Abstract
Aims
Fatty fish and marine omega-3 fatty acids were associated with lower rates of heart failure (HF) among US elderly, but this has not been confirmed in broader age ranges or other populations where source and type of fish may differ. We therefore conducted a population-based, prospective study of 39 367 middle-aged and older Swedish men.
Methods and results
Diet was measured using food-frequency questionnaires. Men were followed for HF through Swedish inpatient and cause-of-death registers from 1 January 1998 to 31 December 2004. We used proportional hazards models adjusted for age and other covariates to estimate hazard ratios (HR). Compared with no consumption, men who ate fatty fish once per week had an HR of 0.88 (95% CI 0.68–1.13). Hazard ratios for consumption two times per week and ≥3 times per week were 0.99 and 0.97, respectively. Hazard ratios across quintiles of marine omega-3 were 1, 0.94 (95% CI 0.74–1.20), 0.67 (95% CI 0.50–0.90), 0.89 (95% CI 0.68–1.16), 1.00 (95% CI 0.77–1.29).
Conclusion
In this population, moderate intake of fatty fish and marine omega-3 fatty acids was associated with lower rates of HF, though the association for fish intake was not statistically significant; higher intake was not associated with additional benefit.
Keywords: Heart failure, Diet, Omega-3 fatty acids, Fish
Introduction
Heart failure (HF) is a clinical syndrome characterized by dyspnoea, fatigue, and fluid retention caused by insufficiencies in the heart’s ability to pump blood.1 Between 0.4 and 2% of the European population have prevalent HF.2 Although age-standardized rates of HF have declined in Sweden, there were 237 first hospitalizations for HF for every 100 000 men in 2000.3 Heart failure treatment consumes ∼2% of Sweden’s healthcare budget.4 Prevention of HF is an important clinical and public health priority,2,5 particularly since population aging and improved treatment of myocardial infarction (MI) and HF are expected to increase HF incidence and prevalence.5
Evidence from clinical trials and cohort studies indicates that fish consumption, particularly fatty fish such as herring and salmon, and supplementation with long-chain marine omega-3 fatty acids reduce rates of cardiovascular diseases, including sudden death and fatal coronary heart disease.6 Omega-3 fatty acids may improve cardiovascular health through anti-arrhythmic effects, reduced triglyceride concentrations, small reductions in blood pressure, and decreased platelet aggregation.7,8 Omega-3 fatty acids may have favourable effects on heart rate,9 heart rate variability,10 and endothelial function which may help prevent HF.11 However, contaminated fish can contain mercury and organic pollutants that may have adverse effects on cardiovascular health.6,12
Both fish and marine omega-3 fatty acids were associated with lower HF rates in a cohort of elderly men and women in the US.13 However, this finding has not been replicated in populations with a broader age range or from regions where the source and types of fish consumed and potential contaminates may differ. We therefore examined the associations of fatty fish and marine omega-3 intake with HF in a cohort of middle-aged and elderly Swedish men.
Methods
Participants
This study included 39 367 participants in the Cohort of Swedish Men. The cohort recruitment process, characteristics, and study methods have been previously described.14 The Cohort of Swedish Men is a population-based cohort of men 45–79 years old in Västmanland and Örebro counties in central Sweden who completed a questionnaire on demographics, weight, height, and intake of foods and beverages in late 1997 and early 1998.14 Of the 48 850 men who returned questionnaires, 2944 were excluded because of missing or incorrect national identification numbers, blank questionnaires, or previous diagnosis of cancer (except non-melanoma skin cancer). For this analysis men with implausible energy intake (>3 standard deviations from the natural logarithm transformed mean) (n = 562) or a history of HF at baseline (n = 743) were also excluded. In the primary analyses men with baseline history of MI (n = 2077) or diabetes (n = 3157) were excluded because they had higher rates of HF and may have changed their diets because of their diagnosis. Baseline HF and MI was determined through the Swedish inpatient register, and diabetes was determined using self-report and the register. The study complies with the Declaration of Helsinki and was approved by the Regional Ethical Review Board at Karolinska Institute, Stockholm, Sweden. Completion and return of the self-administered questionnaire was taken to imply consent.
