Abstract
Cognitive control refers to the ability to flexibly allocate mental resources to guide thoughts and actions in light of internal goals. Given the behavioral inflexibility exhibited by individuals with autism spectrum disorders (ASDs), it would appear they experience cognitive control deficits. Cognitive correlates of this behavioral inflexibility have been elusive in previous investigations. Study goals were to investigate deficits in cognitive control in ASDs; to explore its developmental trajectory; and to test whether control deficits are related to symptoms of inflexible thoughts and/or behaviors, and attention symptoms. Thirty-one children and adolescents aged 8 to17 with ASDs and 32 age, IQ, and gender matched control subjects completed cognitive, diagnostic, and behavorial assessments, as well as a measure of cognitive control involving overcoming a prepotent response tendency. Compared with typically developing control subjects, individuals with ASDs exhibited deficits in cognitive control. Younger children with ASDs did not demonstrate age related improvements in cognitive control. Modest relationships between cognitive control, IQ, and attention problems were found for the sample. Only the relationship between cognitive control and Full Scale IQ survived correction for multiple comparisons.
Keywords: autism, Asperger Syndrome, PDDNOS, executive functions, cognitive control, neuroscience, prefrontal cortex
Autism spectrum disorders (ASDs), including autism, high functioning autism, Asperger’s Disorder, and Pervasive Developmental Disorder NOS (PDDNOS), are neurodevelopmental disorders, with a prevalence of 1 in 150 (CDC MMWR, 2007). One of the most influential cognitive theories of ASD is the executive function hypothesis, which proposes that deficits in planning, inhibitory control, attentional set shifting and working memory are central to the disorder (Bishop, 1993; Ozonoff, 1995, 1997; Pennington and Ozonoff, 1996; Hughes, 2001).
“Cognitive control,” is a conceptual model developing in the field of cognitive neuroscience to describe what traditionally has been referred to as executive functions or executive control. Cognitive control refers to the ability to flexibly allocate mental resources to guide thoughts and actions in light of internal goals. It involves processing of task-relevant information over competing information. Control is not required to perform simple or automatic behaviors, but must be engaged to guide action in novel, difficult or rapidly changing conditions (Braver et al., 2002; Bunge et al., 2002). Impairments in cognitive control cause individuals to perseverate on over-learned behaviors.
One prominent framework for the neural basis of cognitive control has been presented in Miller and Cohen’s (2001) “guided activation hypothesis.” In this framework, prefrontal cortex (PFC) patterns of activity or “context representations” are thought to provide bias signals, which guide the flow of activity along neural pathways that establish correct mappings between inputs, internal states, and outputs. Diverse neural pathways with different sources of information compete for expression in behavior. Behaviors with the strongest sources of “support,” “win” (see Desimone and Duncan, 1995).
A cognitive control based model, provides a mechanistic account of executive functions that maps to neural circuits, and proposes that working memory and response inhibition function interdependently. The need to study the interrelationships between these components of executive functioning also has been suggested by others asserting that the ability to maintain context in the face of interference is the focal and difficulty in the developmental psychopathology field (Joseph, 1999; Roberts and Pennington, 1996).
Exertion of cognitive control has been associated with a reliable network of brain regions including the dorsolateral prefrontal cortex (DLPFC), medial frontal cortex (including the anterior cingulate cortex), and parietal cortex (Yarkoni et al., 2005). The DLPFC is believed to maintain appropriate context for action. The anterior cingulate cortex (ACC) is thought to function as part of a “control loop.” It detects response conflict, and signals the DLPFC to allocate more control-related resources (Egner and Hirsch, 2005; Kerns et al., 2005; MacDonald et al., 2000). The parietal cortex is activated when it is necessary to switch attentional focus (Corbetta et al., 2000; Miller and Cohen, 2001; Posner and Petersen, 1990). It also is thought to act as a repository of learned stimulus-response associations (Bunge et al., 2002; Bunge et al., 2003) from which the DLPFC “selects” the appropriate response.
Several studies have examined the development of cognitive and/or executive control in typically developing children and young adults, as well as those with ASDs. In typical development, more strategic and complex aspects of cognitive control continue to develop well into adolescence. For example task switching involving inhibition (Davidson et al., 2006) and distraction related error rates (Crone et al., 2004) may not reach adult levels until late adolescence or early adulthood. Simple recognition and working memory (Luciana et al., 2005), inhibitory processes as assessed by a stop-signal task (Williams et al., 1999), and simple switch costs on a single-trial counting Stroop Task appear to reach adult levels by approximately age 12 (Cepeda et al., 2001). Developmental findings related to executive control in ASDs have been mixed, with some demonstrating persisting deficiency (Luna et al., 2007; Ozonoff and McEvoy, 1994), and others suggesting developmental progression over time (Happe et al., 2006).
The cognitive control view suggests that working memory and response inhibition are interdependent, and thus has the potential to explain inconsistent findings in the literature. For example, some studies have found deficits in working memory (Bennetto et al. 1996; Landa and Goldberg, 2005; Luna et al. 2007; Williams et al. 2005) while others have not Ozonoff and Strayer, 2001. It often is suggested that individuals with ASDs, exhibit intact inhibition (Brian et al., 2003; Ozonoff and Strayer, 1997; Ozonoff et al., 1994; Ozonoff and Jensen, 1999; Pennington and Ozonoff, 1996). However, other studies employing tasks with increased flexibility requirements, or working memory demands have reported deficits in response inhibition in ASDs (Bishop and Norbury, 2005; Geurts et al., 2004; Nyden et al., 1999; Verte et al., 2006).
In addition to shedding light on contradictory neuropsychological findings, a cognitive control based model may help explain restricted and repetitive and other perseverative behaviors (e.g. poor reciprocal conversation), which involve automatic orientation to salient events and the tendency to repeat responses that are no longer adaptive. Here too, findings have been mixed with Turner (1997) and Lopez et al. (2005) demonstrating relationships between repetitive behaviors and aspects of executive functioning and South et al. (2005) failing to find one. Hughes (2001) has suggested that executive function deficits may be related to everyday problems in communication and social functioning. However, strong clear empirical relationships between executive functions and these types of behaviors also have yet to be found (Joseph and Tager-Flusberg, 2004; Landa and Goldberg, 2005).
The goal of this study was to investigate cognitive control deficits involved in overcoming a prepotent response tendency in children and adolescents with autism spectrum disorders. We hypothesized that children with ASDs would display control deficits related to overcoming a prepotent response tendency. Given recent findings of developmentally related working memory impairments (Luna et al., 2007), and suggestion that executive function deficits may become more prominent over time we hypothesized that control deficits also would follow this developmental pattern. Finally, we hypothesized that context processing deficits would be positively correlated with autism symptoms related to perseveration in reciprocal social and communication behaviors, restricted and repetitive behaviors, and attention symptoms.
