Abstract
Bombyx mori silk fibroin self-assembles on surfaces to form ultrathin nanoscale coatings based on our prior studies using layer-by-layer deposition techniques driven by hydrophobic interactions between silk fibroin protein molecules. In the present study, polylactic-co-glycolic acid (PLGA) and alginate microspheres were used as substrates and coated with silk fibroin. The coatings were visualized by confocal laser scanning microscopy using fluorescein-labeled silk fibroin. On PLGA microspheres the coating was ~1 μm and discontinuous, reflecting the porous surface of these microspheres determined by SEM. In contrast, on alginate microspheres the coating was ~10 μm thick and continuous. The silk fibroin penetrated into the alginate gel matrix. The silk coating on the PLGA microspheres delayed PLGA degradation. The silk coating on the alginate microspheres survived ethylenediamine tetraacetic acid (EDTA) treatment used to remove the Ca+2-cross-links in the alginate gels to solubilize the alginate. This suggests that alginate microspheres can be used as templates to form silk microcapsules. Horseradish peroxidase (HRP) and tetramethylrhodamine-conjugated bovine serum albumin (BSA) as model protein drugs were encapsulated in the PLGA and alginate microspheres with and without the silk fibroin coatings. Drug release was significantly retarded by the silk coatings when compared to uncoated microsphere controls, and was retarded further by methanol-treated silk coating when compared to silk water-based coatings on alginate microspheres. Silk coatings on PLGA and alginate microspheres provide mechanically stable shells as well as a diffusion barrier to the encapsulated protein drugs. This coating technique has potential for biosensor and drug delivery applications due to the aqueous process employed, the ability to control coating thickness and crystalline content, and the biocompatibility of the silk fibroin protein used in the process.
1. Introduction
A sustained drug delivery system, also known as depot delivery, can offer important advantages in the clinic, such as significantly reducing dose frequency and providing efficacy without toxicity [1]. A depot delivery system requires particle sizes above 5 μm, since they should remain at the injection site and slowly degrade and release drug contents over time after subcutaneous or intramuscular administration [1]. Larger size delivery systems may also offer larger drug loading capacities when compared to systems with smaller sizes. Among many different delivery systems, coating microspheres is a common method used to control drug release, since polymer networks of coatings can provide a diffusion barrier to retard the otherwise rapid drug release [2,3]. This is also a beneficial approach since specific ligands can be attached to the surface via the coating to target the delivery system [4]. Coating microspheres with proteins such as gelatin and human serum albumin can improve biocompatibility [2,5]. Aside from coating use for drug delivery, coatings are also of benefit for enzymes to improve stability and selectivity, such as entrapping enzyme in a polymer matrix while allowing substrates, products, and co-factors to diffuse [6,7]. When immobilized enzymes are used in vivo, the coating can also prevent immune recognition, a major impediment to the longevity of such devices [8].
Poly (lactic-co-glycolic acid) (PLGA) copolymer is a synthetic biodegradable and biocompatible polymer extensively used in vivo for applications in sutures and other biomedical devices. Controlled drug release can be achieved with PLGA copolymers because the release of encapsulated drugs is determined by polymer molecular weight and ratio of polylactic acid to polyglycolic acid [9]. A major problem hindering the progression of PLGA microspheres as protein drug carriers is protein stability. The organic solvents used during microencapsulation and the acidic microenvironment generated during polymer degradation can negatively affect the activity of many encapsulated proteins as well as other labile molecules [10].
Alginates are seaweed derived, gel-forming polysaccharides composed of chains of alternating α-L-guluronic acid and β-D-mannuronic acid residues [11]. Gelation is controlled by chelation between the carboxyl groups of α-L-guluronic acid with calcium, or barium ions, or poly(L-lysine) [12,13]. Alginate hydrogels have been extensively investigated due to their biocompatibility, however, they are unstable in physiological environments since phosphate and citrate ions can extract Ca2+ from the alginate and liquefy the system. Another drawback of using alginate gels as drug delivery carriers is their low mechanical strength. One approach to overcome these limitations is to coat the alginate hydrogels with various polymers to stabilize the hydrogels and to slow the release of encapsulated macromolecules [6, 14].
A layer-by-layer (LBL) assembly technique using silk fibroin has recently been reported [15]. Unlike traditional LBL, which is based on the alternate deposition of oppositely charged polyelectrolytes, the driving force for the assembly of silk fibroin was primarily hydrophobic interactions. The deposition process is all-aqueous and relatively simple to conduct, and the assembled nanoscale materials are stable under physiological conditions. Because silk fibroin-based structures, such as fibers, films and three-dimensional scaffolds, have advantages of excellent mechanical properties, biocompatibility and biodegradability [16], silk coatings are expected to impart new and beneficial biointerfacial properties to substrates used for biomedical applications. Most recently, silk fibroin has been used to coat multilamellar liposomes forming composite nanoparticles for sustained drug delivery [17]. Methanol was added to the lyophilized mixture of silk and liposome to insolubilize the coated liposomes. As a result, the silk coating increased the lamellae thickness from 25% to 55% of the overall diameter of liposomes, changed the lamellae structure to more organized, enhanced the adhesion of liposomes to cells, and significantly retarded the release of an anti-tumor drug, emodin, without affecting the drug efficacy [17].
The objective of the present study was to form silk fibroin multilayer coatings on PLGA and alginate microspheres and to examine the impact of such coatings on the microsphere function. The implications for the study are in the control of drug release through the modification of coating layers, thickness and crystalline content.