Diet assessment
Participants reported usual frequency of consumption of 96 foods and beverages over the previous year using self-administered food-frequency items. For commonly consumed foods and beverages such as milk, coffee, cheese, and bread, participants reported their consumption in servings per day or per week. For other foods and beverages there were eight pre-defined responses ranging from never to ≥3 times/day. The questionnaires contained five questions on fish intake: herring/mackerel, salmon/whitefish/char, cod/saithe/fishfingers, caviar, and shellfish including shrimp. Herring/mackerel and salmon/whitefish/char were considered fatty fish. We categorized study participants into categories of no consumption of fatty fish, <1/week, 1/week, 2/week, and ≥3/week. We categorized participants into those who consumed any fish <1/week, 1/week, 2/week, 3–6/week, and ≥1/day.
Portion sizes for most foods were not specified on the questionnaires; mean portion sizes for each food were calculated for men aged 45–52, 53–61, 62–69, and 70–79 years based on 2 weeks of diet records completed during a study of the questionnaire performance. Seven hundred and ninety men from central Sweden who were not Cohort of Swedish Men participants were randomly selected from the population register and invited to participate in the study which included two administrations of the food-frequency questionnaires, 2 weeks of weighed diet records, and fourteen 24 h recalls. Men could choose to participate in the diet record component, the 24 h recall component, or both. One hundred and fifty-two men completed 2 weeks of diet records and contributed to the estimation of portion sizes. Mean portion sizes of herring/mackerel were 127 g (95% CI 107–147) in men 45–52, 110 g (95% CI 92–127) in men 53–61, 94 g (95% CI 82–106) in men 62–69, and 91 g (95% CI 79–102) in men 70–79. Mean portion sizes of salmon/whitefish/char were 77 g (95% CI 55–99) in men 45–52, 118 g (95% CI 90–146) in men 53–61, 114 g (95% CI 89–139) in men 62–69, and 86 g (95% CI 37–134) in men 70–79.
Total consumption of foods and beverages was calculated by multiplying frequency of consumption by age-specific portion sizes. Nutrient values were calculated using food composition data from the Swedish National Food Administration.15 Nutrients were adjusted for energy using the residuals method.16 Marine omega-3 fatty acids were calculated as the sum of eicosapentaenoic acid (C20:5n-3) and docosahexaenoic acid (C22:6n-3) from food sources. Participants also reported the consumption of fish oil supplements in capsules/week. As a secondary exposure, marine omega-3 fatty acids consumed as supplements were included assuming 0.3 g of marine omega-3 fatty acids/capsule.
Spearman correlations between food-frequency questionnaires and fourteen 24 h recalls were 0.64 for eicosapentaenoic acid and 0.57 for docosahexaenoic acid among 248 men who participated in the 24 h recall component of the study of the questionnaires.17 In a second study of this food-frequency questionnaire among 129 women in central Sweden chosen at random from the population register, the correlation between the food-frequency questionnaire and four 1 week weighted diet records was 0.5 for fatty fish.18
Heart failure follow-up
Participants were followed from 1 January 1998 until 31 December 2004 through linkage to Swedish inpatient and cause-of-death registers. The inpatient register captures >99% of inpatient care.19 Hospitalization for or death from HF was identified by codes 428 (International Classification of Disease-9), I50, or I11.0 (International Classification of Disease-10) as the primary diagnosis. In a study of the inpatient register, 95% of people with these codes as primary diagnosis were found to have HF using European Society of Cardiology criteria on medical record review.20 We included the first HF event recorded in the registers for each individual. Incident MI was also assessed using the inpatient register.
Statistical analysis
Because some participants were missing data on BMI (4.6%) and physical activity (21.6%), we used Markov chain Monte Carlo multiple imputation to simulate five complete data sets. Statistical analyses were performed in each of the data sets, the results were averaged, and confidence intervals and P-values were calculated accounting for uncertainty in the imputed estimates.21
We computed means and percentages of demographic, behavioural, and health covariates by intake of fatty fish. To estimate the hazard ratios (HR) associated with fish consumption, we used Cox proportional hazards models that allowed the baseline hazard to vary by age. We adjusted for body mass index (cubed term), physical activity (linear), energy (linear), alcohol (natural logarithm of consumption in grams plus 0.1), fibre (linear), sodium (linear), and red or processed meat consumption (linear), education (less than high school, high school, university), family history of MI at <60 years (yes, no), cigarette smoking (current, past, never), marital status (single, married, divorced, widowed), self-reported history of hypertension (yes, no), and high cholesterol (yes, no). Functional forms for continuous predictors were chosen based on an algorithm which compared the best-fitting 1 and 2 degree fractional polynomials to a linear term.22
We calculated HR associated with quintiles of marine omega-3 fatty acids adjusted as described earlier. We created an additional model adjusted for protein (linear), saturated fat (linear), monounsaturated fat (linear), non-marine omega-3 fatty acids (linear), and omega-6 fatty acids (linear). The additionally adjusted models can be interpreted as the effect of replacing carbohydrate with omega-3 fatty acids. We explored the potentially nonlinear shape of the association between marine omega-3 fatty acids and incidence of HF using a restricted cubic spline with three knots.23 For this model, we excluded individuals with values below the 5th and above the 95th percentiles to avoid modelling were data were sparse.
