Abstract
Aims
To evaluate the associations of myocardial infarction (MI) and major bleeding with 1-year mortality. Both MI and major bleeding predict 1-year mortality in patients presenting with acute coronary syndrome (ACS). However, the risk of each of these events on the magnitude and timing of mortality has not been well studied.
Methods and Results
A multivariable Cox regression model was developed relating 13 independent baseline predictors to 1-year mortality for 13 819 patients with moderate and high-risk ACS enrolled in the Acute Catheterization and Urgent Intervention Triage strategy trial. After adjustment for baseline predictors, Cox models with major bleeding and recurrent MI as time-updated covariates estimated the effect of these events on mortality hazard over time. Within 30 days of randomization, 705 patients (5.1%) had an MI, 645 (4.7%) had a major bleed; 524 (3.8%) died within a year. The occurrence of an MI was associated with a hazard ratio of 3.1 compared with patients not yet having an MI, after adjustment for baseline predictors. However, MI within 30 days markedly increased the mortality risk for the first 2 days after the event (adjusted hazard ratio of 17.6), but this risk declined rapidly post-infarct (hazard ratio of 1.4 beyond 1 month after the MI event). In contrast, major bleeding had a prolonged association with mortality risk (hazard ratio of 3.5) which remained fairly steady over time throughout 1 year.
Conclusion
After accounting for baseline predictors of mortality, major bleeds and MI have similar overall strength of association with mortality in the first year after ACS. MI is correlated with a dramatic increase in short-term risk, whereas major bleeding correlates with a more prolonged mortality risk.
Keywords: Acute coronary syndrome, Myocardial infarction, Mortality, Bleeding, Transfusion
Introduction
Current anti-thrombotic therapy coupled with an early invasive strategy has reduced the incidence of recurrent ischaemic events and death in patients with acute coronary syndromes (ACS).1–3 However, this strategy also increases the likelihood of bleeding complications, especially in subsets of patients at increased risk.4–7
Bleeding complications have been associated with a significant increase in mortality in patients with ACS treated with an invasive strategy.7–11 A recent analysis of the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial showed that both major bleeding and myocardial infarction (MI) were independent predictors of 30-day mortality. With follow-up now complete to 1 year,12 we sought to construct a model to evaluate the associations of major bleeding and MI with the incidence and timing of mortality in the 13 819 patients enrolled in the ACUITY trial presenting with moderate and high-risk ACS, undergoing an early invasive management strategy.
Methods
Study design
The design and primary results of the ACUITY trial have been published.12–14 Briefly, 13 819 patients with moderate and high-risk ACS were randomly assigned in an open-label fashion equally to one of three anti-thrombotic regimens starting immediately after randomization: a heparin (unfractionated or enoxaparin) plus a glycoprotein IIb/IIIa inhibitor (GPI) or bivalirudin plus a GPI or bivalirudin monotherapy, in which GPI administration was permitted only for limited pre-specified indications. Unfractionated heparin was administered as an intravenous bolus of 60 IU/kg plus an infusion of 12 IU/kg/h to achieve an activated partial thromboplastin time of 50–75 s before angiography and an activated clotting time of 200–250 s for patients undergoing percutaneous coronary intervention (PCI). Enoxaparin 1 mg/kg was administered subcutaneously twice daily prior to angiography. An intravenous bolus of an additional 0.3 mg/kg was administered before PCI if the most recent subcutaneous dose had been given >8 h earlier, or an intravenous bolus of an additional 0.75 mg/kg was administered before PCI if the most recent subcutaneous dose had been given >16 h earlier. Bivalirudin was begun before angiography, with an intravenous bolus of 0.1 mg/kg and an infusion of 0.25 mg/kg/h. Before PCI, an additional intravenous bolus of 0.5 mg/kg was administered, and the infusion was increased to 1.75 mg/kg/h.
Patients assigned to a GPI arm were randomized again in a 2 × 2 factorial design to either upstream GPI initiation in all patients immediately after randomization or deferred GPI initiation for selective use in PCI patients only, starting in the catheterization laboratory. As per Food and Drug Administration approved labelling, either eptifibatide or tirofiban was permitted for upstream use and either eptifibatide or abciximab was permitted for deferred selective use. Dosages of all GPI were as per the package insert and were adjusted for renal impairment.
Coronary angiography was required within 72 h of randomization with subsequent triage to PCI, coronary artery bypass graft surgery (CABG), or medical management as per standard of care. Aspirin (300–325 mg orally or 250–500 mg intravenously) was administered prior to angiography. Dosage and timing of clopidogrel were at the discretion of the investigators, but, in patients undergoing PCI, the protocol required 300 mg of clopidogrel in all cases no later than 2 h following PCI. Clopidogrel 75 mg daily was recommended for 1 year in all patients after PCI and aspirin 75–325 mg daily indefinitely. Transfusion of blood product was performed at the investigators' discretion for clinical indications. The study was approved by the institutional review board or Ethics Committee at each participating centre, and all patients signed written informed consent.
