Abstract
Human T-lymphotropic virus type 1 (HTLV-1) infections are associated with varying degrees of HTLV-1 viral load and spasticity. Increased viral load is associated with higher risk of developing HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP). The authors performed a cross-sectional study of 24 people with HAM/TSP in Lima, Perú, to determine if higher HTLV-1 viral load was correlated with increased muscle tone, measured with a device providing quantitative spasticity assessment (QSA). Median HTLV-1 viral load was 17.0 copies/100 peripheral blood mononuclear cells and QSA value was 39.9 Newton-meters/radian. HTLV-1 viral load was significantly correlated with QSA value (Spearman rho = .48, P = .02), suggesting viral load may play a role in expression of symptomatic neurologic disease. Longitudinal studies are needed to determine if treatments that reduce viral load will reduce muscle tone.
Keywords: human T-cell lymphotropic virus type 1 (HTLV-1), myelopathy, tropical spastic paraparesis
Background
Of the estimated 10 to 20 million people worldwide infected with human T-cell lymphotropic virus type 1 (HTLV-1), approximately 3000 are recognized as having HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP) (de The and Bomford, 1993; Leon et al, 1997; Osame, 1999). The lifetime incidence of HAM/TSP in HTLV-1–infected people is between 0.25% and 2.4% (Kaplan et al, 1990; Maloney et al, 1998; Murphy et al, 1997). Symptoms and signs of HAM/TSP usually develop during the fifth decade of life and typically include leg weakness, back pain, and bladder dysfunction. Affected individuals eventually develop spasticity in the lower limbs (Roman and Roman, 1988; Vernant et al, 1987). Compared to HTLV-1–infected people without overt neurologic disease, people with HAM/TSP have higher HTLV-1 viral load in cerebrospinal fluid (CSF) and peripheral blood mononuclear cells (PBMCs) (Lezin et al, 2005; Nagai et al, 1998). In addition, higher HTLV-1 viral load is associated with more rapid progression of motor disability (Matsuzaki et al, 2001).
Spasticity is a state of increased muscle tone producing a velocity-dependent resistance to muscle stretch. A device developed at the University of Washington measures displacement and torque at the ankle and provides a quantitative spasticity assessment (QSA) of muscle tone (Lehmann et al, 1989). In studies of muscle tone, mean QSA value was 24 Newton-meters/radian (N-m/r) in adults without neurologic disease and 98 N-m/r in adults with spasticity due to noninfectious neurologic conditions (Lehmann et al, 1989; Price et al, 1991). In a previous study of women with asymptomatic HTLV-1 infection, muscle tone was significantly higher in these women than in a control group of women without HTLV-1 infection (mean value 27.1 versus 21.6 N-m/r) (Zunt et al, 1999). Muscle tone in people with HAM/TSP (mean value of 62.7 N-m/r) was more than double that measured in women with asymptomatic infection (Zunt et al, 1999, 2005).
In view of the high prevalence of spasticity in people with HAM/TSP, the association of higher viral load with neurologic dysfunction, and the potential benefit of quantitative measurement of spasticity for better understanding the pathogenesis of HAM/TSP and monitoring therapeutic response, we examined the relationship between quantitative HTLV-1 viral load, QSA, and neurologic dysfunction in people with HAM/TSP in Lima, Perú.
Results
Of the 53 patients with myelopathy referred to the study, 43 (81%) were positive for HTLV-1 infection; 2 (4%) were indeterminate; and 8 (15%) were negative. Of the 43 positive subjects, 24 underwent evaluation with the QSA and were included in the analysis. HTLV-1 viral load ranged from 0.1 to 63.8 copies/100 peripheral blood mononuclear cells (PBMCs), and QSA value ranged from 14.09 to 234.3 N-m/r (Table 1). Eleven subjects had QSA performed on both ankles, and values were highly correlated within individuals (ICC = 0.89, P < .001). Demographics, neurologic symptoms, and neurologic signs are detailed in Tables 1 and 2 along with their association with viral load and QSA value.
Table 1.