Because symptoms of HF occurring prior to hospitalization or death may influence behaviour, we performed a sensitivity analysis excluding cases occurring during the first 2 years of follow-up. We repeated the analyses among men with a history of diabetes or MI at baseline and in the combined population. Fatty-fish intake may influence incidence of HF through effects on MI. We therefore constructed models adjusting for incident MI as a time-varying covariate (no MI, MI within the past year, and more distant history of MI). We tested for violations of the proportional hazards assumption by entering the product of fish intake or marine omega-3 intake and the natural logarithm of time in the model. The proportional hazards assumption did not appear to be violated.
Statistical analyses were performed using SAS version 9.1 (Cary, NC) and Stata version 10.0 (College Station, TX). A two-sided P-value <0.05 was considered statistically significant.
Results
Over a median 7 years of follow-up, 597 of 39 367 men without a history of MI or diabetes at baseline developed HF (563 hospitalizations and 34 deaths with HF listed as the primary cause) corresponding to a rate of two cases/1000 person-years. Fatty-fish intake was positively associated with age, family history of MI, history of hypertension and high cholesterol, and intake of alcohol and red and processed meats (Table 1).
Table 1.
Baseline characteristicsa of the population by fatty-fish intake
| Fatty-fish intake |
|||||
|---|---|---|---|---|---|
| Never (n = 5813) | <1 servings/week (n = 10 700) | 1 serving/week (n = 16 588) | 2 servings/week (n = 5340) | ≥3 servings/week (n = 926) | |
| Age (years) | 58.0 ± 9.8 | 59.3 ± 9.6 | 58.9 ± 9.2 | 61.0 ± 9.2 | 64.9 ± 9.3 |
| Physical activity (MET h/day) | 41.9 ± 5.1 | 41.9 ± 5.0 | 41.4 ± 4.8 | 41.5 ± 4.8 | 42.3 ± 5.1 |
| Body mass index (kg/m2) | 25.7 ± 3.5 | 25.6 ± 3.2 | 25.6 ± 3.2 | 25.8 ± 3.3 | 26.0 ± 3.6 |
| Cigarette smoking | |||||
| Current | 1605 (27.6) | 2675 (25.0) | 3808 (23.0) | 1235 (23.1) | 253 (27.3) |
| Past | 1963 (33.8) | 3972 (37.1) | 6375 (38.4) | 2161 (40.5) | 344 (37.2) |
| Never | 2245 (38.6) | 4053 (37.9) | 6405 (38.6) | 1944 (36.4) | 329 (35.5) |
| Marital status | |||||
| Single | 534 (9.2) | 753 (7.0) | 882 (5.3) | 324 (6.1) | 131 (14.2) |
| Married | 4513 (77.6) | 8792 (82.2) | 14 318 (86.3) | 4474 (83.8) | 654 (70.6) |
| Divorced | 524 (9.0) | 764 (7.1) | 963 (5.8) | 367 (6.9) | 73 (7.9) |
| Widowed | 242 (4.2) | 391 (3.7) | 425 (2.6) | 175 (3.3) | 68 (7.3) |
| Education | |||||
| Less than high school | 4218 (72.6) | 7800 (72.9) | 10 683 (64.4) | 3427 (64.2) | 712 (76.9) |
| High school | 773 (13.3) | 1406 (13.1) | 2622 (15.8) | 789 (14.8) | 93 (10.0) |
| University | 822 (14.1) | 1494 (14.0) | 3283 (19.8) | 1124 (21.1) | 121 (13.1) |
| Family history of myocardial infarction | 629 (10.8) | 1254 (11.7) | 2016 (12.2) | 638 (12.0) | 123 (13.3) |
| History of hypertension | 1050 (18.1) | 2129 (19.9) | 3169 (19.1) | 1217 (22.8) | 232 (25.1) |
| History of high cholesterol | 546 (9.4) | 1231 (11.5) | 2046 (12.3) | 706 (13.2) | 120 (13.0) |
| Energy intake (kcal/day) | 2518 ± 862 | 2605 ± 803 | 2719 ± 787 | 2878 ± 819 | 3251 ± 1237 |
| Alcohol (g/day) | 8.7 ± 10.8 | 9.2 ± 9.6 | 11.4 ± 9.9 | 12.6 ± 11.0 | 11.9 ± 14.9 |
| Sodium (g/day)b | 3098 ± 514 | 3139 ± 409 | 3224 ± 387 | 3351 ± 402 | 3825 ± 727 |
| Fibre (g/day)b | 25.0 ± 7.9 | 25.5 ± 6.8 | 25.8 ± 6.6 | 26.6 ± 6.4 | 25.6 ± 7.4 |
| Red and processed meat (servings/day) | 1.1 ± 0.9 | 1.2 ± 0.7 | 1.3 ± 0.7 | 1.4 ± 0.8 | 1.9 ± 1.7 |
| Marine omega-3 (g/day)b | 0.13 ± 0.09 | 0.27 ± 0.08 | 0.43 ± 0.11 | 0.74 ± 0.18 | 1.82 ± 1.08 |
aNumbers are means ± standard deviation or n (%).
