Abstract
Purpose
The Southwest Oncology Group conducted a randomized trial of induction BCG with or without maintenance BCG. In these additional retrospective analyses, our goal was to evaluate the association of a complete response (CR) or remaining with no evidence of disease (NED) versus no CR during induction therapy with subsequent survival after adjusting for other potential confounders. Among all patients randomized to maintenance treatment, we also wanted to identify combinations of baseline covariates in order to define prognostic groups for subsequent worsening-free survival.
Methods
Outcome measures of worsening-free and overall survival were assessed using Kaplan Meier estimates and proportional hazards regression models. For the Classification and Regression Tree (CART) analysis, 434 patients randomized to maintenance vs. no therapy with complete covariate information were included.
Results
Of the 593 evaluable patients, 341 were not randomized to maintenance BCG. Patients who achieved a prior complete response during induction BCG had a 5-year survival probability of 77% compared to 62% for patients who did not [Hazard ratio (HR) 0.60; 95% confidence interval (CI) 0.44, 0.81; p= 0.0008]. Prior CR retained significance when adjusted for age, gender, prior intravesical chemotherapy, and papillary disease versus CIS (HR=0.63; 95% CI: .46, .86; p=0.003). CART analysis identified 4 prognostic groups. Older patients (≥ 62 years old) previously treated with intravesical chemotherapy who failed to achieve a CR had a 5-fold higher risk of a worsening event relative to those who are younger (< 67 years old) and achieve a CR (HR=5.09; 95% CI: 3.37, 7.68; p<0.0001).
Conclusion
Failure to achieve a complete response after induction BCG is associated with a significant risk of a worsening event and death for patients with CIS or Ta or T1 bladder cancer at increased risk of recurrence.
Keywords: bladder cancer, intravesical therapy, BCG, immunotherapy, non-muscle invasive, maintenance
Introduction
Complete response (CR) rates to an induction course of BCG for Ta, T1 and Tis bladder cancer are high and range from 50–70%. Factors affecting response include the presence of high grade papillary disease or CIS, primary vs. recurrent disease, solitary vs. multifocal tumor, granulomatous inflammatory response in the bladder and delayed hypersensitivity skin test response to purified protein derivative [1]. Ten to 22% of patients who fail an initial induction course of BCG may be salvaged with a second induction course [2, 3]. Recently, the addition of a single 3 week maintenance course of BCG increased the complete response rate at 6 months from 68% to 84% compared to patients who receive only a 6-week induction course [4]. The addition of interferon alpha may augment the response to BCG and Phase II studies suggest that salvage of high risk patients who have failed BCG is possible in over one half of patients treated with this combination [5, 6].
Though initial CR rates are similar, the long-term progression and survival rates are significantly lower for patients with CIS associated with T1 disease compared to patients with CIS alone or associated with Ta disease [7]. Patients with high-grade Ta, T1 or CIS are considered at high risk for progression to invasive cancer. In risk stratification models this risk is as high as 40% [8]. As innovative therapies are applied in order to augment the immune response to BCG and to explore salvage therapy options, the risk of progression in high risk patients must be carefully monitored. There is a risk in patients not responding to the initial induction course of BCG that further efforts at salvage therapy may result in a delay in definitive treatment that may have an adverse effect on survival probability. Long-term survival rates for patients treated with radical cystectomy for progression to muscle invasive cancer are significantly lower than for patients with ≤ pT1 at cystectomy [9, 10].
In the present study we asked the question whether failure to achieve a CR after induction BCG is associated with a worse prognosis. SWOG 8507 is one of the largest prospective trials of intravesical BCG with a median follow-up of 7.5 years[4]. This randomized trial tested the hypothesis that the addition of maintenance BCG would be associated with lower recurrence and progression rates compared to an induction course alone. Reporting only on those patients that had an initial CR to induction therapy this study demonstrated a benefit to long-term maintenance therapy. We have queried this data set in a series of secondary analyses in order to determine the long-term outcome regarding worsening-free and overall survival according to the initial response to induction BCG.