Neurologic symptoms with quantitative HTLV-1 viral load (PCR) and quantitative spasticity assessment (QSA)
| Viral load (copies/100 PBMCs) |
QSA (N-m/r) |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Patients with HAM/TSP | Mean (SD) | Median (IQR) | P value* | Mean (SD) | Median (IQR) | P value* |
| All subjects | 24 | 21.8 (15.8) | 17.0 (23.0) | 68.1 (63.2) | 39.9 (81.8) | ||
| Gender | |||||||
| Female | 18 | 24.0 (16.5) | 18.4 (23.7) | 53.0 (46.5) | 33.5 (40.1) | ||
| Male | 6 | 15.1 (12.4) | 12.0 (19.6) | .21 | 113.1 (88.4) | 101.8 (175.2) | .15 |
| Symptoms | |||||||
| Leg pain | |||||||
| Present | 14 | 22.0 (13.6) | 20.0 (23.0) | 68.3 (68.4) | 38.7 (76.2) | ||
| Absent | 9 | 18.9 (18.4) | 14.4 (14.3) | .39 | 59.60 (56.21) | 35.2 (68.2) | .95 |
| Back pain | |||||||
| Present | 16 | 23.2 (17.4) | 17.0 (25.2) | 62.2 (58.7) | 45.2 (68.9) | ||
| Absent | 8 | 18.9 (12.6) | 17.9 (19.5) | .72 | 79.7 (74.3) | 31.0 (141.1) | .86 |
| Arm pain | |||||||
| Present | 4 | 26.3 (16.4) | 24.1 (30.2) | 118.8 (89.6) | 105.4 (170.9) | ||
| Absent | 19 | 21.6 (16.0) | 17.4 (19.8) | .58 | 60.2 (54.5) | 35.2 (72.7) | .15 |
| Joint pain | |||||||
| Present | 14 | 26.2 (16.4) | 21.1 (23.5) | 57.6 (57.8) | 39.9 (40.1) | ||
| Absent | 10 | 15.6 (13.2) | 12.5 (17.6) | .07 | 82.7 (70.6) | 50.3 (126.5) | .78 |
| Arm sensation | |||||||
| Abnormal | 13 | 28.5 (17.8) | 29.4 (29.8) | 73.8 (72.5) | 44.7 (102.5) | ||
| Normal | 11 | 13.8 (8.0) | 14.4 (11.0) | .06 | 61.2 (52.9) | 35.2 (69.4) | .89 |
| Leg sensation | |||||||
| Abnormal | 17 | 26.0 (16.4) | 22.8 (25.2) | 73.0 (64.6) | 45.8 (94.6) | ||
| Normal | 7 | 11.4 (7.8) | 12.7 (13.9) | .05 | 56.1 (63.0) | 30.3 (55.3) | .42 |
| Bladder dysfunction | |||||||
| Present | 21 | 24.4 (15.1) | 19.3 (22.4) | 74.9 (64.8) | 45.8 (93.0) | ||
| Absent | 3 | 3.5 (3.5) | 3.5 (—) | .003 | 19.6 (9.3) | 14.5 (—) | .02 |
| Bowel dysfunction | |||||||
| Present | 18 | 25.1 (15.7) | 21.0 (23.6) | 79.6 (66.6) | 47.7 (104.0) | ||
| Absent | 6 | 11.8 (12.1) | 9.2 (16.7) | .03 | 33.4 (37.3) | 16.6 (35.5) | .01 |
| Signs | |||||||
| Mental status | |||||||
| Abnormal | 9 | 29.3 (14.8) | 34.2 (28.9) | 92.0 (77.2) | 49.6 (132.2) | ||
| Normal | 15 | 17.3 (15.0) | 14.4 (14.3) | .04 | 53.6 (50.7) | 31.9 (52.0) | .40 |
| Cranial Nerves | |||||||
| Abnormal | 1 | 8.3 | — | 133.3 | — | ||
| Normal | 22 | 22.4 (16.3) | 18.4 (25.0) | .53 | 71.3 (65.2) | 45.2 (92.4) | .53 |
| Strength | |||||||
| Abnormal | 17 | 24.6 (16.3) | 19.3 (23.1) | 74.8 (64.1) | 49.6 (93.0) | ||
| Normal | 6 | 15.7 (13.8) | 14.9 (23.9) | .30 | 52.7 (68.6) | 26.5 (54.6) | .27 |
| Reflexes | |||||||
| Abnormal | 20 | 23.3 (15.9) | 17.0 (23.7) | 73.1 (66.9) | 45.2 (103.7) | ||
| Normal | 4 | 14.0 (14.4) | 13.2 (26.8) | .33 | 42.9 (35.8) | 31.0 (60.9) | .41 |
| Coordination | |||||||
| Abnormal | 2 | 25.3 (18.5) | 25.3 (—) | 25.8 (6.0) | 25.8 (—) | ||
| Normal | 21 | 21.7 (16.3) | 17.4 (22.4) | .67 | 73.6 (65.8) | 45.8 (96.7) | .24 |
| Gait | |||||||
| Abnormal | 21 | 23.6 (15.6) | 17.4 (23.1) | 71.1 (65.9) | 44.7 (96.7) | ||
| Normal | 3 | 8.8 (8.8) | 3.5 (—) | .11 | 46.7 (42.8) | 30.3 (—) | .47 |
Note. HAM/TSP = HTLV-1–associated myelopathy/tropical spastic paraparesis; PBMCs = peripheral blood mononuclear cells; N-m/r = Newton-meters/radian; SD = standard deviation; IQR = interquartile range.