bAdjusted for energy using the residuals method.
The association between fatty-fish consumption and HF was U-shaped in age-adjusted analyses (P-value for quadratic trend = 0.04) (Table 2). The association was attenuated in multivariable-adjusted models (HR 1/week vs. no consumption = 0.88, 95% CI 0.68–1.13) and the quadratic trend was no longer statistically significant (P = 0.32). A potentially nonlinear pattern was observed for total fish intake. Compared with consumption <1/week, the multivariable-adjusted HR was 0.78 (95% CI 0.57–1.08) for 1/week, 0.80 (95% CI 0.58–1.11) for 2/week, 0.76 (95% CI 0.54–1.06) for 3–6/week, and 0.89 (95% CI 0.60–1.33) for ≥1/day, though the quadratic trend was not statistically significant (P = 0.26).
Table 2.
Fish intake and incidence of heart failure
| Fatty-fish intake |
|||||
|---|---|---|---|---|---|
| Never | <1 serving/week | 1 serving/week | 2 serving/week | ≥3 serving/week | |
| Cases | 98 | 165 | 211 | 96 | 27 |
| Person-years | 39 189 | 72 331 | 112 700 | 35 898 | 5984 |
| Model 1 HR (95% CI)a | 1 (reference) | 0.84 (0.66–1.08) | 0.76 (0.60–0.97) | 0.87 (0.65–1.15) | 0.95 (0.62–1.45) |
| Model 2 HR (95% CI)b | 1 (reference) | 0.93 (0.72–1.21) | 0.88 (0.68–1.13) | 0.99 (0.73–1.33) | 0.97 (0.61–1.55) |
aCox proportional hazards model. Baseline hazard allowed to vary by age.
bModel 1 additionally adjusted for body mass index, physical activity, energy, alcohol, fibre, sodium, and red or processed meat consumption, education, family history of myocardial infarction at <60 years, cigarette smoking, marital status, self-reported history of hypertension, and high cholesterol.
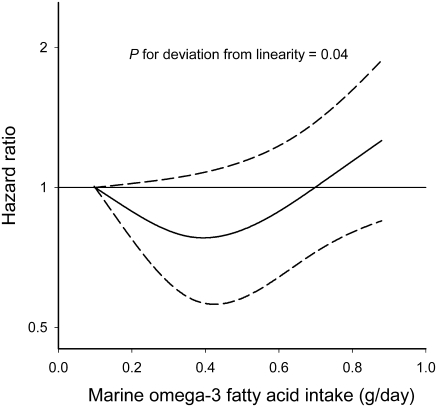
The association between marine omega-3 fatty acids and HF was also U-shaped, with the multivariable-adjusted HR lowest among those in the middle quintile (0.32–0.40 g/day) (P-value for quadratic trend = 0.02) (Table 3). Further adjustment for macronutrients did not materially change the results. A formal analysis of the shape of the association using a restricted cubic spline also suggested a U-shaped relationship (P-value for deviation from linearity = 0.04) (Figure 1).
Table 3.
