Abstract
pH-sensitive polymeric micelles and nanogels have recently been developed to target slightly acidic extracellular pH environment of solid tumors. The pH targeting approach is regarded as a more general strategy than conventional specific tumor cell surface targeting approaches, because the acidic tumor microclimate is most common in solid tumors. When nanosystems are combined with triggered release mechanisms by endosomal or lysosomal acidity plus endosomolytic capability, the nanocarriers demonstrated to overcome multidrug resistance of various tumors. This review highlights recent progress of the pH-sensitive nanotechnology developed in Bae research group.
Keywords: Tumor extracellular pH, endosomal pH, pH double targeting, micelle, nanogel, block copolymers, sulfonamide, histidine
1. Introduction
Amongst various cancer therapies, chemotherapy is one of major treatment modalities along with debulking surgery [1-2]. Major challenges in chemotherapy are linked to toxicity on healthy proliferating cells and multidrug resistance (MDR) against anticancer drugs. The life threatening side effects caused by non-specific tissue distribution of the drugs has restricted the systemic high-dose strategy [3, 4]. Cancer cells except those having intrinsic resistance are sensitive to chemotherapy in the beginning but often develop acquired resistance upon repeated chemotherapy cycles [5-7]. The resistance initiated by a cytotoxic agent also extends cross-resistance to a wide range of drugs having different chemical structures and cellular targets [5-7]. Once the resistance appears, systemic high dose therapy becomes ineffective and more toxic and the resistance is further stimulated [5-7].
Tumor targeting approaches have been developed for improved efficacy and reduced toxicity by altering biodistribution of cancer drugs and by using specific cell surface interactions. Solid tumors are often characterized by overexpression of specific antigens or receptors on cell surfaces [8-13]. Antigens and receptors help in transmitting signals from the surrounding environment that are essential for the growth of tumor cells. Targeting antigens or receptors has been extensively investigated as an important delivery mode by using macromolecular or nanosized carriers to tumor cells. Nanodrug carriers attached with surface ligands or antibodies exploit these receptor-mediated uptake pathways that are recognized and internalized by the tumor cells [14-18]. However, these approaches have achieved limited success in clinic, most likely because of significant heterogeneity in both solid tumor cell types and cell surface markers [8-10]. Additionally, both the presence of antigens and the expression of receptors on surface of these tumor cells are transient and dynamic [8-13]. The heterogeneity of cancer cells may explain the reasons for the unexpected results of targeting strategy [19].
The extracellular pH (pHe) of normal tissues and blood pH are kept constant at pH 7.4 and their intracellular pH (pHi) at 7.2. However, in most tumors the pH gradient is reversed (pHi > pHe). Particularly, tumor pHe is lower than normal tissues [20-23]. Although there is a distribution in in vivo, pHe measurements made by using needle type microelectrodes on human patients having various solid tumors (adenocarcinoma, squamous cell carcinoma, soft tissue sarcoma, and malignant melanoma) and in readily accessible areas (limbs, neck, or chest wall), shows the mean pH value to be 7.0 with a range between 5.7−7.8 [22]. This variation is dependent upon tumor histology, tumor volume, and location inside a tumor. Recent measurements of pHe by noninvasive technology such as 19F, 31P, or 1H probes by magnetic resonance spectroscopy in human tumor xenografts and in animals further proved consistently low pHe [23, 24]. Reported pHe data on human and animal solid tumors either by invasive or noninvasive methods showed that more than 80% of all measured values are below pH 7.2 [23, 24]. The primary reason for this imbalance in cancer pH is the high rate of glycolysis in cancer cells, both in aerobic and anaerobic conditions [25-27]. It is also proposed that the acidic milieu benefits the cancer cells by generating an invasive environment that tears down the extracellular matrix and destroys the surrounding normal tissue cells [28].
There are a variety of mechanisms associated with MDR cells that need to be circumvented for a successful tumor treatment [29-41]. At unicellular level, ATP dependent drug-efflux pumps of P-glycoprotein (Pgp), multidrug resistance protein (MRP), lung resistance protein (LRP), antiapototic (or survival) bcl-2 gene, and altered expression of Topoisomerase II interfere with a sufficient intracellular drug dose and decrease the effectiveness of drug in killing tumor cell [29-36]. In clinical setting, additional tumor microenvironmental factors such as epidermal growth factor, fibroblast growth factor, insulin-like growth factor, and extracellular matrix components are strongly associated with survival mechanisms of cancer cells under cytotoxic drug treatment [37-40].
To tackle the multifaceted MDR mechanisms, it is hypothesized that a focal high dose strategy works at cellular level rather than at systemic level. In addition, local high dose may overwhelm most resistant mechanisms, which might have their intrinsic limitation in defense capability because even extremely resistant experimental MDR cells are killed at high drug concentrations [42-46]. Intracellular organelles in parental drug-sensitive cells are characterized to have somewhat acidic, diffuse pH profiles inside cells [47, 48]. MDR cancer cells develop more acidic organelles (recycling endosome and lysosome) than those in sensitive cells, which are more acidic than cytosolic pH and nucleoplasmic pH [49, 50]. This results in acid-induced sequestration of anticancer drugs. Acidic organelles in MDR cells contribute to developing resistance to chemotherapeutic drugs [51]. Since most anticancer drugs are in an ionizable form, the pH of extracellular matrix and intracellular compartments are critical factors in determining drug partitioning and distribution [52]. The low pH in tumor extracellular space or in various subcellular organelles is a significant signal for targeting.
This review highlights recent progress of the pH-sensitive tumor-targeting nano-carriers developed in Bae research group. For a more general literature overview, interested readers are referred to a most recent review by Oh et al. [53].
2. Tumor extracellular pH (pHe) Targeting
The approaches to target various solid tumors by pHe include micelle systems with a triggered drug release mechanism, and exposing nonspecific cationic TAT (HIV transactivator of transcription) peptide by a shielding/deshielding mechanism or by a pop-up mechanism. These systems have utilized the pH-sensitivity of poly(L-histidine) or polysulfonamide. The imidazole ring of a polyHis (pKb ∼ 7.0; polyHis is the most effective pH-buffering agent in a physiological system) has lone pairs of electrons on the unsaturated nitrogen that endow pH-dependent amphoteric properties [43-46]. Particular polysulfonamides (pKa ∼ 6.8) are negatively charged at blood pH (i.e., pH 7.4) and can be neutralized at acidic pH (e.g., tumor pHe) [41]. In addition, these polymers demonstrated a strong endosomolytic property by proton sponge effect and/or interactions with the anionic phospholipids of endosome [41, 43-46]. These properties of polyHis or polysulfonamide have contributed to the development of smart nanosystems designed for targeting tumor pH.
2.1. Triggered tumor pHe drug release
It has been proposed that pH-induced anticancer drug release from pH-sensitive liposomes, which are stable at neutral pH but leaky under mild acidic condition (pH 4.5−6.0), could be a new mode for tumor treatment. However, due to the lack of response to tumor acidity (pH 6.5−7.2), these carriers are not optimal for pHe targeting [54-58]. Recently, the smart polymeric micelles, which have the capability of responding to tumor pHe, have been designed [59-64]. These polymeric micelles are physically destabilized and thus accelerate the anticancer drug release at tumor pHe.
