Abstract
The tensor tympani is one of two middle ear muscles that regulates the transmission of sound through the middle ear. Contraction of the tensor tympani in response to both auditory and non-auditory stimulation is mediated by the tensor tympani motoneurons (TTMNs). There are interesting differences among species in the acoustic thresholds for contraction of the middle ear muscles, which may be a reflection of underlying anatomical differences such as the number of TTMNs. However anatomical data for mice are lacking, even though the mouse is becoming the most common animal model for auditory and neuroscience research. We investigated the number and morphology of TTMNs in mice using Fluorogold, a retrograde neuronal tracer. After injections of Fluorogold into the tensor tympani muscle, a column of labeled TTMNs was identified ventro-lateral to the ipsilateral trigeminal nucleus. The labeled TTMNs were classified according to their morphological characteristics into three subtypes: “octopus-like”, “fusiform” and “stellate”, suggesting underlying differences in function. All three subtypes formed sparsely branched and radiating dendrites, some longer than 600 μm. Dendrites were longest and most numerous in the dorso-medial direction. In 18 cases, the mean number of mouse TTMNs was 51; the largest numbers were 70, 74 and 90 (n=3 injections). The mean size of mouse TTMNs was 13.0 μm (minor axis) and 23.5 μm (major axis). Compared with studies of TTMNs in larger species (cats and rats), mouse TTMNs are both fewer in number and smaller in size.
Keywords: Middle ear, Pons, Auditory reflex, Trigeminal nucleus, Dendrite, Retrograde tracer, Synaptic input
1. Introduction
The tensor tympani (TT) and the stapedius are two muscles that control the transmission of sound through the middle ear. TT motoneurons (TTMNs) are located near the motor nucleus of the trigeminal nerve (Spangler et al., 1982; Rouiller et al., 1986). They project to the striated muscle fibers of the TT via the “nerve to the tensor tympani”, which is a branch of the mandibular division of the trigeminal nerve. The TT may perform a protective role by preventing overstimulation of the inner ear from self-generated noises during vocalization and chewing (Klockhoff and Anderson, 1960; Stach et al., 1984). The TT is also known to contract during swallowing (Salomon & Starr, 1963) and facial skin contact(Djupesland, 1964). In humans and rabbits, the stapedius plays the major role in middle ear muscle contraction in response to sound (Borg, 1972; Brask, 1979; Ochi et al., 2002;). There is a large, species-dependent range in acoustic threshold for middle ear muscle contraction. Rabbits and rats have very low acoustic thresholds (Borg and Moller, 1968; Relkin et al., 2005) and guinea pigs have very high thresholds (Avan et al., 1992).
Species differences in middle-ear muscle properties almost certainly are a reflection of underlying differences in anatomical characteristics. The light microscopic features of TTMNs have been studied in cats, guinea pigs and rats (Lyon, 1975; Spangler et al., 1982; Mizuno et al., 1982; Friauf and Baker, 1985; Rouiller et al., 1986), but not in mice. The mouse is an important species because of the availability of genetically altered strains in neuroscience research (Ollo and Schwartz, 1979; Vetter et al., 1999). Its relatively short lifespan renders mice preferable for studies on the effects of aging (Zettel et al., 2007). Furthermore, the very high frequency range of mouse hearing (Fay, 1988) vs. the generally low-frequency effects of middle ear muscle contraction (Nuttall, 1974) makes it interesting to speculate on the functional roles of middle ear muscles in this species.
Based on the mouse being a relatively smaller animal, we hypothesize that mouse TTMNs are “downscaled” in both quantity and size compared to other species. Previous morphological studies of mouse auditory pathways have shown that mice have a reduced number of auditory nerve fibers (about 12,350; Ehret, 1979) compared to other animals (Table 1). Relative to other species, mice also have fewer medial olivocochlear (MOC) neurons(Campbell and Henson, 1988) (Table 1), which form a descending pathway from the superior olivary complex to the cochlea and which, like the middle ear muscle system, regulates peripheral auditory processing. In our study, we use Fluorogold (FG), a retrograde tracer, to label TTMNs and their dendrites (Schmued and Fallon, 1986). We concentrated on the dendrites of TTMNs because ultrastructural studies (Lee et al., 2009) reveal that most of the synaptic input to these neurons appears on dendrites. We aim to present the first systematic morphological and dendritic analysis of TTMNs in mice to further our understanding of the anatomy of the TT neural circuitry.
Table 1.