Marine omega-3 intake and incidence of heart failure
| Quintiles of marine omega-3 fatty acids |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Median intake (range) (g/day) | 0.15 (0.01–0.22) | 0.27 (0.24–0.31) | 0.36 (0.32–0.40) | 0.46 (0.41–0.54) | 0.71 (0.55–8.54) |
| Cases | 144 | 122 | 74 | 102 | 155 |
| Person-years | 52 920 | 53 340 | 53 666 | 53 553 | 52 623 |
| Model 1 HR (95% CI)a | 1 (reference) | 0.87 (0.69–1.11) | 0.62 (0.46–0.82) | 0.84 (0.65–1.09) | 0.99 (0.79–1.24) |
| Model 2 HR (95% CI)b | 1 (reference) | 0.94 (0.74–1.20) | 0.67 (0.50–0.90) | 0.89 (0.68–1.16) | 1.00 (0.77–1.29) |
aCox proportional hazards model. Baseline hazard allowed to vary by age.
bModel 1 additionally adjusted for body mass index, physical activity, energy, alcohol, fibre, sodium, and red or processed meat consumption, education, family history of myocardial infarction at <60 years, cigarette smoking, marital status, self-reported history of hypertension, and high cholesterol.
Figure 1.
The solid line represents hazard ratio and the dashed line represents the 95% confidence intervals. The curve was produced from a Cox proportional hazards model where marine omega-3 intake was modelled as a restricted cubic spline with three knots, the baseline hazard was allowed to vary by age, and the hazard ratio was adjusted for body mass index, physical activity, energy, alcohol, fibre, sodium, and red or processed meat consumption, education, family history of myocardial infarction at <60 years, cigarette smoking, marital status, self-reported history of hypertension, and high cholesterol.
Five percent of participants reported consuming ≥1 fish oil capsule/week. When fatty acids from this source were included in the calculation of marine omega-3, the multivariable-adjusted HR across quintiles were 1, 0.99 (95% CI 0.77–1.27), 0.73 (95% CI 0.54–0.97), 0.97 (95% CI 0.75–1.27), 1.05 (95% CI 0.82–1.36). Although the confidence intervals were wider, the pattern of results was similar when we excluded cases of HF occurring during the first 2 years of follow-up. Compared with the lowest quintile, HR adjusted for incident MI as a time-varying covariate in addition to lifestyle and dietary covariates were 0.94 (95% CI 0.74–1.20), 0.67 (95% CI 0.50–0.90), 0.89 (95% CI 0.68–1.16), and 1.00 (95% CI 0.77–1.29) across quintiles.
Among men with a history of MI or diabetes at baseline, multivariable-adjusted HR were 0.84 (95% 0.58–1.21) for consumption <1/week, 0.94 (95% CI 0.66–1.35) for 1/week, 1.32 (95% CI 0.89–1.96) for 2/week, and 1.20 (95% CI 0.67–2.14) for ≥3/week compared with no consumption of fatty fish. Hazard ratios across quintiles of marine omega-3 fatty acids were 1, 1.04 (95% CI 0.72–1.48), 0.87 (95% CI 0.58–1.28), 1.12 (95% CI 0.77–1.62), and 1.30 (95% CI 0.92–1.83). In the combined population of men with and without a history of MI and diabetes at baseline, HR were 0.88 (95% CI 0.72–1.09) for consumption of fatty fish <1/week, 0.89 (0.73–1.09) for 1/week 1.08 (95% CI 0.85–1.36) for 2/week, and 0.99 (95% CI 0.69–1.42) for ≥3/week compared with no consumption of fatty fish. Corresponding HR across quintiles of marine omega-3 fatty acids were 1, 0.98 (95% CI 0.60–1.58), 0.84 (95% CI 0.49–1.43), 1.27 (95% CI 0.77–2.09), and 1.19 (95% CI 0.75–1.91).
Discussion
In this population, the association between fatty-fish consumption and incidence of HF appeared to be U-shaped with men who consumed fatty fish once per week having the lowest rates of HF (12% lower than those who did not consume fatty fish), though the trend was not statistically significant. A statistically-significant U-shaped relationship was observed between marine omega-3 consumption and HF with HR 33% lower in the third compared with the first quintile. In contrast, the rates of HF decreased across the range of intake of fish and marine omega-3 in a previous study of elderly US men and women.13
Fatty fish and omega-3 fatty acids have been shown to have potentially beneficial effects on triglycerides, platelet aggregation, blood pressure, heart rate, and heart rate variability.8–10 High intake seems to reduce propensity to arrhythmia,24–29 though results are not entirely consistent.30 In post-MI patients, supplementation with marine omega-3 fatty acids improved survival.31 In addition, omega-3 fatty acids slightly improve prognosis,32 improve endothelial function,33 and may reduce inflammation34 in patients with HF.