Experimental Procedures
Participants
Two groups of children aged 8 to 18 were recruited for this study. See Table 1 for a summary of the characteristics of this group. They were matched on age, gender, and Full Scale I.Q. Each group included 3 girls. Participants with autism spectrum disorders were recruited from local psychiatrists, neurologists, general practitioners, psychologists, speech and language pathologists, occupational therapists, advocacy groups, regional centers (state agencies that serve individuals with developmental disabilities), and the M.I.N.D. Institute’s Subject Tracking System database.
Table 1.
Participant Characteristics
| ASD Group (n=31) | Typically Developing Group (n=32) | |||
|---|---|---|---|---|
| M (SD) | Range | M (SD) | Range | |
| Age (Yrs) | 12.3 (2.5) | 9–17.3 | 12.2 (2.5) | 8.2–17.2 |
| VIQ | 110 (19) | 69–145 | 116 (15) | 89–143 |
| PIQ | 108 (18) | 79–135 | 112 (11) | 89–141 |
| FSIQ | 110 (20) | 72–142 | 115 (12) | 99–142 |
| SCQ Total | 25 (6) | 15–35 | 3 (2) | 0–8 |
| SCQ Social | ||||
| Behavior Domain | 10 (3) | 2–15 | 1 (1) | 0–4 |
| SCQ Repetitive | ||||
| Behavior Domain | 7 (2) | 2–9 | 1 (1) | 0–7 |
| SCQ Communication | ||||
| Domain | 8 (3) | 2–12 | 1 (1) | 0–5 |
| ADOS Comm + | ||||
| Social Interaction | 12 (4) | 7–23 | — | — |
| ADOS Communication | ||||
| Domain | 4 (2) | 2–9 | — | — |
| ADOS Social | ||||
| Interaction Domain | 8 (2) | 5–14 | — | — |
| ADOS Restricted | ||||
| Interests Domain | 3 (2) | 0–7 | — | — |
Participants with autism spectrum disorder (n=31) met criteria for Autistic Disorder, or Asperger’s Disorder according to DSM-IV-TR (American Psychiatric Association, 2000); autism spectrum disorder or autism according to ADOS-G (Lord et al., 2000); and autism spectrum disorder according to the Social Communication Questionnaire (SCQ; Berument et al., 1999). The final sample included 13 children diagnosed with high functioning autism, 15 diagnosed with Asperger Syndrome, and 3 with PDDNOS. The mean age for subjects with an autism spectrum disorder was 12. 3 years (range = 9.0 –17.3). Typically developing participants (n=32) were included if they had no history of autism spectrum disorder or other neurodevelopmental disorder; and a score of less than 11 on the Social Communication Questionnaire. The mean age of typically developing participants was 12.2 years (range = 8.2–17.2).
Individuals were excluded if they had a co-occurring neurological disorder including seizures or if parents reported they had been given another clinical diagnosis by a mental health or other professional. While all children in the study had primary diagnoses of autism spectrum disorder, diagnostic precedence rules may obscure the existence of co-morbid attention, and mood problems. These were assessed using the parent-report of the Behavioral Assessment Scale for Children (BASC; Reynods and Kamphaus, 1992). Despite parent reports that the children had no other psychiatric diagnoses, on the BASC, 39% of children in the ASD group showed attention and/or hyperactivity problems in the clinical range. These estimates are similar to others reported in the literature (Sturm et al., 2004; Geurts et al., 2004). Typically developing children showed a much lower rate of 4%. In this sample, 21% of children with an ASD and 9% of children with typical development showed clinically significant symptoms of anxiety and depression. Children also were excluded if they had a Full Scale IQ score scores of less than 70 on the Wechsler Abbreviated Scales of Intelligence for Children (WASI; Wechsler, 1999).
The study was approved by the Institutional Review Board of the University of California, Davis. In accordance with their policies, assent was obtained from the participants, and written consent was obtained from their parents/guardians.
Measures
Procedure
Participants came to the lab for two sessions. The first was for qualification into the study, the second, which occurred less than 4 months from the first, involved completion of cognitive control and other measures that were part of a larger battery to assess behavioral symptoms related to cognitive control.
Qualification Measures
Wechsler Abbreviated Scales of Intelligence (WASI; Wechsler, 1999) was developed to provide a short and reliable means of assessing intelligence in individuals aged 6 to 89. The WASI produces the three traditional Verbal, Performance, and Full Scale IQ scores. It consists of four subtests: Vocabulary, Block Design, Similarities, and Matrix Reasoning. These scales were chosen due to their strong association with overall intellectual functioning. These scales provide standard scores with a mean of 100 and a Standard Deviation of 15. The WASI is nationally standardized, and exhibits strong psychometric properties. It has exhibited acceptable levels of internal consistency, test-retest reliability, and validity.
Autism Diagnostic Observation Schedule-Generic (ADOS-G; Lord et al., 2000)
Once qualification based on the WASI was established, participants with ASDs were administered module 3 or 4 of the ADOS-G, a semi-structured interactive session and interview protocol that offers a standardized observation of current social-communication behavior. Each module has approximately 10 standardized interactional “presses.” Participants are rated based on their responses to these social presses, and scored for communication, reciprocal social behavior, and repetitive behaviors and stereotyped interest patterns. An algorithm score, that combines the communication and reciprocal social interaction domains, is the basis for diagnostic classification. Lord et al. (2000) showed that for modules 3 and 4, mean inter-rater agreement was 88% across all items. Inter-rater reliability on all item domains ranged from .82 (restricted and repetitive behaviors) to .93 (social behaviors). Test-retest reliability ranged from .59 (repetitive behaviors) to .78 (social behaviors). Internal consistency reliability was assessed using Cronbach’s alphas, which ranged from .91 to .94 for total social and communication items. Inter-rater agreement in diagnostic classification based on the ADOS-G algorithm for all modules exceeded 90%.