Endpoints
The ACUITY trial was powered for three primary 30 day endpoints: (i) composite ischaemia, defined as death from any cause, non-fatal MI, or unplanned revascularization for ischaemia; (ii) major bleeding (non-CABG related), defined as intracranial or intraocular bleeding, access site haemorrhage requiring intervention, ≥5 cm diameter haematoma, reduction in haemoglobin of ≥4 g/dL without or ≥3 g/dL with an overt bleeding source, reoperation for bleeding, or blood product transfusion; and (iii) net clinical adverse outcome (composite ischaemia or major bleeding). The definition of MI took into consideration the presence or absence of non-ST-elevation MI at baseline, its time of occurrence, and its association with PCI, CABG, or medical treatment. In patients with unstable angina (without non-ST-elevation MI) before angiography or in a medical treatment, MI was defined as any elevation of troponin or creatinine phosphokinase-MB (CPK-MB) (or CPK) greater than the upper limits of normal (ULN). In patients with non-ST-elevation MI before angiography or in a medical treatment, the diagnosis of MI required: (i) recurrent chest pain lasting ≥30 min or new electrocardiographic changes consistent with MI, and the next troponin or CPK-MB (or CPK) level measured ∼8–12 h after the event be elevated by at least 50% above the previous level if the peak troponin or CPK-MB (or CPK) had not yet been reached, (ii) a new elevation of troponin or CPK-MB (or CPK) >ULN if the troponin, CPK-MB or CPK level had returned to <ULN, (iii) a rise by >50% above the previous nadir level if the troponin or CPK-MB (or CPK) level had not returned to <ULN. In patients treated with PCI, MI was defined as: (i) any CPK-MB (or CPK) ≥3 × ULN within 24 h after PCI that was also increased at least 50% over the most recent pre-PCI levels, or new, significant (≥0.04 s) Q waves in two or more contiguous electrocardiographic leads with CPK-MB (or CPK) >ULN if the elevated CPK-MB or CPK levels were falling or normal, (ii) recurrent chest pain ≥30 min or new electrocardiographic changes consistent with a second MI and the next CPK-MB (or CPK level) measured ∼8–12 h after the event is elevated by at least 50% above the previous level or new, significant (≥0.04 s) Q waves in two or more contiguous electrocardiographic leads if the patients had non-ST-elevation MI at baseline but the peak CPK-MB (or CPK) had not yet been reached. In patients treated with CABG, MI was defined as any CPK-MB (or CPK) ≥10 × ULN within 24 h of CABG and increased at least 50% over the most recent pre-CABG levels, or any CPK-MB (or CPK) ≥5 × ULN within 24 h of CABG and increased at least 50% over the most recent pre-CABG levels and new, significant (≥0.04 s) Q waves in two or more contiguous electrocardiographic leads. Definitions for the other components of the composite ischaemia endpoint have been detailed previously.14 All primary and secondary endpoints were adjudicated by a blinded Clinical Events Committee.
Statistical methods
Univariate associations of known baseline predictors and randomized treatment with 1-year mortality were assessed by χ2 tests for categorical variables and by two-sample t-tests for continuous variables. A multivariable Cox proportional hazards model with time to death within 1 year as the outcome was used to express how the baseline variables simultaneously predicted mortality.
The model's goodness of fit was assessed by calculating the risk score for every patient and categorizing these scores into five categories from low risk to very high risk (i.e. bottom 50, 50–75, 75–85, 85–95, and top 5%). The actual observed percentage dying in each category was compared with the expected percentage dying, the latter being the sum of the individual predicted probabilities from the Cox model.
To investigate the associations of MI and major bleeding with the incidence and timing of mortality, Cox models were fitted with each adverse event as a time-updated binary covariate that enters the Cox model from the day of the event (MI or major bleed).15 A few patients had repeat MIs or repeat major bleeds, but our models only include the first of each. These models were fitted both without and with adjustment for the baseline predictor variables. In order to estimate the time-dependent risk on mortality of major bleeding and MI, the Cox models were extended to have different time-updated binary covariates for different time intervals, i.e. days 0–1, days 2–7, days 8–30, and 31 or more days post-event. That is, after each event there are four time-updated binary covariates which are included for these four separately specified time intervals.
All analyses were carried out using STATA version 9.2. All significance levels are two-sided. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Baseline predictors of mortality
Of 13 819 patients enrolled in the ACUITY trial, 705 (5.1%) had an MI and 645 (4.7%) had a major bleed within 30 days of randomization, and 524 (3.8%) died within a year of randomization. The univariate associations of the baseline predictors with the rates of death within a year, and MI and major bleed within 30 days are shown in Table 1. Table 1 also shows the previously published rates of these events by treatment group.12,13
Table 1.
