Abstract
Despite the role of vitamin D3 endocrine system in prevention of mammary gland transformation in animal models, use of 1,25(OH)2D3 in clinical settings is precluded due to its toxicity in vivo. Therefore much effort has been placed in developing relatively non-toxic vitamin D analogs. Recently, with the discovery of the expression of 25-hydroxy vitamin D3 1α-hydroxylase (CYP27B1) in multiple extrarenal organs, the functional role of prohormone, 25-hydroxyvitamin D3 [25(OH)D3], has been redefined. Since 25(OH)D3 does not cause hypercalcemia and maintains relative high concentration in serum, it is possible that the prohormone can be converted to active hormone in mammary epithelial cells to provide chemopreventive effects. In the present study, we evaluated its functional significance using mouse mammary organ culture (MMOC) system. We first showed that 25(OH)D3 1α-hydroxylase is extensively expressed in mammary ductal epithelial cells at both protein and mRNA levels, which is a prerequisite for 25(OH)D3 to function in an autocrine/paracrine manner. However, we also observed that clotrimazol (1α-hydroxylase inhibitor) enhanced 25(OH)D3 -induced CYP24 expression in breast cancer cells. In mammary glands derived from 1α-hydroxylase knockout mice, 25(OH)D3 treatment in organ culture significantly induced CYP24 expression, indicating a potential direct effect of 25(OH)D3. In MMOC, 100–250 nM 25(OH)D3 suppressed both ovarian hormone-dependent and -independent mammary precancerous lesions (induced by DMBA) by more than 50%, while the active hormone 1,25(OH)2D3 (positive control) at 100 nM suppressed alveolar lesions by more than 80%. The inactive vitamin D3 (negative control) at 100 nM suppressed alveolar lesions by only 20% (P > 0.05). We found that 25(OH)D3 inhibits DMBA-induced mammary alveolar lesions (MAL) in a stage-specific manner: 25(OH)D3 mainly inhibits the promotion stage of lesion formation. We conclude that 25(OH)D3 could serve as a non-toxic natural chemopreventive agent for further development for breast cancer prevention.
Keywords: 25(OH)D3, CYP27B1, Chemoprevention, Precancerous lesions, Mouse mammary gland organ culture (MMOC), CYP27B1KO mice, CYP24
Introduction
The role of vitamin D and its analogs in prevention of mammary gland transformation in animal model has been well established, but the majority of the effective vitamin D analogs are precluded in clinical settings due to its toxicity in vivo. Consequently, much attention was paid to developing safe and less calcemic analogs of 1,25(OH)2D3 that would have desirable growth modulatory effects. Outstanding examples of such promising analogs include EB-1089 [1–3], KH-1060 [3], MC-903 [1], Gemini compounds [4] and 1α(OH)D5 [5, 6]. New analogs are continually being synthesized and evaluated in experimental models. Although a few of these analogs have shown potential for use in chemoprevention or therapy of breast cancer, almost all of the synthetic analogs are more or less toxic at a pharmacological dose, limiting its application in clinical use.
Epidemiological studies have supported the negative association of breast, prostate, and colon cancer risk as well as mortality rates with annual sunlight exposure and serum vitamin D3 (25(OH)D3) levels in different geographical areas [7–11]. With the discovery that 25-hydroxy vitamin D3 1α-hydroxylase (CYP27B1) is expressed in multiple vitamin D target tissues including pancreas, colon, breast and prostate [12–15], it is possible that 25(OH)D3, the major vitamin D metabolite present at much higher concentration in serum in comparison to that of 1,25(OH)2D3 [16], may serve as a natural chemoprotective vitamin D in the body. 25(OH)D3 can belocally converted to the active 1,25(OH)2D3 in the cells by 1α-hydroxylase, allowing its function in cell growth modulation and differentiation. The initial step in the activation of vitamin D, whether derived from diet or synthesized in skin, is hydroxylation at the 25 position by the hepatic vitamin D 25-hydroxylase (CYP27A1, CYP2R1, CYP2A4 and CYP2J3) in order to generate 25-hydroxyvitamin D3 [25(OH)D3] [17]. 25(OH)D3 is the major form of vitamin D in circulation, further metabolism of 25(OH)D3 into the high affinity VDR ligand 1,25(OH)2D3 is regulated by the enzyme 25(OH)D3 1α-hydroxylase (CYP27B1), which is highly expressed in renal proximal tubules. CYP27B1 protein is the key enzyme in the two-step activation process of inactive 25(OH)D3 to active 1,25(OH)2D3. CYP27B1 was initially proved to be the 1α-hydroxylase found in proximal renal tubules that carries out the 1α-hydroxylation of 25(OH)D3 and is the enzyme that is defective in vitamin D dependent rickets (VDDR) type 1 [18]. Recently 1α-hydroxylase has been shown to be expressed in various extrarenal sites in the body including mammary gland [12–15]. However, very limited information on CYP27B1 expression and regulation is available for mammary gland. Zinser et al [12] reported that CYP27B1 mRNA was low but detectable in virgin mammary glands from wild-type mice and increased ~ 20-fold by day 9 of pregnancy. CYP27B1 protein was also detectable in MCF-7 and T47D breast cancer cells [12]. The CYP27B1 protein was expressed at comparable levels in mammary glands from WT and VDR knockout mice during pregnancy and lactation. Friedrich et al showed upregulated CYP27B1 mRNA in breast carcinomas as compared to normal tissue [14]. Since CYP27B1 is locally expressed in breast epithelial cells, 25(OH)D3, a natural non-toxic vitamin D metabolite which is present at high concentration in serum, could be developed as an ideal, natural cancer chemoprenventive agent. Here we show the functional significance of 25(OH)D3 at physiological concentration on the expression of 1α-hydroxylase and its chemopreventive effect using mouse mammary organ culture system.
Materials and methods
Vitamin D metabolites
Vitamin D3, 1α(OH)D3, 25(OH)D3 and 1,25(OH)2D3 were purchased from Sigma-Aldrich Corp. (St Louis, MO). The stock solutions of 10 mM 25(OH)D3, 1,25(OH)2D3, 1α(OH)D3 and vitamin D3 in ethanol were stored in −80°C freezer. The appropriate treatments for each experiment consisted of vitamin D treatment and the vehicle ethanol.