Methods
The clinical trial design and analysis of outcomes for complete responders has been reported [4]. Eligible patients were BCG naïve, had completely resected Ta or T1 tumors with or without CIS and were considered to be at increased risk of recurrence. Descriptive factors (prior intravesical chemotherapy yes/no) and disease type (papillary only, carcinoma in situ only or both papillary disease and carcinoma in situ) were recorded. All patients received an induction course of BCG and randomization was to observation or maintenance BCG given weekly for three weeks at 3 and 6 months following induction and at 6-month intervals up to three years. A total of 660 patients were registered to undergo induction BCG and 550 were subsequently randomized. A total of 593 were evaluable for assessment of response to induction therapy and form the basis for the present analysis. The initial cystoscopic evaluation of response to induction BCG was to occur 6 weeks following induction BCG. Follow-up for disease status was discontinued after June 1996 because the clinical trial primary analysis was completed, but overall survival is current. In order for disease and survival status information to match, the survival status was truncated as of June 1996 (i.e., patients alive after June 1996 were censored at that time). Complete response for patients with CIS was defined as no evidence of cancer on biopsy and negative cytology at the first follow-up cystoscopy. Biopsy was not required for patients with papillary disease only (Ta, T1). Treatment for patients who did not achieve an initial CR was up to the discretion of the treating physician.
Statistical methods
Time to a disease-worsening event and survival probabilities were estimated using Kaplan-Meier methods and differences in the distributions were tested using the log rank statistic. Proportional hazards regression models were developed for evaluating event time data. Patients randomized to maintenance BCG were excluded from the analysis of impact of initial complete resonse since subsequent treatment may confound survival results. A total of 434 patients randomized to maintenance vs. no maintenance therapy had complete covariate information and could be used for identifying prognostic groups by implementing Classification and Regression Tree (CART) method in order to determine groups with similar disease worsening-free survival [11]. This tree method allows one to see subsets of covariates working together, which isn’t necessarily seen in proportional hazards models. The tree diagram offers a visual representation of these subsets. Worsening-free survival was measured from randomization to maintenance BCG to first worsening event. Worsening events were defined as any one of the following: cystectomy, systemic chemotherapy, radiation therapy, stage ≥ T2 and/or death. The candidate covariates that the groups could be determined by were selected from the SWOG database and were limited to variables with few missing values. The candidate covariates used in this analysis were complete response to induction BCG (yes/no), age at induction registration, body mass index (bmi) at study entry, prior intravesical chemotherapy (yes/no) and gender. We used SPLUS 2000 (release 3, Insightful Corporation, Seattle, WA) and SAS (version 8, SAS Institute Incorporated, Cary, NC) for these analyses.