Univariate associations of baseline variables with death within 1 year, recurrent MI, and major bleed within 30 days
| Total | Death |
Recurrent MI |
Major bleed |
||||
|---|---|---|---|---|---|---|---|
| n (%) | P-value | n (%) | P-value | n (%) | P-value | ||
| Total | 13 819 | 524 (3.8) | — | 705 (5.1) | — | 645 (4.7) | — |
| Age (years) | |||||||
| Mean (SD) | 62.6 (11.7) | 70.8 (10.9) | <0.001 | 64.3 (11.3) | <0.001 | 67.6 (11.6) | <0.001 |
| White blood cell count (×1000/L) | |||||||
| Mean (SD) | 8.5 (3.0) | 9.9 (4.3) | <0.001 | 8.8 (3.4) | 0.016 | 9.2 (3.6) | <0.001 |
| Diabetes | |||||||
| None | 9857 | 301 (3.1) | 498 (5.1) | – | 418 (4.2) | – | |
| Non-ID diabetes | 2617 | 126 (4.8) | <0.001 | 130 (5.0) | 0.83 | 141 (5.4) | 0.013 |
| ID diabetes | 1193 | 92 (7.7) | <0.001 | 71 (6.0) | 0.19 | 79 (6.6) | <0.001 |
| ST-deviationa | 4825 | 270 (5.6) | <0.001 | 301 (6.2) | <0.001 | 290 (6.0) | <0.001 |
| Left bundle branch block | 423 | 46 (10.9) | <0.001 | 12 (2.8) | 0.037 | 33 (7.8) | 0.002 |
| Gender | |||||||
| Female | 4157 | 152 (3.7) | 199 (4.8) | — | 318 (7.6) | — | |
| Male | 9662 | 372 (3.9) | 0.60 | 506 (5.2) | 0.28 | 327 (3.4) | <0.001 |
| Planned treatment | |||||||
| Medical treatment | 4273 | 134 (3.1) | 40 (0.9) | — | 113 (2.6) | — | |
| PCI | 7754 | 245 (3.2) | 0.96 | 483 (6.2) | <0.001 | 460 (5.9) | <0.001 |
| CABG | 1647 | 124 (7.5) | <0.001 | 181 (11.0) | <0.001 | 64 (3.9) | 0.012 |
| Cerebrovascular event | 803 | 67 (8.3) | <0.001 | 54 (6.7) | 0.034 | 66 (8.2) | <0.001 |
| Creatinine clearance (mL/min) | |||||||
| Mean (SD) | 92 (42) | 72 (38) | <0.001 | 86 (36) | <0.001 | 77 (36) | <0.001 |
| Haemoglobin (g/dL) | |||||||
| Mean (SD) | 14.0 (1.6) | 13.4 (1.9) | <0.001 | 14.0 (1.7) | 0.57 | 13.3 (2.0) | <0.001 |
| Elevated CKMB/troponins | 7552 | 347 (4.6) | <0.001 | 464 (6.1) | <0.001 | 414 (5.5) | <0.001 |
| Current smoker | 3943 | 125 (3.2) | 0.028 | 203 (5.1) | 0.90 | 157 (4.0) | 0.017 |
| Previous MI | 4222 | 189 (4.5) | 0.001 | 239 (5.7) | 0.041 | 185 (4.4) | 0.24 |
| Treatment | |||||||
| Hep/Enox+GPIIb/IIIa | 4603 | 178 (3.9) | — | 228 (5.0) | — | 262 (5.7) | — |
| Bival+GPIIb/IIIa | 4604 | 176 (3.8) | 0.93 | 229 (5.0) | 0.93 | 243 (5.3) | 0.41 |
| Bival alone | 4612 | 170 (3.7) | 0.67 | 248 (5.4) | 0.34 | 140 (3.0) | <0.001 |
There were missing values for the following variables: white blood cells (n = 826), diabetic status (n = 152), ST-deviation (n = 15), left bundle branch block (n = 695), planned treatment (n = 145), cerebrovascular event (n = 192), creatinine clearance (n = 880), haemoglobin (n = 787), cardiac enzymes (n = 1107), smoking status (n = 262), and previous MI (n = 336). ID, insulin dependent.
aUsing definition from main ACUITY paper.
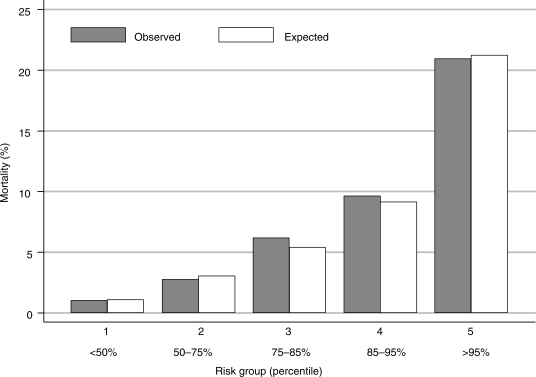
The multivariable Cox model that simultaneously relates these baseline predictors of risk of death within 1 year of randomization is shown in Table 2. The variables are listed in order of their significance in the model. The three strongest predictors of increased mortality were increasing age, increased white blood cell count, and insulin-dependent diabetes. In addition, ST-deviation on ECG, left bundle branch block, male gender, CABG as planned treatment, a prior cerebrovascular event, reduced creatinine clearance, lower haemoglobin, an elevated CKMB or troponin, current smoking, non-insulin-dependent diabetes, and a prior MI were significantly related to an increased risk of mortality. This model had good discrimination. Patients in the bottom 50% of the risk score had a low-mortality risk (1.0%), whereas patients in the top 5% of the risk score distribution had a 21% risk of mortality (Figure 1). The comparison of the observed and expected probabilities of dying demonstrates the model's goodness of fit.
Table 2.
