Abstract
Biomaterials derived from silk fibrion prepared by aqueous (AB) and organic (HFIP) solvent based processes, along with collagen (COL) and poly-lactic acid (PLA) based scaffolds were studied in vitro and in vivo for their utility in adipose tissue engineering strategies. For in vitro studies, human bone marrow and adipose-derived mesenchymal stem cells (hMSCs and hASCs) were seeded on the various biomaterials and cultured for 21 days in the presence of adipogenic stimulants (AD) or maintained as noninduced controls. Alamar Blue analysis revealed each biomaterial supported initial attachment of hMSCs and hASCs to similar levels for all matrices except COL in which higher levels were observed. hASCs and hMSCs cultured on all biomaterials in the presence of AD showed significant upregulation of adipogenic mRNA transcript levels (LPL, GLUT4, FABP4, PPARγ, adipsin, ACS) to similar extents when compared to noninduced controls. Similarly Oil-Red O analysis of hASC or hMSC-seeded scaffolds displayed substantial amounts of lipid accumulating adipocytes following cultivation with AD. The data revealed AB and HFIP scaffolds supported similar extents of lipid accumulating cells while PLA and COL scaffolds qualitatively displayed lower and higher extents by comparison, respectively. Following a 4 week implantation period in a rat muscle pouch defect model, both AB and HFIP scaffolds supported in vivo adipogenesis either alone or seeded with hASCs or hMSCs as assessed by Oil-Red O analysis, however the presence of exogenous cell sources substantially increased the extent and frequency of adipogenesis observed. In contrast, COL and PLA scaffolds underwent rapid scaffold degradation and were irretrievable following the implantation period. The results suggest that macroporous 3D AB and HFIP silk fibroin scaffolds offer an important platform for cell-based adipose tissue engineering applications, and in particular, provide longer-term structural integrity to promote the maintenance of soft tissue in vivo.
INTRODUCTION
Tissue engineering represents an innovative approach for the development of novel clinical modalities for the repair and reconstruction of adipose tissue defects. Each year a variety of medical procedures are required for the repair of adipose tissue traumas and abnormalities, including breast reconstructions following mastectomies[1], cosmetic facial reconstructions of the cheek, chin, and jaw[2,3], and lipodystrophies associated with type II diabetes[4]. Currently, autologous and allogenic adipose tissues represent a ubiquitous source of material for fat reconstructive therapies. However, these approaches are limited, and often accompanied by a 40–60% reduction in graft volume following transplantation[5,6], limited proliferative capacity of mature adipocytes for ex vivo expansion[7], and extensive adipocyte damage encountered when harvested with conventional liposuction techniques[7].
Recently, cell-based approaches utilizing adipogenic progenitor cells in combination with biomaterial carriers for fat tissue engineering have been developed and were reported to promote both short-term in vivo adipogenesis and to repair defect sites[8–14]. For these cell-based applications, human mesenchymal stem cells derived from bone marrow (hMSCs) or human adipose tissue (hASCs) have both been suggested as potential cell sources for adipose repair therapies[13–16]. hMSCs and hASCs are readily isolatable from bone marrow aspirates or adipose stromal vascular fractions, respectively, and can differentiate into a variety of mesenchymal lineages. Both these properties are desirable attributes for incorporation into clinical repair modalities[13, 14, 16]. Currently, however the efficacy of exogenously-delivered stem cell populations to support the generation of long-term volume stable adipose tissue in vivo is limited by suboptimal properties of their biomaterial carriers including insufficient biocompatibility and rapid scaffold degradation rates[15, 16].
Silk-based biomaterials have previously been demonstrated to offer exceptional benefits over conventional synthetic (e.g. poly-glycolic and lactic acid copolymers) and natural (e.g. collagen type I) biomaterials in generating functional tissue replacements for various mesenchymal tissues, such as bone[17], cartilage[18], and ligament[19]. Silk-based biomaterials offer significant advantages for potential adipose tissue engineering applications; they have low immunogenicity[20], an absence of bioburdens[20], slow degradation rates[21], show plasticity during processing[22–24], and have impressive mechanical properties[25].
Here, we report the development of a new adipose tissue engineering strategy that utilizes 3D porous silk-based fibroin scaffolds in combination with exogenously seeded hASCs or hMSCs for in vitro and in vivo adipogenesis. Evaluations of in vitro and in vivo stem cell adipogenesis on silk-based biomaterials were performed in comparison with conventional biomaterial carriers (collagen type I and poly-lactic acid polymers) to ascertain potential advantages of silk fibroin as a scaffold for cell-based adipogenic tissue engineering.
MATERIALS AND METHODS
Biomaterials
Both aqueous (AB) and 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP)-based silk fibroin 3-D scaffolds were prepared according to our previously described procedures[23, 24]. Briefly, cocoons from Bombyx mori were boiled for 20 minutes in an aqueous solution of 0.02 M Na2CO3, and rinsed with water to eliminate sericin and other contaminating proteins. Purified silk fibroin was solubilized in 9 M LiBr solution and dialyzed (Pierce, Woburn, MA) against distilled water for 1 day. Then the solution was diluted to obtain a 6 w/v% silk. Four grams of granular NaCl particles (particle size: 500–600 μm), which was characterized using a sieving tower (Retsch, Arlesheim, Switzerland), were added to 2 ml of a 6 w/v% silk fibroin solution in Teflon cylinder containers at room temperature. Twenty-four hours later the containers were immersed in water to extract the salt from the porous scaffolds for over 2 days. HFIP-derived silk fibroin scaffolds were prepared from lyophilized protein that was redissolved in HFIP to obtain a 17% (w/v) solution. The scaffolds were prepared by adding 3.14 g of granular NaCl (particle size: 500–600 μm) into 1 ml 17 wt% silk fibroin in HFIP. The containers were covered overnight to reduce the evaporation of HFIP for more homogeneous structures. The solvent was then evaporated at room temperature for 3 days. The silk/porogen matrix was then treated in methanol for 30 min to induce the formation of the β-sheet structure and insolubility in aqueous solution. The matrices were then immersed in water for 2 days to remove the NaCl, and then air-dried. Aqueous-derived silk scaffolds and HFIP-derived silk fibroin scaffolds were cut into discs (5 mm in diameter and 2 mm in thickness) and autoclaved for experiments detailed below. The pore size of both HFIP and aqueous-based silk scaffolds was 450±30 μm[23]. In addition, sterile Ultrafoam 3-D collagen type I porous foams (COL) (Davol, Inc.) and 3-D poly-lactic acid woven meshes (Concordia) (PLA) were aseptically punched (5 mm in diameter and 2 mm in thickness) from manufacturer stock materials and were utilized in methods detailed below for cell response comparisons. Both the PLA and COL biomaterials have been previously characterized and utilized as scaffolds for stem cell based musculoskeletal tissue engineering strategies including the generation of adipose tissue in vivo and in vitro [11, 12, 26, 27]. Therefore, these biomaterials were utilized as a baseline point of comparison to evaluate stem cell adipogenic responses to silk-based biomaterials.