The apparent U-shaped relationship of fatty fish and marine omega-3 fatty acids with HF was unexpected. The higher rate of HF in participants who consumed the most fatty fish or marine omega-3 fatty acids compared with moderate consumption may be due to chance. Alternatively, if men in poor health consumed more fatty fish, fatty fish and marine omega-3 fatty acids could appear to be risk factors for HF. In this population, men with the highest intake of fatty fish were more likely to have a history of high cholesterol or hypertension. Although we controlled for presence of these risk factors, we did not have information on severity or treatment which could lead to residual confounding. The ideal level of fish consumption has been the subject of research and debate because contaminated fish are major sources of mercury and organic pollutants including polychlorinated biphenyls and dioxins.6,12 Both mercury and organic pollutants may increase risk of cardiovascular disease,35–39 though the evidence is not consistent.40,41 In Sweden, the concentration of contaminants varies by location, with fish from the Baltic Sea and some lakes having relatively high concentrations.12 We did not have information on the source of fish consumed and could not further evaluated the hypothesis that contaminates may have contributed to the U-shaped relationship observed.
There are several additional limitations of this study. Heart failure is a heterogeneous syndrome, and risk factors may not be identical for all subtypes.1 We were not able to determine HF aetiology or subtype. Although Swedish inpatient and cause-of-death registers are almost complete and the accuracy of HF diagnosis has been shown to be high,20,42 the registers only captured cases that result in hospitalization or death. Therefore, our results may not be generalizable to less severe HF treated on an outpatient basis. Fatty-fish consumption and marine omega-3 fatty acids intake were measured using food-frequency questionnaires; we expect some misclassification of intake. We estimated portion sizes for the calculation of nutrient intakes based on a relatively small number of people, leading to additional uncertainty in nutrient intake. The estimation could potentially lead to biased HR and CI that are too narrow. As with all observational studies, we could not rule out bias due to residual or unmeasured confounding.
In summary, moderate intake of fatty fish and marine omega-3 fatty acids appeared to be associated with lower rates of HF in this population of middle-aged and elderly men, though the association with fatty fish did not reach statistical significance. Higher consumption was not associated with additional benefit.
Funding
Swedish Research Council/Committee for infrastructure; Swedish Foundation for International Cooperation in Research and Higher Education (to E.B.L.); National Heart, Lung, and Blood Institute, National Institutes of Health (F32 HL091683 to E.B.L.).
Conflict of interest: none declared.
References
- 1.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult–Summary Article. Circulation. 2005;112:1825–1852. [Google Scholar]
- 2.Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, Tavazzi L, Smiseth OA. European Society of Cardiology; Guidelines for the diagnosis and treatment of Chronic Heart Failure: full text (update 2005) http://www.escardio.org/NR/rdonlyres/8A2848B4-5DEB-41B9-9A0A-5B5A90494B64/0/guidelines_CHF_FT_2005.pdf. (5 June 2008) [PubMed] [Google Scholar]
- 3.Schaufelberger M, Swedberg K, Koster M, Rosen M, Rosengren A. Decreasing one-year mortality and hospitalization rates for heart failure in Sweden; Data from the Swedish Hospital Discharge Registry 1988 to 2000. Eur Heart J. 2004;25:300–307. doi: 10.1016/j.ehj.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Ryden-Bergsten T, Andersson F. The health care costs of heart failure in Sweden. J Intern Med. 1999;246:275–284. doi: 10.1046/j.1365-2796.1999.00520.x. [DOI] [PubMed] [Google Scholar]