A multivariable Cox regression relating baseline predictors to 1-year mortality
| Risk factor | Hazard ratio | 95% CI | Coefficienta | Z | P-value |
|---|---|---|---|---|---|
| Age | |||||
| Per 5 years | 1.31 | 1.24–1.39 | 0.27 | 9.18 | <0.001 |
| White blood cell count | |||||
| Per 1000/L | 1.07 | 1.06–1.09 | 0.072 | 9.11 | <0.001 |
| Diabetes | |||||
| No | 1 | — | — | ||
| Non-insulin dependent | 1.47 | 1.19–1.82 | 0.39 | 3.61 | <0.001 |
| Insulin dependent | 2.28 | 1.80–2.90 | 0.83 | 6.74 | <0.001 |
| ST-deviation | |||||
| No | 1 | — | — | ||
| Yes | 1.61 | 1.35–1.92 | 0.48 | 5.31 | <0.001 |
| Left bundle branch block | |||||
| No | 1 | — | — | ||
| Yes | 1.90 | 1.50–2.40 | 0.64 | 5.28 | <0.001 |
| Gender | |||||
| Female | 1 | — | — | ||
| Male | 1.66 | 1.36–2.03 | 0.51 | 4.90 | <0.001 |
| Planned treatment | |||||
| Medical | 1 | — | — | ||
| PCI | 0.83 | 0.67–1.01 | −0.19 | −1.83 | 0.067 |
| CABG | 1.76 | 1.38–2.26 | 0.57 | 4.51 | <0.001 |
| Cerebrovascular event | |||||
| No | 1 | — | — | ||
| Yes | 1.79 | 1.38–2.32 | 0.58 | 4.40 | <0.001 |
| Creatinine clearance | |||||
| Per 10 unit decrease < 100 mL/min | 1.12 | 1.06–1.17 | −0.11 | −4.27 | <0.001 |
| Haemoglobin | |||||
| Per g/dL decrease | 1.13 | 1.07–1.20 | −0.12 | −4.27 | <0.001 |
| Elevated CKMB/troponins | |||||
| No | 1 | — | — | ||
| Yes | 1.55 | 1.26–1.90 | 0.44 | 4.23 | <0.001 |
| Current smoker | |||||
| No | 1 | — | — | ||
| Yes | 1.60 | 1.28–1.99 | 0.47 | 4.13 | <0.001 |
| Previous MI | |||||
| No | 1 | — | — | ||
| Yes | 1.33 | 1.11–1.59 | 0.28 | 3.08 | 0.002 |
Model is based on all 13 819 patients with missing values imputed.
aCreatinine clearance and haemoglobin have a negative coefficient as results are the increased risk per unit decrease.
Figure 1.
Observed and expected risk of mortality within 1 year by risk group. The comparison of the observed and expected probabilities of dying demonstrates the model's goodness of fit.
Associations of major bleeding and myocardial infarction with risk and timing of mortality
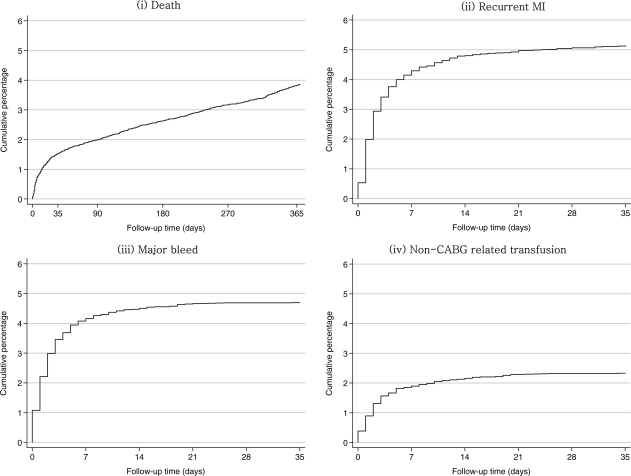
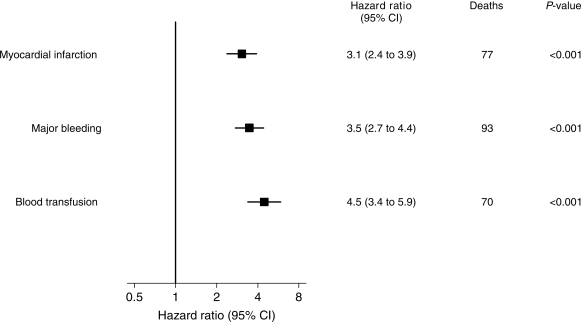
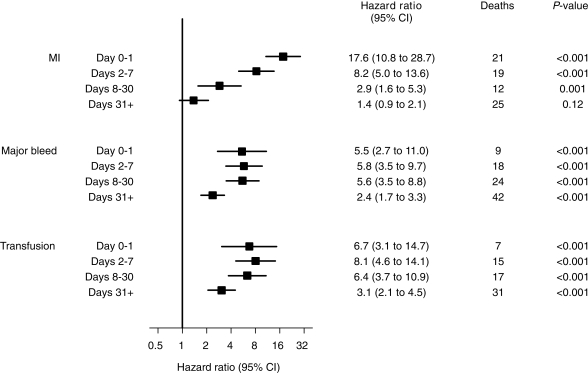
The cumulative incidence of death over 1 year and recurrent MI, major bleeding, and blood transfusion over the first 30 days following randomization are shown in Figure 2. It can be seen that some patients died before others experienced either an MI or a major bleed. Hence in order to investigate the impact of these adverse events on the risk of subsequent mortality, it is necessary to include these events as time-updated covariates in a Cox model. Three separate Cox models for mortality with (i) MI, (ii) major bleed, and (iii) blood transfusion as time-updated covariates demonstrated that each of these events predicts a significantly increased hazard (Figure 3). Each of these Cox models was then extended to demonstrate how the increase in hazard depends on the time since the event (MI, major bleed, blood transfusion) in four time intervals: days 0–1 after, days 2–7 after, days 8–30, and 31 or more days after the event (Figure 4).
Figure 2.
Cumulative risk of (i) death; (ii) recurrent MI, (iii) major bleed; (iv) non-CABG related transfusion. The cumulative risk of mortality (to 1 year), recurrent MI, major bleed, and non-CABG related transfusion (to 35 days).
Figure 3.
Influence of recurrent MI, major bleed, and non-CABG related blood transfusion on mortality to 1 year [three Cox model with MIs, bleeds, and transfusions as time-updated covariates (pre-/post-event) adjusted for baseline predictors]. When included as a time-updated covariate in the Cox model, major bleeding and blood transfusion within 30 days of randomization had similar or slightly greater risk of 1-year mortality compared with MI within 30 days.
Figure 4.