Cells
Human MSCs (hMSCs) were obtained from commercially available bone marrow aspirates from one male donor ≤25 years of age (Clonetics-Poietics, Walkersville, MD) using methods previously reported[28]. Whole bone marrow aspirates were plated at 8–10 μl aspirate/cm2 on 185 cm2 tissue culture plates and cultivated until confluency (~12–14 days) in 40 ml of ex vivo expansion medium consisting of Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum (FCS), 100U/ml penicillin, 100 μg/ml streptomycin, 0.1 mM nonessential amino acids, and 1 ng/ml of basic fibroblast growth factor (bFGF) (Life Technologies, Rockville, MD). hMSCs were maintained in a humidified tissue culture incubator at 37°C with 5%CO2. hMSCs were selected based on their ability to adhere to the tissue culture plastic; non-adherent hematopoietic cells were removed during medium replacement after approximately 5 days in culture. Medium was changed twice per week thereafter. First passage (P1) hMSCs were subsequently detached using 0.25% trypsin/1 mM EDTA, replated at 5×103 cells/cm2, and cultured until confluency to generate second passage (P2) hMSCs. P2 hMSCs were then frozen in liquid nitrogen in DMEM consisting of 10% FCS and 8% dimethylsulfoxide and utilized in experiments detailed below.
For hASC isolation, liposuction aspirates from subcutaneous adipose tissue sites were obtained from a male subject undergoing elective procedures using protocols for hASC isolation reviewed and approved by the Pennington Biomedical Research Center Institutional Review Board prior to this study. Tissues were washed 3–4 times in phosphate buffered saline (PBS) and suspended in an equal volume of PBS supplemented with 1% FCS and 0.1% collagenase type I prewarmed to 37°C. The tissue was placed in an agitated water bath at 37°C with continuous agitation for 60 min and centrifuged for 5 min at 300–500X g at room temperature. The supernatant containing mature adipocytes, was aspirated. The pellet was identified as the stromal vascular fraction (SVF). Portions of the SVF were resuspended and plated at 0.156 ml of tissue digest/cm2 in DMEM/F12 Ham’s medium, 10% FCS, 100U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg of fungizone on 185 cm2 tissue culture plates and maintained in a humidified tissue culture incubator at 37°C with 5%CO2. hASCs were selected based on their ability to adhere to tissue culture plastic and subsequently expanded to 75–90% confluence to generate P1 cells. hASCs were then trypsinized utilizing 0.25% trypsin/1 mM EDTA, replated at 5×103 cells/cm2, and cultured until confluency in DMEM in 10% fetal calf serum (FCS), 100U/ml penicillin, 100 μg/ml streptomycin to generate P2 hASCs. Throughout the expansion period, medium exchange was carried out twice per week. P2 hASCs were subsequently used in experiments detailed below.
Cell Seeding
Scaffolds were prewetted overnight (approximately 12 hours) at 4°C in culture medium consisting of DMEM supplemented with 10% FCS, 100U/ml penicillin, 10 ug/ml streptomycin, and 0.1 mM nonessential amino acids. Scaffolds were transferred aseptically to a 50 ml sterile centrifuge tube (1 scaffold per tube) and were allowed to settle at the bottom of the tube. Following trypsinization, P2 hASCs or hMSCs (1–1.5×106 cells/scaffold) were resuspended into 200 μl of culture medium and 100 μl was applied in two successive applications to opposite faces of the cylindrical biomaterials. The scaffolds were then incubated in the resulting cell solution for 30 min and gently agitated every 15 minutes for 2 hours in a humidified tissue culture incubator at 37°C with 5%CO2. Following primary cell seeding, 5 ml of additional culture medium was added to each cell seeding tube and the scaffolds were further incubated for an additional 8–12 hours to promote cell attachment.
Relative cell numbers
Immediately after cell seeding, the relative number of metabolically active stem cells within each seeded scaffold was determined by the AlamarBlue™ assay according to the manufacturer’s instructions [29]. Seeded scaffolds were incubated in culture medium consisting of DMEM containing 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.1 mM nonessential amino acids supplemented with 10% AlamarBlue reagent for 2 hrs at 37°C with 5% CO2. Aliquots (100 μl) of the culture medium were transferred to 96 well plates and quantified for fluorescence intensity with a microtiter plate reader (Spectromax Gemini XS, Molecular Devices) using an excitation wavelength of 560 nm and an emission wavelength of 590 nm. Non-seeded scaffolds and tissue culture wells were also maintained in culture medium as above and were analyzed similarly as blank controls to adjust for background fluorescence. Scaffolds were then weighed and the relative cell numbers were calculated as the degree of their relative fluorescence intensity (RFU) per mg of scaffold wet weight as previously described [33].
Adipogenic Differentiation
Following cell seeding, scaffolds were washed once with PBS and either analyzed for the extent of attached metabolically active cells using procedures detailed below or transferred to new sterile 50 ml centrifuge tubes for in vitro cultivation as follows. Seeded scaffolds were cultivated for 21 days in adipogenic differentiation medium after one day, consisting of DMEM, 10% FCS, 0.1mM nonessential amino acids, 100 U/mL penicillin, 1000 U/mL streptomycin, 0.2% fungizone antimycotic with adipogenic stimulants (AD) consisting of 0.5 mM 3-isobutyl-1-methyl-xanthine (Sigma-Aldrich, St. Louis, MO), 1 μM dexamethasone (Sigma-Aldrich), 5 μg/ml insulin, and 50 μM indomethacin (Sigma-Aldrich) as previously reported[30]. Similarly, control cultures were maintained in parallel in the absence of adipogenic stimulants. All seeded and nonseeded (control) scaffolds were cultivated in a humidified incubator at 37°C with 5% CO2 and medium exchange was carried out twice per week. Following the in vitro cultivation period, seeded scaffolds were either evaluated for their extent of adipogenesis or utilized in the in vivo studies.
Real-time Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from seeded scaffolds following 21 days of cultivation with AD and in control cultures using Trizol reagent (Life Technologies) according to the single step acid-phenol guanidinium method[31]. cDNA were synthesized using High-Capacity cDNA Archive kit (ABI Biosystems) following the manufacturer’s instructions. Reactions were performed and monitored using the ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). The PCR master mix was based on AmpliTaq Gold DNA polymerase (Applied Biosystems). cDNA samples were analyzed for fatty acid-binding protein-4 (FABP4); lipoprotein lipase (LPL), acyl-CoA synthetase (ACS), adipsin, facilitative glucose transporter-4 (GLUT4), and peroxisome proliferator-activated receptor (PPARγ) using commercially available primers and probes from Assays-on-Demand™ Gene Expression kits (FABP4, Product # Hs00609791_m1; LPL, Product # Hs00173425_m1; ACS, Product # Hs0024252530_m1; Adipsin, Product # Hs00157263_m1; GLUT4, Product # Hs00168966_m1; PPARγ, Product # Hs00234592_m1) following the manufacturer’s instructions while the housekeeping gene GAPDH was analyzed using primers and probes as previously described[32]. cDNA samples (5 μl for a total volume of 50 μl per reaction) were analyzed for the gene of interest and the housekeeping gene in independent reactions. Data analysis was performed using the ABI Prism 7000 Sequence Detection Systems version 1.0 software (Applied Biosystems, Foster City, CA). For each cDNA sample, the Ct value was defined as the cycle number at which the fluorescence intensity reached 0.45 at which amplification of each target gene was within the linear range of the reaction. Relative expression levels for each gene of interest were calculated by normalizing the quantified cDNA transcript level (Ct) to the GAPDH as described previously (2ΔCt formula, Perkin Elmer User Bulletin #2).