Mouse mammary organ culture (MMOC)
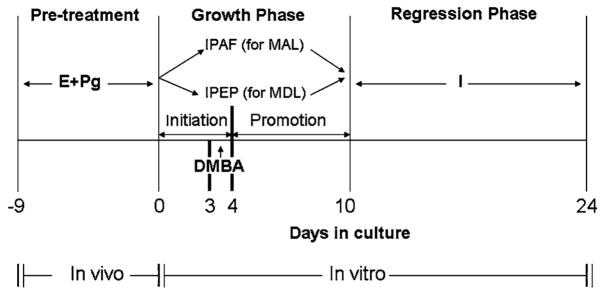
There are two protocols to induce precancerous mammary lesions in the mammary glands of immature mice. The glands can either develop mammary alveolar lesions (MAL) or mammary ductal lesions (MDL), depending upon the steroid hormone combination present in the medium. If the glands are incubated in the presence of 17β-estradiol and progesterone, they develop MDL [19], whereas if estrogen and progesterone are replaced with glucocorticoids, they develop MAL. The entire procedure was described in detail previously [20] and summarized in Fig. 1. Briefly, thoracic pairs of mammary glands from Balb/c mice pretreated with 1 μg estradiol and 1 mg progesterone for 9 days are removed under sterile conditions and floated on silk rafts in Waymouth’s serum-free media supplemented with IPAF (Insulin + prolactin + aldosterone + hydrocortisone) or IPEPg (Insulin + prolactin + estradiol + progesterone). On day 3, glands are treated with 2 lμ/ml DMBA for 24 h to induce precancerous lesions, and continued to culture in the same media for another 6 days. After 10 days’ culture, the glands are transferred to a medium containing insulin alone for additional 14 days. Vitamin D metabolites (100–500 nM 25(OH)D3, 100 nM 1,25(OH)2D3 are included in the culture for various time during the 10 day of growth phase (Fig. 1) depending on treatment protocol. For stage specific effect, vitamin D metabolites were included in culture from day 0–4 to observe the anti-initiation effect, and from day 4–10 to observe the anti-promotion effect. For morphological observation of MAL, the glands were fixed and stained with alum carmine for evaluation of incidence as described previously [19]. The glands for MDL are fixed in formalin for 24 h and processed for histopathological evaluation. The glands are divided into several microscopic fields and each field is analyzed for ductal sections; the ducts containing lesions are compared with the total number of ductal fields counted to determine the incidence of MDL.
Fig. 1.
Protocol for mammary gland organ culture. Thoracic pairs of the mammary glands were removed from female BALB/c mice pretreated for 9 days with estrogen (E) (1 μg/mouse) plus progesterone (Pg) (1 mg/mouse). Glands were incubated with IPAH (5 μg/ml insulin + 5 μg/ml prolactin + 1 μg/ml aldosterone + 1 μg/ml hydrocortisone) or IPEPg (I + P + E + Pg) for 10 days and with 7,12-dimethylbenz[α]-anthracene (DMBA) at a dose of 2 μg/ml for 24 h on the 3rd day. Vitamin D metabolites are included either from day 0 to day 10 or day 0 to day 4 (initiation phase) or day 4 to day 10 (promotion phase). Glands respond to the presence of hormones by developing MAL or MDA. After 10 days in growth promoting hormones, the glands are incubated for an additional 14 days in the medium containing only insulin, which allows the regression of normal structures, whereas glands incubated with DMBA do not fully regress, some area attain altered hormonal requirements and termed MAL or MDL
Mammary glands from CYP27B1KO mice [21] were cultured using standard MMOC protocol as described above with some modifications. The studies were designed to make paired comparisons. A thoracic pair of mammary glands from the mouse were isolated and cultured separately, one served as a control for vehicle treatment, the other one was treated with 25(OH)D3 for 24 h. Paired comparison was made between glands from the same mouse.
Cell culture
T47D was purchased from American Type Tissue Collection (Manassas, VA) and cultured in MEM containing 10% FBS and supplemented with Non-essential amino acids and antibiotics. When cells reached 70% confluence, serum concentration was decreased to 2% and cells were treated with vitamin D metabolites for 24 h and samples were subjected to qRT-PCR analysis as described previously [22].
Immunostaining for 25-hydroxy vitamin D3 1α-hydroxylase
Mammary glands cultured in Waymouth’s serum-free media supplemented with IPEPg and exposed to Vehicle (Control), DMBA, or DMBA + 25(OH)D3 were fixed in buffered formalin, and 5 μm-thick sections were prepared for immunostaining using standard protocol as described previously [5]. Sheep-anti-murine 25-hydroxyvitamin D3-1α-hydroxylase antibody was purchased from the Binding Sites (San Diego, CA). Donkey anti-sheep secondary antibody was purchased form Santa Cruz Biotechnology Inc (Santa Cruz, CA).
qRT-PCR analysis of CYP27B1 and CYP24 mRNA expression in cultured mouse mammary organs
Mouse mammary organs were prepared as described above. The thoracic (for Balb/c and CYP27B1KO C57 mice) and abdominal pairs (for Balb/c) of mammary glands were cultured in medium supplemented with IPAF for 48 h. On the third day the culture was treated with 0.001% Ethanol, 250 nM 25(OH)D3 or 10 nM 1,25(OH)2D3 for 24 h and mammary glands were collected and stored in trizol reagent (Invitrogen) at −80°C. Total RNA was extracted from each individual organ per manufacturer’s instruction. qRT-PCR was conducted as previously described [22]. Mouse hypoxanthine ribosyltransferase (mHPRT) was used as a house keeping gene for normalization [23], which is expressed at relatively stable level from organ to organ. Primers used for real time PCR are: mCYP24 (forward: 5′-CCAAGTGTGCCATTCACAAC-3′, reverse: 5′-GCCCAGCACTTGGGTATTTA-3′), mCYP27B1 (forward: 5′-GTTGCCGACCCTACACTTG-3′, reverse: 5′-CCTCTGCCATTCTTCACCAT-3′), mHPRT (forward: 5′-AGTCCCAGCGTCGTGATTAG-3′, reverse: 5′-TTTCCAAATCCTCGGCATAA-3′).
Statistical analysis
Statistical significance was determined by the χ2 analysis for the incidence of MAL and MDL in MMOC experiments. For qRT-PCR analysis of mammary glands obtained after MMOC, experiments were repeated at least twice, data from individual organs per treatment were analyzed for statistical significance using student’s t-test. P-value < 0.05 was taken as significant. For MMOC samples from CYP27B1KO mice, paired comparison was made using student’s t-test.
Results
Expression of 25-hydroxyvitamin D3 1α-hydroxylase in cultured mouse mammary organ
Since 25-hydroxy vitamin D3 1α-hydroxylase is the key enzyme for conversion of 25(OH)D3 to 1,25(OH)2D3 and therefore may play a key chemopreventive role in mammary carcinogenesis, we first examined its expression in mouse mammary organs cultured in media supplemented with IPEPg as stated in the Materials and Methods. As shown in Fig. 2, regardless of the treatments, mouse mammary ductal epithelial cells displayed extensive staining for 1α-hydroxylase. Both normal ducts (Fig. 2b) and precancerous lesions (Fig. 2c) induced by DMBA demonstrated intensive staining in the epithelial cells. The most intensive staining for 1α-hydroxylase was observed in ductal lesions treated with 25(OH)D3 (Fig. 2d). A control staining without primary antibody was included (Fig. 2a) to show the specificity of the staining.