Influence of recurrent MI, major bleed, and non-CABG related transfusion on mortality to 1 year [three Cox model with recurrent MI, major bleed, and transfusion as binary time-updated covariates (time since event) adjusted for baseline predictors]. The excess risk of death following a major bleed and transfusion within 30 days of randomization remained steady and highly significant over time, whereas the impact of an MI within 30 days on the risk of mortality decreased rapidly over time.
There were 77 deaths that occurred following an MI in 705 patients. The occurrence of an MI was associated with a hazard ratio of 3.1 (95% CI, 2.4–3.9) compared with patients not yet having an MI, after adjustment for baseline predictors (Figure 3). However, this elevated risk of death was highly dependent on time since the MI. That is, on the same day or the day after the MI, the hazard ratio was 17.6, within a week it was 8.2, within 30 days it was 2.9, and thereafter it was 1.4 (no longer a significant excess risk), all baseline adjusted (Figure 4).
There were 93 deaths that occurred following a major bleed in 645 patients. Occurrence of a major bleed had an overall hazard ratio for mortality of 3.5 (95% CI 2.7–4.4) after adjustment for baseline predictors (Figure 3). This excess risk of death following a major bleed remained steady and highly significant over time: on the same day or the day following the bleed the hazard ratio was 5.5, within a week it was 5.8, within 30 days it was 5.6, and thereafter it was 2.4, all baseline adjusted (Figure 4). Blood transfusion occurred in 319 patients following a major bleed, of whom 70 died within 1 year of randomization (overall hazard ratio for mortality of 4.5 (95% CI 3.4–5.9), with a fairly constant and significant hazard ratio for up to 1 year after the transfusion event (Figures 3 and 4).
The ACUITY trial definition of major bleed used here includes ≥ 5 cm diameter haematoma. In the 125 major bleeds (19.4%) for which this was the only criterion, five deaths subsequently occurred, and there was no evidence of excess mortality. Excluding such haematomas, increases the overall hazard ratio for mortality following major bleed to 3.9 (95% CI 3.1–5.0).
Among patients experiencing an MI, 84% of deaths (65/77) were due to a cardiac cause compared with 56% of deaths (251/447) in patients without an MI. In contrast, the percentage of cardiac deaths among those experiencing a major bleed was the same as those without a major bleed [60% (56/93) and 60% (260/431), respectively]. The other 37 deaths after a major bleed were classified as 7 specifically bleeding-related, 24 non-cardiac, 6 unknown cause.
Combined impact of major bleeding and myocardial infarction on mortality risk
A total of 94 patients had both an MI and a major bleed (34 had a major bleed first, 31 had the MI first, and 29 had both events on the same day). The combined influence on risk of 1-year mortality of MI, major bleed (without or prior to transfusion), and blood transfusion within 30 days are shown in Table 3. Once again, MI shows the same pattern of mortality risk, i.e. very high in the first several days with a rapid decline thereafter, but the hazard ratios are somewhat reduced after considering bleeds and transfusions. The mortality risk of major bleeding without transfusion remains reasonably constant over time until 30 days with a hazard ratio ∼3. Post-transfusion, the hazard ratio ∼5, again remaining steady over time. As can be seen, Figure 4 and Table 3 present somewhat different findings: the former is based on three separate models for MI, major bleed, and blood transfusion, respectively, whereas the latter is based on one overall model which simultaneously considers all three types of adverse events. Extension of the latter model to include randomized treatment as an additional covariate had negligible impact on the results. In addition, there was no significant heterogeneity of results between treatment groups.
Table 3.
Hazard ratios for mortality from MI, major bleed, and blood transfusion within 30 days, all simultaneously included in a Cox regression with time-updated covariates (i) time considered as pre-/post-event, (ii) time considered as interval since event, and adjustment for baseline predictors
| Risk factor | Hazard ratio | 95% CI | Z | P-value |
|---|---|---|---|---|
| Pre-/post-event | ||||
| MI | ||||
| Pre-MI | 1 | — | ||
| Post-MI | 2.51 | 1.95–3.25 | 7.06 | <0.001 |
| Major bleeda | ||||
| Pre-bleed | 1 | — | ||
| Post-bleed (before transfusion) | 2.00 | 1.30–3.06 | 3.18 | <0.001 |
| Post-transfusion | 3.93 | 2.95–5.24 | 9.35 | <0.001 |
| Time interval post-event | ||||
| MI | ||||
| Pre-MI | 1 | — | — | |
| 0–1 Day | 14.87 | 8.94–24.72 | 10.40 | <0.001 |
| 2–7 Days | 5.98 | 3.53–10.12 | 6.66 | <0.001 |
| 8–30 Days | 2.08 | 1.12–3.88 | 2.31 | 0.021 |
| 31+ Days | 1.22 | 0.80–1.85 | 0.92 | 0.36 |
| Major bleedb (before or without transfusion) | ||||
| Pre-bleed | 1 | — | ||
| 0–7 Daysc | 2.64 | 1.15–6.04 | 2.29 | 0.022 |
| 8–30 Days | 3.35 | 1.45–7.73 | 2.84 | 0.005 |
| 31+ Days | 1.51 | 0.82–2.77 | 1.32 | 0.19 |
| Transfusion | ||||
| Pre-bleed | 1 | — | ||
| 0–1 Day after transfusion | 3.25 | 1.43–7.36 | 2.82 | 0.005 |
| 2–7 Days | 5.44 | 3.01–9.84 | 5.61 | <0.001 |
| 8–30 Days | 6.00 | 3.45–10.42 | 6.36 | <0.001 |
| 31+ Days | 3.05 | 2.06–4.52 | 5.55 | <0.001 |
aIn this model, there is a binary time-updated covariate for major bleed (without transfusion as yet) which starts on the day of the bleeding event. If there is a transfusion (either immediately or at a later day), this covariate is then subsequently replaced by a binary time-updated covariate for transfusion, which starts on the day of transfusion.
bIn this model, the same principle is extended to include binary time-updated covariates for different intervals post-major bleed (without transfusion as yet) and post-transfusion.
cDays 0–1 and 2–7 combined due to small numbers following a bleed but before transfusion.
