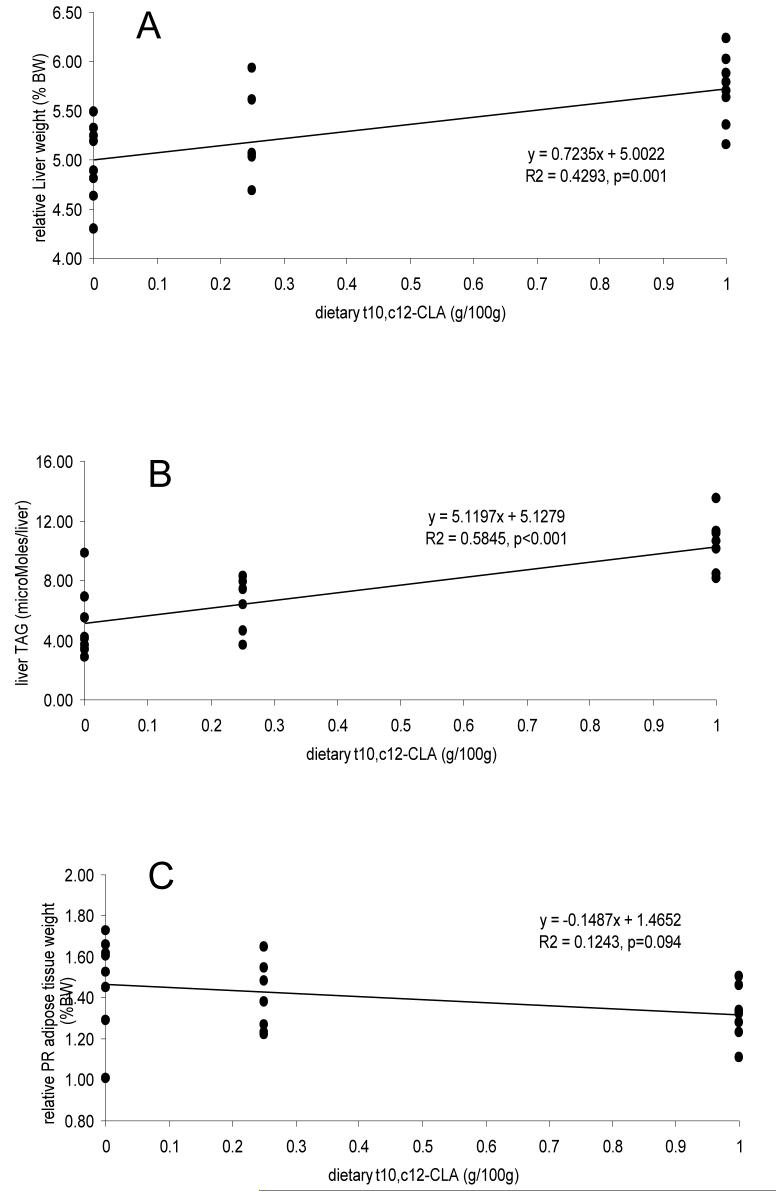
There was a significant reduction in the relative weight of the perirenal adipose tissue depot in animals fed t10, c12-CLA, which was independent of background diet. Further analysis was performed on data from animals fed the HF/HC diet supplemented with 0%, 0.25% and 1% t10, c12-CLA to ascertain whether the effects on perirenal adipose tissue weight were dose dependent (Fig. 1A). Regression analysis failed to demonstrate a statistically significant relationship (p=0.094). By contrast to the perirenal adipose tissue depot, epididymal fat weight was not influenced by CLA feeding, even when t10,c12-CLA was fed at a level of 1% (relative weights of epididymal tissue in animals fed 1% t10,c12-CLAw was 1.79±0.35 (mean±standard deviation) and not significantly different to values for animals fed 0% CLA or 0.25% t10,c12-CLA).
Figure 1.
Groups of 8 hamsters were fed HF/HC supplemented with 0.25% rapeseed oil, 0.25% or 1% t10,c12-CLA. After 6 weeks animals were sacrificed and the weight of perirenal adipose tissue (A) and liver (B) were recorded and expressed relative to total body weight (BW). Liver lipids were extracted and triacylglcerol (TAG) content determined (C).
Fatty acid analysis of perirenal adipose tissue indicated that in animals fed chow, c9,t11-CLA made up 0.24 ± 0.06% of total fatty acids, which increased to 0.37 ± 0.05% in those fed the HF/HC diet. Supplementation of each of these diets with 0.25% c9,t11-CLA increased the content of this fatty acid in perirenal adipose tissue to 1.59 ± 0.18% and 1.24 ± 0.06%, respectively. The t10,c12-CLA isomer was not detectable in the adipose tissue of animals fed the basal chow and high fat diets. Supplementation of the two diets with this isomer increased tissue levels to 0.55 ±0.27% and 0.34 ± 0.04%, respectively.