- 5.Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, Narula J, Shor ES, Young JB, Hong Y. Prevention of heart failure. Circulation. 2008;117:2544–2565. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 8.Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197:12–24. doi: 10.1016/j.atherosclerosis.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Mozaffarian D, Prineas RJ, Stein PK, Siscovick DS. Dietary fish and n-3 fatty acid intake and cardiac electrocardiographic parameters in humans. J Am Coll Cardiol. 2006;48:478–484. doi: 10.1016/j.jacc.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Stein PK, Prineas RJ, Siscovick DS. Dietary fish and omega-3 fatty acid consumption and heart rate variability in US adults. Circulation. 2008;117:1130–1137. doi: 10.1161/CIRCULATIONAHA.107.732826. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, O’Keefe JH, Lavie CJ, Marchioli R, Harris WS. Omega3 fatty acids for cardioprotection. Mayo Clin Proc. 2008;83:324–332. doi: 10.4065/83.3.324. [DOI] [PubMed] [Google Scholar]
- 12.Becker W, Darnerud PO, Petersson-Grawé K. National Food Administration; Risks and benefits of fish consumption. http://www.slv.se/default.aspx?id=231&epslanguage=EN-GB. (27 May 2008) [Google Scholar]
- 13.Mozaffarian D, Bryson CL, Lemaitre RN, Burke GL, Siscovick DS. Fish intake and risk of incident heart failure. J Am Coll Cardiol. 2005;45:2015–2021. doi: 10.1016/j.jacc.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 14.Larsson SC, Rutegard J, Bergkvist L, Wolk A. Physical activity, obesity, and risk of colon and rectal cancer in a cohort of Swedish men. Eur J Cancer. 2006;42:2590–2597. doi: 10.1016/j.ejca.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Bergström L, Kylberg E, Hagman U, Erikson H, Bruce Å. The food composition database KOST: the National Administration’s information system for nutritive values of food. Vår Föda. 1991;43:439–447. [Google Scholar]
- 16.Willett WC. Nutritional Epidemiology. 2 ed. New York: Oxford University Press; 1998. [Google Scholar]
- 17.Messerer M, Johansson SE, Wolk A. The validity of questionnaire-based micronutrient intake estimates is increased by including dietary supplement use in Swedish men. J Nutr. 2004;134:1800–1805. doi: 10.1093/jn/134.7.1800. [DOI] [PubMed] [Google Scholar]
- 18.Wolk A, Larsson SC, Johansson JE, Ekman P. Long-term fatty fish consumption and renal cell carcinoma incidence in women. JAMA. 2006;296:1371–1376. doi: 10.1001/jama.296.11.1371. [DOI] [PubMed] [Google Scholar]
- 19.The Nation Board of Health and Welfare. Stockholm: 2005. The Swedish Hospital Discharge Registry 1964–2003. [Google Scholar]
- 20.Ingelsson E, Arnlov J, Sundstrom J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005;7:787–791. doi: 10.1016/j.ejheart.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Schafer JL. Analysis of Incomplete Multivariate Data. Boca Raton: CRC Press; 1997. [Google Scholar]
- 22.Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: parsimonious parametric modeling. Appl Statist. 1994;43:429–467. [Google Scholar]
- 23.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 24.Den Ruijter HM, Berecki G, Verkerk AO, Bakker D, Baartscheer A, Schumacher CA, Belterman CN, de Jonge N, Fiolet JW, Brouwer IA, Coronel R. Acute administration of fish oil inhibits triggered activity in isolated myocytes from rabbits and patients with heart failure. Circulation. 2008;117:536–544. doi: 10.1161/CIRCULATIONAHA.107.733329. [DOI] [PubMed] [Google Scholar]
- 25.Sakabe M, Shiroshita-Takeshita A, Maguy A, Dumesnil C, Nigam A, Leung TK, Nattel S. Omega-3 polyunsaturated fatty acids prevent atrial fibrillation associated with heart failure but not atrial tachycardia remodeling. Circulation. 2007;116:2101–2109. doi: 10.1161/CIRCULATIONAHA.107.704759. [DOI] [PubMed] [Google Scholar]
- 26.Metcalf RG, Sanders P, James MJ, Cleland LG, Young GD. Effect of dietary n-3 polyunsaturated fatty acids on the inducibility of ventricular tachycardia in patients with ischemic cardiomyopathy. Am J Cardiol. 2008;101:758–761. doi: 10.1016/j.amjcard.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Chrysohoou C, Panagiotakos DB, Pitsavos C, Skoumas J, Krinos X, Chloptsios Y, Nikolaou V, Stefanadis C. Long-term fish consumption is associated with protection against arrhythmia in healthy persons in a Mediterranean region–the ATTICA study. Am J Clin Nutr. 2007;85:1385–1391. doi: 10.1093/ajcn/85.5.1385. [DOI] [PubMed] [Google Scholar]