2. Materials and methods
2.1. Materials
Cocoons of B. mori silkworm silk were kindly supplied by M. Tsukada (Institute of Sericulture, Tsukuba, Japan). End-group uncapped poly (lactide-co-glycolide) 50:50 (PLGA 50:50) with a Mw of approximately 14 kDa was purchased from Boehringer Ingelheim (Resomer® RG502H Ingelheim, Germany). Poly (vinylalcohol) (PVA, Mowiol® 8–88) from Hoechst (Frankfurt, Germany). Pysiogel® (80 mg/ml of succinylated gelatin) from Braun Meidcal (Emmenbrucke, Switzerland). 5-(aminoacetamido)fluorescein (fluoresceinyl glycine amide) and tetramethylrhodamine conjugated bovine serum albumin (Rh-BSA, Mw of approximately 66 KDa) were purchased from Molecular Probes (Carlsbad, CA). Low viscosity alginate, horseradish peroxidase (HRP, Mw of approximately 44 KDa), and other chemicals were obtained from Sigma Aldrich (St. Louis, MO). 3,3′5,5′ Tetramethylbenzidine (TMB) solution was purchased from BioFX laboratories (Owing Mills, MD). 1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide Hydrochloride (EDC), N-hydroxysuccinimide (NHS), and hydroxylamine hydrochloride were purchased from Pierce Biotechnology (Rockford, IL).
2.2. Purification and fluorescent labeling of silk fibroin
Silk fibroin aqueous stock solution was prepared as previously described [18]. Briefly, cocoons of B. mori were boiled for 20 min in an aqueous solution of 0.02 M sodium carbonate, and then rinsed thoroughly with pure water. After drying, the extracted silk fibroin was dissolved in 9.3 M LiBr solution at 60°C for 4 hours, yielding a 20% (w/v) solution. This solution was dialyzed against distilled water using Slide-a-Lyzer dialysis cassettes (MWCO 3,500, Pierce) for 3 days to remove the salt. The resulting solution was centrifuged to remove impurities and the aggregates that formed during dialysis. The final concentration of silk fibroin aqueous solution was approximately 8% (w/v). This concentration was determined by weighing the residual solid of a known volume of solution after drying.
For fluorescent labeling, the silk fibroin stock solution was diluted to 2% (w/v) with water, and 10 ml of the diluted solution was dialyzed against 500 ml of 0.1 M 2-(morpholino)ethanesulfonic acid (MES) solution (pH 5.6) (Pierce, Chemicals, IL) supplemented with 0.9% NaCl overnight. Eighty mg EDC (2 mM) and 220 mg NHS (5 mM) were then added to the buffered silk solution with stirring and the reaction was continued for 15 min. β-mercaptoethanol was then added to a final concentration of 20 mM to quench the unreacted EDC. The carboxyl groups on silk fibroin are then activated for reaction with primary amines. After the reaction, 10 mg of fluoresceinyl glycine amide was added to the solution so that the molar ratio between fluorescent probe and silk fibroin was about 40:1. The coupling reaction went for 2 hours under slow stirring at room temperature, and then 8 mg hydroxylamine hydrochloride was added to quench the reaction. Finally, the solution was dialyzed exhaustively against water to remove all the free compounds. The final concentration of fluorescent silk fibroin was approximately 1.5% (w/v) using the same weighing method.
2.3. Preparation of HRP-loaded PLGA microspheres
The preparation was performed by solvent evaporati on from a water-in-oil-in-water (W1/O/W2) dispersion. For this, 10 μl of 12.5 mg/ml HRP in PBS buffer, pH 7.2 or 10 μl of water (empty microspheres) was mixed with 100 μl Physiogel (W1-Phase). 100 or 300 mg PLGA powder was dissolved in 3 ml of the mixture of dichloromethane and acetone (3:1) (O-Phase). After mixing the W1-Phase with the O-Phase, the mixture was immediately ultrasonicated (Branson 450) (Branson, Danbury, CN) on ice for 30 seconds at 10, 40, 70% of maximal power output (corresponding to 3, 18, 30 Watts, respectively) to obtain the W1/O emulsion. The resulting emulsion was diluted into 50 ml of 5% (w/v) polyvinyl alcohol (Mowiol 8–88, Kuraray Specialities Europe, Frankfurt, Germany) under continuous stirring. The emulsion was kept stirring for 2 minutes before diluting with 600 ml pure water to get the final W1/O/W2 phase. The diluted emulsion was stirred for about 1–2 hours at room temperature and then filtered through a cellulose filter membrane with 0.45 μm pore size (Millipore, Billerica, MA). The filter was rinsed with about 200 ml water and then dried under reduced pressure overnight. The microspheres powder was harvested and stored at 4°C.
2.4. Preparation of alginate microspheres
Five milligrams of tetramethylrhodamine conjugated bovine serum albumin (Rh-BSA) and 2 g of alginate sodium salt were dissolved in 100 ml of distilled water at room temperature with stirring. The solution was pumped through a needle (gauge 22) at a flow rate of 1 ml/min into 500 ml of 25 mM CaCl2 solution under continuous stirring. Rh-BSA-loaded alginate microspheres with a diameter about 700 μm were formed immediately in the solution. The control (empty) microspheres were prepared with the same procedure in the absence of Rh-BSA. Fresh microspheres were incubated in the calcium solutions for 72 h in a shaker at 1.0 Hz at room temperature, filtered out of calcium solution on Whatman #1 filter paper (Whatman, Clifton, NJ), washed with water, and suspended in 70% ethanol. The microsphere suspension was stored at 4°C until use.