Results
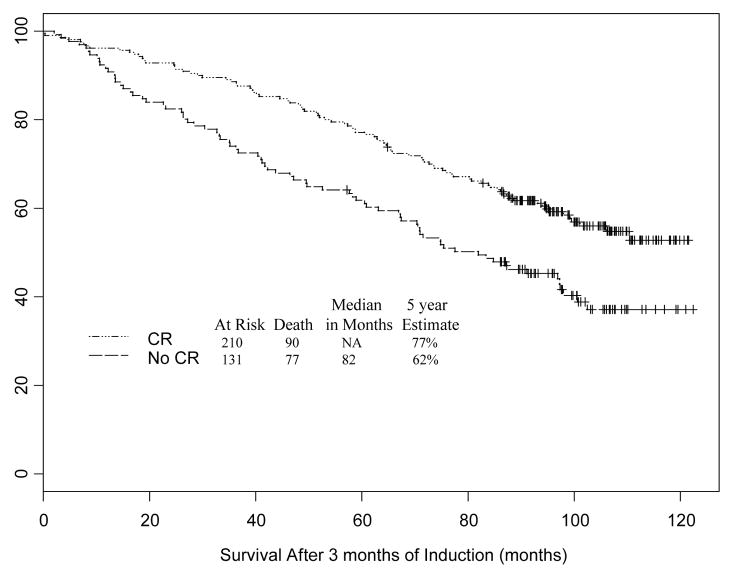
Of the 660 patients registered, 593 were evaluable for response to induction BCG. A total of 341 patients had a compete response to induction BCG and were randomized to receive no maintenance BCG. The median survival was not assessable for patients who achieved a CR compared to 82 months for patients who did not achieve a CR and the five-year survival probabilities were 77% and 62%, respectively (Figure 1). The hazard ratio was 0.60 (95% CI: 0.44, 0.81) indicating that failure to achieve a CR after induction BCG was associated with a 67% higher risk of death. (p = 0.0008). A proportional hazards model was constructed which adjusted for age, gender, whether or not the patient had prior intravesical chemotherapy, and disease type at study entry (papillary disease vs. CIS). Patients who achieved a CR with induction BCG still had a significantly lower risk of death when adjusting for these variables [HR 0.63 (95% CI: 0.46, 0.86; p=0.003)] (Table 1). Papillary disease (Ta or T1 without CIS) had a similar favorable impact on overall survival.
Figure 1.
Kaplan Meier plot of survival following induction BCG stratified by whether the patient had a complete response or not at the initial cystoscopic evaluation following induction therapy.
Table 1.
Proportional hazards model of variables related to complete response following induction BCG and overall survival
| Variable | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Unadjusted Model | ||
| Complete Response | 0.60 (0.44, 0.81) | 0.0008 |
| Adjusted Model | ||
| Complete Response | 0.63 (0.46, 0.86) | 0.003 |
| Age5 | 1.27 (1.16, 1.40) | <0.0001 |
| Male | 1.32 (0.84, 2.08) | 0.23 |
| Papillary | 0.65 (0.45, 0.93) | 0.02 |
| Prior chemotherapy | 0.95 (0.70, 1.30) | 0.76 |
At risk = 341 and deaths = 167
Age5 = age divided by 5. Example: The hazard ratio refers to the risk of death for a 65-year old relative to a 60-year old, thus there is a 32% increased risk of death as age increases 5 years.
CART analysis
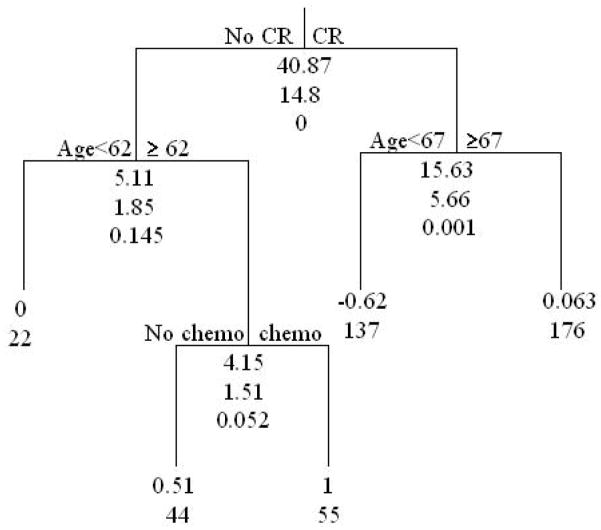
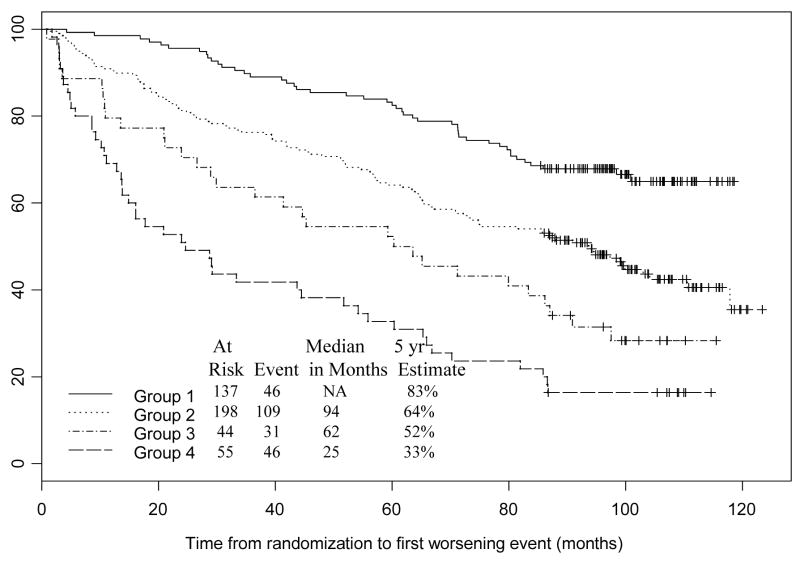
For this analysis, we included 434 patients with no missing data. Patients were placed into risk groups based on response to BCG induction therapy (complete response versus no complete response), whether or not they had received prior intravesical chemotherapy, and age (Table 2). Figure 2 describes the stratification of subsets based on whether or not patients had a complete response to induction BCG. Complete responders were then stratified by age (< 67 years or ≥ 67 years). The no CR group was also