Fig. 2.
Representative examples of expression of 25-hydroxyvitamin D3 1α-hydroxylase in cultured mouse mammary glands and effect of 25(OH)D3 on CYP27B1 mRNA expression at organ level. Mammary glands were cultured using the standard protocol for MDL, then fixed and processed for immunohistochemistry for 25-hydroxyvitamin D3 1α-hydroxylase. Increased expression 1α-hydroxylase was observed after the glands were exposed to DMBA in the presence of 25(OH)D3 (d). (a) Negative control, primary antibody was not included in the immunostaining process; (b) control treatments, mammary glands were not exposed to carcinogen and 25(OH)D3, (c) mammary glands were exposed to DMBA only on day 3 using the standard protocol for MDL; (d) mammary glands were exposed to DMBA on day 3 in the presence of 25(OH)D3 (day 0–10). (e) and (f), CYP24 and CYP27B1 mRNA expression in cultured mouse mammary glands in response to 25(OH)D3. Mammary glands were treated for 24 h with ethanol (control) or 250 nM 25(OH)D3 and total RNA was extracted from whole organs and subjected to qRT-PCR analysis. CYP24 (e) or CYP27B1 (f) mRNA levels in each gland were normalized to HPRT and the lowest basal level in control glands (the lowest basal level in control glands was set as 1). Data are expressed as mean ± SEM, (n = 20) *P < 0.05, ***P < 0.001 in comparison to control
In order to confirm the immunostaining data, we performed qRT-PCR to determine if CYP27B1 is regulated by 25(OH)D3 at organ level. CYP24, a direct target gene of vitamin D, was used as a control gene for response of mammary organs to vitamin D. As shown in Fig 2e, 24 h treatment with 250 nM 25(OH)D3 significantly increased CYP24 by 2100-fold (from arbitrary unit of 1.37 ± 0.2 (SEM) to 2922.2 ± 776.7) (P < 0.001, n = 20), confirming the response of mammary glands to 25(OH)D3; whereas at the same time, 25(OH)D3 increased CYP27B1 expression by 2.2. fold (from 1.1 ± 0.17 to 2.38 ± 0.53) (P < 0.05, n = 20) (Fig. 2f), These results suggest that CYP27B1 expression in mammary glands is upregulated by 25(OH)D3 in the experimental setting and confirm the results obtained by immunohistochemical analyses.
Effect of 25(OH)D3 on CYP24 expression in cultured mammary glands isolated from CYP27B1KO mice and in breast cancer cells in presence of 1α-hydroxylase inhibitor, clotrimazol
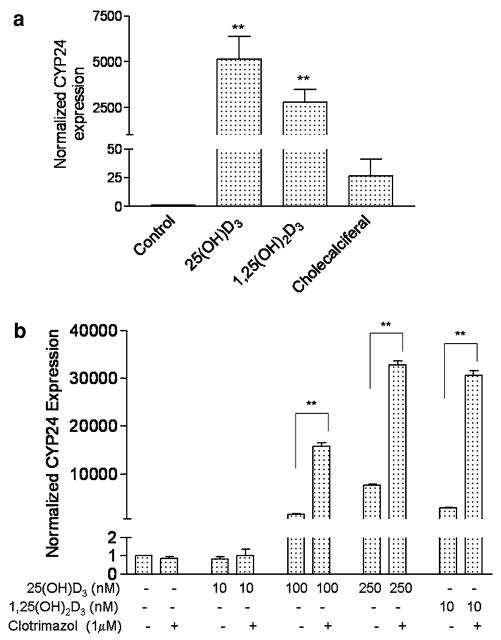
cAlthough 1α-hydroxylase is extensively expressed in ductal epithelial cells, considering the high level of 25(OH)D3 in serum, we asked whether 25(OH)D3, without being converted to 1,25(OH)2D3, has direct interaction with VDR. We conducted MMOC using mammary glands isolated from CYP27B1KO mice. The experiments were conducted to provide results on paired comparisions in order to decrease the variance in the results. As shown in Fig. 3a, 250 nM 25(OH)D3 treatment of CYP27B1KO glands for 24 h increased CYP24 mRNA expression by 5145 ± 1247 (SEM) fold (P < 0.01, n = 9) as evaluated by qRT-PCR; whereas 1,25(OH)2D3 (10 nM) increased CYP24 mRNA expression by 2783 ± 727 (SEM) fold (P < 0.01, n = 9) in the same experimental settings. The inactive vitamin D3 (cholecalciferal) at 250 nM only had marginal effect in induction of CYP24 expression in this model, it increased CYP24 expression by 27 ± 15 fold (P = 0.12, n = 9), which was statistically insignificant. In this experiment, 1,25(OH)2D3 and cholecalciferal served as a positive and a negative control respectively. These results suggest that 25(OH)D3 directly activates VDR without being converted to 1,25(OH)2D3.
Fig. 3.
Direct effect of 25(OH)D3 on the induction of CYP24. (a) CYP24 induction by 25(OH)D3 in MMOC using mammary glands from CYP27B1KO mice. Mammary glands excised from the mice were subjected to various treatments in MMOC as described in Materials and methods. The treatments included ethanol (control), 250 nM 25(OH)D3, 10 nM 1,25(OH)2D3 (positive control) and 250 nM cholecalciferal (negative control) for 24 h. Total RNA was extracted from each individual gland and subjected to qRT-PCR analysis. CYP24 mRNA levels in each gland were normalized to HPRT and the corresponding control gland (the basal level in control gland was set as 1). Data are expressed as mean ± SEM, (n = 9), **P < 0.01 in comparison to control. (b) Effect of 25-hydroxyvitamin D3 1α-hydroxylase inhibitor on CYP24 induction by 25(OH)D3 in breast cancer cells. T47D cells were pre-incubated with clotrimazol (1 μM) for 4 h, then treated with different concentration (10, 100, 250 nM) of 25(OH)D3 or 10 nM 1,25(OH)2D3 in the presence of clotrimazol for 24 h. CYP24 mRNA expression was evaluated using qRT-PCR. CYP24 mRNA level from each sample was normalized to β-actin and basal level of control sample (the basal level of control sample is set as 1). Data are expressed as mean ± SEM from triplicate samples with each sample being subjected to duplicate analyses. Statistical significance was evaluated by Student’s t-test (**P < 0.01)
In order to further confirm the direct interaction between 25(OH)D3 and VDR, we treated T47D breast cancer cells with clotrimazol, a specific 1α-hydroxylase inhibitor to inhibit 1α-hydroxylase activity [24]. Results obtained from these studies would provide evidence whether 25(OH)D3 without conversion to 1,25(OH)2D3 is active in inducing CYP24 expression. Results showed that 25(OH)D3 induced CYP24 mRNA expression in a dose-dependent manner (Fig. 3b); surprisingly, the presence of 1 μM clotrimazol consistently and significantly enhanced the 25(OH)D3 induced CYP24 expression by 10.2-fold (at 100 nM of 25(OH)D3) (from arbitrary unit of 1552 ± 120 (SEM) to 15826 ± 723) and 4.3-fold (at 250 nM of 25(OH)D3) (from 7643 ± 252 to 32768 ± 895) respectively (P < 0.01, n = 3) (Fig. 3b). Clotrimazol also enhanced 1,25(OH)2D3 (10 nM) induced CYP24 expression by 10.6- fold (from 2896 ± 150 to 30574 ± 982) (P < 0.01). Consistent results were also obtained when clotrimazol concentration was increased to 5 μM and in the presence of another 1α-hydroxylase inhibitor, ketoconazol in T47D and MCF-7 cells (data not shown). These results demonstrate that 25(OH)D3 directly binds to VDR and induce CYP24 expression, although 1,25(OH)2D3 is more active than 25(OH)D3. These results do not support the possibility that clotrimazol may be stimulatory for CYP24 expression since treatment with clotrimazol in the absence of vitamin D metabolites did not enhance CYP24 expression.