The HF/HC diet caused a 17% increase in liver weight (P<0.001) which was associated with an accumulation of triacylglycerol and, particularly, cholesterol (Table 1). Thin layer chromatography confirmed that most of the cholesterol was in the esterified form (data not shown). Liver mass was also increased in animals fed t10,c12-CLA with the effect being more pronounced on a chow than a high fat diet (18 vs 4% increase). Divergent effects of CLA isomers were seen on liver triacylglycerol levels with animals fed c9,t11-CLA having lower concentrations than either control or t10, c12-CLA fed animals. In animals fed the HF/HC diet there was a highly significant linear relationship between the amount of t10, c12-CLA in the diet and both relative liver weight (Fig 1A) and TAG content (Fig 1B). Hepatic cholesterol content was not affected by either dose of t10, c12-CLA.
Effect on plasma lipids and lipoproteins
The HF/HC diet significantly increased total plasma cholesterol (p<0.001) and triacylglycerol (p=0.015), however there was no further effects of CLA supplementation (table 2). Both LDL and HDL cholesterol were increased by the HF/HC diet but again no effect of CLA supplementation was seen.
Table 2. Changes in blood lipid profile and liver lipids in hamsters fed a chow or high fat, high cholesterol diet supplemented with 0.25% (wt/wt) CLA isomers (n=8 animals per group).
| CLA | ANOVA1 | |||||||
|---|---|---|---|---|---|---|---|---|
| None | c9,t11 | t10,c12 | mean | SED | P value | |||
| Total Plasma chol (mM) |
Chow HF/HC mean |
3.53 6.58 5.06 |
3.54 6.39 4.97 |
3.09 6.73 4.91 |
3.39 6.57 4.98 |
D C D×C |
0.18 0.22 0.31 |
<0.001 0.794 0.187 |
| LDL chol (mM) |
Chow HF/HC mean |
0.23 0.80 0.52 |
0.24 0.83 0.53 |
0.16 0.83 0.50 |
0.21 0.82 0.52 |
D C D×C |
0.06 0.08 0.11 |
<0.001 0.882 0.759 |
| HDL chol |
Chow HF/HC mean |
2.27 3.08 2.67 |
1.97 3.08 2.52 |
2.17 3.34 2.76 |
2.14 3.16 2.65 |
D C D×C |
0.12 0.15 0.21 |
<0.001 0.284 0.430 |
| Plasma TAG (mM) |
Chow HF/HC mean |
1.26 1.95 1.61 |
1.30 1.55 1.42 |
1.44 1.46 1.45 |
1.33 1.65 1.49 |
D C D×C |
0.13 0.15 0.22 |
0.015 0.424 0.094 |
Statistical values were obtained by two-way ANOVA with background diet (D, chow or HF/HC) as one factor and CLA (C, none, c9,t11 or t10,c12) as a second factor. SED: standard error of the difference
Effect of diet on perirenal adipose tissue mRNA concentrations
Feeding the HF/HC diet suppressed lipogenic gene expression in perirenal adipose tissue (table 3) with levels of FAS, ACC and LPL mRNA all significantly reduced (P<0.001). This was associated with reduced expression of each of the SREBP isoforms. While neither CLA isomer had any effect on FAS, or ACC mRNA, the t10,c12-CLA isomer specifically reduced perirenal adipose LPL mRNA by approximately 50% irrespective of background diet. SREBP-1c mRNA levels were also reduced, by approximately 30%, by t10,c12-CLA independently of background diet.
Table 3. Changes in gene expression in perirenal adipose as a result of feeding either a chow or a high fat, high cholesterol diet supplemented with 0.25% (wt/wt) CLA isomers (n=8 animals per group). Data are expressed in arbitrary unit relative to β-actin mRNA.
| CLA | ANOVA1 | |||||||
|---|---|---|---|---|---|---|---|---|
| None | c9,t11 | t10,c12 | mean | SED | P value | |||
| ACC |
Chow HF/HC mean |
1.70 0.81 1.25 |
1.43 0.62 1.05 |
1.87 0.58 1.23 |
1.67 0.69 1.18 |
D C D×C |
0.16 0.19 0.27 |
<0.001 0.533 0.371 |
| FAS |
Chow HF/HC mean |
1.79 0.72 1.25 |
1.93 0.83 1.38 |
1.55 0.45 1.00 |
1.76 0.66 1.21 |
D C D×C |
0.18 0.22 0.31 |
0.001 0.227 0.995 |
| LPL |
Chow HF/HC mean |
1.78 1.00 1.39a |
1.51 1.14 1.32a |
0.96 0.53 0.74b |
1.41 0.89 1.15 |
D C D×C |
0.14 0.17 0.24 |
<0.001 <0.001 0.427 |
| SREBP-1a |
Chow HF/HC mean |
1.63 0.88 1.26 |
1.25 1.23 1.24 |
1.43 0.69 1.06 |
1.44 0.94 1.19 |
D C D×C |
0.14 0.17 0.24 |
<0.001 0.429 0.056 |
| SREBP-1c |
Chow HF/HC mean |
1.66 1.14 1.40a |
1.55 1.32 1.44a |
1.27 0.64 0.96b |
1.49 1.03 1.26 |
D C D×C |
0.14 0.18 0.25 |
0.003 0.018 0.513 |
| SREBP-2 |
Chow HF/HC mean |
1.44 0.97 1.21 |
1.27 0.77 1.02 |
1.19 0.50 0.84 |
1.30 0.75 1.02 |
D C D×C |
0.15 0.18 0.26 |
<0.001 0.154 0.803 |
Statistical values were obtained by two-way ANOVA with background diet (D, chow or HF/HC) as one factor and CLA (C, none, c9,t11 or t10,c12) as a second factor. SED: standard error of the difference
Effect of diet on hepatic mRNA concentrations
FAS and ACC mRNA levels were decreased, but LPL mRNA concentration was increased, by the HF/HC diet (table 4; P=0.001). Consumption of the HF/HC diet was associated with a decrease in expression of hepatic LDL receptor and SREBP-2 and an increase in ABC-A1 mRNA expression (p<0.001). However no effect of the HF/HC diet was observed on the levels of either SREBP1a or 1c transcripts in the liver. In contrast with the perirenal tissue, none of the mRNAs measured in the liver were affected by CLA consumption. Within liver ACC, FAS, LDLr mRNAs were all positively correlated to SREBP2 mRNA but were not related to either SREBP1c or 1a mRNA (table 5). LPL mRNA was positively correlated with ABCA1 mRNA which in turn was correlated with SREBP1c mRNA.