stratified by age (< 62 years or ≥ 62 years). The no CR and age ≥ 62 years was then stratified by whether they received prior intravesical chemotherapy or not. The risk of a worsening event for those subjects that had a CR of age ≥ 67 relative to those subjects that did not have a CR of age<62 is 1.06. Thus, subjects in these two nodes have approximately the same risk of a worsening event; therefore, these two nodes were combined to form one prognostic group. The tree diagram indicates that for worsening-free survival, complete response is the most important factor. For those patients who did not achieve a CR with induction BCG, age and prior intravesical therapy are important predictive variables. For those patients who achieved a CR with induction BCG, age is an important predictive variable. A proportional hazards model of time to first worsening event was fit with prognostic group (node) indicators as covariates (Table 3). The no CR groups have 3 to 5 times the risk of an event compared to younger, complete responders. In this CART analysis, prior intravesical therapy did not stratify further the group of patients that did not have an initial CR. The estimated probability of a patient in the best prognosis group I (CR and age <67) having a disease-worsening event was 17% compared to 67% for a patient in the worst prognosis group 4 (No CR, age ≥ 62 and prior chemotherapy) (Figure 3).
Table 2.
Description of 4 groups of patients identified by tree diagram
| Group 1 N=137 Median (SD) |
Group 2 N=198 Median (SD) |
Group 3 N=44 Median (SD) |
Group 4 N=55 Median (SD) |
Total N=434 Median (SD) |
|
|---|---|---|---|---|---|
| Age (yrs) | 61 (6.42) | 71 (7.93) | 72 (6.19) | 71 (6.99) | 68 (9.21) |
| Bmi (kg/sq m) | 27 (4.27) | 26 (4.23) | 25 (3.75) | 27 (4.08) | 26 (4.27) |
| Height (cm) | 178 (10.62) | 175 (8.90) | 175 (10.03) | 173 (10.50) | 175 (9.71) |
| Weight (kg) | 84 (16.21) | 78 (14.48) | 76 (14.77) | 79 (14.87) | 80 (15.35) |
| Male | 87% | 86% | 84% | 78% | 85% |
| Papillary | 77% | 81% | 86% | 63% | 78% |
| Prior chemo | 48% | 42% | 0% | 100% | 47% |
| Complete response | 100% | 89% | 0% | 0% | 72% |
| Race | White 94% Black 4% Other 2% |
White 95% Black 2% Other 2% Missing 1% |
White 5% Black 5% Other 0% |
White 94% Black 4% Other 2% |
White 95% Black 3% Other 2% Missing <1% |
| 5-year worsening- free survival estimates | 83% | 64% | 52% | 33% | 65% |
| Proportion without progression that died within 5 yrs | 45% (5/11) | 45% (23/51) | 53% (8/15) | 20% (6/30) | 39%(42/107) |
CR=complete response; prior chemo=prior intravesical chemotherapy; SE = standard error
Figure 2.