A mixed pH-sensitive micelle (PHSM) system with folate (PHSM/f), created from poly(L-histidine) (polyHis) (Mw 5,000)-b-poly(ethylene glycol) (PEG) (Mw 2,000) and poly(L-lactic acid) (PLLA) (Mw 3,000)-b-PEG (Mw 2,000)-folate (0−25 wt%), showed a gradual destabilization below pH 7.0 due to the ionization of the polyHis block in the micelle core [60]. In the drug release studies using PHSM/f containing 25 wt % PLLA-b-PEG showed a favorable pH-dependency; such that within 24 hours 32 wt% of doxorubicin (DOX) was released at pH 7.0, 70 wt% of DOX at pH 6.8, and 82 wt% at pH 5.0. Consequently this enhanced the killing effect on sensitive cancer cells below pH 7.0. Furthermore, the DOX loaded PHSM/f (equivalent DOX=10 mg/kg) exhibited significant inhibition (P<0.05 compared with free DOX or saline solution) on the growth of s.c. MCF-7 xenografts [44]. The tumor volume of mice treated with the PHSM/f (P<0.05 compared with free DOX) was approximately 4.5 to 3.6 times smaller than those treated with saline solution or free DOX after 6 weeks. When tested with DOX loaded polyHis(Mw 5,000)-co-PEG (Mw 3000) micelle, without folate and mixing with PLLA-b-PEG, in MDA 231 MD breast tumor-bearing mice model, the time-dependent DOX accumulation was visualized using skinfold window chamber model (Figure 1). The intensity of DOX fluorescence carried by PHSM was significantly more intense and spread in the tumor site than that carried by a control pH-insensitive (PLLA-b-PEG) micelle [65]. This is because once the micelles are exposed to pHe, the micelles dissociated and released the payload. The micelle dissociation may also help the extravasation of next arriving micelles by providing space. Therefore, the pH induced micelle destabilization and triggered release of DOX by tumor pHe, after the accumulation of the micelles in the tumor sites via enhanced permeability [66], presented a more effective modality of chemotherapy for sensitive tumors by providing higher local concentrations of the drug at tumor sites and minimal release of the drug from micelles during blood circulation (pH 7.4).
Figure 1.
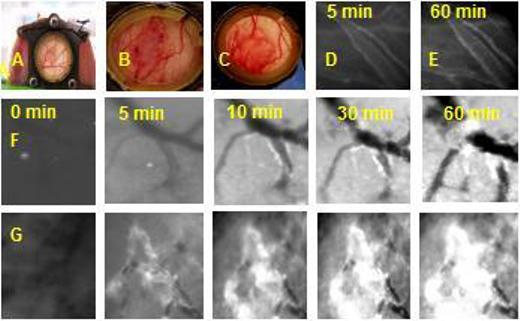
Mouse dorsal skin fold window chamber made of two symmetrical titanium frames. Tumor piece was inoculated into nu/nu mouse window chamber (A). After implanting MDA 213 breast cancer tumor piece, tumor blood vessels were growing in the window chamber Day 1 (B) and Day 15 (C). Normal blood vessel images after IV injection of DOX loaded pH-sensitive micelles (polyHis (Mw 5,000)-b-PEG (Mw 3,000)) 5 min and 60 min were shown in (D) and (E), respectively. The tumor blood vessels after i.v. injection of DOX loaded pH-insensitive and pH-sensitive micelles at the time course for 60 min were present in row (F) and row (G). The bright color is from DOX fluorescence. Reproduced with permission from reference [64].
The detailed physicochemical characteristics of a mixed PHSM, including the size, pH-dependent size change-dissociation kinetics, stability, the compatibility of core forming polymer blocks are reported in reference [67] and PK data using polyHis-b-PEG micelles were described in reference [63]. In addition, the brief toxicity results of the micelles and MTD were reported in reference [68].
2.2. TAT exposure by shield/deshielding mechanism
The shield/deshielding mechanism by positive charges of TAT, a cell penetrating peptide, on micelle surfaces controlled by the pH difference between 7.4 and pHe was designed [69] (Figure 2). Poly(methacryloyl sulfadimethoxine)-b-PEG is negatively charged and interacts electrostatically with TAT molecules (shielding) at pH 7.4. However, charge density on this polymer decreases by decreasing pH. Below pH 6.8, due to destabilized electrostatic interactions, the TAT will be deshielded. The zeta potential measurements on micelle comprising of PLLA-b-PEG-TAT demonstrated the shield/deshielding process. It was shown that the zeta potential is close to zero between pH 8.0 to 6.8, which indicates complete shielding of TAT, and from pH 6.6 to 6.0 it increased to 6.0 mV, which is close to the measured zeta potential for TAT decorated micelles without masking. When the shielded and unshielded TAT-micelles were tested for tumor cell internalization at pHs 7.4 and 6.6 by incubating for an hour, unshielded micelles were internalized into both the cells and its nucleus at pH 7.4 and 6.6. However, the micelle shielded with poly(methacryloyl sulfadimethoxine)-b-PEG was not internalized at 7.4 indicating TAT was masked even as it internalized into cells and the nucleus at pH 6.6. This shield/deshielding mechanism suggests that an optimized pHe targeting system with an appropriate sulfonamide polymer is feasible. The optimized system can be further tested in vivo.
Figure 2.
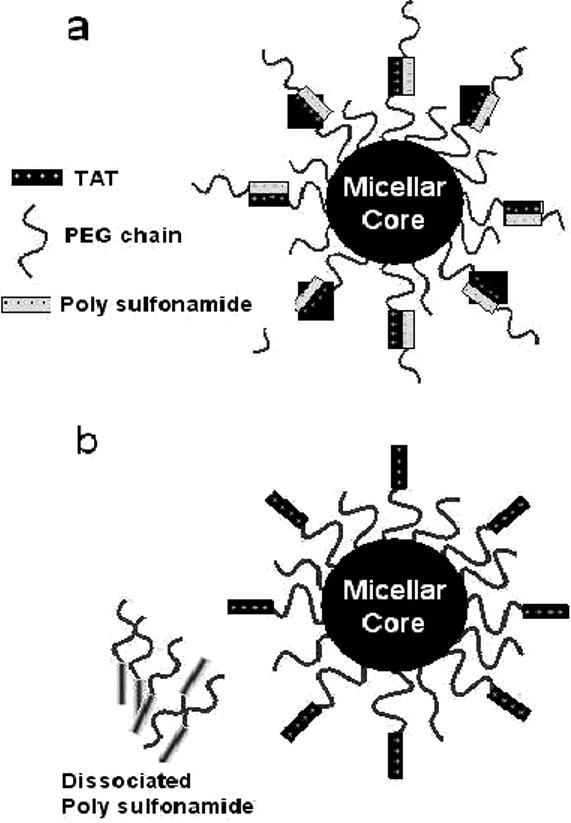
Schematic concept for a proposed drug delivery system: the carrier system consists of two components, poly(L-lactic acid)-b-PEG-TAT micelles and pH-sensitive poly(methacryloyl sulfadimethoxine-b-PEG. a) At normal blood pH, polysulfonamide is negatively charged, and when mixed with TAT, polysulfonamide shields the TAT by electrostatic interaction. Only PEG is exposed to the outside which could make the carrier long circulating; b) when the system experiences a decrease in pH (near tumor) polysulfonamide loses charge and detaches, exposing TAT for interaction with tumor cells. Reproduced with permission from reference [68].
2.3. Ligand exposure by pop up mechanism
This mechanism is described in Section 4.