Table 4. Changes in gene expression in the liver as a result of feeding either a chow or a high fat, high cholesterol diet supplemented with 0.25% (wt/wt) CLA isomers (n=8 animals per group). Data are expressed in arbitrary unit relative to β-actin mRNA.
| CLA | ANOVA1 | |||||||
|---|---|---|---|---|---|---|---|---|
| None | c9,t11 | t10,c12 | mean | SED | P value | |||
| LDLr |
Chow HF/HC mean |
1.81 0.42 1.11 |
1.49 0.54 1.01 |
1.17 0.34 0.75 |
1.49 0.43 0.96 |
D C D×C |
0.13 0.16 0.23 |
<0.001 0.082 0.199 |
| ACC |
Chow HF/HC mean |
1.56 0.49 1.02 |
1.27 0.77 1.02 |
1.35 0.38 0.87 |
1.40 0.54 0.97 |
D C D×C |
0.13 0.16 0.22 |
0.001 0.515 0.159 |
| FAS |
Chow HF/HC mean |
1.77 0.37 1.06 |
1.53 0.70 1.12 |
1.55 0.36 0.95 |
1.61 0.47 1.04 |
D C D×C |
0.15 0.18 0.25 |
<0.001 0.647 0.280 |
| LPL |
Chow HF/HC mean |
0.56 1.81 1.19 |
0.54 1.52 1.03 |
0.46 1.89 1.18 |
0.52 1.74 1.13 |
D C D×C |
0.17 0.21 0.30 |
<0.001 0.695 0.569 |
| SREBP-1a |
Chow HF/HC mean |
1.14 1.24 1.19 |
1.62 1.21 1.41 |
1.24 1.05 1.15 |
1.34 1.17 1.25 |
D C D×C |
0.14 0.17 0.24 |
0.154 0.203 0.493 |
| SREBP-1c |
Chow HF/HC mean |
1.14 1.07 1.10 |
1.19 1.30 1.25 |
1.30 1.07 1.18 |
1.21 1.15 1.18 |
D C D×C |
0.11 0.14 0.19 |
0.587 0.569 0.458 |
| SREBP-2 |
Chow HF/HC mean |
2.32 0.72 1.52 |
2.06 1.37 1.71 |
1.57 0.83 1.20 |
1.98 0.97 1.48 |
D C D×C |
0.21 0.25 0.35 |
<0.001 0.133 0.137 |
| ABC-A1 |
Chow HF/HC mean |
0.81 1.39 1.10 |
1.00 1.49 1.24 |
0.80 1.36 1.08 |
0.87 1.41 1.14 |
D C D×C |
0.12 0.14 0.20 |
<0.001 0.447 0.940 |
Statistical values were obtained by two-way ANOVA with background diet (D, chow or HF/HC) as one factor and CLA (C, none, c9,t11 or t10,c12) as a second factor. SED: standard error of the difference
Table 5. Correlation matrices for mRNA concentrations in liver.