In vivo Implantation
Seeded scaffolds cultivated in the presence of AD as detailed above were evaluated for their ability to support in vivo adipogenesis in a small animal muscle pouch model as previously described[33]. Scaffolds were individually implanted into bilateral muscle pouches within the rectus abdominus muscles of athymic nude male rats (RH-rnu, ~300 g each) and maintained there for 4 weeks. Non-seeded scaffolds served as negative controls and were treated similarly. All animal procedures were approved by Tufts Cummings School of Veterinary Medicine’s Institutional Animal Care and Use Committee (IACUC). In addition, guidelines for the care and use of laboratory animals outlined by the National Institute of Health were also observed. Implant replicates from each experimental group were positioned in multiple animals to account for potential variations in the ability of the host to support production of de novo adipose tissue.
Histology
Following the 4 week implantation period, scaffolds were retrieved, excised of surrounding host tissue, and fixed in 4% formalin for 48 hours. Scaffolds were then embedded in optimal cutting temperature (OCT) tissue freezing mixture and 10 μm sections were cut and subsequently stained with Oil Red-O as previously described [30]. The slides were mounted with glycerol gelatin, and at least 3 surface sections per scaffold were examined with a Zeiss Axiovert S100 light microscope and a Sony Exwave HAD 3CCD color video camera to ascertain the presence of lipid accumulating cells. In addition, scaffolds cultivated in vitro for 21 days prior to implantation were analyzed similarly.
Immunohistochemistry
To assess the species origin of cells within adipose tissue in the silk scaffolds 10 μm sections of matrix were assessed immunohistochemically. For the presence of human cells, an anti-human nuclear antigen (HNA) monoclonal antibody (Chemicon International, Temecula, CA) and a horseradish peroxidase (HRP)-labeled goat anti-mouse antibody were used. The samples were processed with a BenchMark automated histology staining system (Ventana, Tucson, AZ).
Statistical analysis
All measurements for stem cell responses to biomaterials and reverse transcription real-time PCR were collected with N=3 independent determinations per data point and expressed as mean ± standard deviation. Data for these measurements were analyzed with Microsoft Excel software utilizing a Student’s one-tailed t-test assuming equal levels of variance. Statistically significant values were defined as p<0.05. In addition, statistical similarity among groups was analyzed with Microsoft Excel software utilizing ANOVA test single factor.
RESULTS
Analysis of in vitro stem cell responses to biomaterials
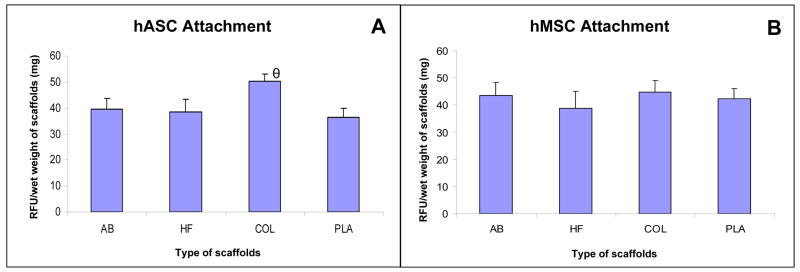
Since initial cell density of adipogenic precursors plays a significant role in determining the extent of adipogenic differentiation[16], the ability of each of the scaffolds utilized in this study to support metabolically active hASCs or hMSCs prior to exposure to AD was evaluated. Alamar blue analysis revealed that similar levels of metabolically active hMSCs or hASCs were capable of adhering to each of the biomaterial scaffolds with the exception of higher levels of attached hASCs observed on COL in comparison to AB, HF, and PLA. (Figure 1).
Figure 1.
Comparison of levels of metabolically active hASCs (A) and hMSCs (B) attached on aqueous silk based scaffolds (AB), HFIP scaffolds (HF), collagen scaffolds (COL) and PLA scaffolds (PLA). hASCs and hMSCs were seeded on AB, HF, COL and PLA scaffolds and were left overnight before analysis. Each point represents the mean and standard deviation of N=3 independent replicates. (A) relative levels of hASCs for AB, HF, and PLA were statistically similar while relative levels of hASCs for COL were significantly different in comparison to levels of hASCs for AB, HF and PL (θ= p<0.01); (B) levels of hASCs for AB, HF and PL were statistically similar. Relative fluorescence intensity (RFU) as determined by the AlamarBlue™ analysis normalized to scaffold wet weight was utilized as a measure of cell attachment levels within each scaffold group as detailed in the Methods.
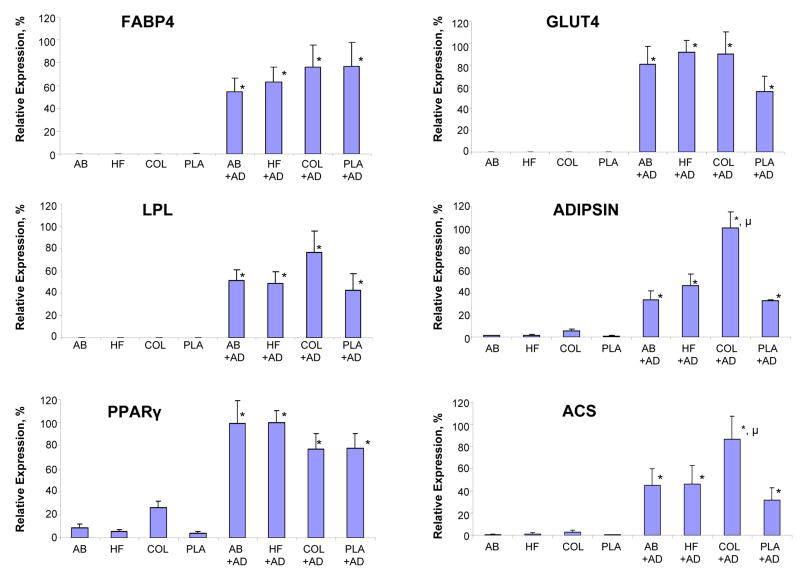
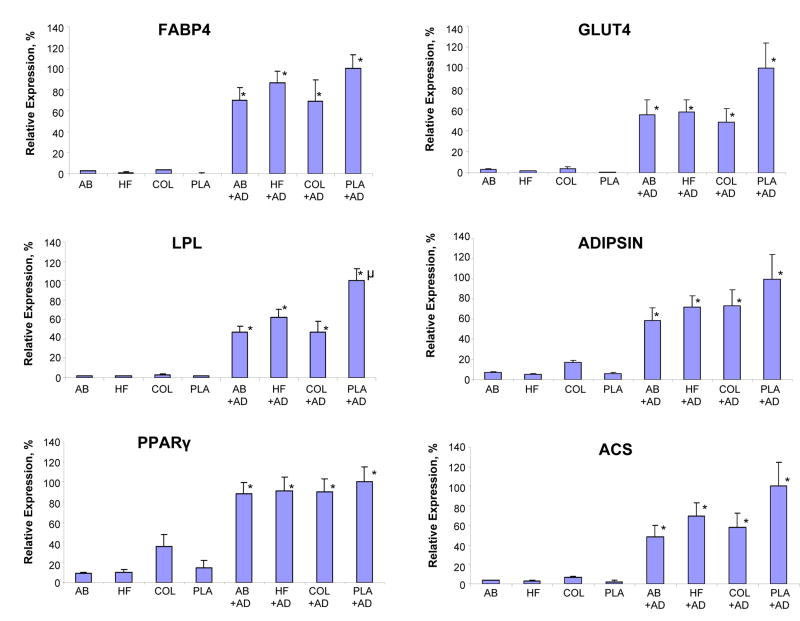
Following 21 days of in vitro cultivation, both hASCs (Figure 2) and hMSCs (Figure 3) cultured on all biomaterials in the presence of AD showed significant upregulation of mRNA transcript levels for six adipogenic markers when compared to noninduced controls. Levels of all adipogenic mRNA transcript levels in hMSCs or hASCs on both types of silk-based biomaterials in the presence of AD were comparable to the expression levels observed in each respective cell type following cultivation on COL or PLA. The exceptions to this observation included mRNA transcript levels of hASC adipisin and ACS on COL and hMSC LPL on PLA which were significantly higher than their respective counterparts on the AB and HFIP matrices.