3. Endosomal pH (pHendo) Targeting
Accelerated anticancer drug release from L-histidine-based polymeric micelles could be triggered by an early endosomal pH of 6.0 [62]. The primary objective of this strategy is to create drug-loaded micelles that destabilizes at an early endosomal pH of 6.0 such that drug release at both the tumor extracellular pH (pHe) and the lysosomal pH of 5.0 can be minimized. This system is shown to be effective for cytosolic high dose drug delivery with minimal drug loss during circulation and in extracellular domain. It is also assumed that the endosolytic activity at early endosomal pH may minimize the leakage of digestive lysosomal enzymes [70].
3.1. Receptor-mediated endocytosis and pHendo targeting
When a mixed PHSM/f micelle with up to 40 wt% PLLA-b-PEG was used, a fraction of this micelle, depending on PLLA-b-PEG content, was destabilized by tumor extracellular pH and the remaining was internalized by folate receptor mediated endocytosis. To eliminate this micelle destabilization before internalization, a micelle system consisted of poly(His-co-phenylalanine (Phe))-b-PEG and PLLA-b-PEG-folate was designed [62]. In particular, the pH sensitivity of the micelle is controlled by the His/Phe block composition, and was fine tuned to target early endosomal pH through blending with PLLA-b-PEG by using anticancer drug, DOX. Since pKb of poly(His-co-Phe (16 mole%))-b-PEG was around 6.3, this block copolymer was blended with 20 wt % of PLLA-b-PEG-folate for targeting endosomal pH of 6.0. This micelle (as denoted ‘EndoPHSM/f’) releases minimal drug above pH 6.0 and demonstrated the triggered release at pH 6.0, indicating tuned micelle destabilization at early endosomal pH. When EndoPHSM/f was internalized into tumor cells via folate receptor-mediated endocytosis, this system effectively killed tumor cells through a focal high dose of DOX in the cytosol, resulting from active internalization, accelerated DOX release triggered by endosomal pH, and disruption of endosomal membrane. Currently, In vivo studies using this micelle system are in progress, and the results will be reported shortly.
3.2. Overcoming MDR
Most antitumor agents are not very effective in tumor chemotherapy: as anticipated that may be because of MDR in various tumor cells. Additionally, premature drug release in blood leads to systemic side effects, and fails to locally concentrate at the site of action. Especially for drug resistant cells, slow drug release kinetics in tumors may decrease the drug efficacy at the site [53]. Consequently, this issue has provoked a strong interest to create a new drug delivery system for the intracellular focal high dose targeting based on triggered release mechanism to achieve maximal therapeutic efficacy [71, 72].
After identification of Pgp as a major cause of MDR in in vitro cell studies and the discovery that verapamil modulates Pgp, tremendous efforts have been made for the identification of more effective Pgp modulators, which have high binding affinity to Pgp without any disruption to normal biological functions and with minimal pharmacokinetic interference [73-78]. Numerous Pgp modulators have been synthesized by chemical modification using existing drugs such as verapamil, cyclosporine A, glibenclamide and many other compounds that are in various stages of clinical studies: phase I, II and even III [73-78]. However, none of these chemical entities has been proven to be effective in the phase III studies yet. MDR, in clinical settings, is multifaceted and only the Pgp inhibition does not seem to be as effective as anticipated [79]. As an alternative to Pgp modulators, various drug carriers have been extensively tested to overcome MDR in vitro and in vivo [80-89]. Nevertheless, none of the approach has been proven to be effective in clinical MDR tumor treatments so far.
EndoPHSM/f micelles are shown to be highly effective in treatment of MDR tumor cells [61]. The endosomal pH triggering anticancer drug release and the endosomal escaping activity of polyHis allows cytosolic delivery of anticancer drug, by avoiding drug sequestration mechanism in MDR cells [43, 61] and through bypassing MDR proteins expression on cellular membrane via folate receptor mediated endocytosis [90]. The EndoPHSM/f demonstrated a similar degree of cytotoxicity against MDR tumor cells (MCF-7/DOXR with Pgp overexpression) when compared with free DOX against the drug-sensitive tumor cells. Investigation on A2780/AD (ovarian carcinoma drug-resistant tumor) xenografts in nude mice for in vivo efficacy demonstrated the tumor regression in mice treated by EndoPHSM/f was extremely promising and superior to PHSM/f (unpublished data).
4. Double Targeting, pHe and pHendo: a universal approach for drug-sensitive and resistant tumors
In an attempt to address the issues related to MDR and tumor heterogeneity simultaneously, one approach is to use tumor cell non-specific interactions that can be activated by tumor microclimate such as pH (pHe targeting) along with triggered release in the endosomes.
Polymeric micelles with pH-induced ligand repositioning on the micelle surface was developed [60]. As shown in Figure 3, the mixed micelle consisted of polyHis-b-PEG and PLLA-b-PEG-b-polyHis (Mw 1,000)-biotin, which is multifunctional; the shorter polyHis block in PLLA-b-PEG-b-polyHis-biotin is located at the interface of the hydrophobic core of PLLA and polyHis and the hydrophilic PEG shell, due to the high water solubility of neighboring PEG and biotin. Biotin was selected in this study to demonstrate the proof of concept. The interfacial polyHis caused PEG chain bending and the biotin burying in the PEG shell, derived from the polyHis-b-PEG block copolymer. As a result, the micelle is stable above pH 7.2 and hides the conjugated biotins. However, as the pH is lowered below pH 7.2, the degree of ionization of polyHis increases. The interfacial short polyHis (Mw 1,000) becomes ionized first and at the critical degree of ionization its hydrophobic interaction with the core phase weakens. As a result, the PEG-b-polyHis-biotin portion expands, exposing biotin out of the PEG shell. The pH 7.0 seems to be the critical point for this expansion as demonstrated by pH-dependent turbidity of the micelle solution containing avidin, which is a tetrameric protein with four biotin-binding sites. Furthermore, when the solution pH was decreased, the relative transparency of the solution is gradually reduced to 10 % between the pH range of 6.8−6.0. This is attributed to the ionization of the polyHis block located in the core and subsequent micelle destabilization by ionized polyHis escaping from the micelle. This process might cause reduced transparency, presumably through certain degree of aggregation of the remaining PLLA-b-PEG block copolymer. In summary, when the environmental pH for the micelle is lowered slightly (pH ∼7.0; tumor acidic pH), biotin is exposed on the micellar surface and can interact with cells, which facilitates biotin receptor-mediated endocytosis. Whereas, when the pH is lowered further (pH<6.5), the micelle destabilizes, resulting in disruption of the endosomal membrane and enhanced cytosolic drug release. This intelligent micelle clearly presented that the hidden biotin at pH 7.4 is exposed at pH 7.0 by the pop-up mechanism, which enhanced the cell cytotoxicity of the DOX loaded micelle at tumor acidic pH.
Figure 3.