| FAS | 0.962*** | ||||||
| LPL | -0.542*** | -0.618*** | |||||
| LDLr | 0.672*** | 0.698*** | -0.615*** | ||||
| ABCA1 | -0.339* | -0.419** | 0.824*** | -0.382** | |||
| SREBP1a | 0.271 | 0.253 | -0.168 | 0.418** | 0.036 | ||
| SREBP1c | 0.379* | 0.283 | 0.053 | 0.203 | 0.450** | 0.385** | |
| SREBP2 | 0.626*** | 0.694*** | -0.453** | 0.691*** | -0.317* | 0.151 | 0.002 |
| ACC | FAS | LPL | LDLr | ABCA1 | SREBP1a | SREBP1c |
Figures represent correlation coefficients, p<0.05
Figures represent correlation coefficients, p<0.01
Figures represent correlation coefficients, p<0.001
Discussion
Initial observations that CLA reduced adipose tissue deposition were originally performed in mice fed high carbohydrate/low fat diets7,9. It is now quite clear that under these conditions, in this species, CLA induces a lipoatrophic syndrome9 in which dramatic adipose tissue loss is associated with severe hyperinsulinemia, insulin resistance and hepatic steatosis. More recently a number of studies have been performed in the Golden Syrian Hamster. In general these studies have explored the impact of CLA isomers against a background of high fat/high cholesterol diets. Thus, one of the aims of the present study was to explore effects on both low and high fat diets. The majority of previous hamster studies have fed highly saturated plant oils as the only source of dietary fat. For example, Portillo and colleagues12,13,15,18,21 use semi-synthetic diets supplemented with 10% palm oil. As a result, total food energy from fat was approximately 22% and that from n-6 PUFA only about 3%. This contrasts with typical intakes in many industrialized countries of over 35% energy from total fat and 5-6% from n-6 PUFA26. Hayes et al.27 have suggested that the responsiveness of animals to changes in fatty acid composition of the diet is exaggerated at low n-6 PUFA intakes. In many previous studies this may be further confounded by the fact that the effect of CLA isomers is directly compared to diets supplemented with equivalent amounts of linoleic acid. For example, Navarro et al.12 showed that adipose tissue mass (epididymal and perirenal) were not significantly different in hamster fed chow compared to those fed a semi-purified diet containing 0.5% t10,c12-CLA. However when compared to a semi-synthetic diet containing 0.5% high-linoleic acid sunflower oil, adipose tissue weights were significantly lower in both the chow-fed animals and those consuming the semi-synthetic diet supplemented with t10, c12-CLA. It is difficult to ascertain whether the changes seen in such studies are a result of CLA or reduced linoleic acid in the diet. In the present study high fat diets were designed to mimic a “Western” diet in terms of both fatty acid composition and quantity. The high fat diet used provided approximately 44% of energy from total fat and 5.6% from n-6 PUFA, intakes which would not be uncommon in a human ‘Western’ diet. Supplementation with CLA isomers has been compared to that with high oleic acid –rape seed oil.
Previous studies of the effects of CLA on lipid metabolism in hamsters have used CLA intakes of 0.5-1% (w/w)11-23. Based on typical food intakes and body weights this represents a daily intake of approximately 30-60mg of CLA/day or 250-500mg/kg body weight/day. This would translate to an intake of 20-40g/day in an 80kg human, which would be clearly unachievable. Alternatively, intake can be expressed as a proportion of total energy intake. Based on an energy content of normal laboratory chow of about 10kJ/g, these levels of supplementation with CLA would represented an approximate intake of 0.5-1mg CLA/kJ. To obtain a similar dose, a human consuming 10MJ/day, would need to consume 5-10g CLA/day. It remains to be established which of these estimations is most appropriate and this probably depends on the extent to which effects are dependent on the accumulation of CLA in the tissue. The amount of CLA used in the present study was approximately equivalent to a human intake of 10g/day (on a body weight basis) or 2.5g/day on an energy intake basis). While still high, this is approaching more achievable human supplementation levels.
As we have previously shown, the high fat/high cholesterol diet increased plasma cholesterol and triacylglycerol28,29. While both LDL and HDL cholesterol were increased, the effect on the former was more significant, resulting in an increase in the ratio of LDL to HDL from 0.1 to 0.25. As previously reported28,29 cholesterol-feeding was also associated with increased hepatic cholesterol ester concentration and a more modest elevation in hepatic triacylglycerol. Also, as previously seen, hepatic LDLr mRNA concentrations were reduced by the high fat/high cholesterol diet29. LDLr expression has been shown to be largely regulated by the activity of SREBP230 and indeed there was a highly significant correlation between SREBP2 and LDLr mRNA concentrations (p<0.001). This diet also had marked effects on adipose tissue and hepatic lipogenic gene expression. ACC and FAS mRNA concentrations were reduced in both tissues while LPL was reduced in adipose tissue but increased in liver. Lipogenic gene expression is regulated by the activity of SREBPs30 and expression of all three isoforms was reduced in adipose tissue. In liver, however, only SREBP2 mRNA was reduced. While it has long been suggested that the expression of lipogenic genes such as ACC and FAS are primarily regulated by SREBP1 isoforms, there is evidence to suggest that SREBP2 may also play a role31,32. In fact, this isoform is a much more potent regulator of gene transcription than SREBP1c (the major SREBP1 isoform in liver)33. In view of a highly significant correlation between hepatic SREBP2 mRNA and both ACC (p<0.001) and FAS (p<0.001) it is interesting to speculate that the reduction in SREBP2 is responsible for the reduction in expression of these two genes. However, it is also possible that nuclear concentrations of SREBP1c mature protein are decreased in the absence of changes in gene expression. LPL mRNA showed tissue specific responses to the HF/HC diet. In adipose tissue, LPL expression was down-regulated and showed a high degree of correlation with ACC, FAS and each of the SREBPs. However, in liver it was actually up-regulated and showed negative associations with ACC, FAS and SREBP2. LPL has been shown to contain a sterol regulatory element within its promoter and therefore might have been expected to change in the same direction as ACC and FAS34. However, it has also been reported to contain response elements for the liver X receptor (LXR)35. LXRα is a nuclear receptor that is activated by oxygenated derivatives of cholesterol and is known to regulate the expression of a range of genes for enzymes and other proteins involved in cholesterol and lipid metabolism36. It is possible that the increased cholesterol content of the diets has led to the generation of increased LXR ligand(s) and this may, in turn lead to the up-regulation of LPL gene expression. The highly significant correlation between ABC-A1 mRNA and LPL mRNA supports this hypothesis. ABC-A1 is well established as a target gene for LXR37. However, it is of note that ACC and FAS have both been reported to have LXR response elements within their promoters38,39, and we observed negative correlations in expression of these genes relative to ABC-A1. How high fat/high cholesterol diets mediate such divergent effects on these genes warrant further investigation.