Figure 2.
Comparison of (FABP4), LPL, PPARgamma, GLUT4, adipsin, and ACS mRNA transcript levels of hASCs) seeded on aqueous based silk scaffolds (AB), HFIP-based silk scaffolds, collagen scaffolds (COL) and poly-lactic acid (PLA) by real-time RT-PCR analysis. hASCs were either left as untreated controls or subjected to adipogenic stimulants (+AD) for 21 days in static condition. Each point represents the mean and standard deviation of N=3 independent determination; (*=p<0.01) significantly different in comparison to respective untreated control. (μ=p<0.05) significantly different in comparison to other groups subjected to adipogenic stimulants.
Figure 3.
Comparison of FABP4, LPL, PPARgamma, GLUT4, adipsin, ACS mRNA transcript levels of hMSCs seeded on aqueous based silk scaffolds (AB), HFIP-based silk scaffolds (HF), collagen scaffolds (COL) and poly-lactic acid (PLA) by real-time RT-PCR analysis. hMSCs were either left as untreated controls or subjected to adipogenic stimulants (+AD) for 21 days in static condition. Each point represents the mean and standard deviation of N=3 independent determination; (*=p<0.005) significantly different in comparison to respective untreated control. (μ=p<0.05) significantly different in comparison to other groups subjected to adipogenic stimulants.
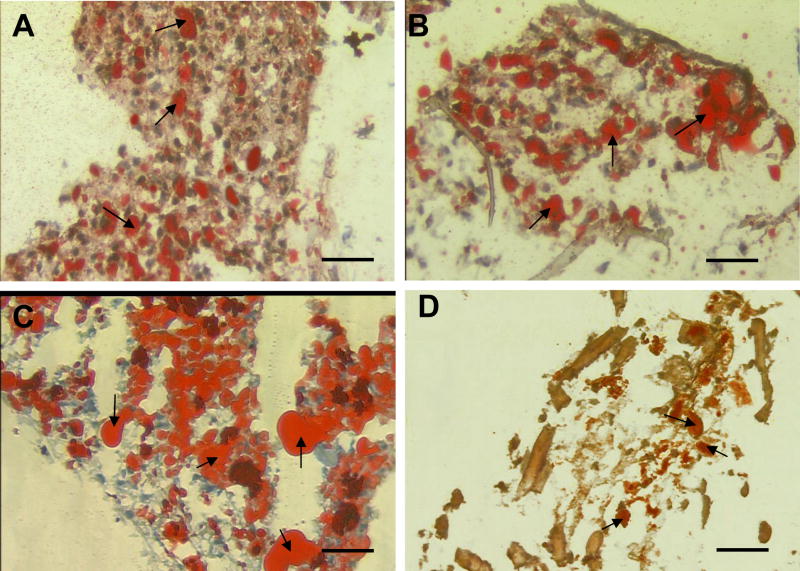
Oil-Red O analysis of hASC or hMSC-seeded scaffolds following 21 days of in vitro cultivation (Figure 4 and Figure 5) in the presence of AD qualitatively displayed substantial amounts of lipid accumulating cells when compared to the noninduced controls. However, both hASCs and hMSCs cultivated in control conditions on COL displayed evidence of lipid accumulating cells, but the extent of Oil Red-O positive cells was qualitatively less in comparison to cultivation with AD. The extent of lipid accumulating hASCs or hMSCs following cultivation with AD on each type of biomaterial was qualitatively similar with the exception of collagen and PLA. Oil-Red O staining of hASCs and hMSCs-seeded on COL showed a slight qualitative increase in of the amount of lipid accumulation when compared to cells seeded on the other biomaterials. In contrast, the degree of lipid accumulating cells observed within the hASC and hMSC-seeded PLA was qualitatively lower when compared to the cells seeded on the other biomaterials.
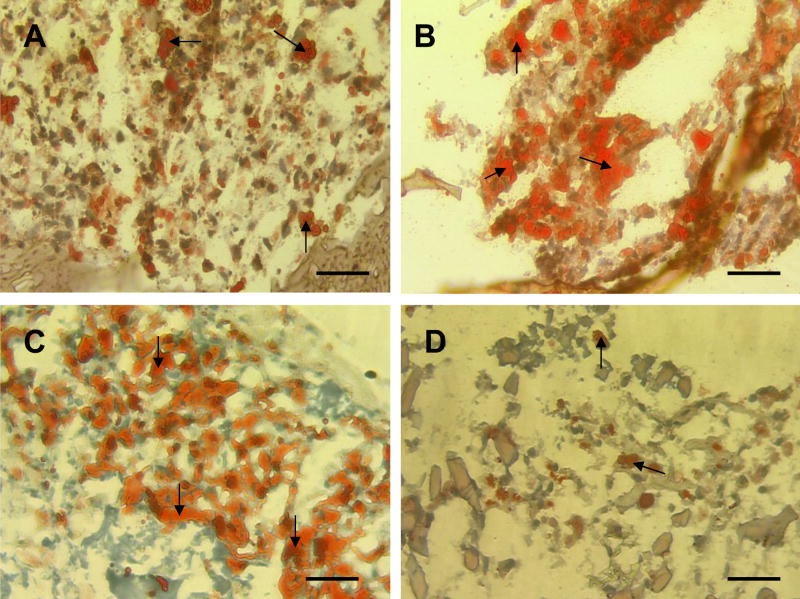
Figure 4.
Oil Red-O analysis of hASC-seeded scaffolds. (A) aqueous based silk scaffold, (B) HFIP based silk scaffold, (C) collagen, (D) PLA was taken after culturing for 21 days. Scale bar = 50 μm. Arrows denote adipocytes stained positive by Oil Red-O.
Figure 5.
Oil Red-O analysis of hMSC-seeded scaffolds: (A) aqueous based silk scaffold, (B) HFIP-based silk scaffold, (C) collagen, (D) PLA after culturing 21 days. Scale bars = 50 μm. Arrows denote adipocytes stained positive by Oil Red-O.