P values based on Mann-Whitney U test.
Table 2.
Association between clinical characteristics with HTLV-1 viral load and quantitative spasticity assessment (QSA)
| HTLV-1 viral load |
QSA |
|||||
|---|---|---|---|---|---|---|
| Measure | Mean (SD) | Median (IQR) | ICC | P value | ICC | P value |
| Age | 43.5 (14.8) | 37.0 (23) | 0.53 | .008 | 0.48 | .03 |
| Tally of neurologic symptoms and signs | 4.3 (1.6) | 5 (2) | 0.56 | .006 | 0.18 | .39 |
| Ashworth (ankle) | 2.2 (1.2) | 2.0 (1.75) | 0.28 | .19 | 0.66 | .0001 |
| Tone at hip | — | — | 0.35 | .09 | 0.48 | .02 |
| Tone at knee | — | — | 0.31 | .13 | 0.52 | .01 |
Note. SD = standard deviation; IQR = interquartile range; ICC = interclass correlation.
P values based on Spearman rho and Mann-Whitney U test.
Higher HTLV-1 viral load was correlated with abnormal leg sensation, abnormal mental status, bowel dysfunction, and bladder dysfunction and higher QSA value was associated with bowel and bladder dysfunction (Table 1). Higher tally of neurologic signs and symptoms was correlated with HTLV-1 viral load but not with QSA value (Table 2). QSA value was significantly correlated with clinical measures of muscle tone at the hip, knee, and ankle but viral load was not (Table 2). Quantitative HTLV-1 viral load was correlated with QSA value (ICC = 0.48, P = .02) (Figure 1).
Figure 1.
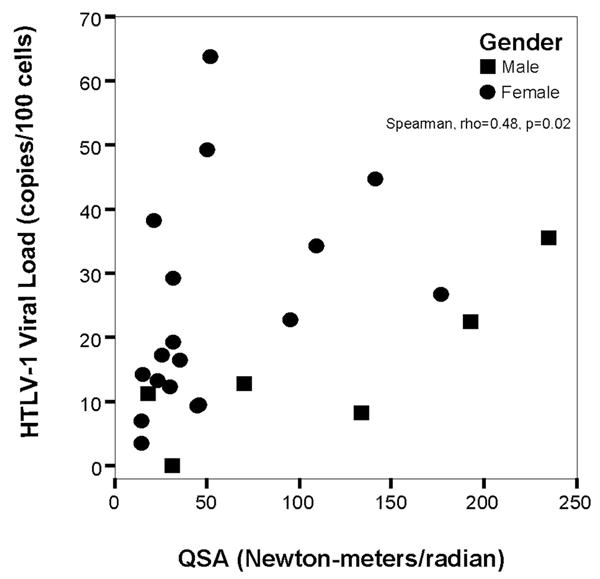
Scatterplot of quantitative Human T-lymphotropic virus type 1 (HTLV-1) PCR assay versus quantitative muscle tone, as measured by quantitative spasticity analysis (QSA).
Discussion
Spasticity eventually develops in all people with HAM/TSP. This study compared quantitative muscle tone assessment with quantitative HTLV-1 viral load and neurologic signs and symptoms in 24 patients with HAM/TSP. We know of no prior study comparing quantitative measurements of muscle tone with viral load in people with HAM/TSP. Our prior study of patients with HAM/TSP found mean QSA value was more than twice that measured in women with asymptomatic HTLV-1 infection (62.5 versus. 27.1 N-m/r), implying that QSA value can differentiate between people with and without HTLV-1–associated neurologic dysfunction. This study expands upon those findings by demonstrating QSA value is also positively correlated with quantitative viral load, suggesting higher viral load is associated with worse neurologic status. In addition, we were able to demonstrate that QSA value is highly reproducible within individuals and correlated well with the Ashworth scale measured at the ankle.