Work in mice has shown that t10,c12 CLA dramatically reduces adipose tissue deposition7,9. Recent studies in hamsters have indicated more modest effects on adipose tissue mass10,11,18,23,40. In this study, the t10,c12-CLA isomer also modestly reduced perirenal, but not epididymal adipose tissue mass. It is of note that even when fed at 1%, t10,c12-CLA (as part of a HF/HC diet) failed to reduce epipidymal fat mass and no dose-dependent effect was seen on the perirenal depot weight. The effect of t10, c12-CLA on perirenal fat was associated with a reduction in LPL mRNA level, which was decreased by up to almost 50% irrespective of the background diet. The t10,c12-CLA isomer also reduced SREBP-1c mRNA concentrations in the perirenal adipose, and potentially represents the mechanism by which t10,c12-CLA is affecting perirenal LPL mRNA levels. This is supported by a highly significant correlation between LPL and SREBP-1c mRNA levels in perirenal adipose. Zabala et al.18 found that 0.5% and 1% t10,c12 –CLA reduced the mass of epididymal, perirenal and subcutaneous fat depots. The reduction in epididymal fat mass was also shown to be associated with reduced LPL mRNA concentration and enzyme activity. They also demonstrated reduced SREBP1c expression, though in this case SREBP1a was also reduced. It is not clear why no effect on epididymal fat mass was seen in the current experiment. However, it is of note that while Zabala et al.18 were substituting linoleic acid rich oil with CLA, in the current experiment, oleic acid –rich, rapeseed oil was used. Another difference in the findings of these two studies was that while Zabala et al.18 found ACC and FAS expression was reduced in epididymal adipose tissue, in the present experiment these were not affected by t10,c12-CLA in the perirenal depot. The differences in findings between these two studies, depot –specific responses and impact of altering the the linoleic acid content of the diets are all worthy of further study.
In the present study we found no evidence of an effect of either CLA isomer on plasma total, LDL or HDL cholesterol, on either background diet. Previous work in the hamster has produced conflicting results. We have previously reported reduced LDL cholesterol levels in hamsters fed butter enriched in c9,t11-CLA and t11-C18:117. While one group has reported reduced LDL in hamsters fed pure c9,t11-CLA22, others have found no effect10,12,16. The results of studies using pure t10,c12-CLA have also produced a range of effects with some authors reporting decreases in LDL cholesterol10,12, one showing an increase19 and others reporting no effect16,22. The response of HDL cholesterol to pure isomers have been equally varied with some reports of increased HDL with c9,t11-CLA41 and t10,c12-CLA16 and others suggesting decreases10,20 or no effect12,17. The variability in responses is likely to be a combination of factors including differences in dose of CLA, background diet and age/strain of hamster. It is of note that in humans no significant effect of supplementation with either CLA isomer was seen when comparing to pre-supplementation levels. There was, however, some suggestion of a divergent effect of the two isomers, with c9,t11-CLA decreasing and t10,c12-CLA increasing the ratio of LDL cholesterol to HDL cholesterol42. Taken together these data suggests that any effects of CLA using dosages achievable in humans will be small.
The t10,c12-CLA isomer increased liver weight in a dose dependent manner. This is consistent with a number of other hamster studies using concentrations of CLA of 0.5-1%10,11,12,15,20. Previous workers15 have suggested that the increase in liver weight is associated with an increase in the number of hepatocytes within the livers of animals fed the t10,c12 isomer. At low concentrations we also found divergent effects of the isomers on liver TAG, with c9,t11-CLA tending to reduce and t10,c12-CLA increase, concentrations. This concurs with previous findings43 which demonstrated divergent effects of these CLA isomers on the level of liver steatosis in apoE knockout mice. One possible theory offered by those authors for this effect is that the c9,t11-CLA may preferentially promote lipolysis in the liver by PPARa activation. Hepatic TAG increased linearly with dose of t10,c12-CLA, with a doubling of the amount in livers from animals fed 1% t10, c12-CLA. It remains to be established whether feeding a mixture of c9, t11-CLA and t10, c12-CLA will offset the steatosis associated with intake of the latter.