Discussion
This analysis demonstrates that major bleeding and MI each have a meaningful and similar associations with the subsequent rate of mortality in the first year in patients with moderate and high-risk ACS managed by an early invasive strategy. These associations persist after adjustment for patient characteristics including baseline demographics, laboratory findings, and treatment strategies. However, the time pattern of mortality risk differed between major bleeding and MI. MI led to a more dramatic very early risk of death, whereas major bleeding carried a more prolonged mortality risk.
In the present study, the risk of mortality over 1 year was strongly associated with major bleeding and transfusion as well as MI after adjustment for baseline and procedural predictors of mortality. When included as a time-updated covariate in the Cox model, major bleeding (hazard ratio 3.5) and blood transfusion (hazard ratio 4.5) had similar or slightly greater risk of 1-year mortality compared with MI (hazard ratio 3.1). Our findings are in agreement with the previous reports assessing the association of bleeding complications and/or blood transfusion with mortality.7–11 Eikelboom et al.10 reported that patients with major bleeding had a five-fold-higher incidence of 30-day mortality. The risk of mortality was increased in a stepwise manner as bleeding severity increased.16 Data from the OASIS-5 trial, which enrolled over 20 000 patients with ACS demonstrated that treatment with fondaparinux, which significantly reduced the rate of early bleeding complications compared with treatment with enoxaparin, resulted in a significantly lower mortality rate at 30 days and 6 months.17 Likewise, Rao et al.8 reported that transfusion was associated with nearly a four-fold increase in the adjusted risk for 30-day mortality in a pooled analysis of 24 112 patients with ACS.
We have identified that the time pattern of elevated mortality risk in patients with major bleeding differs from that of patients with MI. Although, there has been one report that the risk of death in patients with major bleeding was temporally different after 30 days, no analysis was performed to examine the specific time pattern of mortality following the major bleed.10 Peri-procedural MI associated with PCI or CABG has been consistently demonstrated to have a direct correlation with mortality.18–20 The mechanisms of MI during and following revascularization procedures, which have mostly been explained by distal embolization, side-branch or target vessel closure, or flow limiting dissection or vasospasm in PCI and early graft failure, and/or lack of adequate myocardial protection in CABG, lead to immediate myocardial necrosis, which can result in fatal cardiac arrhythmia or reduced ventricular function.18,20,21 This is consistent with our finding that the risk of death after MI was highest within the first several days and steadily decreased thereafter.
In contrast to MI, the mechanisms responsible for the association between bleeding and mortality are more complex, and include hypotension, anaemia, adverse effects of blood product transfusion, ineffective oxygen delivery, vasoconstriction, platelet dysfunction and in some cases, the discontinuation and/or reversal of essential anti-thrombotic therapy or anti-platelet therapy.7,11 These mechanisms likely contribute to the more sustained temporal relation of the risk of mortality after major bleeding compared with that after MI. In addition, major bleed has profound haemodynamic impact that may destabilize a previously tenuous patient with respect to renal and cerebral functions thereby accelerating patient's demise. Finally, a major bleed may also unmask a previously covert ominous diagnosis (e.g. advanced cancer); this should be evaluated in longer term studies and through clinical evaluation of interventions that specifically attenuate major bleeding.
Blood transfusion was also associated with an increased risk of mortality. Although our study protocol did not determine the severity of anaemia that would necessitate a transfusion, the need for transfusion was likely correlated with the severity of bleeding. In addition to being a marker of more severe bleeding, blood transfusion may directly cause adverse outcomes through depletion in nitric oxide and resultant vasoconstriction or decreased oxygen carriage of the blood.8,22 Therefore, it is not surprising that the mortality hazard ratio following blood transfusion was even higher, although its time pattern was similar compared with major bleeding.
In the previous report on 30 day results of ACUITY,11 we identified the independent predictors of major bleeding to be advanced age, female gender, diabetes, hypertension, renal insufficiency, anaemia, no prior PCI, cardiac biomarker elevation, ST-segment deviation, and treatment with heparin plus GPI vs. bivalirudin monotherapy. Major bleeding was an independent predictor of 30-day mortality (odds ratio 7.55, 95% CI 4.68–12.18, P < 0.0001).
Results from a registry conducted in patients with acute MI23 indicated that major bleeding (albeit following a different definition) occurred in ∼3% such patients, accounted for only 10% of all hospital deaths, and the reported low risk of hospital mortality associated with bleeding questioned its importance. These data are in sharp contrast with the recently reported data from our group24 in a large randomized trial of acute MI that indicated that the administration of bivalirudin resulted in lower bleeding and reduced mortality at 30 days, whereas it offered no benefit over control with respect to ischaemic endpoints. Hence, the causal relationship between bleeding and mortality is evolving and can certainly be multifactorial and difficult to definitively prove, especially in studies with low overall mortality such as ACUITY.