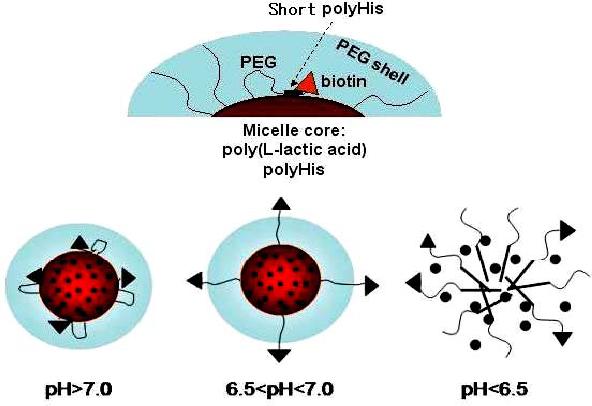
Schematic diagram depicting the central concept of pH induced biotin repositioning on the micelle. While above pH 7.0, biotin that is anchored on the micelle core via a pH-sensitive molecular chain actuator (polyHis) is shielded by PEG shell of the micelle; biotin is exposed on the micelle surface (6.5 < pH < 7.0) and can interact with cells, which facilitates biotin receptor-mediated endocytosis. When the pH is further lowered (pH < 6.5), the micelle destabilizes, resulting in enhanced drug release and disrupting cell membranes such as endosomal membrane. Reproduced with permission from reference [60].
To replace biotin with TAT, a micelle system was constructed with polyHis-b-PEG and PLLA-b-PEG-b-polyHis (Mw 2,000)-TAT [45]. TAT is a non-specific cell penetrating peptide, which has the strong capability to translocate the polymeric micelles into cells.
The pH-dependent micelle uptake by cells is represented in Figure 4. At pH 7.4, micelle uptake was minimized. At pH 7.0, the uptake showed a 30-fold increase compared to pH 7.4, which probably is due to partial TAT expression on micellar surface. At pH 6.8, 70-fold increased micelle cellular uptake was shown as compared to pH 7.4. This observation indicates that at tumor pHe the TAT peptide is exposed on the micellar surface and interacts with cells, which facilitates macropinocytosis. This nanosystem proved to be effective for various in vivo solid tumors including drug-sensitive and drug-resistant phenotypes and is anticipated to replace cumbersome and selective antibody or ligand-based targeting technology.
Figure 4.
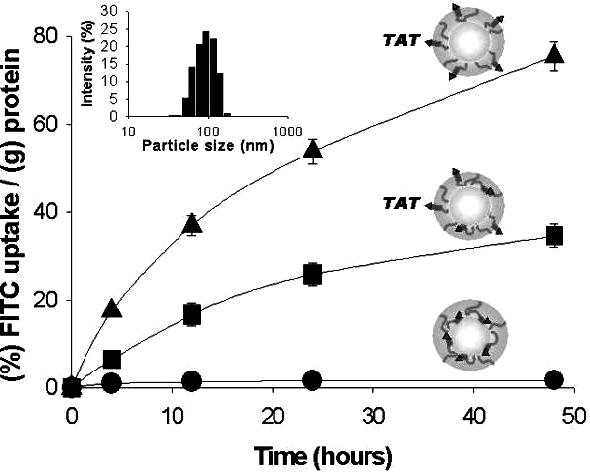
pH-dependent cellular uptake of the micelles in cultured MCF-7 tumor cells caused by TAT exposure: pH 7.4 (•), pH 7.0 (■), pH 6.8 (▲). Particle size and size distributions of micelle suspensions (in PBS) at pH 7.4 were measured by dynamic light scattering. Each data point represents an average with standard deviation (n=3). Reproduced with permission from reference [45].
5. Virus-mimetic (VM) nanogel
Viruses infect specific cells of the host organisms, replicate, destruct the cells and spread from one to another cell causing diseases [91, 92]. They circulate long in the blood and as disease progress they become more pathogenic [93]. Drug delivery vehicles often mimic only few aspects of virus such as size, surface modifications for longer residence in the body before their clearance [94-97], which has attracted many investigators who design delivery vehicles particularly for anticancer agents [98-100]. In particular, the nanosystem including virus-like infectious properties can be spotlighted. This system has a capsid-like protein capsule, able to infect specific cells, injects toxin, destroy infected cells, and migrates to neighboring pathologic cells by repeated cell cycles.
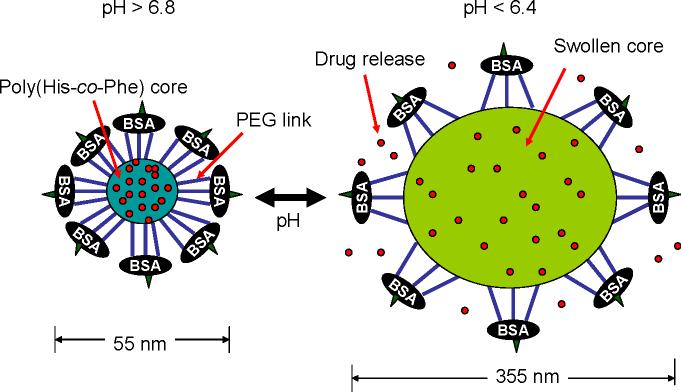
The virus-like infectious nanogel consists of a hydrophobic core (poly(His-co-Phe)) and two layers of hydrophilic shells (PEG and bovine serum albumin (BSA)) (Figure 5) [46]. One end of PEG is linked to the core forming block and other to BSA, which forms a capsid-like outer shell. The structure of core and inner shell was formed by oil-in-water emulsion method [101-103]. At high pH the core of this nanogel is rigid; however, the core swells by the ionization of polyHis at low pH. When these nanogels are exposed to early endosomal pH of 6.4 [45], the size grew abruptly, reaching 355 nm. The size changes by cycling pH between 7.4 and 6.4 are also reversible [46]. This reversible swelling/deswelling by pH of the core is closely linked to the release rate of incorporated DOX (Figure 6). The nanogels release a significant amount of DOX at endosomal pH (e.g., pH 6.4), while reducing DOX release rate at cytosolic [48] or extracellular pH (e.g., pH 7.4−6.8). Furthermore, due to the known proton buffering effect [58] of polyHis and observed substantial nanogel volumetric expansion within cell endosomes, nanogels are proposed to be able to physically disrupt endosomal membranes. This allows the VM nanogels and anticancer drug (released from the nanogels) to transfer from the endosomes to the cytosol, where the VM nanogels rapidly shrink back to their original size with the new, more neutral local pH, and thereby reduce the drug release rate. Free drug released by endosomal pH stimulus and will be in the cytosol, then diffuses into the nucleus, and finally to the pharmacological target site. The drug action on the cells will induce apoptosis and eventually disintegrates, which in turn releases the nanogels from the cell for subsequent infection and action in neighboring cells (Figure 7). This nanogel demonstrated repeated infectious cycles in cultures of drug-resistant tumor cells. Consequently, this appraoch is thought to have a high potential for maximizing drug efficacy in treating tumors, inflamed tissues, and other diseases due to sequential cytotoxic action. Further in vitro evaluations and in vivo investigations are required to confirm their potential.
Figure 5.
Schematic presentation of the virus-like nanogel. See the text for more details.
Figure 6.