Overall our data suggests low dose supplements with c9, t11-CLA has little impact on lipid metabolism in the hamster irrespective of whether this is fed against the background of a high carbohydrate, lipogenic diet or a high fat/high cholesterol diet. The apparent effect of this isomer in reducing liver TAG is worthy of further investigation as it may potentially offset the steatosis induced by the t10, c12 isomer when mixed isomer preparations are fed. The t10,c12 –CLA isomer reduced the size of the perirenal adipose tissue depot, and this is associated with reduced adipose tissue LPL expression which may be the result of reduced SREBP1c expression. However this was also associated with hepatomegaly and increased hepatic TAG accumulation. The study also highlights important differences in the expression of lipogenic genes in response to high fat/high cholesterol diets and tissue specific differences in these responses.
Acknowledgements
The authors would like to acknowledge the excellent technical assistance of Mr Richard Plant and Mr David Bozon. This work was funded by a studentship to Elizabeth Tarling and a project grant from the UK BBSRC. The authors have no conflicts of interest to declare. Elizabeth Tarling and Kevin Ryan performed the majority of the laboratory work and contributed to the analysis of data and the writing of the manuscript. Andrew Bennett was a co-investigator and grant holder and played a significant role in the design of the study, interpretation of results and writing of the manuscript. Andrew Salter was the principle investigator and grant holder and played a major role in the design and performance of the study, analysis and interpretation of the results and the writing of the paper.
Footnotes
References
- 1.Pariza MW. Perspective on safety and effectiveness of conjugated linoleic acid. Am. J. Cin. Nutr. 2004;79:1132S–1136S. doi: 10.1093/ajcn/79.6.1132S. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya A, Banu J, Rahman M, Causey J, Fernandes G. Biological effects of conjugated linoleic acids in health and disease. J. Nutr. Biochem. 2006;17:789–810. doi: 10.1016/j.jnutbio.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Wahle KW, Hey SD, Rotondo D. Conjugated linoleic acids: are they beneficial or detrimental to health. Prog Lipid Res. 2004;43:553–587. doi: 10.1016/j.plipres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Kelly NS, Hubbard NE, Erickson KL. Conjugated linoleic acid isomers and cancer. J Nutr. 2007;137:2599–2607. doi: 10.1093/jn/137.12.2599. [DOI] [PubMed] [Google Scholar]
- 5.Whigham LD, Watras AC, Schoeller DA. Efficacy of conjugated linoleic acid for reducing fat mass: a meta-analysis in humans. Am J Clin Nutr. 2007;85:1203–1211. doi: 10.1093/ajcn/85.5.1203. [DOI] [PubMed] [Google Scholar]
- 6.Griinari JM, Corl BA, Lacy SH, Chuinard PY, Nurmela KVV, Bauman DE. Conjugated linoleic acid is synthesized endogenously in lactating dairy cows by Δ9 –desaturase. J. Nutr. 2000;130:2285–2291. doi: 10.1093/jn/130.9.2285. [DOI] [PubMed] [Google Scholar]
- 7.Park Y, Storkson JM, Albright KJ, Liu W, Pariza M. Evidence that trans-10, cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids. 1999;34:235–241. doi: 10.1007/s11745-999-0358-8. [DOI] [PubMed] [Google Scholar]
- 8.Ip C, Banni S, Angioni E, Carta G, McGinley J, Thompson HJ, Barbano D, Bauman D. Conjugated linoleic acid-rich butter fat alters mammary gland morphogenesis and reduces cancer risk in rats. J. Nutr. 1999;129:2135–2142. doi: 10.1093/jn/129.12.2135. [DOI] [PubMed] [Google Scholar]
- 9.Poirier H, Niot I, Clément L, Guerre-Millo M, Besnard P. Development of conjugated linoleic acid (CLA)-mediated lipoatrophic syndrome in the mouse. Biochimie. 2005;87:73–79. doi: 10.1016/j.biochi.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 10.DeDeckere EAM, van Amelsvoort JMM, McNeill GP, Jones P. Effects of conjugated linoleic acid (CLA) isomers on lipid levels and peroxisome proliferation in the hamster. Brit. J. Nutr. 1999;82:309–317. [PubMed] [Google Scholar]
- 11.Gavino VC, Gavino G, Leblanc M-J, Tuchweber B. An isomeric mixture of conjugated linoleic acids but not pure cis-9,trans-11-octadecadienoic acid affects body weight gain and plasma lipids in hamsters. J. Nutr. 2000;130:27–29. doi: 10.1093/jn/130.1.27. [DOI] [PubMed] [Google Scholar]
- 12.Navarro V, Zabala A, Marcarulla MT, Fernández-Quintela A, Rodríguez VM, Simón E, Portillo MP. Effects of conjugated linoleic acid on body fat accumulation and serum lipids in hamsters fed an atherogenic diet. J. Physiol. Biochem. 2003;59:193–200. doi: 10.1007/BF03179915. [DOI] [PubMed] [Google Scholar]