Social Communication Questionnaire (SCQ: Rutter et al., 2003)
Parents of children believed to be typically developing and parents of children with ASDs were asked to complete the SCQ. The SCQ is a brief 40-item parent-report screening questionnaire to evaluate communication and social skills. It may be used for individuals 4 years of age and older. It contains parallel questions to those included on the ADI-R (Lord et al., 1994), which is the “gold standard” parent report diagnostic measures in the field presented in a briefer yes/no format. The Lifetime Form, which focuses on the child’s entire developmental history, was used in this study. Based on a total sample of 200 (160 with PDD and 40 typically developing children), Berument et al., (1999) reported that the mean SCQ score for non-intellectually impaired individuals was 11.2, while that of individuals with PDD was 22.3 and that of individuals with autism was 24. A cutoff point of 15 or over gave a sensitivity of .96 and a specificity of .80 for autism versus other diagnoses. Thus a score of 15 was used for inclusion. There was high correlation with the ADI algorithm score. Based on this work, a cutoff score of 11 or below was used to screen for exclusion. To construct scores for the autism domains, we aggregated items loading onto ADI domains of reciprocal social interaction, language, and restricted and repetitive patterns of behavior and interests. If items were indicative of the construct assessed by the domain, we assigned a score of “1” to the item. These were tallied to form a composite score for the domain. Internal consistency reliability (Cronbach’s Alpha) across these social, communication, and repetitive behavior scales were .95, .88, and .92, respectively.
Other Behavioral Measures
Behavioral Assessment Scale for Children Parent Rating Scale (BASC; Reynolds and Kamphaus, 1992)
The Behavior Assessment System for Children is a multi-method, multidimensional approach to evaluating the behavior and self-perceptions of children. The Parent Rating Scale (PRS) was used. The BASC measures positive (adaptive) as well as negative (clinical) dimensions of behavior and personality. The clinical behaviors that can be assessed include hyperactivity, aggression, conduct problems, anxiety, depression, somatization, atypicality, withdrawal, and attention problems. In this study, we used the Attention scale. Standard scale scores over 70 are considered clinically significant. The BASC aids in differential diagnosis of specific categories of disorders and aids in the design of treatment plans. The BASC has exhibited acceptable levels of internal consistency, test-retest reliability, and validity (Reynolds and Kamphaus, 1992.)
Yale Special Interest Inventory (YSII; South et al., 1999)
The YSII covers four periods of development including preschool, school age, adolescence, and adulthood. All periods contain identical questions designed to elicit information regarding the presence or absence and quality of circumscribed interests, which are intense and focused interests common in persons with ASDs. It also probes for details of functional and social impairment associated with these interests. Each category of interference is rated on a scale from 0 (no interference related to child’s special interest) to 3 (major disruption caused by an all-encompassing circumscribed interest). Completion time is 40 minutes. Good test-retest reliability has been demonstrated with over 800 participants (South et al., 1999).
Cognitive Control Measures
Preparing to Overcome Prepotency “POP” Task (Barber and Carter, 2005; Rosano et al., 2005; Snitz et al., 2005)
The POP Task was administered using a Compaq Presario 2500 laptop computer with a Pentium 4, 2.66 GHz processor, and the Windows XP operating system. Subjects were seated 60–75 cm from the monitor. Instructions for the POP task, were read by the examiner and presented visually. On incorrect trials there was a beeping tone. The examiner was present throughout the administration of the POP to ensure that subjects were using their best efforts to complete the task.
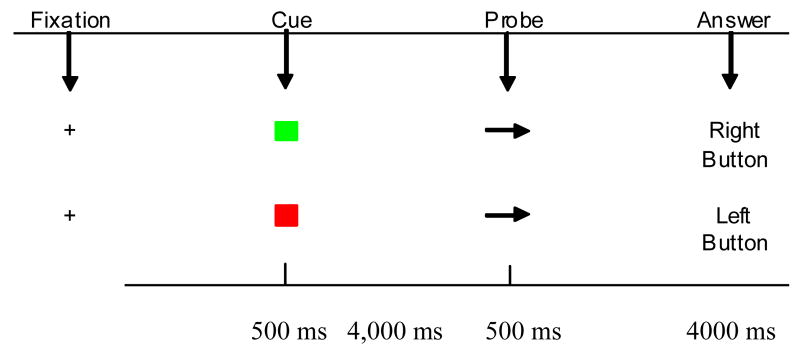
This POP task is designed to study cognitive control involved in context processing (maintaining a cue over a delay and then overcoming a prepotent response tendency). A colored cue, which is presented for 500 ms, instructs subjects to perform one of the two task conditions. A green cue signals the subject to press the key on the same side that the target (an arrow) points to. The target is presented 4,000 ms after the cue and is on the screen for 500 ms. See Figure 1. A red cue signals the subject to press the key on the opposite side that the arrow points to. Green trials involve a response that is compatible with the stimuli (an arrow pointing right means respond with a right button press), occur more often (70% of the time), and are primed at the beginning of each block by three repeated presentations of the green stimulus. Thus, green trials are “prepotent” trials. Red trials involve an incompatible response (an arrow pointing left means respond with a right button press), occur less often (30% of trials), and necessitate inhibition of a prepotent response tendency since green trials are more frequent and are primed. Each subject performed a brief practice block followed by four blocks of 24 trials (9 seconds/trial). Blocks were administered continuously. Trials of the POP Task were presented in a pseudorandom order. Variables of interest include median response times (RTs) as well as error rates for green and red trials. The POP task takes approximately 20 minutes to administer.
Figure 1.
The Pop Task
Data Analysis
Before completing statistical analyses, we screened for skewness, kurtosis, and outliers in the data. Error rates variables were not normally distributed and were transformed using an arcsine transform. Only correct trials were used in analyses involving reaction times. Using only correct trials introduces greater experimental control, because only comparable (e.g. trials done right) are compared across subjects. Incorrect responses also are frequently very rapid (speed accuracy tradeoff), which introduces distortions into aggregated reaction times.
Results
POP Task Group Comparisons
Prepotency
The first set of analyses examined reaction times and error rates on red and green trials. See Table 2. To examine differences in median reaction times for prepotency, we performed a 2×2 analysis of variance (ANOVA) where trial type (red versus green) was the within subjects factor, and diagnosis (ASD versus typical) was the between subjects factor. For median reaction times on red versus green trials, there was a main effect of trial type (F (1, 61) = 6.13, p<.05, ηp2 = .18), however the interaction of trial type and group was not significant. Analysis of simple effects revealed that both groups were significantly slower on red versus green trials.
Table 2.