Although QSA was significantly correlated with clinical measures of muscle tone, it was not significantly correlated with other abnormal neurologic examination findings or with self-reported neurologic symptoms, with the exception of bowel and bladder dysfunction. HTLV-1 viral load was not significantly correlated with clinical measures of muscle tone but was correlated with tally of neurologic symptoms and signs. Thus, in people with HAM/TSP, QSA provides a quantitative and specific measure of muscle tone, whereas viral load reflects broader measures of neurologic dysfunction. By providing more sensitive quantitative measures for detecting and monitoring neurologic dysfunction, QSA and HTLV-1 viral load together could prove useful for studies of natural history and therapeutic efficacy. Long-term follow-up studies will determine if higher viral load and QSA values are associated with development or progression of HAM/TSP, and if fluctuations in viral load correlate with changes in QSA values and with other neurologic dysfunction.
The main limitation of this study is the lack of data regarding variations in viral load and QSA values over time in HTLV-1–infected people with and without neurologic symptoms. If muscle tone and viral load fluctuate markedly from day to day, then QSA and PCR assays would be of less value for studies of pathogenesis and treatment. In addition, we did not have information regarding duration of infection and were therefore unable to examine the relationship between length of infection with viral load or QSA values. Future studies will examine longitudinal changes in QSA and viral load in HTLV-1–infected people with and without neurologic disease.
Methods
The study protocol was approved by the University of Washington, Seattle, and Universidad Nacional Mayor de San Marcos Institutional Review Boards in compliance of all applicable Federal regulations governing the protection of human subjects.
Study design
Patients with clinical evidence of myelopathy were referred to neurologists at the Instituto de Ciencias Neurológicas (ICN) in Lima, Perú, for neurologic evaluation. Between March 1997 and November 1997, 53 patients with an onset in adulthood of progressive spastic paraparesis with pyramidal tract signs and bladder dysfunction not due to trauma, nutritional deficiency, or other defined etiology were referred to the study to screen for HTLV-1 infection and to undergo standardized neurologic evaluations. Sera were tested with enzyme-linked immunosorbent assay (ELISA) for HTLV-1 antibody (Cambridge Bioscience, Worcester, MA) and confirmed with Western blot assay (HTLV-1/2 Blot 2.4,- Genelabs Diagnostics, Singapore). ELISA-positive specimens were considered HTLV-1–seropositive if the Western blot revealed bands representing Gag (p24 and p19), gp46, and Env proteins (GD21 and rgp-46-I). If Gag and Env proteins were absent but other HTLV-specific bands present, the individual was considered indeterminate. Testing of CSF was not performed. Informed consent was obtained from all study subjects. The study protocol was approved by the human subjects committees of the University of Washington in Seattle, and the Universidad Nacional Mayor de San Marcos in Lima, Perú.
Neurologist’s assessment
Study neurologists (SM, JZ) administered a questionnaire and performed a neurologic examination without knowledge of QSA or viral load. Questions addressed pain or sensory dysfunction in the arms or legs, weakness of the limbs, stiffness, impotence, and incontinence of bladder or bowel. The neurologic examination entailed a detailed and standardized assessment of cranial nerve function, muscle strength, deep tendon reflexes, sensory function (light touch, pinprick, and vibration), coordination, and gait. Each section of the neurologic examination was coded as normal or abnormal. Number of neurologic symptoms and signs was tallied for each subject and ranged from 0 to 13. Tone in the lower limbs was assessed at the hip and knee using clinical tests described previously, and coded as either normal or abnormal (Zunt et al, 1999). In addition, the Ashworth scale was used to assess tone at the ankle and was coded between 0 and 4 (Haas et al, 1996).
Quantitative spasticity assessment
The QSA device developed at the University of Washington measures the variation in elastic and viscous stiffness of the gastroc-soleus-achilles tendon unit by oscillating the foot over precise sinusoidal displacements and measuring the resulting torque response of the tendon unit (Lehmann et al, 1989). Assessment involved placing the subject prone on an examining table with the foot attached to the machine by means of a footplate. The computer randomly varied the frequency of sinusoidal oscillation. Displacement and torque were measured simultaneously during the testing, and using Fourier analysis, the torsional stiffness was measured in Newton-meters/radian. This single summary variable was derived for the left ankle, unless there was a previous injury or surgery to that ankle, in which case the right ankle was used. We performed QSA in a subset of subjects to examine the agreement between the measures in both legs of an individual. In previous studies, this device has shown significant and reproducible differences between people with and without spasticity resulting from cerebral palsy, as well as a high degree of test-retest reliability in healthy subjects (correlation coefficient of .907) (Lehmann et al, 1989; Price et al, 1991).