- 13.Zabala A, Churruca I, Marculla MT, Rodríguez VM, Fernández-Quintela A, Martínez JA, Portillo MP. The trans-10, cis-12 isomer of conjugated linoleic acid reduces hepatic triacylglycerol content without affecting lipogenic enzymes in hamsters. Brit. J. Nutr. 2004;92:383–389. doi: 10.1079/bjn20041220. [DOI] [PubMed] [Google Scholar]
- 14.Valeille K, Férézou J, Amsler G, Quignard-Boulangé A, Parquet M, Gripois D, Dorovska-Taran V, Martin J-C. A cis-9, trans-11-conjugated linoleic acid-rich oil reduces the outcome of atherogenic process in hyperlipidemic hamster. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H652–H659. doi: 10.1152/ajpheart.00130.2005. [DOI] [PubMed] [Google Scholar]
- 15.Macarulla MT, Fernández-Quintela A, Zabala A, Navarro V, Echevarría E, Churruca I, Rodríguez VM, Portillo MP. Effects of conjugated linoleic acid on liver composition and fatty acid oxidation are isomer-dependent in hamster. Nutrition. 2005;21:512–519. doi: 10.1016/j.nut.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell PL, Langille MA, Currie DL, McLeod RS. Effect of conjugated linoleic acid isomers on lipoproteins and atherosclerosis in the Syrian Golden hamster. Biochim. Biophys. Acta. 2005;1734:269–276. doi: 10.1016/j.bbalip.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Lock AL, Horne CAM, Bauman DE, Salter AM. Butter naturally enriched in conjugated linoleic acid and vaccenic acid alters tissue fatty acids and improves the plasma lipoprotein profile in cholesterol-fed hamsters. J. Nutr. 2005;135:1934–1939. doi: 10.1093/jn/135.8.1934. [DOI] [PubMed] [Google Scholar]
- 18.Zabala A, Churruca I, Fernández-Quintela A, Rodríguez VM, Macarulla MT, Martínez JA, Portillo MP. trans-10, cis-12 Conjugated linoleic acid inhibits lipoprotein lipase but increases the activity of lipogenic enzymes in adipose tissue from hamsters fed an atherogenic diet. Brit. J. Nutr. 2006;95:1112–1119. doi: 10.1079/bjn20061774. [DOI] [PubMed] [Google Scholar]
- 19.Bissonauth V, Chouinard Y, Marin J, Leblanc N, Richard D, Jacques H. The effects of t10,c12 CLA isomer compared with c9,t11 CLA isomer on lipid metabolism and body composition in hamsters. J. Nutr. Biochem. 2006;17:597–603. doi: 10.1016/j.jnutbio.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Wilson TA, Nicolsi RJ, Saati A, Katyla T, Kritchevsky D. Conjugated linoleic acid isomers reduce blood cholesterol levels but not aortic cholesterol accumulation in hypercholesterolemic hamsters. Lipids. 2006;41:41–48. doi: 10.1007/s11745-006-5068-8. [DOI] [PubMed] [Google Scholar]
- 21.Zabala A, Portillo MP, Macarulla MT, Rodríguez VM, Fernández-Quintela A. Effects of cis-9, trans-11 and trans-10, cis-12 CLA isomers on liver and adipose tissue fatty acid profile in hamsters. Lipids. 2006;41:993–1001. doi: 10.1007/s11745-006-5050-5. [DOI] [PubMed] [Google Scholar]
- 22.LeDoux M, Laloux L, Fontaine J-J, Carpentier YA, Chardigny J-M, Sébédio J-L. Rumenic acid significantly reduces plasma levels of LDL and small dense LDL cholesterol in hamsters fed a cholesterol- and lipid-enriched semi-purified diet. Lipids. 2007;42:135–141. doi: 10.1007/s11745-007-3023-y. [DOI] [PubMed] [Google Scholar]
- 23.Ribot J, Portillo MP, Picó C, Macurulla MT, Palou A. Effects of trans-10, cis-12 conjugated linoleic acid on the expression of uncoupling proteins in hamsters fed an atherogenic diet. 2007. [DOI] [PubMed] [Google Scholar]
- 24.Salter AM, Mangiapane EH, Bennett AJ, Bruce JS, Billett MA, Anderton KL, Marenah CB, Lawson N, White DA. The effect of different dietary fatty acids on lipoprotein metabolism: concentration-dependent effects of diets enriched in oleic, myristic, palmitic and stearic acids. Brit. J. Nutr. 1998;79:195–202. doi: 10.1079/bjn19980031. [DOI] [PubMed] [Google Scholar]
- 25.Major CA, Ryan K, Bennett AJ, Lock AL, Bauman DE, Salter AM. Inhibition of Stearoyl CoEnzyme A Desaturase Activity Induces Hypercholesterolemia in the Cholesterol-Fed Hamster. J. Lipid Res. 2008;49:1456–1465. doi: 10.1194/jlr.M700596-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Henderson L, Gregory J, Irving K, Swan G. The National Diet & Nutrition Survey: adults aged 10 to 64 years. Vol. 2. London: TSO; 2003. pp. 58–78. [Google Scholar]
- 27.Hayes KC, Pronczuk A, Khosla P. A rationale for plasma cholesterol modulation by dietary fatty acids: modeling the human response in animals. J. Nutr. Biochem. 1995;6:188–194. [Google Scholar]