25(OH)D3 inhibits DMBA induced precancerous lesion formation
We first conducted experiments to evaluate the efficacy of 25(OH)D3 in comparison to 1,25(OH)2D3 using MMOC. Table 1 shows the efficacy of 25(OH)D3 for MAL induced by DMBA. MAL were observed in 18 glands out of 24 (75% incidence) after DMBA treatment. Incubation of glands in the presence of 100 nM and 500 nM 25(OH)D3 resulted in 61.2% (P < 0.01) and 57.3% (P < 0.01) suppression respectively, while 1,25(OH)2D3 at 100 nM resulted in 82% (P < 0.001) suppression. Representative photograph showing MAL in DMBA treated glands, control glands not treated with DMBA (no lesions) and vitamin D metabolites- treated glands (with fewer lesions) are shown in Fig. 4a–d. Incubation of glands in the presence of 100 nM inactive vitamin D3 (Cholecalciferol, negative control) only decreased the incidence by 20%, which is not statistically significant (P < 0.05). We also evaluated the efficacy of 1α (OH)D3, which is physiologically not available but served as another control for comparison to test the effect of 1α-hydroxylation. 1α (OH)D3 at the dose of 100 nM resulted in 73% suppression (P < 0.01). Although efficacy of 25(OH)D3 is lower than that of 1,25(OH)D3, the concentration of 25(OH)D3 used is physiological and therefore completely non-toxic, whereas the concentration of 1,25(OH)2D3 used in this study is pharmacological and not achievable in serum. Interestingly, increasing concentration of 25(OH)D3 to 500 nM did not show increase of suppression efficacy, indicating that physiological concentration of 25(OH)D3 is sufficient to play its chemopreventive role in carcinogenesis.
Table 1.
Chemopreventive efficacy of 25(OH)D3 for mammary alveolar lesions (MAL) induced by DMBA in mouse mammary organ culture
| Treatment | Number of glands with lesions (%incidence) | %inhibition | Significance (P valuea) |
|---|---|---|---|
| IPAF control | 0/10 | – | – |
| DMBA | 18/24 (75) | – | – |
| DMBA + 1,25(OH)2D3 —100 nM | 2/15 (13.3) | 82 | 0.0006 |
| DMBA + 25(OH)D3––100 nM | 7/24 (29.1) | 61.2 | 0.0015 |
| DMBA + 25(OH)D3––500 nM | 8/25 (32) | 57.3 | 0.0026 |
| DMBA + vitamin D3––100 nM | 12/20 (60) | 20 | 0.2875 (NS) |
| DMBA + 1α(OH)D3––100 nM | 2/10 (20) | 73 | 0.0030 |
χ2 test, in comparison with DMBA control
IPAF, Waymouth’s medium supplemented with insulin + prolactin + aldosterone + hydrocortisone Vitamin D3 = Cholecalciferol
Fig. 4.
Representative examples of MAL (left panel) and MDL (right panel) in response to DMBA and efficacy of vitamin D metabolites. Mouse mammary glands were cultured using the standard protocol as shown in Fig. 1 for MAL and MDL. Vitamin D metabolites were included from day 0 to 10. (a) Control mammary glands cultured in IPAF medium without any treatments; (b) mammary glands cultured in IPAF medium were exposed to DMBA on day 3 (MAL) in the presence of 0.001% ethanol (control); arrows show MAL. (c) Mammary glands cultured in IPAF medium were exposed to DMBA in the presence of 25(OH)D3 (250 nM) from day 0 to 10; (d) whole-mounts of the mammary glands cultured in IPAF medium were exposed to DMBA on day 3 in the presence of 1,25(OH)2D3 (100 nM) from day 0 to 10 (positive control); (e) histopathological sections of mammary organs cultured in IPEPg medium without any treatments; (f) mammary organs cultured in IPEPg medium exposed to DMBA on day 3 (MDL); (g) mammary organs cultured in IPEPg medium exposed to DMBA on day 3 in the presence of 25(OH)D3 (250 nM) from day 0 to 10
We next evaluated the efficacy of 25(OH)D3 for MDL. The MDL were induced by DMBA by including 0.001 μg/ml estradiol and 1 μg/ml progesterone in the medium. These steroid hormones replaced aldosterone and hydrocortisone in the medium. As shown in Table 2, 88 of 112 ductal fields examined in the DMBA treated glands contained hyperproliferative and atypical ductal lesions. The incidence of MDL in the absence of 25(OH)D3 was 78.6%, while in the presence of 250 nM 25(OH)D3, the incidence of MDL was only 32.3%, indicating that 25(OH)D3 inhibited MDL incidence by 58.9% (P < 0.001). Representative photographs showing MDL and effects of 25(OH)D3 are shown in Fig. 4e–g. These results demonstrate that 25(OH)D3 inhibits both hormone-independent MAL and hormone-dependent MDL.
Table 2.