Analysis of in vivo cell responses to biomaterials
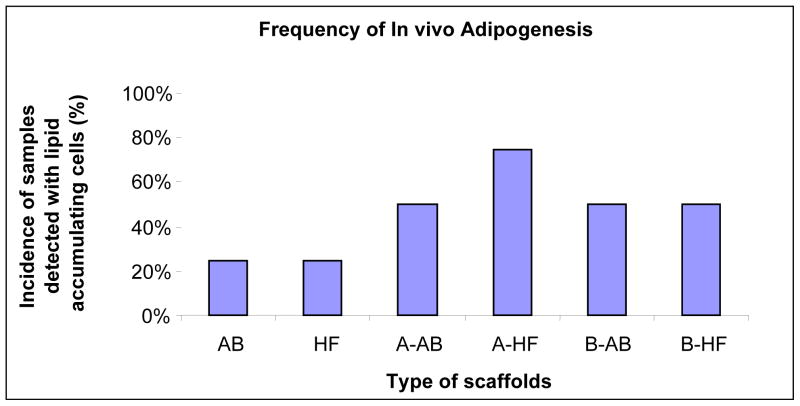
Figures 6 and 7 summarize the Oil Red-O histological findings of the ability of the various biomaterial carriers to support in vivo adipogenesis with and without hASCs or hMSCs. Experimental groups containing either non-seeded AB or HFIP scaffolds alone displayed a 25% incidence in the presence of lipid accumulating cells. The extent of lipid accumulating cells in both HFIP and AB scaffolds observed was qualitatively similar. In contrast, all samples containing nonseeded COL and PLA scaffolds had completely degraded following the 4 week implantation period and therefore were not subjected to further analyses. Similarly, experimental groups containing hASC or hMSC-seeded COL and PGA scaffolds were also irretrievable from the host defect site due to their significant extent of degradation. In contrast, experimental groups containing either hASC or hMSCs seeded on HFIP or AB scaffolds displayed a substantial increase in the frequency of lipid accumulating cells in comparison to their nonseeded controls. This resulted in at least a two-fold increase within the percentage of each experimental group to support in vivo adipogenesis. In addition, the extent of lipid accumulating cells for both hASC and hMSC-seeded HFIP and AB scaffolds was also observed to be qualitatively similar, but higher in comparison to their respective non-seeded controls.
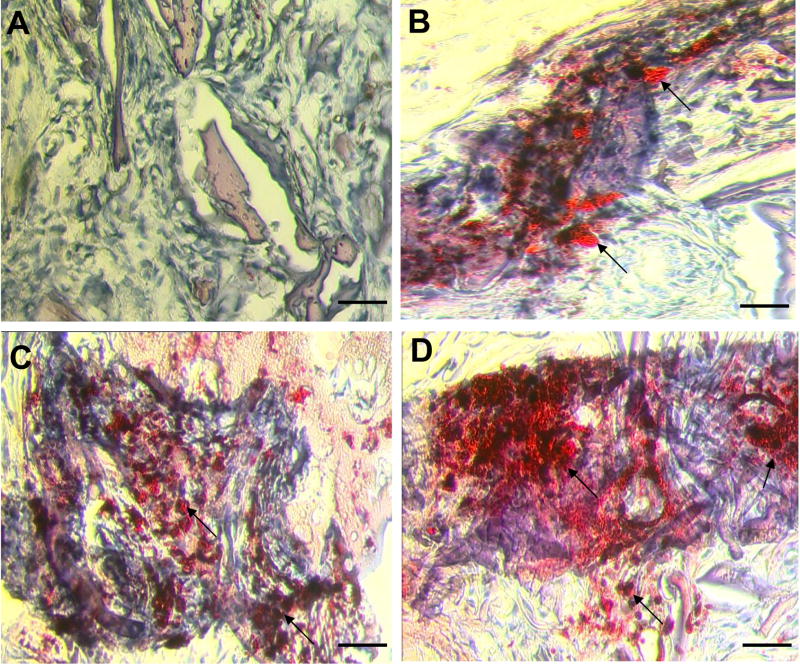
Figure 6.
Oil Red-O analysis of in vivo adipogenesis following 4 weeks of implantation: (A) nonseeded HFIP based silk scaffold which stained negative for adipocytes, control, (B) aqueous based silk scaffold demonstrating positive staining for adipocytes; representative sample indicative of the extent of adipogenesis observed in both nonseeded HFIP and aqueous based silk scaffolds, (C) hMSC-seeded aqueous based silk scaffold demonstrating positive staining for adipocytes; representative sample indicative of the extent of adipogenesis observed in both hMSC-seeded HFIP and aqueous based silk scaffolds, (D) hASC-seeded HFIP-based silk scaffold demonstrating positive staining for adipocytes; representative sample indicative of the extent of adipogenesis observed in both hASC-seeded HFIP and aqueous based silk scaffolds. Arrows denote adipocytes stained positive by Oil Red-O. Scale bars = 50 μm.
Figure 7.
Frequency (percent of total) of samples, which are seeded or non-seeded with hASCs (A on the x-axis scaffold types) and hMSCs (B on the x-axis scaffold types), detected with lipid accumulating cells by Oil-Red O staining. hASCs and hMSCs were seeded on silk water-based scaffolds (AB), silk-HFIP scaffolds (HF), collagen scaffolds (COL) and poly-lactic acid (PLA) scaffolds and were cultivated for 21 days before implantation in vivo. After 4 weeks, COL scaffolds and PLA scaffolds were irretrievable. AB and HF scaffolds were analyzed by Oil-Red O histology. N=4. See note in Methods regarding how the frequency (%) is defined.
Analysis of the origin of adipose tissue within silk scaffolds in vivo
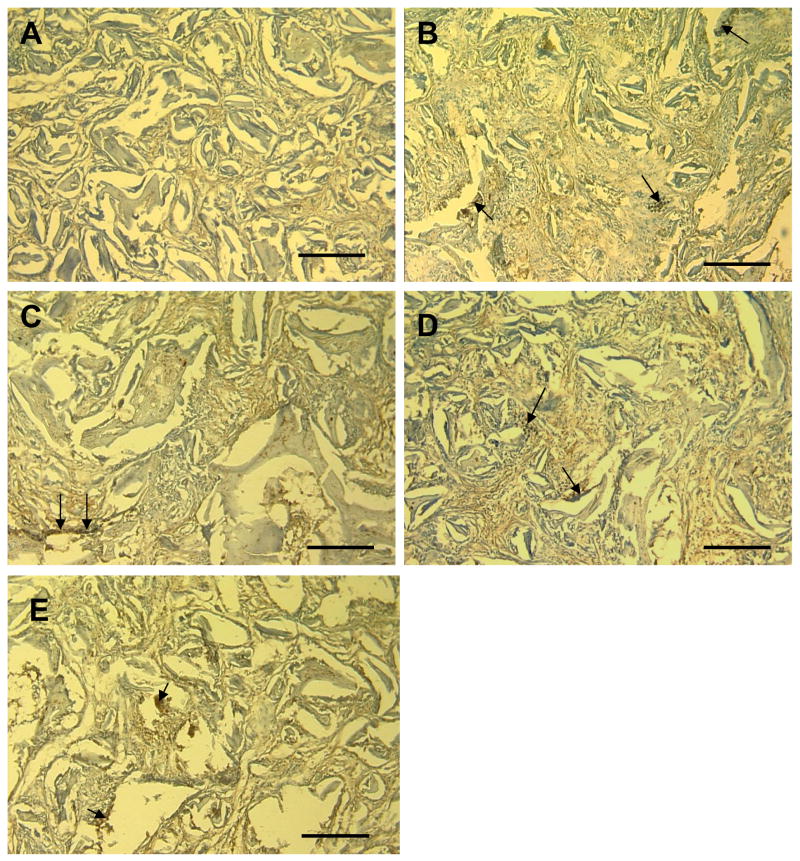
Immunohistochemistry based on positive staining for human nuclear antigen was used to document the contribution of human cells to the adipose phenotype observed within hASC and hMSC seeded scaffolds (Figure 8). Human nuclear antigen positive cells persisted within scaffolds for up to 4 weeks in vivo, documenting their contribution to the increased adipose deposits observed within hASC and hMSC seeded scaffolds.