Inter species comparison of numbers of auditory-nerve fibers (ANF), medial olivocochlear (MOC) neurons and tensor tympani moto neurons (TTMNs)
| Mouse | Rat | Guinea Pig | Cat | |
|---|---|---|---|---|
| ANF | 12350a | 15800b | 24011c | 51000c |
| MDC neurons | 166d | 398e | 1025f | 415g |
| TTMN | 78h | 165i | 530j | 756j |
(Keithley and Feldman, 1961)
(Present Study)
2. Results
2.1 Distribution and number of labeled TTMNs
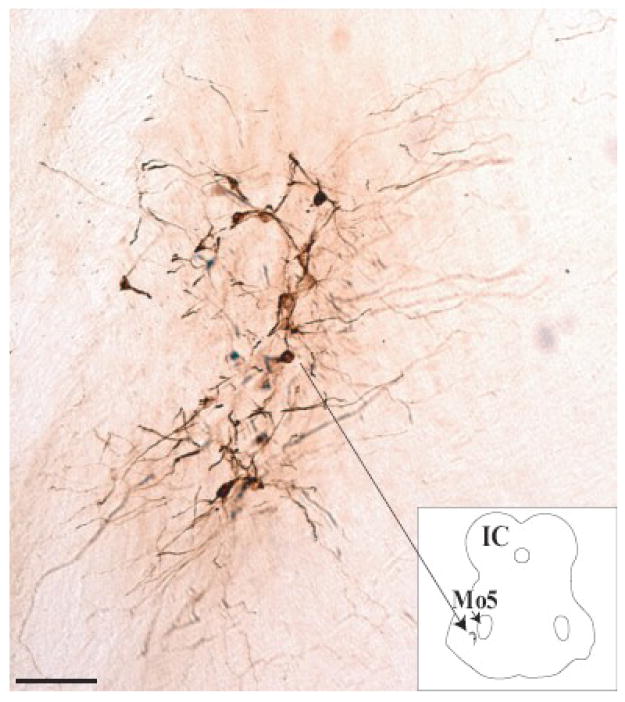
FG labeled TTMNs were stained a dark brown color. They were arranged as a narrow curvi-linear column located ventro-lateral to the ipsilateral trigeminal motor nucleus (Fig. 1). In all 18 injections, the dorsal horn of the column curved more than the ventral horn. The column began in proximity to the rostral pole of the facial motor nucleus and extended to the level of the inferior colliculus. Labeled TTMNs were found on the left side of the brainstem, which was the same side as the injection. In 13 of the 18 cases, incidental labeling of non-TTMN structures was found caudal to the trigeminal motor nucleus, within the ipsilateral facial motor nucleus, the inferior salivatory nucleus and the parvicellular reticular tract. We did not include this labeling in our counts.
Fig. 1.
Micrograph of representative FG labeled TTMNs: Labeled TTMNs were organized as a curvi-linear column ventro-lateral to the trigeminal motor nucleus: Mo5 (see inset). IC: Inferior Colliculus. Scale bar: 100 μm.
The average number of labeled TTMNs in 18 injected cases was 51 (n=920 total neurons). In our three cases with the most labeling, there were 70, 74, and 90 (avg.= 78) TTMNs.
2.2 TTMN subtypes
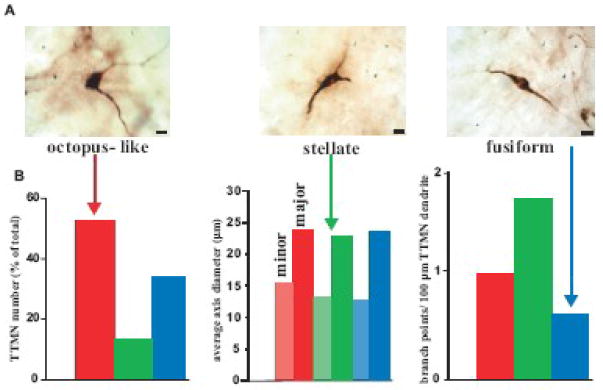
Labeled TTMNs exhibited different morphologies (Fig. 2A). Each TTMN was categorized into one of three subtypes, based on the number and orientation of the primary dendrites that projected from the cell body. In our study, we used the following criteria: “Stellate” TTMNs had three or more dendrites projecting from the cell body. “Octopus-like” TTMNs had two dendrites projecting from one side of the cell body. “Fusiform” TTMNs had two dendrites projecting from opposing poles of the cell body. The distinction between the octopus-like and fusiform subtypes was usually clear but we acknowledge the presence of a small number of intermediates. Stellate TTMNs were very distinct from the other subtypes. The average number of primary dendrites originating from the cell body of a stellate TTMN was 3.5 (n=30 stellate TTMNs). The highest number of primary dendrites that projected from a cell body was 5. From our total sample of 945 classified TTMNs, octopus-like TTMNs were the most common (52% of the total sample), followed by fusiform (35%) and stellate (13%) (Fig. 2B, left graph).
Fig. 2.
A - Micrographs of the three TTMN subtypes; “octopus- like”, “fusiform” and “stellate”. Scale bars: 10 μm. B - Left graph shows the percentage of each TTMN subtype in 729 labeled TTMNs from 14 cases; 52% were octopus-like (red bar), 7% were stellate (green bar) and 41% were fusiform (blue bar). Middle graph shows the average minor axis diameter (light color) and major axis diameter (dark color) of each TTMN subtype; octopus- like: 15.0 μm (n=50) and 24.0 μm (n=122), stellate: 13.5 μm (n=20) and 22.8 μm (n=34), fusiform: 12.6 μm (n=50) and 23.6 μm (n=102). Right graph shows the average number of branch points in the first 100 μm of primary dendritic length for each TTMN subtype; octopus like: 0.98 (n=50), stellate: 1.67 (n=50) and fusiform: 0.6 (n=20).