CART analysis of worsening-free survival from randomization date for 434 subjects with no missing data based on complete response to induction therapy (yes/no), age, body mass index (BMI), prior chemo (yes/no), and gender. At each splitting point in the tree diagram there are three numbers listed. The first number indicates the unadjusted log rank statistic, the second number is the bootstrap log rank and the third number is the p-value corresponding to the bootstrap log rank which takes into account that the same individuals are used to make multiple splits (i.e., tests are not independent). The two numbers listed at each node correspond to the log hazard ratio for worsening-free survival relative to the reference node (whose log hazard ratio = 0), and the sample size for the node, respectively.
Table 3.
Proportional hazards model for risk of disease worsening event following treatment with BCG: (At risk=434 with no missing data; events=232)
Group 1 – Complete responders (CR) and age < 67 (n = 137)
Group 2 – Complete responders and age ≥ 67, or No CR and age < 62 (n = 198)
Group 3 – No CR, age ≥ 62 and no prior chemotherapy (n = 44)
Group 4 – No CR, age ≥ 62 and prior chemotherapy (n = 55)
| Variable | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Group 1 | 1.00 (reference group) | --- |
| Group 2 | 1.96 (1.39, 2.77) | 0.0001 |
| Group 3 | 3.08 (1.95, 4.86) | <0.0001 |
| Group 4 | 5.09 (3.37, 7.68) | <0.0001 |
At risk = 434 and events = 232
Figure 3.
Kaplan Meier plot of worsening-free survival following randomization. Groups were defined as in Table 3 and stratified by the CART tree diagram (Figure 2)
Discussion
SWOG 8507 demonstrated the superiority of induction plus maintenance BCG over induction BCG alone for patients at increased risk of recurrence and progression to invasive cancer [4]. The final report focused only on those patients who achieved a complete response after induction BCG. In the present study we examined long-term outcome for all evaluable patients and endeavored to identify prognostic groups using a classification and regression tree (CART) analysis tool.
Using conventional Kaplan Meier and Cox proportional hazards analysis, whether or not the patient achieved a complete response to induction BCG was the most important stratification variable associated with long-term survival. Failure to achieve a CR was associated with a 67% higher risk of death and a 5-year survival probability of 62% compared to 77% for patients with a complete response to induction BCG. We also confirmed the important favorable prognostic impact of papillary only disease without CIS. Solsona recently reported that failure to achieve a complete response at 3 months following induction intravesical therapy was the only independent factor associated with progression to invasive cancer in patients with stage T1 grade 3 tumors or CIS of the bladder, prostatic urethra or prostatic ducts [12].
Many studies have addressed the treatment options for patients with BCG refractory bladder cancer and the standard of care remains definitive local therapy with cystectomy, particularly for patients with high risk features including CIS and T1G3 tumors. Five year survival probabilities for patients with Ta, Tis or T1 cancer in contemporary cystectomy series range from 80–88% when there is no evidence of muscle invasion [13]. Recent studies have also clearly demonstrated the important of re-staging TUR for patients with clinical T1G3 cancers as the understaging rate in cystectomy series is as high as 40% and muscle invasion is associated with a significant worse prognosis[10, 14, 15]. For patients who refuse cystectomy or who are not medically fit options may include intravesical Valrubicin, dose reducing BCG and combining this with interferon, or investigational drugs such as gemcitabine and keyhole limpet hemocyanin [16–19].