Figure 8.
Immunohistochemistry of silk scaffolds for human-origin cells following 4 weeks of in vivo implantation. For the presence of human cells, an anti-human nuclear antigen (HNA) monoclonal antibody and a horseradish peroxidase (HRP)-labeled goat anti-mouse antibody were used. (A) non-seeded HFIP silk scaffold as control, (B) aqueous based silk scaffold seeded with hASCs, (C) HFIP silk scaffold seeded with hASCs, (D) aqueous based silk scaffold seeded with hMSCs, (E) HFIP silk scaffold seeded with hMSCs (scale bars=200 μm)
DISCUSSION
The development of novel adipose tissue engineered constructs based on silk fibroin based-biomaterials in combination with various adipogenic stem cell populations is reported in the present study. The results demonstrate that both aqueous- and HFIP-based silk fibroin 3D porous scaffolds support in vitro adipogenic differentiation of hASCs and hMSCs to extents comparable to conventional natural and synthetic biomaterials such as COL and PLA scaffolds.
However, the in vivo results provide evidence that silk-based biomaterials also support in vivo adipogenesis either alone or in combination with hASCs or hMSCs to greater levels when compared with the same conventional biomaterial systems, such as COL and PLA. The collagen and poly-lactic acid-based polymers utilized in this study were unable to support the production of stable fat tissue volume during the implantation period, presumably due to their rapid degradation rates. Therefore, the ability of the silk scaffolds to support adipose tissue was potentially due to the combination of a porous and stable structure with relatively slow degradation rates.
Qualitative Oil red-O analysis demonstrated the presence of hASCs or hMSCs contributed not only to an increased frequency of implants exhibiting in vivo adipogenesis, but also an increased extent of adipose tissue formation when compared to non seeded controls. In the control (non seeded) scaffolds, adipose deposits were primarily observed along the periphery of the implants, suggesting ingrowth and differentiation of rat preadipocytes from the neighboring host tissue. However, the extent of adipose deposition was qualitatively higher and more homogenous in the silk scaffold systems initially seeded with the hASCs and hMSCs. The immunohistochemistry results also confirmed the presence of human cells within the hASC and hMSC seeded scaffolds. These cells exhibited a rounded morphological phenotype typical of mature adipocytes suggesting the higher degrees of adipogenesis observed within these samples was partly due to the direct differentiation of the human adipose progenitors. However, the number of human cells observed within the silk scaffolds was qualitatively lower than the extent of lipid accumulating cells seen following Oil red-O staining. This data suggests that the increased extent of adipogenesis was not only a function of direct differentiation of human adipose progenitors, but that the exogenous cell sources also promoted increased migration and/or adipogenic differentiation of host preadipocytes within the scaffolds. Although the exact mechanisms of how the exogenous cells influenced host adipogenesis are unknown, previous studies have demonstrated the ability of adipocytes to secrete various paracrine factors which can positively influence both the migration and differentiation of preadipocytes[34, 35]. Therefore, these mechanisms may also be involved in our system, however further experimentation is warranted.
Long-term maintenance of adipose tissue formation within adipose tissue engineered constructs has been suggested to require a number of factors including a stable scaffold support structure[15] and vascularization necessary to support de novo adipogenesis [15]. Previous studies emphasized the importance of a stable scaffold by correlating the ability of poly-lactic/glycolic acid-based polymers to support long-term maintenance (>5 months of implantation) of adipose tissue formation with extent of scaffold degradation. On the other hand, related studies have reported the ability of collagen and PLA-based scaffolds to support in vivo adipogenesis for periods up to 1–4 months [11, 13, 15, 36–38]. However, long-term production of adipose tissue has remained elusive due to the use of rapidly degrading biomaterials such as collagen and PLA. However, differences such as collagen and PLA scaffold processing methods, scaffold porosity and size and implantation site (muscle pouch versus subcutaneous implantation) may have contributed to the faster degradation rates observed with the collagen and poly-lactic acid-based polymers utilized in this study.
When cultured on biomaterials in the presence of adipogenic medium, both hASCs and hMSCs expressed adipogenic related markers (FABP4, LPL, PPARγ, GLUT4, adipsin, ACS) and accumulated lipid droplets within differentiated adipocytes. Peroxisome proliferator-activated receptor gamma (PPARγ), which plays a key role in regulating several genes critical to adipogenesis and therefore induces adipogenic differentiation [39], was upregulated in all cell seeded scaffolds cultivated in AD conditions. Similarly, other adipose specific markers such as lipoprotein lipase (LPL), adipsin, fatty acid binding protein-4 (FABP4), and acetyl coA synthase[40] were induced after adipogenic differentiation. The glucose transporter GLUT4, which is one member of a family of glucose transporter proteins, was also upregulated. The extent of lipid accumulating hASCs following cultivation with AD on collagen was slightly higher compared to that of the other materials, in agreement with the higher level of attached hASCs on COL in comparison to levels of hASCs on AB, HF and PLA. This can be explained in part by the presence of collagen in adipose tissue networks which may mediate optimal cellular attachment and adipogenic differentiation. Adipose tissue growth could therefore potentially be further improved by seeding cells on collagen coated silk scaffolds. In addition, it should be noted that although both hASCs and hMSCs cultured in vitro on PLA in the presence of AD displayed similar levels of adipogenic mRNA transcripts in comparison to cells cultivated on the silk biomaterials, the levels of Oil Red-O stained adipocytes observed in the PLA constructs were overall qualitatively lower by comparison. This discrepancy can be explained by the fact that the PLA scaffolds underwent significant degradation following the 21 day in vitro cultivation period. Therefore the number of adipocytes observed in the PLA scaffolds appeared to be qualitatively lower than in the silk scaffolds due to the loss of scaffold integrity; however the extent of adipogenic differentiation measured in the remaining cell populations in the PLA scaffolds was similar to cells cultivated on the silk scaffolds as assessed by real time RT-PCR.
PPARγ is a member of the intracellular receptor family of transcription factors and one of the two isoforms of PPAR which is highly expressed in adipocytes and specific to adipose tissue [41, 42]. PPARγ plays a key role in regulating several genes critical to adipogenesis, lipid uptake and lipid metabolism and therefore induces adipocyte differentiation [39, 43–46]. Thus, upregulation in the cell seeded scaffolds cultivated in AD conditions in comparison to those cultivated in control conditions is a positive indication of activation of adipogenic differentiation pathways in the present study. LPL is an adipogenic marker which is expressed in early stages of adipocyte development[47]. During the adipogenesis process, transcript levels of LPL are upregulated and plateau when adipocytes mature [48]. FABP4 is a member of fatty acid binding protein super family and is a key regulator of intracellular transport and metabolism of fatty acids in adipose tissue. FABP4 binds fatty acids and transports them to different regions of the cell [49, 50]. Adipsin, complement factor D, is expressed at high levels in adipose tissue and is a regulator of lipid accumulation in adipocytes[51, 52]. ACS is an enzyme that converts free fatty acids to long chain acyl-coA for further metabolism [53]. GLUT4 is a integral membrane protein in adipose tissue regulates glucose uptake in adipocytes[54]. The upregulation of all of these markers (LPL, FABP4, adipsin, ACS and GLUT4) in the cell seeded scaffolds exposed to adipogenic differentiation medium is clear indication of a physiologically relevant progression of these systems toward adipogenic outcomes.