The average minor axis diameter of 334 labeled TTMNs was 13.0 μm, and the average major axis diameter of 258 labeled TTMNs was 23.5 μm. The minor and major axis average sizes are plotted according to subtype in Fig. 2B, middle graph. There was no statistical difference in cell size (one way ANOVA; F=5.469, p = 0.005) between TTMN subtypes. The average minor axis diameter of octopus-like shaped TTMNs (15.5 μm; n=53 octopus-like TTMNs) was only slightly larger than the average minor axis diameters of both fusiform (12.7 μm; n=53 fusiform TTMNs) and stellate TTMNs (13.0 μm; n=20 stellate TTMNs). The average major axis diameter of octopus-like TTMNs (24 μm; n=122 octopus-like TTMNs) was similar to the average major axis diameters of fusiform (23.6 μm; n=102 fusiform TTMNs) and only slightly larger than the average major axis diameter of stellate TTMNs (22.8 μm; n=34 TTMNs). Plots of major vs. minor axis did not separate the TTMNs into subtypes (data not shown). There was also no clear relationship between a TTMN subtype and its position along the rostro-caudal axis of labeled TTMNs. Instead, the three TTMN subtypes were distributed evenly within the populations of TTMNs throughout the sections (data not shown).
2.3 TTMN dendrites
Labeled TTMN dendrites were long, radiating and sparsely branched (Fig. 3). They never entered the borders of the trigeminal motor nucleus, instead touching only the most lateral and dorsal edges.
Fig. 3.
FG –labeled TTMNs with dendrites extending medially beyond the pool of labeled TTMNs. These TTMNs were located on the left side of the brainstem so the medial direction is toward the right. This section is located along the rostral-caudal distribution of labeled TTMNs at the position about 1/3 from the caudal end. Micrograph is a montage of two images taken of a single section at different focal planes. Scale bar: 50 μm.
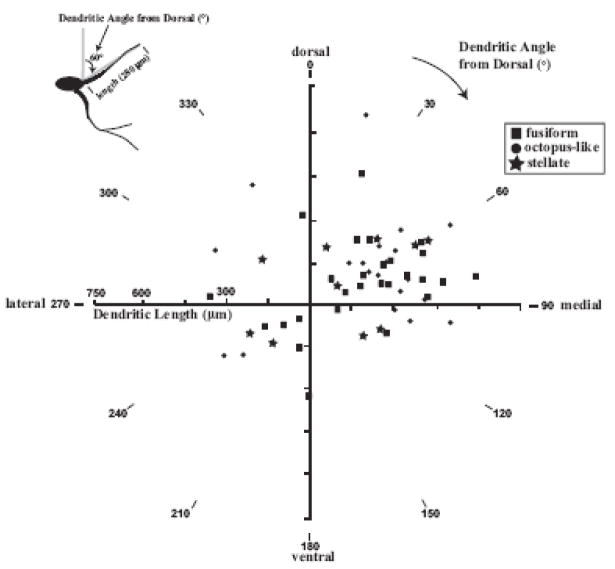
We reconstructed 57 dendrites from 18 cases across all three TTMN subtypes that could be measured in their entirety from soma to tip without fading. The lengths are plotted as a function of their orientation in polar format (Fig. 4). The lengths of all 57 reconstructed TTMN dendrites ranged from 154 μm to 650 μm, with an average length of 362 μm. Most of the reconstructed dendrites traveled dorso-medially (n=37 reconstructed TTMN dendrites) compared to the other quadrants; ventro-medial (n=7), ventro-lateral (n=8) and dorso-lateral (n=5).
Fig. 4.
Polar plot showing the lengths (μm) and dendritic angles (°) of 58 reconstructed FG- labeled dendrites across the three TTMN subtypes; octopus- like (circles), stellate (stars) and fusiform (squares). Dendrites directed dorso-medially were more numerous and were the longest in length. Inset shows measurement of dendritic angle by drawing a line from soma to dendrite tip and measuring the angle of this line with respect to the dorsal direction.
The amount of branching of the primary dendrite varied with each TTMN subtype. In 120 labeled TTMN dendrites, the average number of branch points within the first 100 μm of primary dendrite length was counted (Fig. 2B, right graph). Stellate TTMN dendrites branched the most with an average of 1.67 branch points (n=50 stellate TTMN dendrites) per 100 μm of primary dendritic length. Fusiform TTMN dendrites exhibited the least branching with an average of 0.6 branch points (n=20 fusiform TTMN dendrites). Octopus-like TTMN dendrites had 0.98 branch points (n=50 octopus-like TTMN dendrites).