2.5. Silk fibroin coating
Wang et al. have recently reported in their silk fibroin-based layer-by-layer (LBL) coating study that the amount of silk fibroin deposited on a planar surface increased as a function of protein concentration in the dipping solution. The maximum deposition was achieved once the protein concentration reached 0.1% (w/v) [15]. Therefore, all the coating experiments presented in this study were done with 0.1% (w/v) silk fibroin. For this, approximately 20 mg of PLGA microspheres or 100 mg of alginate microspheres after removing the 70% ethanol was suspended in 1 ml of 0.1% (w/v) silk fibroin or fluorescein-labelled silk fibroin and the suspension was gently shaken for 2 min at room temperature. In a control sample, 1 ml of water was used instead of silk fibroin solution for coating. The microspheres suspension was then bath-ultrasonicated (Branson 3510) (Branson, Danbury, CN) for 1 min to disperse the clustered microspheres. The suspension was then shaken for another 2 min. To obtain a water-based silk coating, the silk solution was removed by centrifugation for 2 min at 14,000 rpm, 4°C (Eppendorf 5417R centrifuge), and the microspheres were washed twice with 1 ml of pure water with 1 min shaking time and the same centrifugation. To obtain a MeOH-based silk coating on alginate microspheres, the silk-coated alginate microspheres were further immersed in 90% (v/v) MeOH for 15 min to induce silk crystalline β-sheet structure formation [15]. The washed microspheres were then dried by a flow of nitrogen gas and subjected to the next coating procedure. In the present work a total of three coatings were deposited by the above process and then the dried microspheres were suspended in PBS buffer, pH 7.2 for characterization.
2.6. Scanning electron microscopy (SEM)
For PLGA microspheres coated and not coated (control) with silk fibroin, dried powder was directly mounted and sputter-coated with Au using a Ploaron SC502 Sputter Coater (Fison Instruments, UK). Specimens were then examined using a JEOL JSM 840A Scanning Electron Microscope (Peabody, MA) at 15 KV.
2.7. Phase contrast and confocal laser scanning microscopy
Microspheres were suspended in pure water and approximately 20 μl of suspension was put on a glass slide and covered with a cover-slip. The samples were analyzed by a phase contrast light and fluorescence microscope (Carl Zeiss, Jena, Germany) equipped with a Sony Exwave HAD 3CCD color video camera, or a confocal laser scanning microscope (TCS Leica SP2, Welzlar, Germany) with Leica Confocal Software, version 2.5 (Leica Microsystems, Heidelberg, Germany).
2.8. Determination of HRP loading and release from PLGA microspheres
To determine the HRP loading in PLGA microspheres, 10–15 mg of microspheres with and without silk coating were weighed and separately dissolved in Eppendorf tubes containing 0.3 ml of dichloromethane and 0.7 ml of acetone. The solution was centrifuged for 5 min at 14,000 rpm, 4°C (Eppendorf 5417R centrifuge). The supernatant was then removed and the pellet was washed twice with 0.9 ml of the mixture of acetone and dichloromethane (3:1) at the same centrifugation conditions. The pellet obtained was dried with a gentle flow of nitrogen gas and further dissolved in 150 μl PBS buffer, pH 7.2. After a short centrifugation, the HRP content in the supernatant was measured either by activity assay or high pressure liquid chromatography (HPLC) assay. For activity assay, 5 μl of the solution was mixed with 100 μl of TMB solution in 96-well standard microplate wells for 1 min at room temperature and the reaction was stopped by addition of 100 μl 0.1 M sulfuric acid. Absorbance was detected at 450 nm using a VersaMax microplate reader (Molecular devices, Sunnyvale, CA). The HRP content was obtained using a HRP standard curve generated under the same condition. The remaining microspheres were spun down, dried and weighed. Loading in micrograms HRP per milligram of PLGA was obtained by dividing the HRP content (in micrograms) by the weight of the microspheres (in milligrams). Loading efficiency (percent) was calculated by dividing the total of actual HRP loading (in micrograms per milligram) × total microsphere weight (in milligrams) by the theoretical HRP content (in micrograms, calculated based on 125 micrograms of HRP mixed with 100 or 300 milligrams of DOPC during the microsphere preparation).
For HPLC assay, a Waters HPLC system (Milford, MA) consisting of a pump (2690), a UV detector (996) and a computer interface (Millennium32) was used. Separation was performed on an YMC HPLC column (Waters) with two eluents: solvent A consisting of 0.1% (v/v) trifluoroacetic acid (TFA) in water and solvent B consisting of 90% (v/v) acetonitrile and 0.1% (v/v) TFA in water. The flow rate was kept constant at 3 ml/min. After loading the sample (50 μl), 100% solvent A was changed to 60% solvent B and 40% solvent A in 3 min, and further to 100% solvent B in 0.5 min. The column was washed with 100% solvent B for 1 min, and the solvent condition was brought back to the initial 100% solvent A in 0.5 min. HRP was detected at 280 nm and the peak area was integrated. The HRP solutions with known concentrations were assayed first, and the obtained peak areas were used to calculate the amount of HRP in a sample.
To determine HRP release, 10 mg of PLGA microspheres were incubated in 1 ml PBS buffer, pH 7.2 at room temperature. At desired time points, the suspension was centrifuged at 10,000 rpm for 2 min. The supernatant was carefully moved to another tube and the pellet was resuspended in 1 ml fresh PBS buffer, pH 7.2. The pH of the supernatant was monitored and held constant at pH 7.2. The HRP content in the withdrawn supernatant was determined by activity assay as described and the percentage of accumulative release was obtained by comparing with the actual loading level.
2.9. Determination of Rh-BSA loading and release from alginate microspheres
To determine loading, 10–15 mg of dry alginate microspheres with and without silk coating, were separately suspended in 1 ml of PBS buffer, pH 7.2 supplemented with 10 mM EDTA, and the suspension was shaken for 30 min at room temperature. After a short centrifugation, 200 μl of the supernatant was moved to the 96-well standard microplate wells and absorbance at 570 nm was determined. The Rh-BSA content in the microspheres was obtained using an Rh-BSA standard curve generated under the same conditions. To determine the release, the 1 ml suspensions were incubated at room temperature and at desired time points the suspension was centrifuged at 10,000 rpm for 2 min. The supernatant was carefully moved to another tube and the pellet was resuspended in 1 ml fresh PBS buffer, pH 7.2. Rh-BSA content in the supernatant was determined as described and the percentage of accumulative release was obtained by comparing with the loading level.