What to do when for patients when BCG fails is a vexing problem. The ability to prospectively identify those patients at highest risk for subsequent progression would be valuable. Using CART analysis we identified four prognostic groups for risk of a disease-worsening event following induction BCG. Again, whether or not a complete response was achieved to induction BCG was the most important stratification variable further supporting the importance of this event. Patients with the worst prognosis did not achieve a CR, were older, and had received prior intravesical chemotherapy. The risk of a worsening event was 5-fold higher for these patients compared to patients who achieved a CR and were younger than 67 years of age. Age has been identified as a significant variable that affects survival probability in other studies [20]. The presumption is that older patients have a shorter life expectancy and may not respond as well to immunotherapy.
The strengths of these data reported in this paper are that the patients came from 81 different institutions around the U.S. and many of the top accruers were local CCOPs and not major research institutions. As such this is a fairly representative sample of the patient population at risk that most urologists deal with. The potential limitations include the lack of follow-up treatment information, no accurate cause of death information, and the retrospective nature of the analysis.
Conclusion
Failure to achieve a complete response following induction BCG for patients with CIS or Ta or T1 tumors at increased risk of recurrence is associated with a significant risk of death or a disease-worsening event. Older patients who did not achieve a CR and received prior intravesical therapy had the worst prognosis.
Acknowledgments
Supported by grants from the National Cancer Institute
1. N01-CN-85186
2. 1UO1CA77150-01
3. 1R01CA71921-01
4. 5 R01 CA098897 04
5. 5 R01 CA074880 08
6. NCI (SWOG)
7. 2PC50CA091846-06S2
8. NCI (SWOG0353)
9. CA38926
10. CA32102
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Kelley DR, Ratliff TL, Catalona WJ, Shapiro A, Lage JM, Bauer WC, et al. Intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer: effect of bacillus Calmette-Guerin viability on treatment results. J Urol. 1985;134(1):48–53. doi: 10.1016/s0022-5347(17)46976-x. [DOI] [PubMed] [Google Scholar]
- 2.Herr HW, Schwalb DM, Zhang ZF, Sogani PC, Fair WR, Whitmore WF, Jr, et al. Intravesical bacillus Calmette-Guerin therapy prevents tumor progression and death from superficial bladder cancer: ten-year follow-up of a prospective randomized trial. J Clin Oncol. 1995;13(6):1404–8. doi: 10.1200/JCO.1995.13.6.1404. [DOI] [PubMed] [Google Scholar]
- 3.Cookson MS, Sarosdy MF. Management of stage T1 superficial bladder cancer with intravesical bacillus Calmette-Guerin therapy. J Urol. 1992;148(3):797–801. doi: 10.1016/s0022-5347(17)36724-1. [DOI] [PubMed] [Google Scholar]
- 4.Lamm DL, Blumenstein BA, Crissman JD, Montie JE, Gottesman JE, Lowe BA, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163(4):1124–9. [PubMed] [Google Scholar]
- 5.Esuvaranathan K, Kamaraj R, Mohan RS, Cheng C, Tan PK, Tay KP, et al. A phase IIB trial of BCG combined with interferon alpha for bladder cancer. J Urol. 2000;163(4 Supplement) Abstract # 675. [Google Scholar]
- 6.Joudi FN, Smith BJ, O’Donnell MA. Final results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alpha-2B for reducing recurrence of superficial bladder cancer. Urol Oncol. 2006;24(4):344–8. doi: 10.1016/j.urolonc.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Griffiths TR, Charlton M, Neal DE, Powell PH. Treatment of carcinoma in situ with intravesical bacillus Calmette-Guerin without maintenance. J Urol. 2002;167(6):2408–12. [PubMed] [Google Scholar]