3. DISCUSSION
3.1 Distribution and number of TTMNs
The distribution of labeled TTMNs in mice was similar to that of other species (Lyon, 1978; Strominger et al., 1981; Spangler et al., 1982; Rouiller et al., 1986). They were located ventro-laterally to the ipsilateral trigeminal motor nucleus as a spatially restricted, narrow and well defined curvi-linear column that was distinct from the trigeminal motor nucleus. The column extended along the rostro-caudal axis of the trigeminal motor nucleus. Our findings agree with past experiments showing that labeled TTMNs are found just outside the trigeminal motor nucleus(Lyon, 1975; Keller et al., 1983; Shaw and Baker, 1983;Spangler et al., 1982). Previous studies have referred to this pool of labeled TTMNs as being distinct from the trigeminal motor nucleus in terms of cell body size, location and function(Friauf and Baker, 1985). The pool of TTMNs has therefore been termed as being a separate “tensor tympani motor nucleus of V” rather than an accessory of the trigeminal motor nucleus). We attribute the incidental labeling of other brainstem structures to the uptake of FG by structures closely related to either the TT or the trigeminal nerve. Incidental labeling was seen in non-TTMN structures such as the ipsilateral facial motor nucleus, inferior salivatory nucleus and the parvicellular reticular tract. In previous TTMN labeling studies using HRP in rats, labeling in the salivatory nucleus was explained by the incidental uptake of FG by the lesser petrosal nerve (a branch of the 9th cranial nerve) which travels in close proximity to the TT (Rouiller et al., 1986). The lesser petrosal nerve passes through the same bony canal as the TT muscle, en route to the otic ganglion(Spangler et al., 1982). Labeling in the facial motor nucleus may be due to the uptake of FG by either the 7th cranial nerve or the visceral efferent fibers in the chorda tympani nerve, a branch of the 7th cranial nerve.
There are fewer mouse TTMNs compared to other larger species (Table 1). The number of labeled TTMNs from mouse, for the cases with the most labeling that are probably the most representative, was between 70 and 90. Mice have 45% less TTMNs than rat, 83% less TTMNs than guinea pig and 88% less TTMNs than cat (see Table 1 for references). Smaller numbers of TTMNs in mice is paralleled by smaller numbers of auditory nerve fibers and MOC neurons. Additionally, the decline may be partly explained by the middle ear muscle system acting on low frequencies but the mouse’s hearing range being biased toward high frequencies. Fewer neurons do not necessarily reflect diminished importance, and anatomical studies such as the present one are restricted in terms of the limitation of extrapolations that can be made. Finally, our results might predict large numbers of TTMNs in humans given their large body and brain size.
3.2 Three TTMN Subtypes
Based on certain morphological characteristics, we separate labeled mouse TTMNs into distinct subtypes, called octopus-like, fusiform, and stellate TTMNs. A few intermediates between octopus-like and fusiform subtypes may exist. Octopus-like TTMNs were the most common and stellate TTMNs were the least common. We used this classification scheme because it fit our results better than previous schemes used in studies of other species. Fusiform, “elongated” and “lens-shaped” TTMNs were more common in cats and “ovoidal” and “pyramidal” TTMNs were the least common in cats (Friauf and Baker, 1985). “Stellate” TTMNs were more commonly seen in rats. These subtypes may reflect differences in TTMN function similar to that seen in the cochlear nucleus (Kiang et al., 1973; Rhode et al., 1983; Rouiller and Ryugo, 1984; Adams, 1986). For example, varying morphologies might account for the multiple functions of TTMNs in response to auditory vs. non-auditory stimuli. Alternatively, the different subtypes might innervate different types of muscle fibers, such as the slow, fast and medium twitch fibers. On histochemical analysis, the TT of rats is known to consist mainly of fast oxidative glycolytic fibers (van den Berge and Wirtz, 1989), but mouse TT fibers have not been studied.
3.3 TTMNs in mice are relatively small
Our study shows that mouse TTMNs are smaller than TTMNs in other species such as rats and cats. The average major axis diameters of our labeled TTMNs was about 5% smaller than rat (average major axis diameter = 25 μm; Billig et al., 2007) and about 35% smaller than cat (average major axis diameter=34.87 μm; Friauf and Baker, 1985). Our octopus-like TTMNs were comparable to TTMNs in other species but our stellate and fusiform TTMNs were much smaller.
3.4 TTMN dendrites are long and sparsely branched
The study of dendrites is important because the orientation and distribution of dendritic spread have been to shown to influence neuronal response properties (Sotnikov, 2005; Berkowitz et al., 2006; Hickmott and Ethell, 2006; Saxon and Hopkins, 2006; Torres-Fernandez et al., 2007; Bergquist and Ludwig, 2008). Furthermore, proximal dendrites of TTMNs receive abundant synaptic input, but there is sparse innervation of the TTMN soma(Lee et al., 2009). Distal dendrite synapses have not been investigated, but since the distal dendrites project extensively (Friauf and Baker, 1985), they present a large surface area on which to receive inputs. Such inputs come from the cochlear nucleus (Billig et al., 2007), from serotoninergic sources(Thompson et al., 1998), and presumably from motor control areas. This may be reflected in the diversity of TT activity in response to both auditory and non-auditory stimuli.