2.10. Statistical analysis
All experiments were performed with a minimum of N = 3 for each data point. Statistical analysis was performed by one-way analysis of variance (ANOVA) and Student-New-man-Keuls Multiple Comparisons Test. Differences were considered significant when p ≤0.05.
3. Results
3.1. Microspheres before and after silk coatings
3.1.1. PLGA microspheres
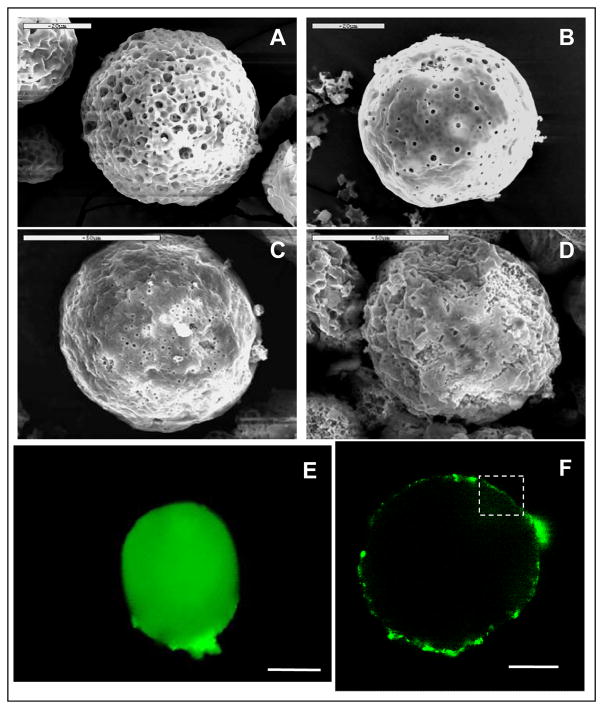
PLGA microspheres were coated with water (control) or 0.1% (w/v) silk fibroin three times. The average diameter of the control microspheres was 53 ± 8 μm as determined by SEM on 50 individual microspheres. The surface of microspheres was porous (Fig. 1A and B). After coating with the silk fibroin the microspheres had a similar size but exhibited a rougher surface than the uncoated microspheres but with fewer and smaller pores (Fig. 1C and D). Similar sizes and surface morphology were observed with the microspheres when HRP was loaded (data not shown). When fluorescein-labeled silk fibroin was used for the coating, the PLGA microspheres were surrounded by a fluorescent layer as seen under fluorescence microscope and confocal laser scanning microscope (Fig. 1E and F). The fluorescent layer was nonhomogeneous in fluorescence intensity (Fig. 1F), which may be a reflection of the porous surface morphology of the PLGA microspheres. The silk fibroin layers deposited on the PLGA microspheres was estimated to be approximately 1 μm thick in the relatively homogeneous regions (see the region highlighted with white frame in Fig. 1F). When only one layer of coating was used only a weak fluorescent layer was observed (data not shown), while at the three layer point this more consistent layer was observed and thus selected for the remainder of the study.
Fig. 1.
Microscopic imaging of silk fibroin coated PLGA microspheres. Surface morphology of uncoated microspheres (A,B) and silk fibroin-coated microspheres (C,D) were determined by scanning electron microscopy (SEM). Fluorescein-labeled silk coating was visualized by fluorescence microscopy (E) and confocal laser scanning microscopy (F). The membrane thickness was estimated in the region highlighted by the white frame. Bar indicates 20 μm in A, B; 50 μm in C, D, E; 23.2 μm in F.
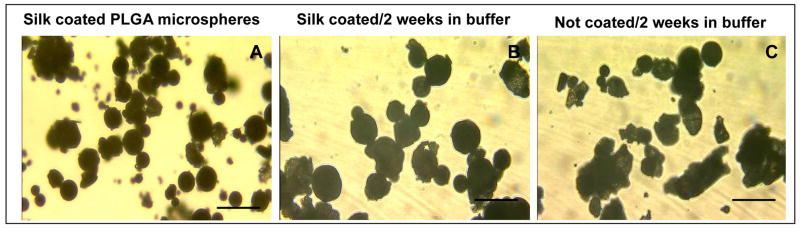
The influence of silk coating on the stability of PLGA microspheres were studied microscopically. The PLGA microspheres used in this experiment had a lager size and broader size distribution (mean diameter 80–120 μm) as compared to those used in the SEM experiment (45–65 μm), probably due to slight variation of preparation conditions (e.g., sonication, solvent evaporation). PLGA microspheres remained in their spherical shapes after three layers of silk coating (Fig. 2A). Uncoated and coated microspheres were incubated in PBS buffer, pH 7.2, at 37°C for two weeks and the morphologies were compared under the microscope (Fig. 2B and C). Most silk-coated microspheres remained in their spherical shapes while uncoated ones were significantly aggregated and deformed, indicating that silk coating can prevent PLGA microspheres from aggregation and decomposition.
Fig. 2.
Stabilization of PLGA microspheres by silk coatings. Silk coated PLGA microspheres had a spherical shape when suspended in PBS buffer, pH 7.2 (A). Most microspheres remained spherical shape after two weeks incubation at 37°C (B). Most control microspheres without coating lost their spherical morphology due to degradation after two weeks incubation at 37°C (C). Bar indicates 200 μm.