Chemopreventive efficacy of 25(OH)D3 for mammary ductal lesions (MDL) induced by DMBA in mouse mammary organ culture
| Treatment | Number of glands with lesions (%incidence) | %inhibition | Significance (P valuea) |
|---|---|---|---|
| IPEPg control | 0/10 | – | – |
| DMBA control | 88/112 (78.6) | – | – |
| DMBA + 25(OH)D3––250 nM | 20/62 (32.3) | 58.9 | <0.0001 |
χ2 test, in comparison with DMBA control
IPEPg, Waymouth’s medium supplemented with insulin (I) + prolactin (P) + oestrodiol (E) + progesterone (Pg)
25(OH)D3 preferentially inhibits MAL at the promotion stage of carcinogenesis
In order to evaluate the stage-specific efficacy of 25(OH)D3 on the development of DMBA-induced lesion formation, 14–15 glands per group were incubated with a MAL-promoting hormone combination with or without 250 nM 25(OH)D3. 25(OH)D3 was included in the medium during either the initiation phase from day 0 to day 4 of culture or the promotion phase from day 4 to day 10 of the culture period (Fig. 1). For comparison, 25(OH)D3 was also included in the medium during both initiation and promotion phase (day 0–day 10) in a group. 1,25(OH)2D3 served as a positive control as anti-promotion agent, whereas aspirin served an anti-initiation agent in the experiments. As shown in Table 3, the DMBA treated glands in this series of experiments developed MAL in 86.7% (13 out of 15 glands) of the glands. This incidence is consistent with our previous results evaluating effects of DMBA on more than 1500 mammary glands, where the incidence of DMBA induced mammary lesions range from 60 to 100% [5, 19, 20]. The treatment of glands with 25(OH)D3 during the initiation phase resulted in 8 out of 14 (57.1%) glands developing MAL, with an inhibition rate of 34.1%. This anti-initiation effect of 25(OH)D3 is not statistically significant (P < 0.1), whereas aspirin as an anti-initiation agent significantly inhibited MAL development by 84.7% (P < 0.001). Treatment with 25(OH)D3 during the promotion phase significantly inhibited MAL development by 75.3% (P < 0.01), while treatment with 1,25(OH)2D3 during the promotion phase in this series of experiments completely inhibited MAL formation. In addition, treatment with 25(OH)D3 covering both initiation and promotion phase significantly inhibited MAL development by 92.3% (P < 0.001). These results suggest that 25(OH)D3 mainly targets the promotion phase of carcinogenesis, but treatment covering both initiation and promotion phases may have better effect in suppression of carcinogenesis.
Table 3.
Stage-specific inhibition of DMBA-induced mammary alveolar lesions (MAL) by 25(OH)D3 in mouse mammary organ culture
| Treatment | Days of treatment | Number of glands with lesions (%incidence) | %inhibition | Significance (P valuea) |
|---|---|---|---|---|
| IPAF control | – | 0/10 | – | – |
| DMBA | – | 13/15 (86.7) | – | – |
| DMBA + 25(OH)D3––250 nM | 0–4 | 8/14 (57.1) | 34.1 | 0.1732 |
| DMBA + 25(OH)D3––250 nM | 4–10 | 3/14 (21.4) | 75.3 | 0.0016 |
| DMBA + 25(OH)D3––250 nM | 0–10 | 1/15 (6.7) | 92.3 | <0.0001 |
| DMBA + 1,25(OH)2D3––100 nM | 4–10 | 0/15 (0) | 100 | <0.0001 |
| DMBA + Aspirin––100 nM | 0–4 | 2/15 (13.3) | 84.7 | 0.0003 |
χ2 test, in comparison with DMBA control
1,25(OH)2D3 served as a positive control for inhibition of promotion of MAL; aspirin served as a positive control for inhibition of initiation of MAL. 25(OH)D3 mainly exhibited inhibition at the promotion stage of DMBA-induced MAL, similar to that of 1,25(OH)2D3. But treatment during day 1–10 with 25(OH)D3 generated best inhibitory effect on MAL development
Discussion
Low level of 25-hydroxy vitamin D3 (25(OH)D3), as a prohormone of the active 1,25(OH)2D3, has been found to be associated with increased risk of breast cancer [7, 25]; however, there is no direct experimental evidence to demonstrate that 25(OH)D3 is efficacious as a chemopreventive vitamin D metabolite. On the other hand, it is well established that active vitamin D metabolites or analogs are efficacious in inhibiting cell proliferation and inducing cell differentiation [26–29]. Here we use an unique mouse mammary organ culture system to demonstrate the chemopreventive role of 25(OH)D3 in DMBA-induced carcinogenesis of mouse mammary glands at its physiological concentration.
We first demonstrate the extensive expression of 25-hydroxyvitamin D 1α-hydroxylase in the target cells––mammary ductal epithelial cells. 1α-hydroxylase is the key enzyme to convert the prohormone 25(OH)D3 to active hormone 1,25(OH)2D3 for 25(OH)D3 to function in extrarenal tissues. Although the immunostaining data are generally not very quantitative, overall analysis of immunostaining data indicates that 25(OH)D3 treatment seems to increase the expression of 1α-hydroxylase. To our knowledge, this is the first report to demonstrate 1α-hydroxylase expression in cultured normal and preneoplastic mouse mammary glands and enhanced expression in the presence of 25(OH)D3. It was reported that 1α-hydroxylase is expressed in both normal human breast tissue and breast cancer tissue, but the expression of 1α-hydroxylase in breast cancer tissue is either higher or similar to normal breast epithelial cells [24, 30, 31]. These results from human samples are in agreement with our observation in MMOC. Our RT-PCR analysis of mRNA extracted from cultured mammary glands also confirmed CYP27B1 expression at mRNA level and it was upregulated by 25(OH)D3. It should be noted that multiple negative VDRE were identified in the promoter region of CYP27B1 in kidney HEK-293 cells, in which CYP27B1 is downregulated by 1,25(OH)2D3 [32, 33]. However, in breast cancer MCF-7 cells, the altered expression of CYP27B1 by 1,25(OH)2D3 is not significant, suggesting a tissue/cell type specific regulation of CYP27B1 expression [32, 34]. In agreement with the literature, our data from breast cancer cell line T47D also demonstrate insignificant regulation of CYP27B1 mRNA expression by 25(OH)D3 or 1,25(OH)2D3 (data not shown). At organ level, the regulation of CYP27B1 is more complex considering the interaction between stromal and epithelial cells as well as different culture conditions. However, the regulation of direct VDR target gene––CYP24 by vitamin D metabolites seems to be more consistent. Although multiple negative VDRE binding sites in CYP27B1 promoter region have been identified, CYP27B1 gene is also regulated by PKA signaling activated by liganded PTH/TPHrP receptor [33]. Therefore the final regulation of CYP27B1 expression could be time, tissue (cell type) and condition specific. It is possible that 25(OH)D3 directly or indirectly stimulates CYP27B1 expression in order to convert 25(OH)D3 to 1,25(OH)2D3 in the experimental setting. This is the first time to show CYP27B1 response to 25(OH)D3 at organ level in vitro. The extensive expression of 1α-hydroxylase in both normal epithelial cells and precancerous lesions strongly support that 25(OH)D3 plays a functional role in mammary organ and that 25(OH)D3 could be developed for both chemopreventive and therapeutical agent for carcinogenesis of mammary glands.