The prominent dendritic labeling that was achieved using FG allowed us to appreciate specific characteristics of TTMN dendrites in mice. Mouse TTMN dendrites extended for long distances beyond the region of labeled cell bodies. This was similar to TTMN dendrites in cats (Friauf and Baker, 1985). In both species, TTMN dendrites avoided crossing into the trigeminal motor nucleus (Friauf and Baker, 1985). Perhaps the distinct organization of TTMN dendrites reflects underlying physiological differences between the pool of TTMNs and the motoneurons of the trigeminal motor nucleus (Friauf and Baker, 1985).
Our study is the first to systematically quantify the TTMN dendrites and also to measure their lengths and angle of orientation. The dendrites extended in a radial pattern in all directions from the column of labeled TTMNs. However, dendrites directed dorso-medially were more numerous and were the longest in length. TTMN dendrites in cats were longest in the ventro-medial direction(Friauf and Baker, 1985). A similar numerical and length bias is present for dendrites of MOC neurons(Brown and Levine, 2008). MOC neurons receive dominant inputs from the opposite side onto their medially projecting dendrites; the similar numerical and length bias suggest that this is also true for TTMNs. Future work will determine the types and richness of the synaptic inputs onto the distal dendrites of TTMNs.
4. Experimental Procedures
4.1 Animals
A total of 18 mice were used. The CBA/CaJ mouse strain was used because of their excellent hearing thresholds (Yoshida et al., 2000). The mice were 3–6 months old and weighed between 25–35 grams. All experimental procedures on animals were performed in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals and were performed under approved protocols at the Massachusetts Eye & Ear Infirmary.
4.2 Retrograde labeling
Mice were anesthetized with Ketamine (100 mg/kg) and Xylazine (20 mg/kg). Boosters of Ketamine and Xylazine were administered as needed. The level of anaesthesia was monitored by paw-pinch withdrawal reflex and response to surgical manipulation. In order to label TTMNs, a left postauricular surgical approach with partial removal of the bulla was used for exposure of the middle ear. Flourogold (FG, hydroxystilbamidine, Fluorochrome, Denver, CO) was injected into the muscle belly of the left TT. Fifteen injections were made with glass pipettes (inner diameter=0.6 mm) filled with 1–2 μl FG. The tip of the glass pipette was inserted into the muscle and remained there for 1–2 minutes to allow for passive infiltration of FG into the TT. To test a different method of injection, another 3 mice were injected with the 10 μl Hamilton syringe (inner diameter=0.33 mm) filled with 1 μl FG. The tip of the syringe was inserted through the muscular sheath and into the medial belly of the TT muscle. The FG was then pressure injected slowly and the muscle was seen to swell with the administration of FG. The numbers of labeled TTMNs from the syringe-injected cases was generally smaller than the pipette cases, but this difference was not statistically significant (t-test; t = 0.219). Dental pledgets were used to minimize secondary spread into the surrounding structures of the middle ear.
Following surgical closure and recovery, the animals underwent a 7–9 day survival period prior to perfusion. After the survival period, the animals were re-anesthetized and perfused transcardially with physiological saline followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.2). After post fixing for approximately 1 h, brainstems were dissected out and blocked immediately rostral to the inferior colliculus and caudal to the cochlear nuclei. A pin was used to mark the right side of the dissected brainstem. The brainstem was placed in fixative solution for another 1 hr, before it was put into 30% sucrose for 1–2 days.
The following day, transverse sections of 80 μm thickness were cut on a freezing microtome and placed into phosphate buffer solution (PBS). All sections were collected from a point caudal to the dorsal cochlear nucleus extending to just rostral to the inferior colliculus. Sections were incubated with 1:100,000 anti-FG rabbit IgG fraction (Fluorochrome, Denver, CO) for 24 hours. Sections were then incubated in 1:1000 biotinylated goat anti-rabbit secondary antibody solution (Vector Labs). Standard immunohistochemistry technique using DAB (diaminobenzidine) solution was used to visualize reaction product.
4.3 Morphological measurements
A light microscope fitted with a camera lucida was used to identify and draw the labeled neurons. Images (Figs. 1, 2A, 3) were acquired using a Hamamatsu CCD (Charged-Coupled Device) digital camera. A labeled neuron was counted if the cell body was judged entirely or for the most part within that section, and every labeled neuron was counted. No correction was made for the possibility of counting the same labeled neuron multiple times in adjacent sections. Labeled neurons were traced onto paper using the camera lucida tube on a light microscope with 40X objectives. The major axis diameter was measured along the longest axis of the labeled cell body. The minor axis diameter was measured as a line perpendicular to the major axis of the labeled cell body.
The sample of dendrites was composed of dendrites that were not intertwined with other labeled elements. Dendrites that split into two processes within 10 μm of the soma were considered two separate dendrites. Dendrites were not used if their reaction product became very light towards the ends of the dendrite, giving the dendritic tips a faded appearance. Dendrites were not used if they became entangled with other dendrites or exited the plane of section. The reconstructed dendrites were traced onto paper and the dendritic angle was measured using a protractor by drawing a line from soma to dendrite tip, using the angle of this line with respect to the dorsal direction (Fig. 4, inset). For all sections, the dorsal/ventral direction was defined as parallel to the midline of the section. Dendrite length was taken as the length of the entire course of the dendrite from soma to tip measured using a flexible contour against a ruler. Statistical tests were conducted at the 5% level of significance.