HTLV-1 real-time PCR
Genomic DNA was extracted from isolated PBMCs using the Puregene DNA Isolation Kit (Gentra, Minneapolis, MN). HTLV-1 provirus load was measured by a real-time polymerase chain reaction (PCR) assay (Nagai et al, 2001). The primer set for the HTLV-1 (pX) region was 5′-ACACGTAGACTGGGTAT CCGAA-3′ and 5′-ACAAAGTTAACCATGCTTATTAT CAGC-3′. The Taqman fluorescent probe was 5′-FAM-TTCCCAGGGTT-TGGACAGAGTCTTCT-TAMRA-3′. One hundred nanogram of sample DNA was added to a PCR mixture containing 1 × Taqman PCR master mix (Applied Biosystems, Foster city, CA), 200 nM of each primer, and 100 nM probe. Thermal cycler conditions were 2 min at 50°C, 10 min at 95°C, and 45 cycles of 15 s at 95°C, followed by 1 min at 60°C. Dilutions of HTLV-1 DNA obtained from TARL-2, an HTLV-1–infected rat lymphoid cell line, were used to prepare a standard curve. To control for variations in the input DNA, each sample was also analyzed by a β-globin real-time PCR assay using primers 5′-TGAAGGCTCATGGCAAGAAA-3′ and 5′-GCTCACTCAGTGTGGCAAAGG-3′ and the fluorescent probe 5′-FAM-TCCAGGTGAGCCAGGCCATCACTATAMRA-3′. The reaction and thermal conditions were identical to the HTLV-1 assay. DNA extracted from the cell line A3.01, an uninfected human T-cell line obtained from the NIH AIDS Research and Reference Reagent Program, was used to prepare a β-globin standard curve. All standards and samples were assayed in triplicate in the iCycler IQ Real-Time Detection System (Biorad, Hercules, CA). The HTLV-1 proviral DNA load was calculated by the following formula: copy number of HTLV-1 (pX) per 100 cells = (copy number of pX)/(copy number of β-globin/2) × 100.
Statistical analysis
Associations were assessed using non-parametric tests: Spearman-rank test and Mann-Whitney U, providing interclass correlation (ICC). All P values were two-sided, with significance set at P < .05. Statistical analyses were performed with STATA v.8.0 (Stata Corporation, College Station, TX) and SPSS v.11 (Advanced Statistics, Chicago, IL).
Acknowledgments
This work was supported by NIH grants K23-AI01600, TW00679; AI0714P; Fogarty International grant T22-TW00001; and University of Washington Center for AIDS Research (CFAR) grant AI27757.
Footnotes
This work was presented in part at the 12th International Conference on Human Retrovirology, Montego Bay, Jamaica, June 2005; abstract P18.
Disclaimer. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, the U.S. Government, University of Washington nor Universidad Nacional Mayor de San Marcos.
References
- de Thé G, Bomford R. An HTLV-1 vaccine: why, how, for whom? AIDS Res Hum Retroviruses. 1993;9:381–386. doi: 10.1089/aid.1993.9.381. [DOI] [PubMed] [Google Scholar]
- Haas BM, Bergstrom E, Jamous A, Bennie A. The inter rater reliability of the original and of the modified Ashworth scale for the assessment of spasticity in patients with spinal cord injury. Spinal Cord. 1996;34:560–564. doi: 10.1038/sc.1996.100. [DOI] [PubMed] [Google Scholar]
- Kaplan JE, Osame M, Kubota H, Igata A, Nishitani H, Maeda Y, Khabbaz RF, Janssen RS. The risk of development of HTLV-I-associated myelopathy/tropical spastic paraparesis among persons infected with HTLV-1. J Acquir Immune Defic Syndr. 1990;3:1096–1101. [PubMed] [Google Scholar]
- Lehmann JF, Price R, deLateur BJ, Hinderer S, Traynor C. Spasticity: quantitative measurements as a basis for assessing effectiveness of therapeutic intervention. Arch Phys Med Rehabil. 1989;70:6–15. [PubMed] [Google Scholar]
- Leon SF, Costa CM, Gaffga N. Discrepancy, coincidence or evidence in chronic idiopathic spastic paraparesis throughout the world. A meta-analysis on 2811 patients. Arq Neuropsiquiatr. 1997;55:530–535. doi: 10.1590/s0004-282x1997000400002. [DOI] [PubMed] [Google Scholar]