Interestingly, we observed the direct effect of 25(OH)D3 in induction of CYP24 in MMOC using CYP27B1 knockout model. In this model, 25(OH)D3 cannot be converted to 1,25(OH)2D3 due to the absence of the enzyme 1α-hydroxylase [21]. Thus, the significant induction of CYP24 observed in the mammary glands obtained from CYP27B1KO mice must be directly from 25(OH)D3. These results suggest that 25(OH)D3 directly binds to VDR and activates CYP24 transcription. Our observation differs from the previous study using mammary cells derived from CYP27B1 null mice [34], in which, no CYP24 induction by 25(OH)D3 was observed. These differences could be attributed to 1) the use of different model systems (organ culture vs. cell culture); 2) difference in concentrations of vitamin D metabolites (250 nM 25(OH)D3 in the present study vs. 100 nM 25(OH)D3 in the published report) and 3) different measurements (CYP24 mRNA detection in our studies as compared to CYP24 protein detection in the published report). We preferred using sensitive qRT-PCR to detect CYP24 mRNA expression after 25(OH)D3 since VDR is a transcription factor and directly acts on CYP24 promoter. In addition, we further observed that 1α-hydroxylase inhibitor clotrimazol enhanced CYP24 expression induced by both 25(OH)D3 and 1,25(OH)2D3. It is realized that these results using clotrimazol is not conclusive since it might not be a specific 1α-hydroxylase inhibitor. Yet, our results indicate that the inhibition of the activity of both 1α-hydroxylase and 24-hydroxylase did not affect the expression of CYP24, indicating that 25(OH)D3 activates CYP24 expression by directly binding to VDR. These results also suggest that clotrimazol probably inhibits both metabolism and catabolism of 25(OH)D3, extend the half life of 25(OH)D3, and in turn increases its direct effect in CYP24 induction. Similar results were also reported with other 1α-hydroxylase inhibitors such as VID400 or SDZ88-357 [16]. These data suggest that 25(OH)D3 mediates its functional role through both activation of VDR by direct binding to VDR and via activation of VDR by the formation of active metabolite 1,25(OH)2D3 after 1α-hydroxylation. Therefore in the absence of 1α-hydroxylase inhibitor clotrimazol, what we observed could be the mixed role of both 25(OH)D3 and 1,25(OH)2D3 Indeed, it was previously reported that 25(OH)D3 binds to VDR with low affinity, but the binding affinity has been reported to be tissue specific [35, 36]. In agreement with our observation, 25(OH)D3 has recently been reported to suppresses PTH synthesis by parathyroid cells, possibly by direct activation of the VDR [37].
Our data also show that 25(OH)D3 at concentration of 100–250 nM is efficacious in inhibiting both ovarian hormone independent MAL and estrogen and progesterone dependent MDL development. Since 25(OH)D3 is nontoxic in comparison to other active vitamin D analogs, it can serve as an ideal chemopreventive agent. It is also possible to utilize MMOC to determine if 25(OH)D3 is selectively effective against the initiation or promotion stage by exposing the glands to 25(OH)D3 either before or after the carcinogen treatment. In the present study, we found that 25(OH)D3 showed efficacy selectively against the promotion stage of lesion formation: there was more suppression of MAL during the promotion stage than during the initiation, however, the treatment with 25(OH)D3 covering both stages generated better effects. This is consistent with our previous report indicating that 1α(OH)D5, a synthetic analog of vitamin D also mainly inhibited the promotion stage of MAL development in mice and DMBA induced mammary adenocarcioma in rats [6, 19]. These results suggest that vitamin D metabolites and analogs are efficacious during the promotion stage of carcinogenesis.
Although the efficacy of 25(OH)D3 is lower than 1,25(OH)2D3, considering the concentration, toxicity and bioavailability of these vitamin D metabolites, 25(OH)D3 appears to be a better option for cancer chemoprevention among all the vitamin D metabolites. These data are also in agreement with the results reported in the literature that 25(OH)D3 inhibits the growth of human mammary epithelial cells [34]. It should be noted that the concentration of 25(OH)D3 used in some of the current experiments is within the physiological range. Interestingly, higher concentration (500 nM, Table 1) did not enhance the inhibition over that of 100 nM physiological concentration. These results suggest that maintenance of normal physiological concentration of 25(OH)D3 in the serum will generate protective effect against carcinogenesis, providing direct evidence in support of the epidemiological research [7]. Regarding the physiological concentration of 25(OH)D3, generally 80–200 nmol/l (32–80 ng/ml) is considered as vitamin D sufficient physiological concentration [38]. In our studies we used 250 nM 25(OH)D3 which is considered physiologically achievable and not toxic concentration for this prohormone. Studies performed on subpopulations living or working in sun-rich environments have shown that exposure to sunlight without dietary supplementation can raise 25(OH)D3 above 200 nM [39].
It should also be noted that in serum in vivo, as much as 99% of 25(OH)D3 is bound to the vitamin D binding protein (DBP), and cellular uptake of this complex in the kidney via receptor-mediated endocytosis is essential for vitamin D homeostasis in vivo [40, 41]. Vitamin D is transduced by two separate routes of uptake; active uptake in the presence of DBP and passive uptake in the absence of DBP. In our system, it appears that the passive uptake plays a major role, since vitamin D is lipid-soluble, it can directly diffuse into the cells and mediate its functional role. Active endocytosis seems to take place in the kidney based on literature It has also been reported that normal murine mammary tissue, non-transformed human mammary epithelial cells and some established breast cancer cell lines express megalin and cubilin, which are involved in active uptake of 25(OH)D3-DBP complex. These results suggest that endocytosis of DBP is associated with activation of VDR by 25(OH)D3 in human mammary epithelial cells [42]. However more studies are needed to define the endocytosis of vitamin D metabolites.
Two distinct vitamin D3 endocrine systems have recently been proposed [16]. The first one is the classical system involved in calcium and phosphorus regulation based on 1,25(OH)2D3. The formation of 1,25(OH)2D3 is tightly controlled in kidney and the concentration in serum varies with an extremely narrow range. The alternative vitamin D3 endocrine system involved in cell proliferation and differentiation regulation and maintenance of homeostasis is based on 25(OH)D3, which is mainly generated in the liver and maintained at high levels in circulation and fluctuates within a wide range. The prohormone 25(OH)D3 can be metabolized to 1,25(OH)2D3 in extrarenal tissues. Our data support this hypothesis in relation to 25(OH)D3. However, we also propose that 25(OH)D3 plays a direct functional role in maintaining homeostasis in mammary gland and can serve as a natural preventive agent for carcinogenesis. A modified hypothesis of action of 25(OH)D3 in relation to breast cancer chemoprevention is presented in Fig. 5.
Fig. 5.