Acknowledgments
This study was supported by NIDCD grants RO1 DC01089, K08 DCO6285 and The Norwegian State Lending Agency.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature references
- Adams JC. Neuronal morphology in the human cochlear nucleus. Arch Otolaryngol. 1986;112:1253–1261. doi: 10.1001/archotol.1986.03780120017003. [DOI] [PubMed] [Google Scholar]
- Aschoff A, Ostwald J. Different origins of cochlear efferents in some bat species, rats and guinea pigs. J Comp Neurol. 1987;264:56–72. doi: 10.1002/cne.902640106. [DOI] [PubMed] [Google Scholar]
- Avan P, Loth D. Hypothetical roles of middle ear muscles in guinea-pig. Hear Res. 1992;59:59–69. doi: 10.1016/0378-5955(92)90102-s. [DOI] [PubMed] [Google Scholar]
- Bergquist F, Ludwig M. Dendritic transmitter release: a comparison of two model systems. J Neuroendocrinol. 2008;20:677–686. doi: 10.1111/j.1365-2826.2008.01714.x. [DOI] [PubMed] [Google Scholar]
- Berkowitz A, Yosten GL, Ballard RM. Somato-dendritic morphology predicts physiology for neurons that contribute to several kinds of limb movements. J Neurophysiol. 2006;95:2821–2831. doi: 10.1152/jn.01246.2005. [DOI] [PubMed] [Google Scholar]
- Billig I, Yeager MS, Blikas A, Raz Y. Neurons in the cochlear nuclei controlling the tensor tympani muscle in the rat: a study using pseudorabies virus. Brain Res. 2007;1154:124–136. doi: 10.1016/j.brainres.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg E, Moller AR. The acoustic middle ear reflex in unanesthetized rabbits. Acta Otolaryngol. 1968;65(6):575–585. doi: 10.3109/00016486809119292. [DOI] [PubMed] [Google Scholar]
- Borg E. On the use of acoustic middle ear muscle reflexes in studies of auditory function in nonanesthetized rabbits. Acta Otolaryngol. 1972;74(4):240–247. doi: 10.3109/00016487209128445. [DOI] [PubMed] [Google Scholar]
- Brask T. The noise protection effect of the stapedius reflex. Acta Otolaryngol Suppl. 1979;360:116–117. doi: 10.3109/00016487809123490. [DOI] [PubMed] [Google Scholar]
- Brawer JR, Morest DK, Kane EC. The neuronal architecture of the cochlear nucleus of the cat. J Comp Neurol. 1974;155:251–300. doi: 10.1002/cne.901550302. [DOI] [PubMed] [Google Scholar]
- Brown MC, Levine JL. Dendrites of medial olivocochlear neurons in mouse. Neuroscience. 2008;154:147–159. doi: 10.1016/j.neuroscience.2007.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JP, Henson MM. Olivocochlear neurons in the brainstem of the mouse. Hear Res. 1988;35:271–274. doi: 10.1016/0378-5955(88)90124-4. [DOI] [PubMed] [Google Scholar]
- Djupesland G. Middle ear muscle reflexes elicited by acoustic and non-acoustic stimulation. Acta Otolaryngol Suppl. 1964;188:287–288. doi: 10.3109/00016486409134578. [DOI] [PubMed] [Google Scholar]
- Ehret G. Quantitative analysis of nerve fibre densities in the cochlear of the house mouse (Mus musculus) J Comp Neurol. 1979;183:73–88. doi: 10.1002/cne.901830107. [DOI] [PubMed] [Google Scholar]
- Evans EF. Cochlear Nerve and Cochlear Nucleus. In: Keidel WD, Neff WD, editors. Handbook of Sensory Physiology. 2. Vol. 5. Springer Verlag; Berlin: 1975. pp. 1–108. [Google Scholar]
- Fay RR. Hearing in Vertebrates: a Psychophysics Databook. Worcester, MA: Heffernan Press; 1988. [Google Scholar]
- Friauf E, Baker R. An intracellular HRP-study of cat tensor tympani motoneurons. Exp Brain Res. 1985;57:499–511. doi: 10.1007/BF00237837. [DOI] [PubMed] [Google Scholar]
- Gacek RR, Rasmussen GL. Fiber analysis of the statoacoustic nerve of guinea pig, cat, and monkey. Anat Rec. 1961;139:455–463. doi: 10.1002/ar.1091390402. [DOI] [PubMed] [Google Scholar]
- Hackney CM, Osen KK, Kolston J. Anatomy of the cochlear nuclear complex of the guinea pig. Anat Embryol. 1990;182:123–149. doi: 10.1007/BF00174013. [DOI] [PubMed] [Google Scholar]
- Hickmott PW, Ethell IM. Dendritic plasticity in the adult neocortex. Neuroscientist. 2006;12:16–28. doi: 10.1177/1073858405282417. [DOI] [PubMed] [Google Scholar]
- Howe HA. The reaction of cochlear nerves to destruction of its end organs: a study on deaf albino cats. J Comp Neurol. 1935;62:73–79. [Google Scholar]