POP Task Variables Summary
| ASD Group (n=31) | Typically Developing Group (n=32) | |||||
|---|---|---|---|---|---|---|
| M (SD) | Range | M (SD) | Range | F statistic | p-value | |
| Green Trials | ||||||
| Median RT | 611 (271) | 324–1325 | 562 (229) | 351–1067 | 1.870 | 0.349 |
| Mean ER | 0.02 (0.02) | 0.00–0.08 | 0.02 (0.03) | 0.00–0.14 | 0.701 | 0.925 |
| Red Trials | ||||||
| Median RT | 632 (276) | 334–1335 | 586 (213) | 354–1210 | 2.920 | 0.419 |
| Mean ER | 0.26 (0.20) | 0.00–0.75 | 0.17 (0.14) | 0.00–0.46 | 3.017 | 0.036 |
A similar 2×2 ANOVA analysis of error rates revealed both a main effect of trial type (F (1, 61) = 86.85, p<.05, ηp2 = .59) and a group by trial type interaction (F (1, 61) = 4.86, p<.05, ηp2 = .074). A post hoc comparison showed that the error rate for red trials in the ASD group was significantly higher than that for the control group (25.9% versus 16.9%; t (61) = 2.08, p< .05).
To ensure that differences in performance and/or motivation over time did not confound results of analyses, we examined performance on red trials by block for RTs and error rates, and whether this differed between the groups. Repeated measures ANOVAs were conducted with block as the within subjects factor (1,2,3,4) and diagnostic group (ASD vs. typical) as the between-subjects factor for both median RT and error rates for red trials. There were no significant main or interaction effects of block on reaction time. For red error rates, the main effect of block approached significance (F (3, 60) = 2.5, p = .066, ηp2 = .11). However, the interaction of block and group was not significant, meaning that reaction times for both groups slowed comparably, and not quite significantly, over the course of the task. See Table 3.
Table 3.
POP Task Variables by Block Summary
| ASD Group (n=31) |
Typically Developing Group (n=32) |
|||
|---|---|---|---|---|
| M (SD) | Range | M (SD) | Range | |
| Median RT ms | ||||
| Red Trials | ||||
| Block 1 | 623.2 (247.2) | 374–1190 | 573.0 (208.1) | 344–1082 |
| Block 2 | 645.7 (277.0) | 328–1366 | 610.1 (236.5) | 339–1239 |
| Block 3 | 632.9 (285.0) | 332–1314 | 621.9 (257.3) | 357–1290 |
| Block 4 | 651.3 (299.8) | 331–1330 | 601.7 (232.1) | 325–1127 |
| Mean ER | ||||
| Red Trials | ||||
| Block 1 | 0.19 (0.21) | 0.0–0.67 | 0.14 (0.15) | 0.0–0.50 |
| Block 2 | 0.20 (0.23) | 0.0–0.83 | 0.19 (0.18) | 0.0–0.50 |
| Block 3 | 0.26 (0.26) | 0.0–0.83 | 0.16 (0.21) | 0.0–0.83 |
| Block 4 | 0.29 (0.30) | 0.0–1.00 | 0.19 (0.17) | 0.0–0.67 |
| Median RT ms | ||||
| Green Trials | ||||
| Block 1 | 572.4 (247.2) | 321–1241 | 539.9 (190.6) | 350–1027 |
| Block 2 | 629.3 (312.0) | 295–1341 | 563.2 (228.2) | 331–1087 |
| Block 3 | 632.1 (316.4) | 332–1398 | 579.6 (231.7) | 333–1222 |
| Block 4 | 628.7 (271.4) | 346–1317 | 564.9 (214.7) | 347–1059 |
| Mean ER | ||||
| Green Trials | ||||
| Block 1 | 0.02 (0.03) | 0.0–0.11 | 0.02 (0.03) | 0.0–0.11 |
| Block 2 | 0.03 (0.05) | 0.0–0.22 | 0.02 (0.05) | 0.0–0.17 |
| Block 3 | 0.02 (0.03) | 0.0–0.11 | 0.02 (0.05) | 0.0–0.22 |
| Block 4 | 0.02 (0.03) | 0.0–0.11 | 0.02 (0.03) | 0.0–0.11 |
Developmental Analyses
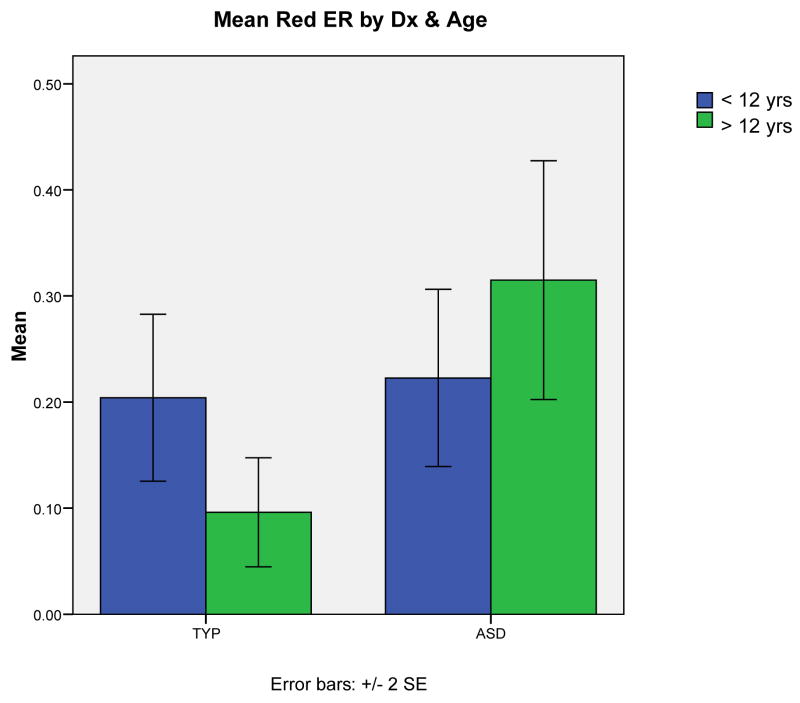
A next series of analyses investigated developmental effects in children with ASDs and typical development. Experimental groups were divided into a younger (8–12: n = 35 with 18 children with ASDs and 17 with typical development) and an older (13–18: n = 28 with 14 children with typical development and 14 with ASD) group. See Figure 2. We also conducted a similar 2×2 ANOVA analysis for green and red reaction times and error rates. There were no significant age related effects for reaction times. On the analysis of red error rates, age, and group we found a main effect of diagnostic group (F (3, 59) = 7.93, p<.01, ηp2 = 0.119) and that the interaction of age group and diagnostic group also was significant (F (3, 59) = 6.02, p<.05, ηp2 = 0.093. An analysis of green error rates by time period showed a trend level main effect of time suggesting that both groups’ performance improved somewhat by the time they were older (F(3, 59) = 3.36, p = .072).
Figure 2.