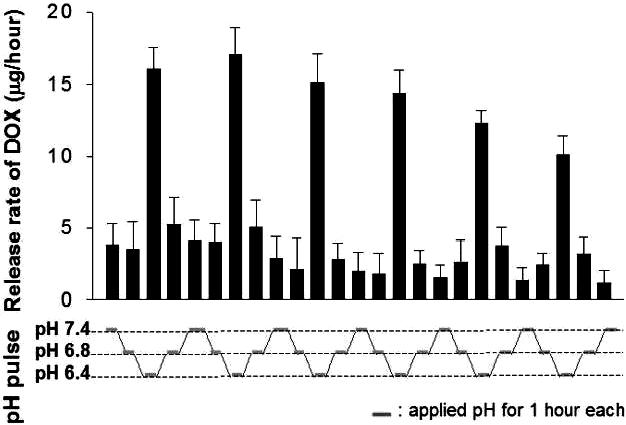
Release rate of DOX from DOX-loaded virus-like infectious nanogels. 150 μg of DOX was encapsulated into 1 mg of DOX-loaded VM-nanogels. The pH of the solution is stepwise adjusted to pH 7.4, pH 6.8 and pH 6.4 at one-hour intervals. Each data point represents an average with standard deviation (n=3). Reproduced with permission from reference [46].
Figure 7.

Virus-like nanogels infect cells selectively depending on specific interactions, kill the host cells, and migrate to neighboring cells as virus does to repeat the cycle.
6. Conclusion
Tumor extracellular pH- and/or endosomal pH-responsive micelles, TAT shield/deshield nanosystem, virus-like infectious nanogels, and pop-up micelles are the examples of the novel anticancer drug delivery systems for overcoming limitations of conventional drug delivery systems. These systems increase target drug accumulation at tumor sites or at intercellular cytosolic compartments in tumor cells with less drug distribution to normal tissues and organs. In particular, the pH-sensitive micelles or nanogels presented here are the unique delivery systems in the treatment of MDR. The constituting polymer components also have no apparent cytotoxicity and systemic toxicity in small animal model. These outlooks motivate us to carry out a preclinical study, although more detailed studies are need to further prove the hypotheses. In the future, these technologies will be extended by using various anticancer drugs and molecular tools for active internalization to achieve a general platform for solid cancer chemotherapy.
8. Acknowledgments
The works covered in this review article are supported by NIH CA 101850 and 122356.
Footnotes
Publisher's Disclaimer: This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2−3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
References
- 1.Matei D. Novel agents in ovarian cancer. Expert Opin. Investig. Drugs. 2007;16:1227–1239. doi: 10.1517/13543784.16.8.1227. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv. Exp. Med. Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 3.Cavaletti G, Bogliun G, Marzorati L, Zincone A, Marzola M, Colombo N, Tredici G. peripheral neurotoxicity of taxol in patients previously treated with cisplatin. Cancer. 1995;75:1141–1150. doi: 10.1002/1097-0142(19950301)75:5<1141::aid-cncr2820750514>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Carelle N, Piotto E, Bellanger A, Germanaud J, Thuillier A, Khayat D. Changing patient perceptions of the side effects of cancer chemotherapy. Cancer. 2002;95:155–163. doi: 10.1002/cncr.10630. [DOI] [PubMed] [Google Scholar]
- 5.Fojo T, Coley HM. The role of efflux pumps in drug-resistant metastatic breast cancer: new insights and treatment strategies. Clin. Breast Cancer. 2007;7:749–756. doi: 10.3816/CBC.2007.n.035. [DOI] [PubMed] [Google Scholar]
- 6.O'Connor R. The pharmacology of cancer resistance. Anticancer Res. 2007;27:1267–1272. [PubMed] [Google Scholar]
- 7.Higgins CF. Multiple molecular mechanisms for multidrug resistance transports. Nature. 2007;446:749–757. doi: 10.1038/nature05630. [DOI] [PubMed] [Google Scholar]
- 8.Scholler N, Fu N, Yang Y, Ye Z, Goodman GE, Hellstrom KE, Hellstom I. I. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc. Natl. Acad. Sci. USA. 1999;96:11531–11536. doi: 10.1073/pnas.96.20.11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaidarun SS, Eggo MC, Sheppard MC, Stewart PM. Expression of epidermal growth factor (EGF), its receptor, and related oncoprotein (erbB-2) in human pituitary tumors and response to EGF in vitro. Endocrinology. 1994;135:2012–2021. doi: 10.1210/endo.135.5.7956924. [DOI] [PubMed] [Google Scholar]
- 10.Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005;338:284–293. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 11.Muss HB, Thor AD, Berry DA, Kute T, Liu ET, Koerner F, Cirrincione CT, Budman DR, Wood WC, Barcos M, Henderson IC. c-erbB-2 expression and response to adjuvant therapy in women with node-positive early breast cancer. N. Engl. J. Med. 1994;330:1260–1266. doi: 10.1056/NEJM199405053301802. [DOI] [PubMed] [Google Scholar]
- 12.Daniels RA, Turley H, Kimberley FC, Liu XS, Mongkolsapaya J, Ch'En P, Xu XN, Jin BQ, Pezzella F, Screaton GR. Expression of TRAIL and TRAIL receptors in normal and malignant tissues. Cell Res. 2005;15:430–438. doi: 10.1038/sj.cr.7290311. [DOI] [PubMed] [Google Scholar]
- 13.Muller C, Schubiger PA, Schibli R. In vitro and in vivo targeting of different folate receptor-positive cancer cell lines with a novel 99mTc-radiofolate tracer. Eur. J. Nucl. Med. Mol. Imaging. 2006;33:1162–1170. doi: 10.1007/s00259-006-0118-2. [DOI] [PubMed] [Google Scholar]
- 14.Cirstoiu-Hapca A, Bossy-Nobs L, Buchegger F, Gurny R, Delie F. Differential tumor cell targeting of anti-HER2 (Herceptin) and anti-CD20 (Mabthera) coupled nanoparticles. Int. J. Pharm. 2007;331:190–196. doi: 10.1016/j.ijpharm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Rapoport N, Gao Z, Kennedy A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J. Natl. Cancer Inst. 2007;99:1095–1106. doi: 10.1093/jnci/djm043. [DOI] [PubMed] [Google Scholar]
- 16.Ponce AM, Vujaskovic Z, Yuan F, Needham D, Dewhirst MW. Hyperthermia mediated liposomal drug delivery. Int. J. Hyperthermia. 2006;22:205–213. doi: 10.1080/02656730600582956. [DOI] [PubMed] [Google Scholar]
- 17.Ko J, Park K, Kim YS, Kim MS, Han JK, Kim K, Park RW, Kim IS, Song HK, Lee DS, Kwon IC. Tumoral acidic extracellular pH targeting of pH-responsive MPEG-poly(beta-amino ester) block copolymer micelles for cancer therapy. J. Control. Release. 2007;123:109–115. doi: 10.1016/j.jconrel.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Bae Y, Fukushima S, Harada A, Kataoka K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew. Chem. Int. Ed. Engl. 2003;42:4640–4643. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhry A, Carrasquillo JA, Avis IL, Shuke N, Reynolds JC, Bartholomew R, Larson SM, Cuttitta F, Johnson BE, Mulshine JL. Phase I and imaging trial of a monoclonal antibody directed against gastrin-releasing peptide in patients with lung cancer. Clin. Cancer Res. 1999;5:3385–3393. [PubMed] [Google Scholar]
- 20.Engin K, Leeper DB, Cater JR, Thistlethwaite AJ, Tupchong L, McFarlane JD. Extracellular pH distribution in human tumours. Int. J. Hyperthermia. 1995;11:211–216. doi: 10.3109/02656739509022457. [DOI] [PubMed] [Google Scholar]