- Horvath M, Kraus KS, Illing RB. Olivocochlear neurons sending axon collaterals into the ventral cochlear nucleus of the rat. J Comp Neurol. 2000;422:95–105. [PubMed] [Google Scholar]
- Hutson KA, Glendenning KK, Masterton RB. Accessory abducens nucleus and its relationship to the accessory facial and posterior trigeminal nuclei in cat. J Comp Neurol. 1979;188:1–16. doi: 10.1002/cne.901880102. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Feldman ML. Spiral ganglion cell counts in an age-graded series of rat cochleas. J Comp Neurol. 1979;188:429–442. doi: 10.1002/cne.901880306. [DOI] [PubMed] [Google Scholar]
- Keller JT, Saunders MC, Ongkiko CM, Johnson J, Frank E, Van Loveren H, Tew JM., Jr Identification of motoneurons innervating the tensor tympani and tensor veli palatini muscles in the cat. Brain Res. 1983;270:209–215. doi: 10.1016/0006-8993(83)90594-2. [DOI] [PubMed] [Google Scholar]
- Klockhoff I, Anderson H. Reflex activity in the tensor tympani muscle recorded in man; preliminary report. Acta Otolaryngol. 1960;51:184–188. doi: 10.3109/00016486009124480. [DOI] [PubMed] [Google Scholar]
- Kiang NYS, Morest DK, Godfrey DA, Guinan JJ, Kane EC. Stimulus coding at caudal levels of the cat’s auditory system: Response characteristics of single units. In: Moller AR, editor. Basic Mechanisms in Hearing. Academic Press, Inc; New York: 1973. pp. 455–478. [Google Scholar]
- Lee DJ, Brown MC, Benson TE. Ultrastructure features of inputs to tensor tympani motoneurons in rats. Assoc for Res in Otolaryngol. 2009 Abstract # 552. [Google Scholar]
- Lyon MJ. Localization of the efferent neurons of the tensor tympani muscle of the newborn kitten using horseradish peroxidase. Exp Neurol. 1975;49:439–455. doi: 10.1016/0014-4886(75)90100-4. [DOI] [PubMed] [Google Scholar]
- Lyon MJ. The central location of the motor neurons to the stapedius muscle in the cat. Brain Res. 1978;143:437–444. doi: 10.1016/0006-8993(78)90355-4. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Nomura S, Konishi A, Uemura-Sumi M, Takahashi O, Yasui Y, Takada M, Matsushima R. Localization of motoneurons innervating the tensor tympani muscles: a horseradish peroxidase study in the guinea pig and cat. Neurosci Lett. 1982;31:205–208. doi: 10.1016/0304-3940(82)90020-9. [DOI] [PubMed] [Google Scholar]
- Nadol JB., Jr Comparative anatomy of the cochlea and auditory nerve in mammals. Hear Res. 1988;34:253–266. doi: 10.1016/0378-5955(88)90006-8. [DOI] [PubMed] [Google Scholar]
- Nuttall AL. Measurements of the guinea-pig middle-ear transfer characteristic. J Acoust Soc Am. 1974;56:1231–1238. doi: 10.1121/1.1903413. [DOI] [PubMed] [Google Scholar]
- Ochi K, Ohashi T, Kinoshita H. Acoustic tensor tympani response and vestibular-evoked myogenic potential. Laryngoscope. 2002;112:2225–2229. doi: 10.1097/00005537-200212000-00018. [DOI] [PubMed] [Google Scholar]
- Ollo C, Schwartz IR. The superior olivary complex in C57BL/6 mice. J Comp Neurol. 1979;155:349–373. doi: 10.1002/aja.1001550306. [DOI] [PubMed] [Google Scholar]
- Osen KK. Cytoarchitecture of the cochlear nuclei in the cat. J Comp Neurol. 1969;136:453–484. doi: 10.1002/cne.901360407. [DOI] [PubMed] [Google Scholar]
- Rasmussen AT. Studies of the VIIIth cranial nerve of man. Laryngoscope. 1940;50:67–83. [Google Scholar]
- Relkin EM, Sterns W, Azeredo WJ, Prieve BA, Woods CI. Physiological mechanisms of onset adaptation and contralateral suppression of DPOAEs in the rat. J Assoc Res Otolaryngol. 2005;6:119–135. doi: 10.1007/s10162-004-5047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode WS, Smith PH, Oertel D. Physiological response properties of cells labeled intracellularly with horseradish peroxidase in cat dorsal cochlear nucleus. J Comp Neurol. 1983;213:426–447. doi: 10.1002/cne.902130407. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Ryugo DK. Intracellular marking of physiologically characterized cells in the ventral cochlear nucleus of the cat. J Comp Neurol. 1984;225:167–186. doi: 10.1002/cne.902250203. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Capt M, Dolivo M, de Ribaupierre F. Tensor tympani reflex pathways studied with retrograde horseradish peroxidase and transneuronal viral tracing techniques. Neurosci Lett. 1986;72:247–252. doi: 10.1016/0304-3940(86)90521-5. [DOI] [PubMed] [Google Scholar]