Correlations with Measures of Behavioral Inflexibility
To ascertain whether cognitive control deficits were associated with behavioral symptoms we correlated error rates on red trials with variables related to IQ, autism symptoms, and attention problems. Given the preliminary nature of this analysis, we examined correlations both with and without correction for multiple comparisons. Correction for multiple comparisons consisted of adjusting P values based on the false discovery rate (FDR) controlling procedure (Benjamini and Hochberg, 1995). In the entire sample, POP red error rates were significantly negatively correlated with FSIQ before (r = −.359, p = .004) and after correction (p = .032). Although they were no longer significant after FDR correction, red error rates were significantly negatively correlated with Verbal IQ (r = .285, p = .025) as well as Full Scale IQ for the autism group (r = −.439, p = .012) and significantly positively correlated with the SCQ social behavior score (r = .265, p = .037), the SCQ communication score (r = .27, p = .033) and BASC Attention problems (r = .318, p = .013).
Discussion
Consistent with our first hypothesis, children with ASDs had significantly more difficulty holding a cue in mind and inhibiting an incorrect prepotent motor response tendency on red trials. The ASD group showed generally longer, but not significantly greater, response latencies on all trials. Group differences in cognitive control were manifest on red trial errors, with children with autism showing significantly higher error rates. A series of analyses examining the groups’ relative fatigue and motivation on the different trial blocks found that while both groups showed a trend towards more red errors over time, there was no interaction of block and group, indicating that both groups tired at a similar rate.
To test our second hypothesis, we divided the sample at age 12, an age by which development of simple cognitive processes has been achieved (Luciana et al., 2005). We examined reaction time differences as well as those associated with accuracy. We found a trend to faster RTs for both groups on green trials. We also found that red error rates in the typically developing group were considerably lower for the older participants. Red error rates for the ASD group increased slightly. These results are consistent with a developmental effect in which there is no improvement, and even a slight worsening, in cognitive control in individuals with ASDs.
Finally we examined the relationship between red error rates, autism symptoms, and attention symptoms in both the whole sample and the autism and typically developing groups. The only correlation that survived FDR correction was that between red error rates and Full Scale IQ for the entire sample. We did not find a significant relationship between red error rates and repetitive behaviors. However, the relationships between red error rates and autism symptoms as assessed by the SCQ and BASC Attention Problems Scales were significant before correction, and should be investigated in a larger study.
Findings that individuals with ASDs showed difficulty inhibiting a prepotent motor response tendency are at odds with the influential hypothesis that autism does not involve impaired response inhibition (Pennington and Ozonoff, 1996). We would argue however, that this assertion, as well as many made in the current debate about spared and impaired component processes in ASDs, is difficult to verify given the multiple different tasks that have been used to assess response inhibition. For example, several studies finding inhibition deficits in autism have employed a classic pencil and paper version of the Stroop task (e.g. Ozonoff and Jensen, 1999). This version puts lower demands on context processing than single trial Stroop tasks or the POP because different trial types are presented simultaneously in blocks, thereby reducing demands to maintain context (Cohen and Servan-Schreiber, 1992). Another measure used in the autism literature with null findings, the Stop Signal task (Ozonoff and Strayer, 1997), does not involve overcoming a motor response tendency like the POP. Furthermore, the Stop Signal task includes a perceptual cue (a tone), with one study reporting that performance in a group with autism was better than in typical controls raising questions about the alerting effects of the tone on performance (Raymaekers et al., 2006). Truly meaningful comparisons across studies of executive control can be made, only if task format, task “level” (e.g. perceptual or motor), and task difficulty or load (which may result from response competition) of the both context processing and response phases of the tasks are equated. A similar argument recently has been made in the schizophrenia literature (MacDonald and Carter, 2002).
From the results of this study, we were not able to ascertain whether developmental findings of cognitive control impairments in 12–18 year olds with ASDs can be interpreted as a sign of delay or as a marker of persistent impairment. Based on the results of a study of 2 occulomotor executive control tasks including an oculomotor delayed response task and antisaccade task in individuals aged 8 to 33, Luna et al., (2007) argued that while some attention related executive processes improved over time in individuals with ASDs, the general picture was one of persistent impairment. This corroborated the findings of Ozonoff and Jensen, (1999) who also failed to find age related improvements in cognitive flexibility tasks in their sample. However, Happe et al. (2006) compared the development of executive functions in three groups of children with Attention Deficit Hyperactivity Disorder (ADHD), ASD, and typical development and found that children with ASD improved over time so that by age 11–16 they more closely resembled the typical group. Children with ADHD remained the most impaired. In both children with ADHD and those with ASD, executive functions deficits were related to attention problems.
We found modest relationships between variables related to cognitive control and behavioral symptoms. Other studies of the relationship between executive functions and behavior, and especially those in older children, also have produced few results. For example, Joseph and Tager-Flusberg (2004) concluded that executive functions did not predict variance in social interaction or repetitive behaviors. Happe et al. (2006) found that executive functions composite scores only were related to social functions after covarying age.
We found that cognitive control deficits were related to parent reported attention symptoms before correction for multiple comparisons. These results, although hypothesis-driven, must be interpreted with caution given the relatively large number of comparisons made in this study and the consequent high potential to commit Type I errors. Our result that cognitive control deficits were related to parent reported attention symptoms is consistent with the findings of Happe et al. (2006) that attention symptoms were associated with executive functions problems in children with ADHD and children with ASD. Increasingly, the high co-morbidity of ASDs and ADHD is being noted in the literature (Corbett and Constantine, 2006; Sturm et al., 2004). Diagnostic precedence rules do not allow a diagnosis of ADHD once a diagnosis of autism is made, further complicating the picture. Writing about co-morbidities, Caron and Rutter (1991) point out that co-occurring disorders may be viewed as categories or dimensions; contain overlapping diagnostic criteria; be artificial subdivisions of syndromes; represent early manifestations of the other; and/or be part of each other. According to this framework, we believe that ADHD and ASDs contain overlapping symptoms. When attention problems are present in ASDs, they are not necessarily co-morbid ADHD symptoms, but likely are evidence of symptomatic overlap with the attention abnormalities inherent in autism. Attention abnormalities in both disorders appear to be associated with executive control symptoms. In ASDs, these attention symptoms may be less likely to be associated with core symptoms of autism per se (Geurts et al., 2004). The group of children with autism and clinically significant attention symptoms may represent an endophenotype of ASD worthy of further study.