Proposed hypothesis for the action of 25(OH)D3 in extrarenal organs specifically mammary gland. Circulating 25(OH)D3 is converted to 1,25(OH)2D3 by 1α-hydroxylase (CYP27B1) in mammary gland, then exerts its inhibitory effect on mammary epithelial cells in an autocrine/paracrine manner and therefore is a natural chemopreventive agent in the serum. In addition, 25(OH)D3 also directly interacts with VDR to exert its effect on epithelial cells as evidenced by MMOC experiment using CYP27B1KO mouse model and cell model using 1α-hydroxylase inhibitors. In kidney, 25(OH)D3 is converted to 1,25(OH)2D3 by 1α-hydroxylase to play its functional role in maintaining calcium and phosphorus homeostasis
In summary, we found that 1α-hydroxylase is expressed in mouse mammary epithelial cells and that 25(OH)D3 could directly activate VDR. At physiological concentration, 25(OH)D3 inhibits mammary preneoplastic lesion formation induced by DMBA. These results suggest that 25(OH)D3, the major circulating form of vitamin D metabolite could be a natural chemopreventive agent against transformation of breast epithelial cells.
Acknowledgments
This work was supported by NIH Public Health Service Grant R03 CA121365-02 (XP) and R01 CA82316 (RGM). Support for engineering of the CYP27B1K0 mice was provided by a grant from shriners of North America to R. St-Arnaud.
References
- 1.Berry DM, Meckling-Gill KA. Vitamin D analogs, 20-Epi-22-oxa-24a,26a,27a,-trihomo-1alpha,25(OH)2-vitamin D3, 1,24 (OH)2-22-ene-24-cyclopropyl-vitamin D3 and 1alpha,25(OH) 2-lumisterol3 prime NB4 leukemia cells for monocytic differentiation via nongenomic signaling pathways, involving calcium and calpain. Endocrinology. 1999;140:4779–4788. doi: 10.1210/endo.140.10.7041. [DOI] [PubMed] [Google Scholar]
- 2.Milliken EL, Zhang X, Flask C, Duerk JL, MacDonald PN, Keri RA. EB1089, a vitamin D receptor agonist, reduces proliferation and decreases tumor growth rate in a mouse model of hormone-induced mammary cancer. Cancer Lett. 2005;229:205–215. doi: 10.1016/j.canlet.2005.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryhanen S, Jaaskelainen T, Mahonen A, Maenpaa PH. Inhibition of MG-63 cell cycle progression by synthetic vitamin D3 analogs mediated by p27, Cdk2, cyclin E, and the retino-blastoma protein. Biochem Pharmacol. 2003;66:495–504. doi: 10.1016/s0006-2952(03)00283-1. [DOI] [PubMed] [Google Scholar]
- 4.Spina CS, Ton L, Yao M, Maehr H, Wolfe MM, Uskokovic M, Adorini L, Holick MF. Selective vitamin D receptor modulators and their effects on colorectal tumor growth. J Steroid Biochem Mol Biol. 2007;103:757–762. doi: 10.1016/j.jsbmb.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 5.Mehta RG, Moriarty RM, Mehta RR, Penmasta R, Lazzaro G, Constantinou A, Guo L. Prevention of preneoplastic mammary lesion development by a novel vitamin D analogue, 1alpha-hydroxyvitamin D5. J Natl Cancer Inst. 1997;89:212–218. doi: 10.1093/jnci/89.3.212. [DOI] [PubMed] [Google Scholar]
- 6.Mehta RG, Hawthorne M, Uselding L, Albinescu D, Moriarty R, Christov K, Mehta RR. Prevention of N-methyl-N-nitro-sourea-induced mammary carcinogenesis in rats by 1alpha-hydroxyvitamin D5. J Natl Cancer Inst. 2000;92:1836–1840. doi: 10.1093/jnci/92.22.1836. [DOI] [PubMed] [Google Scholar]
- 7.Lowe LC, Guy M, Mansi JL, Peckitt C, Bliss J, Wilson RG, Colston KW. Plasma 25-hydroxy vitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur J Cancer. 2005;41:1164–1169. doi: 10.1016/j.ejca.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Grant WB, Garland CF. A critical review of studies on vitamin D in relation to colorectal cancer. Nutr Cancer. 2004;48:115–123. doi: 10.1207/s15327914nc4802_1. [DOI] [PubMed] [Google Scholar]
- 9.Lipkin M, Newmark HL. Vitamin D, calcium and prevention of breast cancer: a review. J Am Coll Nutr. 1999;8:392S–397S. doi: 10.1080/07315724.1999.10718903. [DOI] [PubMed] [Google Scholar]
- 10.Ainsleigh HG. Beneficial effects of sun exposure on cancer mortality. Prev Med. 1993;22:132–140. doi: 10.1006/pmed.1993.1010. [DOI] [PubMed] [Google Scholar]
- 11.Garland FC, Garland CF, Gorham ED, Young JF. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med. 1990;19:614–622. doi: 10.1016/0091-7435(90)90058-r. [DOI] [PubMed] [Google Scholar]
- 12.Zinser GM, Welsh J. Accelerated mammary gland development during pregnancy and delayed postlactational involution in vitamin D3 receptor null mice. Mol Endocrinol. 2004;18:2208–2223. doi: 10.1210/me.2003-0469. [DOI] [PubMed] [Google Scholar]
- 13.Cross HS, Kallay E, Farhan H, Weiland T, Manhardt T. Regulation of extrarenal vitamin D metabolism as a tool for colon and prostate cancer prevention. Recent Results Cancer Res. 2003;164:413–425. doi: 10.1007/978-3-642-55580-0_30. [DOI] [PubMed] [Google Scholar]
- 14.Friedrich M, Rafi L, Mitschele T, Tilgen W, Schmidt W, Reichrath J. Analysis of the vitamin D system in cervical carcinomas, breast cancer and ovarian cancer. Recent Results Cancer Res. 2003;164:239–246. doi: 10.1007/978-3-642-55580-0_17. [DOI] [PubMed] [Google Scholar]
- 15.Cross HS, Kallay E, Lechner D, Gerdenitsch W, Adlercreutz H, Armbrecht HJ. Phytoestrogens and vitamin D metabolism: a new concept for the prevention and therapy of colorectal, prostate, and mammary carcinomas. J Nutr. 2004;134:1207S–1212S. doi: 10.1093/jn/134.5.1207S. [DOI] [PubMed] [Google Scholar]
- 16.Lou YR, Laaksi I, Syvala H, Blauer M, Tammela TL, Ylikomi T, Tuohimaa P. 25-hydroxyvitamin D3 is an active hormone in human primary prostatic stromal cells. FASEB J. 2004;18:332–344. doi: 10.1096/fj.03-0140fje. [DOI] [PubMed] [Google Scholar]
- 17.Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004;29:664–73. doi: 10.1016/j.tibs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Fu GK, Lin D, Zhang MY, Bikle DD, Shackleton CH, Miller WL, Portale AA. Cloning of human 25-hydroxyvitamin D-1 alpha-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol Endocrinol. 1997;11:1961–1970. doi: 10.1210/mend.11.13.0035. [DOI] [PubMed] [Google Scholar]