- 28.Brouwer IA, Zock PL, Camm AJ, Bocker D, Hauer RN, Wever EF, Dullemeijer C, Ronden JE, Katan MB, Lubinski A, Buschler H, Schouten EG. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: the Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized trial. JAMA. 2006;295:2613–2619. doi: 10.1001/jama.295.22.2613. [DOI] [PubMed] [Google Scholar]
- 29.Leaf A, Albert CM, Josephson M, Steinhaus D, Kluger J, Kang JX, Cox B, Zhang H, Schoenfeld D. Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation. 2005;112:2762–2768. doi: 10.1161/CIRCULATIONAHA.105.549527. [DOI] [PubMed] [Google Scholar]
- 30.Raitt MH, Connor WE, Morris C, Kron J, Halperin B, Chugh SS, McClelland J, Cook J, MacMurdy K, Swenson R, Connor SL, Gerhard G, Kraemer DF, Oseran D, Marchant C, Calhoun D, Shnider R, McAnulty J. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: a randomized controlled trial. JAMA. 2005;293:2884–2891. doi: 10.1001/jama.293.23.2884. [DOI] [PubMed] [Google Scholar]
- 31.GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 32.GISSI-HF Investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 33.Morgan DR, Dixon LJ, Hanratty CG, El-Sherbeeny N, Hamilton PB, McGrath LT, Leahey WJ, Johnston GD, McVeigh GE. Effects of dietary omega-3 fatty acid supplementation on endothelium-dependent vasodilation in patients with chronic heart failure. Am J Cardiol. 2006;97:547–551. doi: 10.1016/j.amjcard.2005.08.075. [DOI] [PubMed] [Google Scholar]
- 34.Lennie TA, Chung ML, Habash DL, Moser DK. Dietary fat intake and proinflammatory cytokine levels in patients with heart failure. J Card Fail. 2005;11:613–618. doi: 10.1016/j.cardfail.2005.06.434. [DOI] [PubMed] [Google Scholar]
- 35.Guallar E, Sanz-Gallardo MI, van’t Veer P, Bode P, Aro A, Gomez-Aracena J, Kark JD, Riemersma RA, Martin-Moreno JM, Kok FJ. Mercury, fish oils, and the risk of myocardial infarction. N Engl J Med. 2002;347:1747–1754. doi: 10.1056/NEJMoa020157. [DOI] [PubMed] [Google Scholar]
- 36.Gustavsson P, Hogstedt C. A cohort study of Swedish capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs) Am J Ind Med. 1997;32:234–239. doi: 10.1002/(sici)1097-0274(199709)32:3<234::aid-ajim8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 37.Virtanen JK, Voutilainen S, Rissanen TH, Mursu J, Tuomainen TP, Korhonen MJ, Valkonen VP, Seppanen K, Laukkanen JA, Salonen JT. Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arterioscler Thromb Vasc Biol. 2005;25:228–233. doi: 10.1161/01.ATV.0000150040.20950.61. [DOI] [PubMed] [Google Scholar]
- 38.Dalton TP, Kerzee JK, Wang B, Miller M, Dieter MZ, Lorenz JN, Shertzer HG, Nerbert DW, Puga A. Dioxin exposure is an environmental risk factor for ischemic heart disease. Cardiovasc Toxicol. 2001;1:285–298. doi: 10.1385/ct:1:4:285. [DOI] [PubMed] [Google Scholar]
- 39.Vena J, Boffetta P, Becher H, Benn T, Bueno-de-Mesquita HB, Coggon D, Colin D, Flesch-Janys D, Green L, Kauppinen T, Littorin M, Lynge E, Mathews JD, Neuberger M, Pearce N, Pesatori AC, Saracci R, Steenland K, Kogevinas M. Exposure to dioxin and nonneoplastic mortality in the expanded IARC international cohort study of phenoxy herbicide and chlorophenol production workers and sprayers. Environ Health Perspect. 1998;106(Suppl. 2):645–653. doi: 10.1289/ehp.98106645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshizawa K, Rimm EB, Morris JS, Spate VL, Hsieh CC, Spiegelman D, Stampfer MJ, Willett WC. Mercury and the risk of coronary heart disease in men. N Engl J Med. 2002;347:1755–1760. doi: 10.1056/NEJMoa021437. [DOI] [PubMed] [Google Scholar]
- 41.Svensson BG, Mikoczy Z, Stromberg U, Hagmar L. Mortality and cancer incidence among Swedish fishermen with a high dietary intake of persistent organochlorine compounds. Scand J Work Environ Health. 1995;21:106–115. doi: 10.5271/sjweh.17. [DOI] [PubMed] [Google Scholar]
- 42.McCullough PA, Philbin EF, Spertus JA, Kaatz S, Sandberg KR, Weaver WD. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol. 2002;39:60–69. doi: 10.1016/s0735-1097(01)01700-4. [DOI] [PubMed] [Google Scholar]