Given our findings of no relationship between cognitive control, and other modest findings in attempts to link executive functions with symptoms from the third autism domain, it is likely that prefrontally mediated cognitive control deficits are not the only neural substrates of restricted and repetitive behaviors, and/or that behavioral assays of executive functions and cognitive control lack the sensitivity to detect relationships. The basal ganglia and cerebellum likely also are involved in aspects of cognitive control as well as restricted and repetitive behaviors. Repetitive behaviors are thought to result from disinhibition of response selection in the motor system (Garner et al., 2003), and alterations in the dopamine, serotonin, and opiate systems and their interactions in basal ganglia (Lewis and Bodfish, 1998). Townsend et al. (1999) have demonstrated that, cerebellar abnormalities affect temporal properties of cognitive processing in autism. This may also affect the tendency to engage in preservative behaviors. Thus, future studies of repetitive behavior symptoms should more directly assess the effects of motor requirements of tasks, as well as cerebellar and basal ganglia function. Studies that utilize fMRI hold the promise of being able to better tease apart the relationships between these various brain regions and their role in cognitive control and repetitive behaviors. The repetitive behavior domain also is heterogeneous (Bodfish et al., 2006; Cuccaro et al., 2003). Relationships may not be found across its four constituent symptom classes, and the continued development of psychometrically valid measures of each is warranted.
The current study has several limitations. First our sample was relatively small, limiting statistical power. Inclusion of more subjects would make it possible to look at developmental findings in a more fine-grained way. Of course a longitudinal versus a cross sectional approach also would be preferable. Inclusion of additional measures of cognitive control matched for task format, task “level” (e.g. perceptual or motor), and task difficulty of the both context processing and response phases of the tasks with the POP would strengthen the findings of this study. Inclusion of another clinical group, and/or a “cleaner” control group (i.e. one without parent reported psychopathology) against which specificity of cognitive control deficits found in ASDs could be assessed, also will be an important next step in this line of research. Finally, it bears mention that this is a sample of highly able individuals with ASDs, and findings may not generalize to lower functioning individuals with the disorders.
Temporal properties of cognitive processing in autism may be altered, and the duration and delay of the cue and probe may be critical in shaping responses to stimuli (Muller, Cauich, Rubio, Mizuno & Courchesne, 2004; Townsend et al., 1999). While this version of the POP task did not reveal statistically significant group differences in RTs, there has been considerable work demonstrating slowed responding in individuals with ASDs which may be the result of abnormalities in the cerebellum, motor regions or both. It would be instructive to develop versions of the POP task that manipulate inter-stimulus and inter-trial intervals, as well as motor demands of tasks to tease apart relative contributions of the cerebellum and basal ganglia (e.g. Ravizza and Ivry, 2001).
Cognitive control has been associated with a reliable network of brain regions including the dorsolateral prefrontal cortex (DLPFC), medial prefrontal cortex (including the anterior cingulate cortex), and parietal cortex (Carter et al., 1998) Numerous studies have investigated the activation of these regions in typically developing individuals during tasks requiring cognitive control. Thus, a cognitive control based model provides a theoretical roadmap for study of the neural substrates of executive deficits using functional neuroimaging (fMRI). Furthermore, a cognitive control based model with its emphasis on the roles of the DLPFC, ACC, and parietal cortex offers theoretical direction for studies of functional connectivity in ASDs. Advancing this area is critical given the few existing neuroimaging studies, and the importance of this area of autism research (Frith, 2003).
Table 4.
Age Group Comparisons
| ASD Group |
Typically Developing Group |
|||
|---|---|---|---|---|
| Ages 8–12 (n=18) | Ages 13–18 (n=14) | Ages 8–12 (n=17) | Ages 13–18 (n=14) | |
| Age | ||||
| M (SD) | 11 (1.2) | 15.2 (1.3) | 10.4 (1.3) | 14.4 (1.7) |
| Range | 9–12.6 | 13.4–17.3 | 8.25–12.8 | 12.9–17.3 |
| FSIQ | ||||
| M (SD) | 113.3 (18.0) | 104.3 (23.1) | 119 (11.8) | 111 (11.2) |
| Range | 76–139 | 72–142 | 102–142 | 99–130 |
| Green Trials | ||||
| Median RT ms (SD) | 637 (314) | 558 (206) | 540 (220) | 570 (205) |
| Range | 324–1325 | 353–1008 | 370–1067 | 351–985 |
| Mean ER (SD) | 0.02 (0.02) | 0.02 (0.02) | 0.02 (0.03) | 0.01 (0.02) |
| Range | 0.00–0.08 | 0.00–0.08 | 0.00–0.14 | 0.00–0.07 |
| Red Trials | ||||
| Median RT ms (SD) | 667 (310) | 566 (222) | 587 (233) | 578 (174) |
| Range | 334–1335 | 354–1053 | 370–1210 | 356–919 |
| Mean ER (SD) | 0.22 (0.18) | 0.32 (0.21) | 0.20 (0.16) | 0.10 (0.09) |
| Range | 0.00–0.54 | 0.13–0.75 | 0.00–0.46 | 0.00–0.33 |
Table 5.
Correlations between POP Error Rates on Red Trials and Demographic Variables
| All Subjects | ASD (n=31) | TYP (n=32) | ||||
|---|---|---|---|---|---|---|
| Pearson r | p-value | Pearson r | p-value | Pearson r | p-value | |
| VIQ | −0.285* | 0.025 | −0.337 | 0.06 | −0.088 | 0.644 |
| PIQ | −0.219 | 0.088 | −0.287 | 0.111 | −0.002 | 0.992 |
| FSIQ | −0.359*** | 0.004 | −0.439* | 0.012 | −0.064 | 0.736 |
| SCQ Total | 0.235 | 0.066 | −0.069 | 0.706 | −0.260 | 0.166 |
| SCQ Social Behavior Domain | 0.265* | 0.037 | 0.036 | 0.845 | −0.202 | 0.285 |
| SCQ Communication Domain | 0.270* | 0.033 | 0.107 | 0.561 | −0.140 | 0.461 |
| SCQ Repetitive Behavior Domain | 0.117 | 0.363 | −0.317 | 0.077 | −0.194 | 0.303 |
| BASC Attention Problems | 0.318** | 0.013 | 0.180 | 0.333 | 0.070 | 0.712 |
p < .05 before false discovery rate (FDR) correction.
p < .05 before FDR and .052 < p < .059 after FDR correction.
p<.05 before and after FDR correction.
Acknowledgments
The authors would like to thank Danh V. Nguyen for statistical assistance and the families participating in the study. During the preparation of this manuscript, Dr. Solomon was supported by NIH K12 HD051958, MIND Institute, and Autism Speaks. Dr. Ozonoff was supported by R01 MH068398 and U19-HD35468. Dr. Carter was supported by MH64190. Statistical support was provided by UL1 RR024146 from National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research (Danh V. Nguyen, UCD Clinical and Translational Research Center).