- 28.Session VA, Salter AM. The effects of different dietary fats and cholesterol on serum lipoprotein concentrations in hamsters. Biochim. Biophys. Acta. 1994;1211:207–214. doi: 10.1016/0005-2760(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 29.Billett MA, Bruce JS, White DA, Bennett AJ, Salter AM. Interactive effects of dietary cholesterol and different saturated fatty acids on lipoprotein metabolism. Brit. J. Nutr. 2000;84:439–447. doi: 10.1017/s0007114500001744. [DOI] [PubMed] [Google Scholar]
- 30.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magana MM, Osborne TF. Two tandem binding sites for sterol regulatory element binding proteins are required for sterol regulatory element binding proteins are required for sterol regulation of fatty-acid synthase promoter. J. Biol. Chem. 1996;271:32689–32694. doi: 10.1074/jbc.271.51.32689. [DOI] [PubMed] [Google Scholar]
- 32.Bennett MK, Toth JI, Osborne TF. Selective association of sterol regulatory element-binding protein isoforms with target promoters in vivo. J. Biol. Chem. 2004;279:37360–37367. doi: 10.1074/jbc.M404693200. [DOI] [PubMed] [Google Scholar]
- 33.Amemiya-Kudo M, Shimano H, Hasty AH, Yahagi N, Yoshikawa T, Matsuzaka T, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, Osuga J-I, Harada K, Gotoda T, Sato R, Kimura S, Ishibashi S, Yamada N. Transcriptional activities of nuclear SREBP-1a, -1c, and -2 to different target promoters of lipogenic and cholesterogenic genes. J. Lipid Res. 2002;43:1220–1235. [PubMed] [Google Scholar]
- 34.Schoonjans K, Gelman L, Haby C, Briggs M, Auwerx J. Induction of LPL gene expression by sterols is mediated by a sterol regulatory element and is independent of the presence of multiple E boxes. J. Mol. Biol. 2000;304:323–334. doi: 10.1006/jmbi.2000.4218. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Repa JJ, Gauthier K, Mangelsdorf DJ. Regulation of lipoprotein lipase by the oxysterol receptors LXRα and LXRβ. J. Biol. Chem. 2001;276:43018–43024. doi: 10.1074/jbc.M107823200. [DOI] [PubMed] [Google Scholar]
- 36.Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Gu J, Sundseth SS, Winegar DA, Blanchard DE, Spencer TA, Wilson TM. Activation of the nuclear receptor LXRα by oxysterols defines a new hormone response pathway. J. Biol. Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 37.Costet P, Luo Y, Wang N, Tall AR. Sterol-dependent transactivation of the the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol Chem. 2000;275:28240–28245. doi: 10.1074/jbc.M003337200. [DOI] [PubMed] [Google Scholar]
- 38.Talukdar S, Hillgartner FB. The mechanism mediating the activation of acetyl-coenzyme A carboxylase-alpha gene transcription by the liver X receptor agonist T0-901317. J. Lipid Res. 2006;47:2451–2461. doi: 10.1194/jlr.M600276-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Joseph SB, Laffitte BA, Patel PH, Watson MA, Matsukuma KE, Walczak R, Collins JL, Osborne TF, Tontonoz P. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J. Biol. Chem. 2002;277:11019–11025. doi: 10.1074/jbc.M111041200. [DOI] [PubMed] [Google Scholar]
- 40.Simón E, Macarulla MT, Churruca I, Fernández-Quintela A, Portillo MP. trans-10,cis-12 Conjugated linoleic acid prevents adiposity but not insulin resistance induced by an atherogenic diet in hamsters. J. Nutr. Biochem. 2006;17:126–131. doi: 10.1016/j.jnutbio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Valeille K, Gripois D, Blouquit M-F, Souidi M, Riottot M, Bouthegourd J-C, Sérougne C, Martin J-C. Lipid atherogenic risk markers can be more favourably influenced by cis-9,trans-11-octadecadienoate isomer than a conjugated linoleic acid mixture or fish oil in hamsters. Brit. J. Nutr. 2004;91:191–199. doi: 10.1079/BJN20031057. [DOI] [PubMed] [Google Scholar]
- 42.Tricon S, Burdge GC, Kew S, Banerjee T, Russell JJ, Jones EL, Grimble RF, Williams CM, Yaqoob P, Calder PC. Opposing effects of cis-9,trans-11 and trans-10,cis-12 conjugated linoleic acid on blood lipids in healthy humans. Am. J. Clin. Nutr. 2004;80:614–20. doi: 10.1093/ajcn/80.3.614. [DOI] [PubMed] [Google Scholar]
- 43.Arbonés-Mainar JM, Navarro MA, Acín S, Guzmán MA, Arnal C, Surra JC, Carnicer R, Roche HM, Osada J. Trans-10, cis-12- and cis-9, trans-11-conjugated linoleic acid isomers selectively modify HDL-apolipoprotein composition in apolipoprotein E knockout mice. J. Nutr. 2006;136:353–359. doi: 10.1093/jn/136.2.353. [DOI] [PubMed] [Google Scholar]