Limitations
This report has several potential limitations. First, the current analysis was not pre-specified in the original protocol of the ACUITY trial. Secondly, although multivariable analysis was performed to adjust for baseline predictors of mortality, there might be unmeasured confounders that affect the observed association between bleeding, transfusion, MI, and mortality. Hence, one cannot directly infer from these analyses that the observed associations establish a causal link between bleeding events and mortality risk. Thirdly, the patients enrolled in this study had moderate and high-risk ACS and underwent an early invasive management strategy. Therefore, these results may not apply to patients with lower-risk ACS and those who do not undergo early angiography.
Conclusions
Our analysis from the ACUITY trial demonstrates that both major bleeding and MI have important and similar associations with mortality in the first year following presentation with an ACS. MI, which accounted for 10% of deaths, and major bleeding, which accounted for 13% of deaths, carry a similar and substantial risk. An MI is associated with a more dramatic short-term risk of death, whereas a major bleed correlates with a more prolonged mortality risk. Contemporary treatment of patients with ACS should take into careful consideration hazards of both major bleeding and MI to provide best possible patient management.
Funding
The ACUITY trial was sponsored by the Medicines Company.
Conflict of interest: R.M. is on the speaker's bureau for the Medicines Company, Cordis, and Boston Scientific; has received research grants from sanofi-aventis, TMC, Boston Scientific, Abbott Vascular; and honoraria/consulting fees from Boston Scientific, Lilly/Diachi Sankyo, Medtronic Vascular, Abbott Vascular, Cordis Corp., Flow Medica. S.J.P. has received consulting fees from the Medicines Company. G.W.S. has received consulting fees from the Medicines Company, Boston Scientific, Guidant, Abbott, Volcano, St Jude, and BMS Imaging, and lecture fees from the Medicines Company, Nycomed, Guidant, Medtronic, and Abbott. T.C.C. has received consulting fees from the Medicines Company. F.F. has equity interests in the Medicines Company, Johnson & Johnson, and Millennium Pharmaceuticals, and receiving consulting fees from the Medicines Company. S.V.M. has received lecture fees from the Medicines Company and Nycomed. E.N. has received lecture fees from Abbott. A.K. is on the speaker's bureau for the Medicines Company. H.D.W. has received consulting fees and lecture fees from sanofi-aventis and the Medicines Company, and grant support from Alexion, sanofi-aventis, Eli Lilly, Merck Sharpe and Dohme, the Medicines Company, Neuren Pharmaceuticals, National Institutes of Health, GlaxoSmithKline, Pfizer, Roche, Fournier Laboratories, Johnson & Johnson, Proctor & Gamble, and Schering Plough. J.H.W. has received consulting fees from InfraReDX, Biogen, the Medicines Company, Pfizer, Schering Plough, and Proctor & Gamble. J.W.M. has received consulting fees from Johnson & Johnson and is on the speaker's bureau for Astra Zeneca. E.M.O. has received consulting fees from Inovise Medical, Response Biomedical, and Savacor; has equity/ownership in Medtronic and Savacor; has received lecture fees from Schering Plough, Bristol Myers Squibb, and Datascope; and grant support from Bristol Myers Squibb, sanofi-aventis, Schering Plough, Millenium, and Berlex. G.D.D., A.J.L., and A.C. none declared.
References
- 1.Bavry AA, Kumbhani DJ, Rassi AN, Bhatt DL, Askari AT. Benefit of early invasive therapy in acute coronary syndromes: a meta-analysis of contemporary randomized clinical trials. J Am Coll Cardiol. 2006;48:1319–1325. doi: 10.1016/j.jacc.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, Jones RH, Kereiakes D, Kupersmith J, Levin TN, Pepine CJ, Schaeffer JW, Smith EE, III, Steward DE, Theroux P, Gibbons RJ, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Smith SC, Jr American College of Cardiology; American Heart Association. Committee on the Management of Patients with Unstable Angina. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction—summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients with Unstable Angina) J Am Coll Cardiol. 2002;40:1366–1374. doi: 10.1016/s0735-1097(02)02336-7. [DOI] [PubMed] [Google Scholar]
- 3.Bertrand ME, Simoons ML, Fox KA, Wallentin LC, Hamm CW, McFadden E, de Feyter PJ, Specchia G, Ruzyllo W. Management of acute coronary syndromes: acute coronary syndromes without persistent ST-segment elevation; recommendations of the Task Force of the European Society of Cardiology. Eur Heart J. 2000;21:1406–1432. doi: 10.1053/euhj.2000.2301. [DOI] [PubMed] [Google Scholar]
- 4.The PRISM-PLUS Study Investigators. Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q-wave myocardial infarction. N Engl J Med. 1998;338:1488–1497. doi: 10.1056/NEJM199805213382102. [DOI] [PubMed] [Google Scholar]
- 5.The PURSUIT Trial Investigators. Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. N Engl J Med. 1998;339:436–443. doi: 10.1056/NEJM199808133390704. [DOI] [PubMed] [Google Scholar]