3.1.2. Alginate microspheres
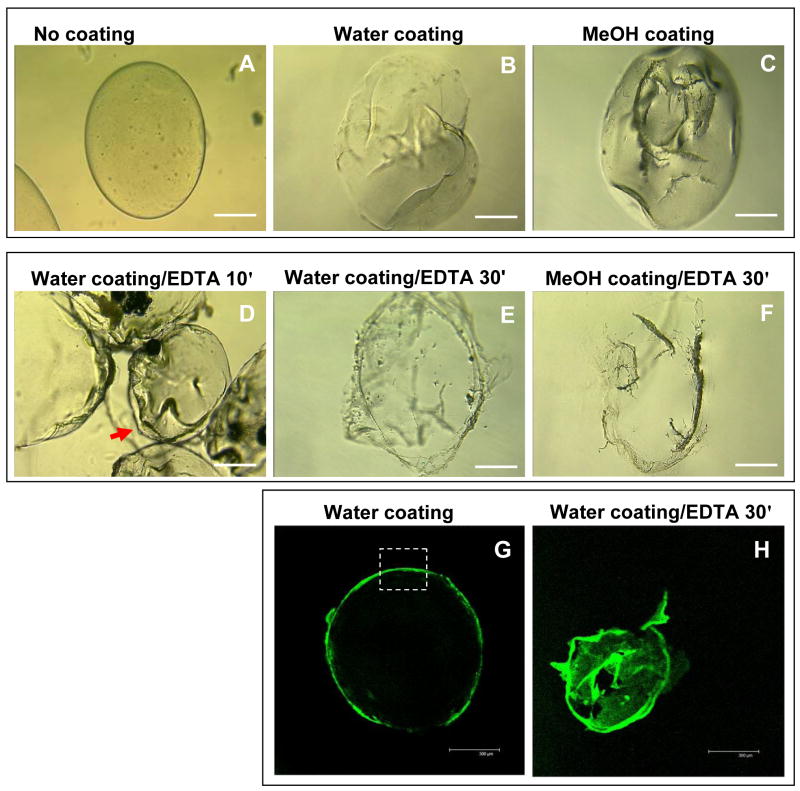
Alginate microspheres exhibited an average diameter 700 ± 80 μm as determined by phase contrast microscopy on 50 individual microspheres. The morphology of alginate microspheres changed upon coating with the silk fibroin as imaged by phase contrast microscopy, and the coated microspheres suspended in water were less transparent than those uncoated (Fig. 3A–C). Water-based coating had a more homogenous surface morphology than the MeOH-based coating (Fig. 3B and C). When the alginate microspheres were suspended in 10 mM EDTA solution, the Ca2+-cross-linked alginate core was liquefied and the free alginate was released to the surrounding environment, leading to the collapse of the microspheres (Fig. 3D). After 30 min of incubation, only the transparent silk fibroin film still remained and kept the shape of the original microsphere in EDTA solution, indicating that most of the alginate content had been released (Fig. 3E and F). When the alginate microspheres were coated by fluorescein-labeled silk fibroin and resuspended in water, a strong fluorescent layer could be observed by confocal laser scanning microscope (Fig. 3G). The coating was approximately 10 μm thick, estimated in the highlighted region on the image. One layer of coating resulted in a weaker fluorescent layer outside the microspheres which could not be clearly imaged. After suspension in EDTA solution for 30 min, the alginate was dissolved and probably released through the pores on silk coating, resulting in the collapse of silk coating into a non-spherical shape (Fig. 3H). Silk coating was not dissolved and remained the shape even after overnight incubation in EDTA solution.
Fig. 3.
Microscopic imaging of silk fibroin-coated alginate microspheres. The morphology of uncoated alginate microspheres in water (A), silk-coated microspheres in water (B), and the coated microspheres after MeOH treatment in water (C) were compared by phase contrast microscopy. The coated microspheres were suspended in 10 mM EDTA and incubated for 10 min (D) and 30 min (E) for water-based coating and 30 min (F) for MeOH-based coating. The water-based fluorescein-labeled silk coating (G) was visualized by confocal laser scanning microscopy. The membrane thickness was estimated in the region highlighted by the white frame. The same sample was suspended in 10 mM EDTA for 30 min to remove the alginate content, leaving green fluorescent silk layer (H). Bars indicate 200 μm in A–F and 300 μm in G and H.
3.2. Drug loading and release
3.2.1. PLGA microspheres
Ultrasonication used in the preparation of PLGA microspheres can affect the activity of protein to be encapsulated [19]. HRP loading in PLGA microspheres was therefore optimized by changing the energy output during the ultrasonication step and the amount of PLGA used in the process. As shown in Table 1, HRP loading reached maximal level of 0.6 μg per mg of PLGA with an energy output of 30 w. The percentage of loaded HRP in the total HRP (loading efficiency) was about 25%. When energy output was decreased from 30 to 18 to 3 w, HRP loading efficiency significantly decreased (p<0.001 between all groups) while HRP loading decreased less significantly (p>0.05 between all groups). The loading and loading efficiency determined by enzyme activity and HPLC assay were consistent. When the amount of PLGA used was increased three times while the energy output was kept 30 w, HRP loading decreased to approximately 0.3 μg per mg of PLGA, although the loading efficiency increased to approximately 35%. The microspheres with the highest loading of HRP (0.6 μg/mg of PLGA microspheres) were used for the coating and release studies.
Table 1.
Optimization of HRP loading in PLGA microspheres
| Power output (sonicator) | Amount of PLGA in prep. (mg) | Loading (μg/mg PLGA microspheres) | Loading efficiency (%) | ||
|---|---|---|---|---|---|
| activity assay | HPLC | activity assay | HPLC | ||
| 3 W | 100 | 0.27 ± 0.02 | 0.24 ± 0.04 | 10.83 ± 0.77 | 9.51 ± 1.46 |
| 18 W | 100 | 0.47 ± 0.03 | 0.48 ± 0.04 | 18.89 ± 1.2 | 19.23 ± 1.60 |
| 30 W | 100 | 0.62 ± 0.07 | 0.62 ± 0.06 | 25.07 ± 2.83 | 24.74 ± 2.19 |
| 30 W | 300 | 0.32 ± 0.05 | 0.27 ± 0.04 | 38.15 ± 5.96 | 32.44 ± 4.93 |
Note: (1) all experiments were done with a minimum of N = 3, and data represent the average value ± standard deviation. (2) the numbers in bold indicate that the prepared microspheres were used for the silk coating and drug release studies.