Abbreviations
- ASDs
autism spectrum disorders
- PDDNOS
Pervasive Developmental Disorder NOS
- DLPFC
dorsolateral prefrontal cortex
- PFC
prefrontal cortex
- ACC
anterior cingulate cortex
- ADHD
attention deficit hyperactivity disorder
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association.; 2000. Text Revision. [Google Scholar]
- Barber AD, Carter CS. Cognitive control involved in overcoming prepotent response tendencies and switching between tasks. Cerebral Cortex. 2005;15(7):899–912. doi: 10.1093/cercor/bhh189. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B. 1995;57:289–300. [Google Scholar]
- Bennetto L, Pennington B, Rogers S. Intact and impaired memory functions in autism. Child Development. 1996;67 (4):1816–1835. [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. British Journal of Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Bishop DV. Annotation: autism, executive functions, and theory of mind: a neuropsychological perspective. Journal of Child Psychology and Psychiatry. 1993;34(3):279–293. doi: 10.1111/j.1469-7610.1993.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Norbury CF. Executive functions in children with communication impairments, in relation to autistic symptomatology. 2: Response inhibition. Autism. 2005;9 (1):29–43. doi: 10.1177/1362361305049028. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Lam KSL, Lewis MH. Empirically-derived phenotypes of repetitive behavior in autism spectrum disorders. International Meeting for Autism Meeting; Montreal, Quebec, Canada. 2006. p. 83. [Google Scholar]
- Braver TS, Cohen JD, Barch DM. The role of prefrontal cortex in normal and disordered cognitive control: A cognitive neuroscience perspective. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford University Press; 2002. pp. 428–447. [Google Scholar]
- Brian JA, Tipper SP, Weaver B, Bryson SE. Inhibitory mechanisms in autism spectrum disorders: typical selective inhibition of location versus facilitated perceptual processing. Journal of Child Psychology and Psychiatry. 2003;44(4):552–560. doi: 10.1111/1469-7610.00144. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33 (2):301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits subserving the retrieval and maintenance of abstract rules. Journal of Neurophysiology. 2003;90 (5):3419–3428. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- Caron C, Rutter M. Comorbidity in child psychopathology: concepts, issues, and research strategies. Journal of Child Psychology and Psychiatry. 1991;32 (7):1063–1080. doi: 10.1111/j.1469-7610.1991.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280 (5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. . Prevalence of Autism Spectrum Disorders-Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2002. Surveillance Summaries, 2007. MMWR. 2007;56 (No. SS-1) [PubMed] [Google Scholar]
- Cepeda NJ, Kramer AF, Gonzalez de Sather JC. Changes in executive control across the life span: examination of task-switching performance. Developmental Psychology. 2001;37 (5):715–730. [PubMed] [Google Scholar]
- Cohen JD, Servan-Schreiber D. Context, cortex and dopamine: A connectionist approach to behavior and biology in schizophrenia. Psychological Review. 1992;99:45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Constantine LJ. Autism and attention deficit hyperactivity disorder: assessing attention and response control with the integrated visual and auditory continuous performance test. Child Neuropsychology. 2006;12 (4–5):335–348. doi: 10.1080/09297040500350938. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3 (3):292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Crone EA, Ridderinkhof KR, Worm M, Somsen RJ, van der Molen MW. Switching between spatial stimulus-response mappings: a developmental study of cognitive flexibility. Developmental Science. 2004;7 (4):443–455. doi: 10.1111/j.1467-7687.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- Cuccaro ML, Shao Y, Grubber J, Slifer M, Wolpert CM, Donnelly SL, Abramson RK, Ravan SA, Wright HH, DeLong GR, Pericak-Vance MA. Factor analysis of restricted and repetitive behaviors in autism using the Autism Diagnostic Interview-R. Child Psychiatry and Human Development. 2003;34 (1):3–17. doi: 10.1023/a:1025321707947. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amos D, Anderson LC, Diamond A. Development of cognitive control and executive function from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44 (11):2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nature neuroscience. 2005;8 (12):1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Frith C. What do imaging studies tell us about the neural basis of autism? In: Bock G, Goode J, editors. Autism: Neural basis and treatment possibilities: Novartis Foundation symposium. Chichester, UK: John Wiley & Sons, Ltd.; 2003. pp. 149–166. [PubMed] [Google Scholar]
- Garner JP, Meehan CL, Mench JA. Stereotypies in caged parrots, schizophrenia and autism: evidence for a common mechanism. Behavioural Brain Research. 2003;145 (1–2):125–134. doi: 10.1016/s0166-4328(03)00115-3. [DOI] [PubMed] [Google Scholar]
- Georgiades S, Szatmari P, Zwaigenbaum L, Duku E, Bryson S, Roberts W, Goldberg J, Mahoney W. Structure of the autism symptom phenotype: A proposed multidimensional model. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46 (2):188–196. doi: 10.1097/01.chi.0000242236.90763.7f. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Verte S, Oosterlaan J, Roeyers H, Sergeant JA. How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism? Journal of Child Psychology and Psychiatry. 2004;45 (4):836–854. doi: 10.1111/j.1469-7610.2004.00276.x. [DOI] [PubMed] [Google Scholar]
- Happe F, Booth R, Charlton R, Hughes C. Executive function deficits in autism spectrum disorders and attention-deficit/hyperactivity disorder: examining profiles across domains and ages. Brain and Cognition. 2006;61 (1):25–39. doi: 10.1016/j.bandc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA. Task feedback effects on conflict monitoring and executive control: relationship to subclinical measures of depression. Emotion. 2007;7 (1):68–76. doi: 10.1037/1528-3542.7.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. Executive dysfunction in autism: its nature and implications for the everyday problems experienced by individuals with autism. In: Burack AA, Charman T, Yirmiya N, Zelazo PR, editors. The Development of Autism. Mahwah, NJ: Lawrence Erlbaum Associates; 2001. pp. 255–275. [Google Scholar]
- Joseph RM. Neuropsychological Frameworks for Understanding Autism. International Review of Psychiatry. 1999;11 (4):309–324. doi: 10.1080/09540269974195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RM, Tager-Flusberg H. The relationship of theory of mind and executive functions to symptom type and severity in children with autism. Development and Psychopathology. 2004;16 (1):137–155. doi: 10.1017/S095457940404444X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Johnson MK, Stenger VA, Aizenstein H, Carter CS. Decreased conflict- and error-related activity in the anterior cingulated cortex in subjects with schizophrenia. American Journal of Psychiatry. 2005;162 (10):1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Goldberg MC. Language, social, and executive functions in high functioning autism: a continuum of performance. Journal of Autism and Developmental Disorders. 2005;35 (5):557–573. doi: 10.1007/s10803-005-0001-1. [DOI] [PubMed] [Google Scholar]