- Salomon G, Starr A. Electromyography of middle ear muscles in man during motor activities. Acta Neurol Scand. 1963;39:161. doi: 10.1111/j.1600-0404.1963.tb05317.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gonzalez MA, Warr WB, Lopez DE. Anatomy of olivocochlear neurons in the hamster studied with FluoroGold. Hear Res. 2003;185:65–76. doi: 10.1016/s0378-5955(03)00213-2. [DOI] [PubMed] [Google Scholar]
- Saxon DW, Hopkins DA. Ultrastructure and synaptology of the paratrigeminal nucleus in the rat: primary pharyngeal and laryngeal afferent projections. Synapse. 2006;59:220–234. doi: 10.1002/syn.20233. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Fallon JH. Fluoro-Gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res. 1986;377:147–154. doi: 10.1016/0006-8993(86)91199-6. [DOI] [PubMed] [Google Scholar]
- Shaw MD, Baker R. The locations of stapedius and tensor tympani motoneurons in the cat. J Comp Neurol. 1983;216:10–19. doi: 10.1002/cne.902160103. [DOI] [PubMed] [Google Scholar]
- Sotnikov OS. Primary sensory neurons in the central nervous system. Morfologia. 2005;127:75–81. [PubMed] [Google Scholar]
- Spangler KM, Henkel CK, Miller IJ., Jr Localization of the motor neurons to the tensor tympani muscle. Neurosci Lett. 1982;32:23–7. doi: 10.1016/0304-3940(82)90223-3. [DOI] [PubMed] [Google Scholar]
- Stach BA, Jerger JF, Jenkins HA. The human acoustic tensor tympani reflex. A case report. Scand Audiol. 1984;13:93–9. doi: 10.3109/01050398409043046. [DOI] [PubMed] [Google Scholar]
- Strominger NL, Silver SM, Truscott T, Goldstein JC. Horseradish peroxidase identification of the motoneurons to the tensor tympani muscle in rhesus and owl monkeys. Soc Neurosci Abstr. 1981;7:54. [Google Scholar]
- Thompson AM, Thompson GC, Britton BH. Serotoninergic innervation of stapedial and tensor tympani motoneurons. Brain Res. 1998;787:175–178. doi: 10.1016/s0006-8993(97)01020-2. [DOI] [PubMed] [Google Scholar]
- Torres-Fernandez O, Yepes GE, Gomez JE. Neuronal dentritic morphology alterations in the cerebral cortex of rabies-infected mice: a Golgi study. Biomedica. 2007;27:605–613. [PubMed] [Google Scholar]
- Van den Berge H, Wirtz P. Detailed morphology of the tensor tympani muscle of the rat. An integrated light microscopical, morphometrical, histochemical, immunohistochemical and electron microscopical study in relation to function. J Anat. 1989;164:215–228. [PMC free article] [PubMed] [Google Scholar]
- Vetter DE, Liberman MC, Mann J, Barhanin J, Boulter J, Brown MC, Saffiote-Kolman J, Heinemann SF, Elgoyhen AB. Role of a9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron. 1999;23:93–103. doi: 10.1016/s0896-6273(00)80756-4. [DOI] [PubMed] [Google Scholar]
- Warr WB. Organization of olivocochlear efferent systems in mammals. In: Webster DB, Popper AN, Fay RR, editors. The Mammalian Auditory Pathway: Neuroanatomy. Springer-Verlag; New York: 1992. pp. 410–448. [Google Scholar]
- Warr WB, Beck Boche JE, Ye Y, Kim DO. Organization of olivocochlear neurons in the cat studied with the retrograde tracer cholera toxin-B. J Assoc Res Otolaryngol. 2002;3:457–478. doi: 10.1007/s10162-002-2046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren EH, III, Liberman MC. Effects of contralateral sound on auditory-nerve responses I. Contributions of cochlear efferents. Hear Res. 1989;37:89–104. doi: 10.1016/0378-5955(89)90032-4. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Hequembourg SJ, Atencio CA, Rosowski JJ, Libeman MC. Acoustic injury in mice: 129/SvEv is exceptionally resistant to noise-induced hearing loss. Hear Res. 2000;141:97–106. doi: 10.1016/s0378-5955(99)00210-5. [DOI] [PubMed] [Google Scholar]
- Zettel ML, Zhu X, O’Neill WE, Frisina RD. Age-related decline in Kv3.1b expression in the mouse auditory brainstem correlates with functional deficits in the medial olivocochlear efferent system. J Assoc Res Otolaryngol. 2007;8:280–293. doi: 10.1007/s10162-007-0075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]