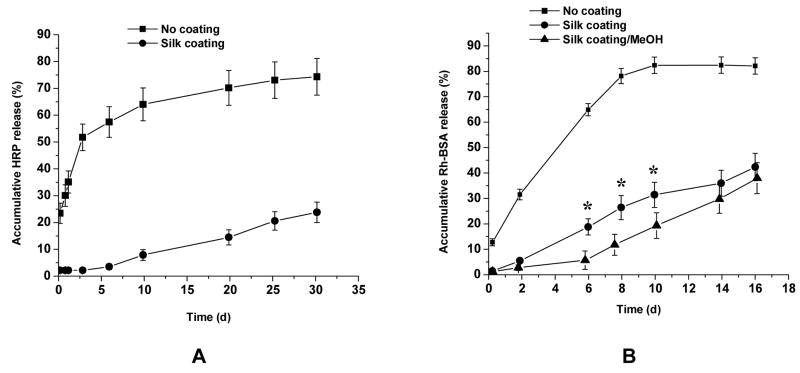
After coating, the HRP content in the control and silk fibroin-coated sample did not change compared to samples before coating. HRP release from the coated samples, however, was significantly retarded compared to that from the control samples (significant difference, p<0.001, for all time points) (Fig. 4A). In fact, almost no HRP was released from the coated samples in the first 5 days, and a slow linear release was observed over the following 25 days.
Fig. 4.
Release of model protein drugs from silk coated microspheres. (A) HRP encapsulated PLGA microspheres were coated with silk fibroin (●) and water as a control (■) and HRP release were determined after suspending the microspheres in PBS buffer, pH 7.2. Silk coated PLGA microspheres had a much slower HRP release than the control sample (p<0.001 for all time points). Experiment was done with a minimum of N = 3, and data represent the average value. ± standard deviation. (B) Rh-BSA encapsulated alginate microspheres were coated with silk fibroin (●) and further treated with MeOH (▲), and water instead of silk as a control (■) and Rh-BSA release were done under the same condition. Compared to the control sample, silk/water and silk/MeOH-coated alginate microspheres had a much slower HRP release (p<0.001 for all time points). The difference between silk/water and silk/MeOH-coated PLGA microspheres was significant for day 6, 8, and 10 (*, p<0.001) but not significant for the other time points (p > 0.05). Experiment was done with a minimum of N = 3, and data represent the average value ± standard deviation.
3.2.2. Alginate microspheres
The control and silk fibroin-coated alginate microspheres contained approximately 15.5 μg Rh-BSA per mg of alginate with loading efficiency of approximately 15%. As compared to water-based silk coating, the MeOH-treated silk coating showed significantly retarded Rh-BSA release from 6 to 10 days (p<0.001) but no significant difference at the early and late stage of release (p > 0.05) (Fig. 4B). Therefore, high content of silk β-sheet structure network in the coating that was induced by MeOH treatment further retarded drug release, as reported previously [15,20,21]. The slow degradation of silk polymer from the surface or the dissolution of alginate polymer after longer time incubation (more than 10 days) might have caused loss of β-sheet structure and increase of drug release rate. Compared to the control sample, the Rh-BSA release from the silk coated samples with and without MeOH treatment was significantly retarded (p<0.001 for all time points) (Fig. 4B). The alginate microspheres degraded in time during the release determination. The control sample had almost no microspheres remained at the end (16 days) but the silk coated sample had more than 50% (by dry weight) remained. Silk coating therefore significantly stabilized the alginate microspheres resulting in slower degradation.
4. Discussion
We have recently exploited the self-assembly of silk fibroin to form nanoscale thin films on a planar surface using stepwise LBL deposition [15]. This technique was applied for the first time to coating PLGA and alginate microspheres in the present study. The surface of PLGA microspheres is hydrophobic [22] while alginate microspheres exhibit negative surface charge [23]. The mechanisms behind the silk coating of PLGA and alginate microspheres are therefore different, as previously described in the ultrathin film silk study [15]. The silk fibroin interacts with the PLGA microspheres via hydrophobic interactions whereas electrostatic interactions most likely mediate interactions with the alginate microspheres. Multiple layers of silk coating were stacked via hydrophobic interactions between the individual silk fibroin layers. With the same coating process (3-layer silk fibroin coating), the thickness of the coatings were about 1 μm on the PLGA microspheres but about 10 μm on the alginate microspheres. The hydrogel nature of cross-linked alginate system allowed the silk fibroin to penetrate into the matrix and electrostatic interactions between silk fibroin and alginate may promote this process.
Silk fibroin coatings significantly changed the surface properties of PLGA and alginate microspheres. The ultrastructure of silk coated layer is not yet elucidated, but from previous atomic force microscopic study on a silk coated flat surface, the coating is composed of nanoscale granules or fibers and possibly pores [15]. Due to its robust material property, silk coating helped maintain the shape of PLGA and alginate microspheres and prevented them from aggregation and decomposition. Once the microspheres are loaded with protein drugs, both diffusion barrier and stabilization effect provided by silk coating may attribute to the retardance of drug release. Compared to the Rh-BSA release from alginate microspheres, HRP release from PLGA microspheres showed an initial lag phase and overall slower release (Figure 4A and B). This might be due to either the difference in generic properties of the two coatings (e.g., pore size, hydrophobicity, charge distribution) or the non-specific interaction between HRP and silk materials as reported previously [20,21] or both effects. This will need to be elucidated in the future studies.