- 6.Newby LK, Gibson CM, Allen-LaPointe NM, Pollack C, Gibler WB, Ohman EM, Peterson ED CRUSADE Investigators. Excess dosing of antiplatelet and antithrombin agents in the treatment of non-ST-segment elevation acute coronary syndromes. J Am Med Assoc. 2005;294:3108–3116. doi: 10.1001/jama.294.24.3108. [DOI] [PubMed] [Google Scholar]
- 7.Rao SV, Eikelboom JA, Granger CB, Harrington RA, Califf RM, Bassand J-P. Bleeding and blood transfusion issues in patients with non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1193–1204. doi: 10.1093/eurheartj/ehm019. [DOI] [PubMed] [Google Scholar]
- 8.Rao SV, Jollis JG, Harrington RA, Granger CB, Newby LK, Armstrong PW, Moliterno DJ, Lindblad L, Pieper K, Topol EJ, Stamler JS, Califf RM. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. J Am Med Assoc. 2004;292:1555–1562. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 9.Rao SV, O'Grady K, Pieper KS, Granger CB, Newby LK, Van de Werf F, Mahaffey KW, Califf RM, Harrington RA. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96:1200–1206. doi: 10.1016/j.amjcard.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 10.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KAA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–782. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 11.Manoukian SV, Feit F, Mehran R, Voeltz MD, Ebrahimi R, Hamon M, Dangas GD, Lincoff AM, White HD, Moses JW, King SB, III, Ohman EM, Stone GW. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY trial. J Am Coll Cardiol. 2007;49:1362–1368. doi: 10.1016/j.jacc.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 12.Stone GW, Ware JH, Bertrand ME, Lincoff AM, Moses JW, Ohman EM, White HD, Feit F, Colombo A, McLaurin BT, Cox DA, Manoukian SV, Fahy M, Clayton TC, Mehran R, Pocock SJ ACUITY Investigators. Antithrombotic strategies in patients with acute coronary syndromes undergoing early invasive management: one-year results from the ACUITY trial. J Am Med Assoc. 2007;298:2497–2506. doi: 10.1001/jama.298.21.2497. [DOI] [PubMed] [Google Scholar]
- 13.Stone GW, McLaurin BT, Cox DA, Bertrand ME, Lincoff AM, Moses JW, White HD, Pocock SJ, Ware JH, Feit F, Colombo A, Aylward PE, Cequier AR, Darius H, Desmet W, Ebrahimi R, Hamon M, Rasmussen LH, Rupprecht HJ, Hoekstra J, Mehran R, Ohman EM ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355:2203–2216. doi: 10.1056/NEJMoa062437. [DOI] [PubMed] [Google Scholar]
- 14.Stone GW, Bertrand M, Colombo A, Dangas G, Farkouh ME, Feit F, Lansky AJ, Lincoff AM, Mehran R, Moses JW, Ohman M, White HD. Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial: study design and rationale. Am Heart J. 2004;148:764–775. doi: 10.1016/j.ahj.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 15.Altman DG, De Stavola BL. Practical problems in fitting a proportional hazards model to data with updated measurements of the covariates. Stat Med. 1994;13:301–341. doi: 10.1002/sim.4780130402. [DOI] [PubMed] [Google Scholar]
- 16.Rao SV, O'Grady K, Pieper KS, Granger CB, Newby LK, Mahaffey KW, Moliterno DJ, Lincoff AM, Armstrong PW, Van de Werf F, Califf RM, Harrington RA. A comparison of the clinical impact of bleeding measured by two different classifications among patients with acute coronary syndromes. J Am Coll Cardiol. 2006;47:809–816. doi: 10.1016/j.jacc.2005.09.060. [DOI] [PubMed] [Google Scholar]
- 17.The Fifth Organization to Assess Strategies in Acute Ischemic Syndromes I. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med. 2006;354:1464–1476. doi: 10.1056/NEJMoa055443. [DOI] [PubMed] [Google Scholar]
- 18.Ricciardi MJ, Wu E, Davidson CJ, Choi KM, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation. 2001;103:2780–2783. doi: 10.1161/hc2301.092121. [DOI] [PubMed] [Google Scholar]
- 19.Cavallini C, Savonitto S, Violini R, Arraiz G, Plebani M, Olivari Z, Rubartelli P, Battaglia S, Niccoli L, Steffenino G, Ardissino D Italian ‘Atherosclerosis, Thorombosis, Vascular Biology’, ‘Society for Invasive Cardiology-GISE’ Investigators. Impact of the elevation of biochemical markers of myocardial damage on long-term mortality after percutaneous coronary intervention: results of the CK-MB and PCI study. Eur Heart J. 2005;26:1494–1498. doi: 10.1093/eurheartj/ehi173. [DOI] [PubMed] [Google Scholar]
- 20.Brener SJ, Lytle BW, Schneider JP, Ellis SG, Topol EJ. Association between CK-MB elevation after percutaneous or surgical revascularization and three-year mortality. J Am Coll Cardiol. 2002;40:1961–1967. doi: 10.1016/s0735-1097(02)02538-x. [DOI] [PubMed] [Google Scholar]
- 21.Herrmann J. Peri-procedural myocardial injury: 2005 update. Eur Heart J. 2005;26:2493–2519. doi: 10.1093/eurheartj/ehi455. [DOI] [PubMed] [Google Scholar]
- 22.Wallis JP. Nitric oxide and blood: a review. Transfus Med. 2005;15:1–11. doi: 10.1111/j.1365-3148.2005.00542.x. [DOI] [PubMed] [Google Scholar]
- 23.Spencer FA, Moscucci M, Granger CB, Gore JM, Goldberg RJ, Steg PG, Goodman SG, Budaj A, FitzGerald G, Fox KA GRACE Investigators. Does comorbidity account for the excess mortality in patients with major bleeding in acute myocardial infarction? Circulation. 2007;116:2793–2801. doi: 10.1161/CIRCULATIONAHA.107.694273. [DOI] [PubMed] [Google Scholar]
- 24.Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Kirtane AJ, Parise H, Mehran R HORIZONS-AMI Trial Investigators. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218–2230. doi: 10.1056/NEJMoa0708191. [DOI] [PubMed] [Google Scholar]