- 21.Volk T, Jahde E, Fortmeyer HP, Glusenkamp KH, Rajewsky MF. pH in human tumour xenografts: effect of intravenous administration of glucose. Br. J. Cancer. 1993;68:492–500. doi: 10.1038/bjc.1993.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Sluis R, Bhujwalla ZM, Raghunand N, Ballesteros P, Alvarez J, Cerdan S, Galons JP, Gillies RJ. In vivo imaging of extracellular pH using 1H MRSI. Magn. Reson. Med. 1999;41:743–750. doi: 10.1002/(sici)1522-2594(199904)41:4<743::aid-mrm13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 23.Ojugo AS, McSheehy PM, McIntyre DJ, McCoy C, Stubbs M, Leach MO, Judson IR, Griffiths JR. Measurement of the extracellular pH of solid tumours in mice by magnetic resonance spectroscopy: a comparison of exogenous (19)F and (31)P probes. NMR Biomed. 1999;12:495–504. doi: 10.1002/(sici)1099-1492(199912)12:8<495::aid-nbm594>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 24.Leeper DB, Engin K, Thistlethwaite AJ, Hitchon HD, Dover JD, Li DJ, Tupchong L. Human tumor extracellular pH as a function of blood glucose concentration. Int. J. Radiat. Oncol. Biol. Phys. 1994;28:935–943. doi: 10.1016/0360-3016(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 25.Tannockand IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373–4384. [PubMed] [Google Scholar]
- 26.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stubbs M, Mcsheehy RMJ, Griffiths JR, Bashford L. Causes and consequences of tumour acidity and implications for treatment. Opinion. 2000;6:15–19. doi: 10.1016/s1357-4310(99)01615-9. [DOI] [PubMed] [Google Scholar]
- 28.Yamagata M, Hasuda K, Stamato T, Tannock IF. The contribution of lactic acid to acidification of tumours: studies of variant cells lacking lactate dehydrogenase. Br. J. Cancer. 1998;77:1726–1731. doi: 10.1038/bjc.1998.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borst P, Evers R, Kool M, Wijnholds J. A Family of drug transporters: the multidrug resistance-associated proteins. J. Natl. Cancer Inst. 2000;92:1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- 30.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naoraand H, Montell DJ. Ovarian cancer metastasis: intergrating insights from disparate model organisms. Nat. Rev. Cancer. 2005;5:355–366. doi: 10.1038/nrc1611. [DOI] [PubMed] [Google Scholar]
- 32.Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, Brangi M, Greenberger L, Dean M, Fojo T, Bates SE. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 33.Scheffer GL, Wijngaard PLJ, Flens MJ, Izquierdo MA, Slovak ML, Pinedo HM, Meijer CJLM, Clevers HC, Scheper RJ. The drug resistance-related protein LRP is the human major vault protein. Nat. Med. 1995;1:578–582. doi: 10.1038/nm0695-578. [DOI] [PubMed] [Google Scholar]
- 34.Izquierdo MA, Shoemaker RH, Flens MJ, Scheffer GL, Wu L, Prather TR, Scheper RJ. Overlapping phenotypes of multidrug resistance among panels of human cancer-cell lines. Int. J. Cancer. 1996;65:230–237. doi: 10.1002/(SICI)1097-0215(19960117)65:2<230::AID-IJC17>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 35.Izquierdo MA, Scheffer GL, Flens MJ, Giaccone G, Broxterman HJ, Meijer CJ, van der Valk P, Scheper RJ. Broad distribution of the multidrug resistance-related vault lung resistance protein in normal human tissues and tumors. Am. J. Pathol. 1996;148:877–887. [PMC free article] [PubMed] [Google Scholar]
- 36.Stavrovskaya AA. Cellular mechanisms of multidrug resistance of tumor cells. Biochemistry (Mosc) 2000;65:95–106. [PubMed] [Google Scholar]
- 37.Hynes NE, Lane HA. Erbb receptors and cancer: The complexity of targeted inhibitors. Nat. Rev. Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 38.Henning T, Kraus M, Brischwein M, Otto AM, Wolf B. Relevance of tumor microenvironment for progression, therapy and drug development. Anticancer Drugs. 2004;15:7–14. doi: 10.1097/00001813-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat. Rev. Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 40.Shain KH, Dalton WS. Cell adhesion is a key determinant in de novo multidrug resistance (mdr): New targets for the prevention of acquired mdr. Mol. Cancer Ther. 2001;1:69–78. [PubMed] [Google Scholar]
- 41.Kang HC, Bae YH. pH-tunable endosomolytic oligomers for enhanced nucleic acid delivery. Adv. Funct. Mater. 2007;17:1263–1272. [Google Scholar]
- 42.Kobayashi T, Ishida T, Okada Y, Ise S, Harashima H, Kiwada H. Effect of transferrin receptor-targeted liposomal doxorubicin in P-glycoprotein-mediated drug resistant tumor cells. Int. J. Pharm. 2007;329:94–102. doi: 10.1016/j.ijpharm.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 43.Mohajer G, Lee ES, Bae YH. Enhanced intercellular retention activity of novel pH-sensitive polymeric micelles in wild and multidrug resistant MCF-7 cells. Pharm. Res. 2007;24:1618–1627. doi: 10.1007/s11095-007-9277-5. [DOI] [PubMed] [Google Scholar]
- 44.Lee ES, Na K, Bae YH. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J. Control. Release. 2005;103:405–418. doi: 10.1016/j.jconrel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 45.Lee ES, Gao Z, Kim D, Park K, Kwon IC, Bae YH. Super pH-sensitive multifunctional polymeric micelle for tumor pHe specific TAT exposure and multidrug resistance: in vivo efficacy. J. Control. Release. doi: 10.1016/j.jconrel.2008.04.024. (under revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee ES, Kim D, Youn YS, Oh KT, Bae YH. A novel virus-mimetic nanogel vehicle. Angew. Chem. Int. Ed. 2008;47:2418–2421. doi: 10.1002/anie.200704121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliver. Rev. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz MA, Both G, Lechene C. The effect of cell spreading on cytoplasmic pH in normal and transformed fibroblasts. Proc. Natl. Acad. Sci. USA. 1989;86:4525–4529. doi: 10.1073/pnas.86.12.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Busa WB, Nuccitelli R. Metabolic regulation via intracellular pH. Am. J. Physiol. 1984;246:R409–R438. doi: 10.1152/ajpregu.1984.246.4.R409. [DOI] [PubMed] [Google Scholar]
- 50.Simon SM, Roy D, Schindler M. Intracellular pH and the control of multidrug resistance. Proc. Natl. Acad. Sci. USA. 1994;91:1128–1132. doi: 10.1073/pnas.91.3.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belhoussine R, Morjani H, Millot JM, Sharonov S, Manfait M. Confocal scanning microspectrofluorometry reveals specific anthracycline accumulation in cytoplasmic organelles of multidrug-resistant cancer cells. J. Histochem. Cytochem. 1998;46:1369–1376. doi: 10.1177/002215549804601205. [DOI] [PubMed] [Google Scholar]