CONCLUSION
In summary, the results demonstrate the feasibility to grow adipose tissue on silk fibroin based biomaterials in combination with various stem cell populations. The slow degradation and mechanical integrity of silk scaffolds in comparison with other conventional biomaterials such as collagen and PLA, especially for long-term in vivo studies, suggest that silk-fibroin based scaffolds would be an efficient biomaterial for long-term adipose tissue growth and function. Further research into the optimization of these systems for soft tissue reconstruction is warranted in light of the biocompatibility and processing versatility of silk fibroin for biomaterials needs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patrick CW., Jr Adipose tissue engineering: the future of breast and soft tissue reconstruction following tumor resection. Semin Surg Oncol. 2000;19:302–11. doi: 10.1002/1098-2388(200010/11)19:3<302::aid-ssu12>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 2.Powers MP, Bosker H. Functional and cosmetic reconstruction of the facial lower third associated with placement of the transmandibular implant system. J Oral Maxillofac Surg. 1996;54:934–42. doi: 10.1016/s0278-2391(96)90386-9. [DOI] [PubMed] [Google Scholar]
- 3.Tzikas TL. Lipografting: autologous fat grafting for total facial rejuvenation. Facial Plast Surg. 2004;20(2):135–43. doi: 10.1055/s-2004-861754. [DOI] [PubMed] [Google Scholar]
- 4.Rooney DP, Ryan MF. Diabetes with partial lipodystrophy following sclerodermatous chronic graft vs. host disease Diabet Med. 2006;23:436–40. doi: 10.1111/j.1464-5491.2006.01855.x. [DOI] [PubMed] [Google Scholar]
- 5.Patrick CW, Jr, Chauvin PB, Robb GL. Tissue engineered adipose. In: Patrick CW Jr, Mikos AG, McIntire LV, editors. Frontiers in tissue engineering. Oxford: Elsevier Science; 1998. pp. 369–382. [Google Scholar]
- 6.Lee KY, Halberstadt CR, Holder WD, Mooney DJ. Breast reconstruction. In: Lanza RP, Langer R, Vacanti J, editors. Principles of tissue engineering. San Diego: Academic Press; 2000. pp. 409–423. [Google Scholar]
- 7.Patrick CW., Jr Tisse engineering strategies for adipose tissue repair. Anat Rec. 2001;263:361–6. doi: 10.1002/ar.1113. [DOI] [PubMed] [Google Scholar]
- 8.Patrick CW, Jr, Chauvin PB, Hobley J, Reece GP. Preadipocyte seeded PLGA scaffolds for adipose tissue engineering. Tissue Eng. 1999;5:139–51. doi: 10.1089/ten.1999.5.139. [DOI] [PubMed] [Google Scholar]
- 9.Borges J, Mueller MC, Padron NT, Tegtmeier F, Lang EM, Stark GB. Engineered adipose tissue supplied by functional microvessels. Tissue Eng. 2003;9:1263–70. doi: 10.1089/10763270360728170. [DOI] [PubMed] [Google Scholar]
- 10.Walton RL, Beahm EK, Wu L. De novo adipose formation in a vascularized engineered construct. Microsurgery. 2004;24:378–84. doi: 10.1002/micr.20056. [DOI] [PubMed] [Google Scholar]
- 11.Fischbach C, Spruss T, Weiser B, Neubauer M, Becker C, Hacker M, Gopferich A, Blunk T. Generation of mature fat pads in vitro and in vivo utilizing 3-D long-term culture of 3T3-L1 preadipocytes. Exp Cell Res. 2004;300:54–64. doi: 10.1016/j.yexcr.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 12.Fischbach C, Seufert J, Staiger H, Hacker M, Neubauer M, Gopferich A, Blunk T. Three-dimensional in vitro model of adipogenesis: comparison of culture conditions. 1. Tissue Eng. 2004;10:215–29. doi: 10.1089/107632704322791862. [DOI] [PubMed] [Google Scholar]
- 13.Cho SW, Kim SS, Rhie JW, Cho HM, Choi CY, Kim BS. Engineering of volume-stable adipose tissues. Biomaterials. 2005;26:3577–85. doi: 10.1016/j.biomaterials.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Kang X, Xie Y, Kniss DA. Adipose tissue model using three-dimensional cultivation of preadipocytes seeded onto fibrous polymer scaffolds. Tissue Eng. 2005;11:458–68. doi: 10.1089/ten.2005.11.458. [DOI] [PubMed] [Google Scholar]
- 15.Patrick CW, Jr, Zheng B, Johnston C, Reece GP. Long-term implantation of preadipocyte-seeded PLGA scaffolds. Tissue Eng. 2002;8:283–93. doi: 10.1089/107632702753725049. [DOI] [PubMed] [Google Scholar]
- 16.Neubauer M, Hacker M, Bauer-Kreisel P, Weiser B, Fischbach C, Schulz MB, Goepferich A, Blunk T. Adipose tissue engineering based on mesenchymal stem cells and basic fibroblast growth factor in vitro. Tissue Eng. 2005;11:1840–51. doi: 10.1089/ten.2005.11.1840. [DOI] [PubMed] [Google Scholar]
- 17.Meinel L, Karageorgiou V, Hofmann S, Fajardo R, Snyder B, Li C, Zichner L, Langer R, Vunjak-Novakovic G, Kaplan DL. Engineering bone-like tissue in vitro using human bone marrow stem cells and silk scaffolds. J Biomed Mater Res A. 2004;71:25–34. doi: 10.1002/jbm.a.30117. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Blasioli D, Kim HJ, Kim HS, Kaplan DL. Cartilage tissue engineering with silk scaffolds and human articular chondrocytes. Biomaterials. 2006;27:4434–4442. doi: 10.1016/j.biomaterials.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 19.Vunjak-Novakovic G, Meinel L, Altman G, Kaplan D. Bioreactor cultivation of osteochondral grafts. Orthod Craniofac Res. 2005;8:209–18. doi: 10.1111/j.1601-6343.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 20.Meinel L, Hofmann S, Karageorgiou V, Kirker-Head C, McCool J, Gronowicz G, Zichner L, Langer R, Vunjak-Novakovic G, Kaplan DL. The inflammatory responses to silk films in vitro and in vivo. Biomaterials. 2005;26:147–55. doi: 10.1016/j.biomaterials.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 21.Horan RL, Antle K, Collette AL, Wang Y, Huang J, Moreau JE, Volloch V, Kaplan DL, Altman GH. In vitro degradation of silk fibroin. Biomaterials. 2005;26:3385–93. doi: 10.1016/j.biomaterials.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Jin HJ, Fridrikh SV, Rutledge GC, Kaplan DL. Electrospinning Bombyx mori silk with poly(ethylene oxide) Biomacromolecules. 2002;3:1233–9. doi: 10.1021/bm025581u. [DOI] [PubMed] [Google Scholar]
- 23.Nazarov R, Jin HJ, Kaplan DL. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules. 2004;5:718–26. doi: 10.1021/bm034327e. [DOI] [PubMed] [Google Scholar]
- 24.Kim UJ, Park J, Kim HJ, Wada M, Kaplan DL. Three dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26(15):2775–85. doi: 10.1016/j.biomaterials.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 25.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24:401–16. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 26.Meinel L, Hofmann S, Karageorgiou V, Zichner L, Langer R, Kaplan D, Vunjak-Novakovic G. Engineering cartilage-like tissue using human mesenchymal stem cells and silk protein scaffolds. Biotechnol Bioeng. 2004;88:379–91. doi: 10.1002/bit.20252. [DOI] [PubMed] [Google Scholar]