- Lewis MH, Bodfish JW. Mental Retardation & Developmental Disabilities Research Reviews. Special Autism. 1998;4 (2):80–89. doi: 10.1002/mrdd.20045. [DOI] [PubMed] [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of Autistic Disorder. Journal of Autism and Developmental Disorders. 2005;35 (4):445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule--Generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism & Developmental Disorders. 2000;30 (3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-- Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism & Developmental Disorders. 1994;24 (5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Development. 2005;76 (3):697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biological Psychiatry. 2007;61 (4):474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Carter CS. Cognitive experimental approaches to investigating impaired cognition in schizophrenia: a paradigm shift. Journal of Clinical and Experimental Neuropsychology. 2002;24 (7):873–882. doi: 10.1076/jcen.24.7.873.8386. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288 (5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Muller RA, Cauich C, Rubio MA, Mizuno A, Courchesne E. Abnormal activity patterns in premotor cortex during sequence learning in autistic patients. Biological Psychiatry. 2004;56:323–332. doi: 10.1016/j.biopsych.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Nyden A, Gilberg C, Hjelmquist E, Heiman M. Executive function/attention deficits in boys with Asperger syndrome, attention disorder and reading/writing disorder. Autism. 1999;3 (3):213–228. [Google Scholar]
- Ozonoff S. Executive functions in autism. In: Schopler E, Mesibov GB, editors. Learning and cognition in autism. Current issues in autism. New York: Plenum Press.; 1995. pp. 199–219. [Google Scholar]
- Ozonoff S. Components of executive function in autism and other disorders. In: Russell J, editor. Autism as an executive disorder. London: Oxford University Press.; 1997. [Google Scholar]
- Ozonoff S, Jensen J. Specific executive function profiles in three neurodevelopmental disorders. Journal of Autism & Developmental Disorders. 1999;29 (2):171–177. doi: 10.1023/a:1023052913110. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, McEvoy RE. A longitudinal study of executive function and theory of mind development in autism. Development and Psychopathology. 1994;6 (3):415–431. [Google Scholar]
- Ozonoff S, Strayer DL. Inhibitory function in nonretarded children with autism. Journal of Autism & Developmental Disorders. 1997;27 (1):59–77. doi: 10.1023/a:1025821222046. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Strayer DL. Further evidence of intact working memory in autism. Journal of Autism & Developmental Disorders. 2001;31 (3):257–263. doi: 10.1023/a:1010794902139. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Strayer DL, McMahon WM, Filloux F. Executive function abilities in autism and Tourette syndrome: an information processing approach. Journal of Child Psychology & Psychiatry & Allied Disciplines. 1994;35 (6):1015–1032. doi: 10.1111/j.1469-7610.1994.tb01807.x. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff SJ. Executive functions and developmental psychopathology. Journal of Child Psychology & Psychiatry & Allied Disciplines Special Issue: Annual research review. 1996;37 (1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Ivry RB. Comparison of the basal ganglia and cerebellum in shifting attention. Journal of Cognitive Neuroscience. 2001;13:285–297. doi: 10.1162/08989290151137340. [DOI] [PubMed] [Google Scholar]
- Raymaekers R, van der Meere J, Roeyers H. Response inhibition and immediate arousal in children with high-functioning autism. Child Neuropsychology. 2006;12 (4–5):349–259. doi: 10.1080/09297040600760457. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW. Behavior assessment system for children. Circle Pines, MN: American Guidance Service.; 1992 . [Google Scholar]
- Roberts RJ, Jr, Pennington BF. An interactive framework for examining prefrontal cognitive processes. Developmental Neuropsychology. 1996;12 (1):105–126. [Google Scholar]
- Rosano C, Aizenstein H, Cochran J, Saxton J, De Kosky S, Newman AB, Kuller LH, Lopez OL, Carter CS. Functional neuroimaging indicators of successful executive control in the oldest old. Neuroimage. 2005;28 (4):881–889. doi: 10.1016/j.neuroimage.2005.05.059. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. SCQ: Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services.; 2003. [Google Scholar]
- Snitz BE, MacDonald A, III, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naïve state and effects of short-term atypical antipsychotic treatment. American Journal of Psychiatry. 2005;162:2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- South M, Klin A, Ozonoff S. Yale Special Interests Interview. 1999 Unpublished manuscript. [Google Scholar]
- South M, Ozonoff S, McMahon WM. Repetitive behavior profiles in Asperger syndrome and high-functioning autism. Journal of Autism & Developmental Disorders. 2005;35 (2):145–158. doi: 10.1007/s10803-004-1992-8. [DOI] [PubMed] [Google Scholar]
- Sturm H, Fernell E, Gillberg C. Autism spectrum disorders in children with normal intellectual levels: associated impairments and subgroups. Developmental Medicine and Child Neurology. 2004;46:444–447. doi: 10.1017/s0012162204000738. [DOI] [PubMed] [Google Scholar]
- Townsend J, Courchesne E, Covington J, Westerfield M, Singer Harris N, Lyden P, Lowry TP, Press GA. Spatial orienting deficits in patients with acquired or developmental cerebellar abnormality. Journal of Neuroscience. 1999;19(13):5632–5643. doi: 10.1523/JNEUROSCI.19-13-05632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. Towards an executive dysfunction account of repetitive behavior in autism. In: Russell J, editor. Autism as an executive disorder. New York: Oxford University Press.; 1997. pp. 57–100. [Google Scholar]
- Verte S, Geurts HM, Roeyers H, Oosterlaan J, Sergeant JA. Executive functioning in children with an Autism Spectrum Disorder: can we differentiate within the spectrum? Journal of Autism & Developmental Disorders. 2006;36 (3):351–372. doi: 10.1007/s10803-006-0074-5. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment.; 1999. [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Developmental Psychology. 1999;35(1):205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Williams DL, Goldstein G, Carpenter PA, Minshew NJ. Verbal and Spatial Working Memory in Autism. Journal of Autism and Developmental Disorders. 2005;35 (6):747–756. doi: 10.1007/s10803-005-0021-x. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Gray JR, Chrastil ER, Barch DM, Green L, Braver TS. Sustained neural activity associated with cognitive control during temporally extended decision making. Cognitive Brain Research. 2005;23:71–84. doi: 10.1016/j.cogbrainres.2005.01.013. [DOI] [PubMed] [Google Scholar]