- 52.Simon SM. Role of organelle pH in tumor cell biology and drug resistance. Drug Discovery Today. 1999;4:32–38. doi: 10.1016/s1359-6446(98)01276-8. [DOI] [PubMed] [Google Scholar]
- 53.Oh KT, Yin H, Lee ES, Bae YH. Polymeric nanovehicles for anticancer drugs with triggering release mechanisms. J. Mater. Chem. 2007;17:3987–4001. [Google Scholar]
- 54.Drummond DC, Zignani M, Leroux JC. Current status of pH-sensitive liposomes in drug delivery. Prog. Lipid Res. 2000;39:409–460. doi: 10.1016/s0163-7827(00)00011-4. [DOI] [PubMed] [Google Scholar]
- 55.Gerasimov OV, Boomer JA, Qualls MM, Thompson DH. Cytosolic drug delivery using pH- and light-sensitive liposomes. Adv. Drug Del. Rev. 1999;38:317–338. doi: 10.1016/s0169-409x(99)00035-6. [DOI] [PubMed] [Google Scholar]
- 56.Shi G, Guo W, Stephenson SM, Lee RJ. Efficient intracellular drug and gene delivery using folate receptor-targeted pH-sensitive liposomes composed of cationic/anionic lipid combinations. J. Control. Release. 2002;80:309–319. doi: 10.1016/s0168-3659(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 57.Simoes S, Slepushkin V, Duzgunes N, Pedroso de Lima MC. On the mechanisms of internalization and intracellular delivery mediated by pH-sensitive liposomes. Biochim. Biophys. Acta. 2001;1515:23–37. doi: 10.1016/s0005-2736(01)00389-3. [DOI] [PubMed] [Google Scholar]
- 58.Tachibana R, Harashima H, Shono M, Azumano M, Niwa M, Futaki S, Kiwada H. Intracellular regulation of macromolecules using pH-sensitive liposomes and trafficking. Biochem. Biophys. Res. Commun. 1998;251:538–544. doi: 10.1006/bbrc.1998.9460. [DOI] [PubMed] [Google Scholar]
- 59.Lee ES, Shin HJ, Na K, Bae YH. Poly(L-histidine)-PEG block copolymer micelles and pH-induced destabilization. J. Control. Release. 2003;90:363–374. doi: 10.1016/s0168-3659(03)00205-0. [DOI] [PubMed] [Google Scholar]
- 60.Lee ES, Na K, Bae YH. Polymeric micelle for tumor pH and folate-mediated targeting. J. Control. Release. 2003;91:103–113. doi: 10.1016/s0168-3659(03)00239-6. [DOI] [PubMed] [Google Scholar]
- 61.Lee ES, Na K, Bae YH. Super pH-sensitive multifunctional polymeric micelle. Nano Lett. 2005;5:325–329. doi: 10.1021/nl0479987. [DOI] [PubMed] [Google Scholar]
- 62.Kim D, Lee ES, Oh KT, Gao Z, Bae YH. Doxorubicin-loaded polymeric micelle overcomes multidrug resistance of cancer by double-targeting folate receptor and early endosomal pH. Small. doi: 10.1002/smll.200701275. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao ZG, Lee DH, Kim DI, Bae YH. Doxorubicin loaded pH-sensitive micelle targeting acidic extracellular pH of human ovarian A2780 tumor in mice. J. Drug Target. 2005;13:391–397. doi: 10.1080/10611860500376741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim GM, Bae YH, Jo WH. pH-induced micelle formation of poly(histidinecophenylalanine)-block-poly(ethylene glycol) in aqueous media. Macromol. Biosci. 2005;5:1118–1124. doi: 10.1002/mabi.200500121. [DOI] [PubMed] [Google Scholar]
- 65.Gao Z, Kim DI, Lee ES, Bae YH. Visualization of pH-sensitive nanoparticle extravasation from breast cancer tumor microvessel in window chamber model. Microvascular Research. (submitted) [Google Scholar]
- 66.Lyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discovery Today. 2006;11:812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 67.Yin H, Lee ES, Kim D, Lee KH, Oh KT, Bae YH. Physicochemical characteristics of pH-sensitive poly(L-Histidine)-b-poly(ethylene glycol)/poly(L-lactic acid)-b-poly(ethylene glycol) mixed micelles. J. Control. Release. 2008;126:130–138. doi: 10.1016/j.jconrel.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oh KT, Lee ES, Kim D, Bae YH. L-Histidine based pH-sensitive anticancer drug carrier micelle: Reconstitution and brief evaluation of its systemic toxicity. Int. J. Pharm. doi: 10.1016/j.ijpharm.2008.03.003. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sethuraman VA, Bae YH. TAT peptide-based micelle system for potential active targeting of anti-cancer agents to acidic solid tumors. J. Control. Release. 2007;118:216–224. doi: 10.1016/j.jconrel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duncan R. Polymer conjugates for tumor targeting and intracytoplasmic delivery. The EPR effect as a common gateway. Pharm. Sci. Tech. Today. 1999;2:441–449. doi: 10.1016/s1461-5347(99)00211-4. [DOI] [PubMed] [Google Scholar]
- 71.Wong HL, Bendayan R, Rauth AM, Xue HY, Babakhanian K, Wu XY. A mechanistic study of enhanced doxorubicin uptake and retention in multidrug resistant breast cancer cells using a polymer-lipid hybrid nanoparticle system. J. Pharmacol. Exp. Ther. 2006;317:1372–1381. doi: 10.1124/jpet.106.101154. [DOI] [PubMed] [Google Scholar]
- 72.Wang T, Jiang X, Yang DC, Elliott RL, Head JF. Doxorubicin-gallium-transferrin conjugate overcomes multidrug resistance: evidence for drug accumulation in the nucleus of drug resistant MCF-7/ADR cells. Anticancer Res. 2000;20:799–808. [PubMed] [Google Scholar]
- 73.Cheng C, Wei H, Shi BX, Cheng H, Li C, Gu ZW, Cheng SX, Zhang XZ, Zhuo RX. Biotinylated thermoresponsive micelle self-assembled from double-hydrophilic block copolymer for drug delivery and tumor target. Biomaterials. 2008;29:497–505. doi: 10.1016/j.biomaterials.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 74.Golstein PE, Boom A, van Geffel J, Jacobs P, Masereel B, Beauwens R. P-glycoprotein inhibition by glibenclamide and related compounds. Pflugers Arch. 1999;437:652–660. doi: 10.1007/s004240050829. [DOI] [PubMed] [Google Scholar]
- 75.Wu CP, Shukla S, Calcagno AM, Hall MD, Gottesman MM, Ambudkar SV. Evidence for dual mode of action of a thiosemicarbazone, NSC73306: a potent substrate of the multidrug resistance linked ABCG2 transporter. Mol. Cancer Ther. 2007;6:3287–3296. doi: 10.1158/1535-7163.MCT-07-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.mei Y, Qian F, Wei D, Liu J. Reversal of cancer multidrug resistance by green tea polyphenols. J. Pharm. Pharmacol. 2004;56:1307–1314. doi: 10.1211/0022357044364. [DOI] [PubMed] [Google Scholar]
- 77.Chen LM, Wu XP, Ruan JW, Liang YJ, Ding Y, Shi Z, Wang XW, Gu LQ, Fu LW. Screening novel, potent multidrug-resistant modulators from imidazole derivatives. Oncol. Res. 2004;14:355–362. doi: 10.3727/0965040041292378. [DOI] [PubMed] [Google Scholar]
- 78.Lee BD, French KJ, Zhuang Y, Smith CD. Development of a syngeneic in vivo tumor model and its use in evaluating a novel P-glycoprotein modulator, PGP-4008. Oncol. Res. 2003;14:49–60. doi: 10.3727/000000003108748603. [DOI] [PubMed] [Google Scholar]