The present study provides new options to modify microspheres for use in a diverse array of controlled drug delivery applications. PLGA microspheres have been previously coated with a poly (vinyl alcohol) (PVA) membranes. The hydrophilic surface upon coating permitted the incorporation of PLGA microspheres into tissue-engineered scaffolds [22]. However, the PVA coating quickly dissolved in an aqueous environment which limited its function as a drug diffusion barrier. For the microspheres used in the body as either biosensor matrices or drug delivery carriers, silk coatings provide a barrier not only to slow the release of encapsulated macromelcules but also to slow the decomposition of microspheres relative to uncoated microspheres. Silk polymers in material formats will degrade from the surface, thus the extent of degradation and release of drugs encapsulated in the coated systems will depend on the number of layers used. In the present work we focused on three layers as this was the minimal coating to attain a relatively uniform coverage of the microspheres of PGLA and alginate. The results proved the principle that the drug release rates can be controlled by varying the number of silk coated layers and type of material matrix used (e.g., PLGA or alginate), which is very important for the future biomedical applications using silk as a coating material. For example, the silk-coated microspheres that release drugs very slowly (e.g., 20% HRP release from silk coated PLGA microspheres in 30 days) can be used as implantable systems for the treatment of chronic disease, where therapeutic drugs are expected to be released for more than a year. The problem of initial lag phase of release in this case can be overcome by loading drugs directly to the silk coatings. On the other hand, those microspheres releasing drug faster (e.g., 40% Rh-BSA release from silk coated alginate microspheres in 16 days) might be used for tissue engineering or tissue repair purposes, where the drug release rates will match that of tissue growth and material degradation.
In addition, silk fibroin coatings also impart mechanical strength to the microspheres, especially to the alginate microspheres with the hydrogel nature. Since silks in material forms are the strongest natural polymers known, the coating layers should elevate the mechanical profiles of the microspheres with predictable outcomes, as we have previously seen with silk fiber degradation [24]. MeOH treatment of silk coating may enhance the effect and, therefore, would be an important option in processing silk coatings in the future applications.
Alginate microspheres have been extensively studied as templates to form polyelectrolyte-coated microcapsules in which bioactive macromolecules are loaded. Multlayer polyelectrolyte coatings can be chitosan/alginate, poly (lysine)/alginate, and poly (allylamine hydrochloride)/poly (styrene sulfonate) (PAH/PSS) [25–27]. Alginate-templated microcapsules with four layers of PAH/PSS coating were prepared by partially releasing the alginate content under EDTA treatment. Positively charged macromolecules could be efficiently loaded into the microcapsules and the driving force for this spontaneous loading was believed to be the electrostatic interactions between the macromolecules and the alginate molecules remaining in the microcapsules [25]. The microcapsules kept their (spherical) shape after the alginate was released because the alginate microspheres were small in size (average diameter about 4 μm). In the present study, the silk microcapsules collapsed after releasing the alginate content because the alginate microspheres used were larger (average diameter 480 ± 80 μm). Future work will therefore focus on reducing the size of the alginate microspheres in obtain stable silk microcapsules in which larger amounts of protein drugs could be loaded. Furthermore, a second protein drug or enzyme could be encapsulated in the multilayers of silk coatings so that different drugs can be sequentially released from one carrier system or different steps of enzymatic reactions can be accomplished in one reaction system. This is particularly relevant to the silk systems since the degradation process is a surface, enzyme mediated process, thus predictable and controllable release profiles can be attained for multiple components.
The silk-coated microspheres in this study have distinct features as compared to the reported silk-coated liposomes [17]: (I) MeOH is not necessarily used in the coating process, so that the system is suitable for delivering protein drugs. (II) The thickness of coatings can be controlled by the addition of different silk layers, leading to rate-controllable drug release. (III) Silk thin layer coatings did not change the overall morphology of microspheres as well as the drug loading, but did retard the releases. (IV) Larger size of PLGA and alginate microspheres offers large drug loading capacities. Therefore, instead of being a targeting nanoparticle system, silk-coated microspheres are more suitable as a depot drug delivery device to provide long-term sustained drug release through local subcutaneous or intramuscular administration.
5. Conclusions
This is the first paper to demonstrate that layer-by-layer silk fibroin coating on PLGA and alginate microspheres can be achieved. Silk coating on the PLGA microsphere surface was heterogeneous with an average thickness of about 1 μm, whereas it was homogeneous with a thickness of about 10 μm on the alginate microsphere surface, as determined by SEM and confocal laser scanning microscope. Silk fibroin coatings not only stabilized microspheres from degradation but also sustained protein drug release from the microspheres by providing an effective diffusion barrier. Protein drug loading was not changed by silk coating in either case. Therefore, the silk coatings provide new attributes to the microspheres in terms of sustained drug release and improved mechanical strength, suggesting new and useful options for these systems in many applications.