- Lezin A, Olindo S, Oliere S, Varrin-Doyer M, Marlin R, Cabre P, Smadja D, Cesaire R. Human T lymphotropic virus type 1 (HTLV-1) proviral load in cerebrospinal fluid: a new criterion for the diagnosis of HTLV-1-associated myelopathy/tropical spastic paraparesis? J Infect Dis. 2005;191:1830–1834. doi: 10.1086/429962. [DOI] [PubMed] [Google Scholar]
- Maloney EM, Cleghorn FR, Morgan OS, Rodgers-Johnson P, Cranston B, Jack N, Blattner WA, Bartholomew C, Manns A. Incidence of HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) in Jamaica and Trinidad. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:167–170. doi: 10.1097/00042560-199802010-00011. [DOI] [PubMed] [Google Scholar]
- Matsuzaki T, Nakagawa M, Nagai M, Usuku K, Higuchi I, Arimura K, Kubota H, Izumo S, Akiba S, Osame M. HTLV-1 proviral load correlates with progression of motor disability in HAM/TSP: analysis of 239 HAM/TSP patients including 64 patients followed up for 10 years. J NeuroVirol. 2001;7:228–234. doi: 10.1080/13550280152403272. [DOI] [PubMed] [Google Scholar]
- Murphy EL, Fridey J, Smith JW, Engstrom J, Sacher RA, Miller K, Gibble J, Stevens J, Thomson R, Hansma D, Kaplan J, Khabbaz R, Nemo G. HTLV-associated myelopathy in a cohort of HTLV-1 and HTLV-2-infected blood donors. The REDS investigators. Neurology. 1997;48:315–320. doi: 10.1212/wnl.48.2.315. [DOI] [PubMed] [Google Scholar]
- Nagai M, Kubota R, Greten TF, Schneck JP, Leist TP, Jacobson S. Increased activated human T cell lymphotropic virus type 1 (HTLV-1) Tax11-19-specific memory and effector CD8+ cells in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis: correlation with HTLV-1 provirus load. J Infect Dis. 2001;183:197–205. doi: 10.1086/317932. [DOI] [PubMed] [Google Scholar]
- Nagai M, Usuku K, Matsumoto W, Kodama D, Takenouchi N, Moritoyo T, Hashiguchi S, Ichinose M, Bangham CR, Izumo S, Osame M. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-1 carriers: high proviral load strongly predisposes to HAM/TSP. J NeuroVirol. 1998;4:586–593. doi: 10.3109/13550289809114225. [DOI] [PubMed] [Google Scholar]
- Osame M. The recent advances of HAM/TSP research. Rinsho Shinkeigaku. 1999;39:1200–1202. [PubMed] [Google Scholar]
- Price R, Bjornson KF, Lehmann JF, McLaughlin JF, Hays RM. Quantitative measurement of spasticity in children with cerebral palsy. Dev Med Child Neurol. 1991;33:585–595. doi: 10.1111/j.1469-8749.1991.tb14928.x. [DOI] [PubMed] [Google Scholar]
- Roman GC, Roman LN. Tropical spastic paraparesis. A clinical study of 50 patients from Tumaco (Colombia) and review of the worldwide features of the syndrome. J Neurol Sci. 1988;87:121–138. doi: 10.1016/0022-510x(88)90059-7. [DOI] [PubMed] [Google Scholar]
- Vernant JC, Maurs L, Gessain A, Barin F, Gout O, Delaporte JM, Sanhadji K, Buisson G, de Thé G. Endemic tropical spastic paraparesis associated with human T-lymphotropic virus type 1: a clinical and seroepidemiological study of 25 cases. Ann Neurol. 1987;21:123–130. doi: 10.1002/ana.410210204. [DOI] [PubMed] [Google Scholar]
- Zunt JR, Alarcon JO, Montano S, Longstreth WT, Jr, Price R, Holmes KK. Quantitative assessment of subclinical spasticity in human T-cell lymphotropic virus type 1 infection. Neurology. 1999;53:386–390. doi: 10.1212/wnl.53.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunt JR, Montano SM, Alarcon JO, Longstreth WT, Jr, Price R, Holmes KK. Quantitative assessment of spasticity in human T-cell lymphotropic virus type 1-associated myelopathy/tropical spastic paraparesis. J NeuroVirol. 2005;11:70–73. doi: 10.1080/13550280590900571. [DOI] [PubMed] [Google Scholar]