- 79.Pierre A, Dunn TA, Kraus-Berthier L, Leonce S, Saint-Dizier D, Regnier G, Dhainaut A, Berlion M, Bizzari JP, Atassi G. In vitro and in vivo circumvention of multidrug resistance by servier 9788, a novel triazinoaminopiperidine derivative. Invest. New Drugs. 1992;10:137–148. doi: 10.1007/BF00877238. [DOI] [PubMed] [Google Scholar]
- 80.Soma CE, Dubernet C, Bentolila D, Benita S, Couvreur P. Reversion of multidrug resistance by co-encapsulation of doxorubicin and cyclosporin a in polyalkylcyanoacrylate nanoparticles. Biomaterials. 2000;21:1–7. doi: 10.1016/s0142-9612(99)00125-8. [DOI] [PubMed] [Google Scholar]
- 81.van Vlerken LE, Duan Z, Seiden MV, Amiji MM. Modulation of intracellular ceramide using polymeric nanoparticles to overcome multidrug resistance in cancer. Cancer Res. 2007;67:4843–4850. doi: 10.1158/0008-5472.CAN-06-1648. [DOI] [PubMed] [Google Scholar]
- 82.Kabanov AV, Alakhov VY. Pluronic block copolymers in drug delivery: From micellar nanocontainers to biological response modifiers. Crit. Rev. Ther. Drug Carrier Syst. 2002;19:1–72. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.10. [DOI] [PubMed] [Google Scholar]
- 83.Chavanpatil MD, Patil Y, Panyam J. Susceptibility of nanoparticle-encapsulated paclitaxel to p-glycoprotein-mediated drug efflux. Int. J. Pharm. 2006;320:150–156. doi: 10.1016/j.ijpharm.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 84.Deng WJ, Yang XQ, Liang YJ, Chen LM, Yan YY, Shuai XT, Fu LW. Fg020326-loaded nanoparticle with PEG and PDLLA improved pharmacodynamics of reversing multidrug resistance in vitro and in vivo. Acta Pharmacol. Sin. 2007;28:913–920. doi: 10.1111/j.1745-7254.2007.00565.x. [DOI] [PubMed] [Google Scholar]
- 85.Batrakova E, Lee S, Li S, Venne A, Alakhov V, Kabanov A. Fundamental relationships between the composition of pluronic block copolymers and their hypersensitization effect in mdr cancer cells. Pharm. Res. 1999;16:1373–1379. doi: 10.1023/a:1018942823676. [DOI] [PubMed] [Google Scholar]
- 86.Advani R, Lum BL, Fisher GA, Halsey J, Chin DL, Jacobs CD, Sikic BI. A phase I trial of liposomal doxorubicin, paclitaxel and valspodar (psc-833), an inhibitor of multidrug resistance. Ann. Oncol. 2005;16:1968–1973. doi: 10.1093/annonc/mdi396. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y, Yu L, Han L, Sha X, Fang X. Difunctional Pluronic copolymer micelles for paclitaxel delivery: synergistic effect of folate-mediated targeting and Pluronic-mediated overcoming multidrug resistance in tumor cell lines. Int. J. Pharm. 2007;337:63–73. doi: 10.1016/j.ijpharm.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 88.Wu J, Lu Y, Lee A, Pan X, Yang X, Zhao X, Lee RJ. Reversal of multidrug resistance by transferrin-conjugated liposomes co-encapsulating doxorubicin and verapamil. J. Pharm. Pharm. Sci. 2007;10:350–357. [PubMed] [Google Scholar]
- 89.Mayer LD, Shabbits JA. The role for liposomal drug delivery in molecular and pharmacological strategies to overcome multidrug resistance. Cancer Metastasis Rev. 2001;20:87–93. doi: 10.1023/a:1013108524062. [DOI] [PubMed] [Google Scholar]
- 90.Kim SH, Jeong JH, Joe CO, Park GT. Folate receptor mediated intracellular protein delivery using PLL-PEG-FOL conjugate. J. Control. Release. 2005;103:625–634. doi: 10.1016/j.jconrel.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 91.Sailaja G, Skountzou I, Quan FS, Compans RW, Kang SM. Human immunodeficiency virus-like particles activate multiple types of immune cells. Virology. 2007;362:331–341. doi: 10.1016/j.virol.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Young KR, McBurney SP, Karkhanis LU, Ross TM. Virus-like particles: designing an effective AIDS vaccine. Methods. 2006;40:98–117. doi: 10.1016/j.ymeth.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 93.Liu J, Zeng F, Allen C. In vivo fate of unimers and micelles of a poly(ethylene glycol)-block-poly(caprolactone) copolymer in mice following intravenous administration. Eur. J. Pharm. Biopharm. 2007;65:309–319. doi: 10.1016/j.ejpb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 94.Kajiwara E, Kawano K, Hattori Y, Fukushima M, Hayashi K, Maitani Y. Long-circulating liposome-encapsulated ganciclovir enhances the efficacy of HSV-TK suicide gene therapy. J. Control. Release. 2007;120:104–110. doi: 10.1016/j.jconrel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 95.Kommareddy S, Amiji M. Biodistribution and pharmacokinetic analysis of long-circulating thiolated gelatin nanoparticles following systemic administration in breast cancer-bearing mice. J. Pharm. Sci. 2007;96:397–407. doi: 10.1002/jps.20813. [DOI] [PubMed] [Google Scholar]
- 96.Huang M, Wu W, Qian J, Wan DJ, Wei XL, Zhu JH. Body distribution and in situ evading of phagocytic uptake by macrophages of long-circulating poly (ethylene glycol) cyanoacrylate-co-n-hexadecyl cyanoacrylate nanoparticles. Acta Pharmacol. Sin. 2005;26:1512–1518. doi: 10.1111/j.1745-7254.2005.00216.x. [DOI] [PubMed] [Google Scholar]
- 97.Stolnik S, Daudali B, Arien A, Whetstone J, Heald CR, Garnett MC, Davis SS, Illum L. The effect of surface coverage and conformation of poly(ethylene oxide) (PEO) chains of poloxamer 407 on the biological fate of model colloidal drug carriers. Biochim. Biophys. Acta. 2001;1514:261–279. doi: 10.1016/s0005-2736(01)00376-5. [DOI] [PubMed] [Google Scholar]
- 98.Wong HL, Bendayan R, Rauth AM, Li Y, Wu XY. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2007;59:491–504. doi: 10.1016/j.addr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 99.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 100.Kommareddy S, Tiwari SB, Amiji MM. Long-circulating polymeric nanovectors for tumor-selective gene delivery. Technol. Cancer Res. Treat. 2005;4:615–625. doi: 10.1177/153303460500400605. [DOI] [PubMed] [Google Scholar]
- 101.Vinogradov SV, Zeman AD, Batrakova EV, Kabanov AV. Polyplex Nanogel formulations for drug delivery of cytotoxic nucleoside analogs. J. Control. Release. 2005;107:143–157. doi: 10.1016/j.jconrel.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vinogradov SV, Bronich TK, Kabanov AV. Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv. Drug Deliv. Rev. 2002;54:135–147. doi: 10.1016/s0169-409x(01)00245-9. [DOI] [PubMed] [Google Scholar]
- 103.Oishi M, Hayashi H, Iijima M, Nagasaki Y. Endosomal release and intracellular delivery of anticancer drugs using pH-sensitive PEGylated nanogels. J. Mater. Chem. 2007;17:3720–3725. [Google Scholar]