- 8.Kurth KH, Denis L, Bouffioux C, Sylvester R, Debruyne FM, Pavone-Macaluso M, et al. Factors affecting recurrence and progression in superficial bladder tumours. Eur J Cancer. 1995;31A(11):1840–6. doi: 10.1016/0959-8049(95)00287-s. [DOI] [PubMed] [Google Scholar]
- 9.Dinney CP, Babkowski RC, Antelo M, Perrotte P, Liebert M, Zhang HZ, et al. Relationship among cystectomy, microvessel density and prognosis in stage T1 transitional cell carcinoma of the bladder. J Urol. 1998;160(4):1285–90. [PubMed] [Google Scholar]
- 10.Gupta A, Lotan Y, Nielsen M, Bastian P, Palapattu G, Rogers C, et al. Outcomes of patients with clinical T1 grade 3 bladder urothelial carcinoma treated with radical cystectomy. J Urol. 2007;177 doi: 10.1016/j.urology.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 11.Crowley J, LeBlanc M, Jacobson J, Salmon S. Exploratory Methods Survival Analysis in First Seattle Symposium. Springer-Verlag; 1997. [Google Scholar]
- 12.Solsona E, Iborra I, Dumont R, Rubio-Briones J, Casanova J, Almenar S. The 3-month clinical response to intravesical therapy as a predictive factor for progression in patients with high risk superficial bladder cancer. J Urol. 2000;164(3 Pt 1):685–9. doi: 10.1097/00005392-200009010-00016. [DOI] [PubMed] [Google Scholar]
- 13.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19(3):666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 14.Dutta SC, Smith JA, Jr, Shappell SB, Coffey CS, Chang SS, Cookson MS. Clinical under staging of high risk nonmuscle invasive urothelial carcinoma treated with radical cystectomy. J Urol. 2001;166(2):490–3. [PubMed] [Google Scholar]
- 15.Herr HW, Donat SM, Dalbagni G. Can restaging transurethral resection of T1 bladder cancer select patients for immediate cystectomy? J Urol. 2007;177(1):75–9. doi: 10.1016/j.juro.2006.08.070. discussion 79. [DOI] [PubMed] [Google Scholar]
- 16.Jurincic-Winkler CD, Metz KA, Beuth J, Klippel KF. Keyhole limpet hemocyanin for carcinoma in situ of the bladder: a long-term follow-up study. Eur Urol. 2000;37(Suppl 3):45–9. doi: 10.1159/000052392. [DOI] [PubMed] [Google Scholar]
- 17.O’Donnell MA, Krohn J, De Wolf W. Salvage intravesical therapy with interferon-alpha2b plus low dose bacillus calmette-guerin is effective in patients with superficial bladder cancer in whom bacillus calmette-guerin alone previously failed. J Urol. 2001;166(4):1300–5. [PubMed] [Google Scholar]
- 18.Dalbagni G, Russo P, Sheinfeld J, Mazumdar M, Tong W, Rabbani F, et al. Phase I trial of intravesical gemcitabine in bacillus Calmette-Guerin-refractory transitional-cell carcinoma of the bladder. J Clin Oncol. 2002;20(15):3193–8. doi: 10.1200/JCO.2002.02.066. [DOI] [PubMed] [Google Scholar]
- 19.Steinberg G, Bahnson R, Brosman S, Middleton R, Wajsman Z, Wehle M. Efficacy and safety of valrubicin for the treatment of Bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. J Urol. 2000;163(3):761–7. [PubMed] [Google Scholar]
- 20.Takashi M, Wakai K, Hattori T, Furuhashi K, Ono Y, Ohshima S, et al. Multivariate evaluation of factors affecting recurrence, progression, and survival in patients with superficial bladder cancer treated with intravesical bacillus Calmette-Guerin (Tokyo 172 strain) therapy: significance of concomitant carcinoma in situ. Int Urol Nephrol. 2002;33(1):41–7. doi: 10.1023/a:1014444601158. [DOI] [PubMed] [Google Scholar]