- 19.Mehta RG. Stage-specific inhibition of mammary carcinogenesis by 1alpha-hydroxyvitamin D5. Eur J Cancer. 2004;40:2331–2337. doi: 10.1016/j.ejca.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Mehta RG, Hawthorne ME, Steele VE. Induction and prevention of carcinogen-induced precancerous lesions in mouse mammary gland organ culture. Methods Cell Sci. 1997;19:19–24. [Google Scholar]
- 21.Dardenne O, Prud’homme J, Arabian A, Glorieux FH, St-Arnaud R. Targeted inactivation of the 25-hydroxyvitamin D(3)-1(alpha)-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology. 2001;142:3135–3141. doi: 10.1210/endo.142.7.8281. [DOI] [PubMed] [Google Scholar]
- 22.Peng X, Mehta R, Wang S, Chellappan S, Mehta RG. Prohibitin is a novel target gene of vitamin D involved in its antiproliferative action in breast cancer cells. Cancer Res. 2006;66:7361–7369. doi: 10.1158/0008-5472.CAN-06-1004. [DOI] [PubMed] [Google Scholar]
- 23.de Kok JB, Roelofs RW, Giesendorf BA, Pennings JL, Waas ET, Feuth T, Swinkels DW, Span PN. Normalization of gene expression measurements in tumor tissues: comparison of 13 endogenous control genes. Lab Invest. 2005;85:154–159. doi: 10.1038/labinvest.3700208. [DOI] [PubMed] [Google Scholar]
- 24.Friedrich M, Diesing D, Cordes T, Fischer D, Becker S, Chen TC, Flanagan JN, Tangpricha V, Gherson I, Holick MF, Reichrath J. Analysis of 25-hydroxyvitamin D3–1alpha-hydroxylase in normal and malignant breast tissue. Anticancer Res. 2006;26:2615–2620. [PubMed] [Google Scholar]
- 25.Colston KW, Lowe LC, Mansi JL, Campbell MJ. Vitamin D status and breast cancer risk. Anticancer Res. 2006;26:2573–2580. [PubMed] [Google Scholar]
- 26.Flynn G, Chung I, Yu WD, Romano M, Modzelewski RA, Johnson CS, Trump DL. Calcitriol (1,25-dihydroxychole-calciferol) selectively inhibits proliferation of freshly isolated tumor-derived endothelial cells and induces apoptosis. Oncology. 2006;70:447–457. doi: 10.1159/000098872. [DOI] [PubMed] [Google Scholar]
- 27.Swami S, Krishnan AV, Feldman D. 1alpha,25-Dihydroxyvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF-7 human breast cancer cells. Clin Cancer Res. 2000;6:3371–3379. [PubMed] [Google Scholar]
- 28.Colston KW, Hansen CM. Mechanisms implicated in the growth regulatory effects of vitamin D in breast cancer. Endocr Relat Cancer. 2001;9:45–59. doi: 10.1677/erc.0.0090045. [DOI] [PubMed] [Google Scholar]
- 29.Welsh J. Vitamin D and prevention of breast cancer. Acta Pharmacol Sin. 2007;28:1373–1382. doi: 10.1111/j.1745-7254.2007.00700.x. [DOI] [PubMed] [Google Scholar]
- 30.Townsend K, Banwell CM, Guy M, Colston KW, Mansi JL, Stewart PM, Campbell MJ, Hewison M. Autocrine metabolism of vitamin D in normal and malignant breast tissue. Clin Cancer Res. 2005;11:3579–3586. doi: 10.1158/1078-0432.CCR-04-2359. [DOI] [PubMed] [Google Scholar]
- 31.Segersten U, Holm PK, Bjorklund P, Hessman O, Nordgren H, Binderup L, Akerstrom G, Hellman P, Westin G. 25-Hydroxyvitamin D3 1alpha-hydroxylase expression in breast cancer and use of non-1alpha-hydroxylated vitamin D analogue. Breast Cancer Res. 2005;7:R980–R986. doi: 10.1186/bcr1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turunen MM, Dunlop TW, Carlberg C, Vaisanen S. Selective use of multiple vitamin D response elements underlies the 1 alpha,25-dihydroxyvitamin D3-mediated negative regulation of the human CYP27B1 gene. Nucleic Acids Res. 2007;35:2734–2747. doi: 10.1093/nar/gkm179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murayama A, Kim MS, Yanagisawa J, Takeyama K, Kato S. Transrepression by a liganded nuclear receptor via a bHLH activator through co-regulator switching. EMBO J. 2004;23:1598–1608. doi: 10.1038/sj.emboj.7600157. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Kemmis CM, Salvador SM, Smith KM, Welsh J. Human mammary epithelial cells express CYP27B1 and are growth inhibited by 25-hydroxyvitamin D-3, the major circulating form of vitamin D-3. J Nutr. 2006;136:887–892. doi: 10.1093/jn/136.4.887. [DOI] [PubMed] [Google Scholar]
- 35.Bouillon R, Okamura WH, Norman AW. Structure-function relationships in the vitamin D endocrine system. Endocr Rev. 1995;16:200–257. doi: 10.1210/edrv-16-2-200. [DOI] [PubMed] [Google Scholar]
- 36.Skowronski RJ, Peehl DM, Feldman D. Actions of vitamin D3, analogs on human prostate cancer cell lines: comparison with 1,25-dihydroxyvitamin D3. Endocrinology. 1995;136:20–26. doi: 10.1210/endo.136.1.7530193. [DOI] [PubMed] [Google Scholar]
- 37.Ritter CS, Armbrecht HJ, Slatopolsky E, Brown AJ. 25-Hydroxyvitamin D(3) suppresses PTH synthesis and secretion by bovine parathyroid cells. Kidney Int. 2006;70:654–659. doi: 10.1038/sj.ki.5000394. [DOI] [PubMed] [Google Scholar]
- 38.Masuda S, Jones G. Promise of vitamin D analogues in the treatment of hyperproliferative conditions. Mol Cancer Ther. 2006;5:797–808. doi: 10.1158/1535-7163.MCT-05-0539. [DOI] [PubMed] [Google Scholar]
- 39.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842–856. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 40.Nykjaer A, Fyfe JC, Kozyraki R, Leheste JR, Jacobsen C, Nielsen MS, Verroust PJ, Aminoff M, de la Chapelle A, Moestrup SK, Ray R, Gliemann J, Willnow TE, Christensen EI. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3) Proc Natl Acad Sci USA. 2001;98:13895–13900. doi: 10.1073/pnas.241516998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 42.Rowling MJ, Kemmis CM, Taffany DA, Welsh J. Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxycholecalciferol actions in human mammary cells. J Nutr. 2006;136:2754–2759. doi: 10.1093/jn/136.11.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]