Acknowledgments
We are grateful to Dr. Fuxin Shi, Eaton-Peabody Laboratory, Department of Otology and Laryngology, Harvard Medical School, for assistance with confocal laser scanning microscopic measurements. The study was supported by the NIH P41 Tissue Engineering Resource Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Katre NV. Liposome-based depot injection technologies. Am J Drug Deliv. 2004;2:213–27. [Google Scholar]
- 2.Hurteaux R, Edwards-Lévy F, Laurent-Maquin D, Lévy M. Coating alginate microspheres with a serum albumin-alginate membrane: application to the encapsulation of a peptide. Eur J Pharmaceut Sci. 2005;24:187–97. doi: 10.1016/j.ejps.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Chan LW, Liu X, Heng PW. Liquid phase coating to produce controlled-release alginate microspheres. J Microencapsul. 2005;22:891–900. doi: 10.1080/02652040500273936. [DOI] [PubMed] [Google Scholar]
- 4.Scholes PD, Coombes AGA, Davies MC, Illum I, Davis SS. Particle engineering of biodegradable colloids for site-specific drug delivery. In: Park K, editor. Controlled drug delivery: challenges and strategies. 1997. [Google Scholar]
- 5.Tsung MJ, Burgess DJ. Preparation and characterization of gelatin surface modified PLGA microspheres. AAPS PharmSci. 2001;3:1–11. doi: 10.1208/ps030211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srivastava R, Brown JQ, Zhu H, McShane MJ. Stable encapsulation of active enzyme by application of multilayer nanofilm coatings to alginate microspheres. Macromol Biosci. 2005;5:717–27. doi: 10.1002/mabi.200500061. [DOI] [PubMed] [Google Scholar]
- 7.Gemeiner P. Enzyme engineering: immobilized biosystems. Chichester, UK: Ellis Horwood, Ltd; 1992. [Google Scholar]
- 8.Liang J, Li Y, Yang V. Biomedical application of immobilized enzymes. J Pharm Sci. 2000;89:979–90. doi: 10.1002/1520-6017(200008)89:8<979::aid-jps2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 9.Watts PJ, Davies MC, Melia CD. Microencapsulation using emulsification/solvent evaporation: an overview of techniques and applications. Crit Rev Ther Drug Carrier Syst. 1990;7:235–59. [PubMed] [Google Scholar]
- 10.Tamber H, Johansen P, Merkle HP, Gander B. Formulation aspects of biodegradable polymeric microspheres for antigen delivery. Adv Drug Deliv Rev. 2005;57:357–76. doi: 10.1016/j.addr.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Sriamornsak P. Preliminary investigation of some polysaccharides as a carrier for cell entrapment. Eur J Pharm Biopharm. 1998;46:233–36. doi: 10.1016/s0939-6411(98)00021-6. [DOI] [PubMed] [Google Scholar]
- 12.Draget KI, Skjak-Braek G, Smidsrod O. Alginate based new materials. Int J Biol Macromol. 1997;21:47–55. doi: 10.1016/s0141-8130(97)00040-8. [DOI] [PubMed] [Google Scholar]
- 13.Smidsrod O, Skak-Braek G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990;8:71–8. doi: 10.1016/0167-7799(90)90139-o. [DOI] [PubMed] [Google Scholar]
- 14.Taqieddin E, Amiji M. Enzyme immobilization in novel alginate–chitosan core-shell microcapsules. Biomaterials. 2004;25:1937–45. doi: 10.1016/j.biomaterials.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Kim HJ, Xu P, Matsumoto A, Kaplan DL. Biomaterial coatings by stepwise deposition of silk fibroin. Langmuir. 2005;21:11335–41. doi: 10.1021/la051862m. [DOI] [PubMed] [Google Scholar]
- 16.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24:401–16. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 17.Gobin AS, Rhea R, Newman RA, Mathur AB. Silk-fibroin-coated liposomes for long-term and targeted drug delivery. Int J Nanomed. 2006;1:81–7. doi: 10.2147/nano.2006.1.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. Functionalized silk-based biomaterials for bone formation. J Biomed Mater Res. 2001;54:139–48. doi: 10.1002/1097-4636(200101)54:1<139::aid-jbm17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Meinel L, Illi OE, Zapf J, Malfanti M, Merkle HP, Gander B. Stabilizing insulin-like growth factor-I in poly(D,L-lactide-co-glycolide) microspheres. J Control Release. 2001;70:193–202. doi: 10.1016/s0168-3659(00)00352-7. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann S, Wong Po Foo CT, Rossetti F, Textor M, Vunjak-Novakovic G, Kaplan DL, Merkle HP, Meinel L. Silk fibroin as an organic polymer for controlled drug delivery. J Control Release. 2006;111:219–27. doi: 10.1016/j.jconrel.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Wenk E, Matsumoto A, Meinel L, Li C, Kaplan DL. Silk microspheres for encapsulation and controlled release. J Control Release. 2007;117:360–70. doi: 10.1016/j.jconrel.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Meese TM, Hu Y, Nowak RW, Marra KG. Surface studies of coated polymer microspheres and protein release from tissue-engineered scaffolds. J Biomater Sci Polym Ed. 2002;13:141–51. doi: 10.1163/156856202317414339. [DOI] [PubMed] [Google Scholar]
- 23.Wee S, Gombotz WR. Protein release from alginate matrices. Adv Drug Delivery Rev. 1998;31:267–85. doi: 10.1016/s0169-409x(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 24.Horan RL, Antle K, Collette AL, Wang Y, Huang J, Moreau JE, Volloch V, Kaplan DL, Altman GH. In vitro degradation of silk fibroin. Biomaterials. 2005;26:3385–93. doi: 10.1016/j.biomaterials.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 25.Schneider S, Feilen PJ, Slotty V, Kampfner D, Preuss S, Berger S, Beyer J, Pommersheim R. Multilayer capsules: a promising microencapsulation system for transplantation of pancreatic islets. Biomaterials. 2001;22:1961–70. doi: 10.1016/s0142-9612(00)00380-x. [DOI] [PubMed] [Google Scholar]
- 26.Robitaille R, Pariseau JF, Leblond FA, Lamoureux M, Lepage Y, Halle JP. Studies on small (<350 microm) alginate-poly-L-lysine microcapsules. III. Biocompatibility of smaller versus standard microcapsules. J Biomed Mater Res. 1999;44:116–20. doi: 10.1002/(sici)1097-4636(199901)44:1<116::aid-jbm13>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Zhu H, Srivastava R, McShane MJ. Spontaneous loading of positively charged macromolecules into alginate-templated polyelectrolyte multilayer microcapsules. Biomacromolecules. 2005;6:2221–8. doi: 10.1021/bm0501656. [DOI] [PubMed] [Google Scholar]