- 27.Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, Zichner L, Kaplan D, Langer R, Vunjak-Novakovic G. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32:112–22. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- 28.Altman GH, Horan RL, Martin I, Farhadi J, Stark PR, Volloch V, Richmond JC, Vunjak-Novakovic G, Kaplan DL. Cell differentiation by mechanical stress. FASEB J. 2002;16:270–2. doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
- 29.Voytik – Harbin SL, Brightman AO, Waisner B, Lamar CH, Badylak SF. Application and evaluation of the alamar Blue assay for cell growth and survival of fibroblasts. In vitro. Cell Dev Biol Anim. 1998;34:239–46. doi: 10.1007/s11626-998-0130-x. [DOI] [PubMed] [Google Scholar]
- 30.Mauney JR, Volloch V, Kaplan DL. Matrix-mediated retention of adipogenic differentiation potential by human adult bone marrow-derived mesenchymal stem cells during ex vivo expansion. Biomaterials. 2005;26:6167–75. doi: 10.1016/j.biomaterials.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 31.Chomezynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 32.Frank O, Heim M, Jakob M, Barbero A, Schafer D, Bendik I, Dick W, Heberer M, Martin I. Real-time quantitative RT-PCR analysis of human bone marrow stromal cells during osteogenic differentiation in vitro. J Cell Biochem. 2002;85:737–46. doi: 10.1002/jcb.10174. [DOI] [PubMed] [Google Scholar]
- 33.Mauney JR, Jaquiery C, Volloch V, Heberer M, Martin I, Kaplan DL. In vitro and in vivo evaluation of differentially demineralized cancellous bone scaffolds combined with human bone marrow stromal cells for tissue engineering. Biomaterials. 2005;26:3173–85. doi: 10.1016/j.biomaterials.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 34.Shillabeer G, Forden JM, Lau DC. Induction of preadipocyte differentiation by mature fat cells in the rat. J Clin Invest. 1989;84:381–387. doi: 10.1172/JCI114177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim CS, Kawada T, Yoo H, Kwon BS, Yu R. Macrophage inflammatory protein-related protein-2, a novel CC chemokine, can regulate preadipocyte migration and adipocyte differentiation. FEBS Lett. 2003;548:125–30. doi: 10.1016/s0014-5793(03)00728-2. [DOI] [PubMed] [Google Scholar]
- 36.Kimura Y, Ozeki M, Inamoto T, Tabata Y. Adipose tissue engineering based on human preadipocytes combined with gelatin microspheres containing basic fibroblast growth factor. Biomaterials. 2003;24:2513–21. doi: 10.1016/s0142-9612(03)00049-8. [DOI] [PubMed] [Google Scholar]
- 37.Choi YS, Cha SM, Lee YY, Kwon SW, Park CJ, Kim M. Adipogenic differentiation of adipose tissue derived adult stem cells in nude mouse. Biochem Biophys Res Commun. 2006;345:631–7. doi: 10.1016/j.bbrc.2006.04.128. [DOI] [PubMed] [Google Scholar]
- 38.von Heimburg D, Zachariah S, Heschel I, Kuhling H, Schoof H, Hafemann B, Pallua N. Human preadipocytes seeded on freeze-dried collagen scaffolds investigated in vitro and in vivo. Biomaterials. 2001;22:429–38. doi: 10.1016/s0142-9612(00)00186-1. [DOI] [PubMed] [Google Scholar]
- 39.Schoonjans K, Staels B, Auwerx J. The peroxisome proliferator activated receptors (PPARs) and their effects on lipid metabolism and adipocyte differentiation. Biochim Biophys Acta. 1996;1302:93–109. doi: 10.1016/0005-2760(96)00066-5. [DOI] [PubMed] [Google Scholar]
- 40.Cowherd RM, Lyle RE, McGehee RE., Jr Molecular regulation of adipocyte differentiation. Semin Cell Dev Biol. 1999;10:3–10. doi: 10.1006/scdb.1998.0276. [DOI] [PubMed] [Google Scholar]
- 41.Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal β-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68:879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- 42.Lazar MA. PPAR gamma, 10 years later. Biochimie. 2005;87:9–13. doi: 10.1016/j.biochi.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 43.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 44.Kubota N, Teauchi T, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Kadowaki T, et al. PPARγ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 45.Auwerx J. PPARγ, the ultimate thrifty gene. Diabetologia. 1999;42:1033–1049. doi: 10.1007/s001250051268. [DOI] [PubMed] [Google Scholar]
- 46.Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002;16:22–6. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dani C, Amri EZ, Bertrand B, Enerback S, Bjursell G, Grimaldi P, Ailhaud G. Expression and regulation of pOb24 and lipoprotein lipase genes during dipocyte conversion. J Cell Biochem. 1990;43:103–110. doi: 10.1002/jcb.240430202. [DOI] [PubMed] [Google Scholar]
- 48.Samuelsson L, Stromberg K, Vikman K, Bjursell G, Enerback S. The CCAAT/enhancer binding protein and its role in adipocyte differentiation: evidence for direct involvement in terminal adipocyte development. EMBO J. 1991;10:3787–3793. doi: 10.1002/j.1460-2075.1991.tb04948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Makowski L, Hotamisligil GS. Fatty acid binding proteins – the evolutionary crossroads of inflammatory and metabolic responses. J Nutr. 2004;134:2464–8. doi: 10.1093/jn/134.9.2464S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmer JS, Dyckes DF, Bernlohr DA, Murphy RC. Fatty acid binding protein stabilize leukotriene A4. J Lipid Res. 2004;45:2138–44. doi: 10.1194/jlr.M400240-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.White RT, Damm D, Hancock N, Rosen BS, Lowell BB, Usher P, Flier JS, Spiegelman BM. Human adipsin is identical to complement factor D and is expressed at high levels in adipose tissue. J Biol Chem. 1992;267:9210–9213. [PubMed] [Google Scholar]
- 52.Miner JL. The adipocyte as an endocrine cell. J Anim Sci. 2004;82:935–41. doi: 10.2527/2004.823935x. [DOI] [PubMed] [Google Scholar]
- 53.Shimomura I, Tokunaga K, Jiao S, Funahashi T, Keno Y, Kobatake T, Kotani K, Suzuki H, Yamamoto T, Tarui S, et al. Marked enhancement of acyl-CoA synthetase activity and mRNA, paralleled to lipoprotein lipase mRNA, in adipose tissues of Zucker obese rats (fa/fa) Biochim Biophys Acta. 1992;1124:112–8. doi: 10.1016/0005-2760(92)90086-b. [DOI] [PubMed] [Google Scholar]
- 54.Czech MP, Corvera S. Signaling mechanisms that regulate glucose transport. J Biol Chem. 1999;274:1865–8. doi: 10.1074/jbc.274.4.1865. [DOI] [PubMed] [Google Scholar]