Summary
The organ of Corti, the auditory organ of the inner ear, contains two types of sensory hair cells and at least seven types of supporting cells. Most of these supporting cell types rely on Notch-dependent expression of Hes/Hey transcription factors to maintain the supporting cell fate. Here we show that Notch signaling is not necessary for the differentiation and maintenance of pillar cell fate, that pillar cells are distinguished by Hey2 expression, and that – unlike other Hes/Hey factors – Hey2 expression is Notch-independent. Hey2 is activated by FGF and blocks hair cell differentiation, while mutation of Hey2 leaves pillar cells sensitive to the loss of Notch signaling and allows them to differentiate as hair cells. We speculate that co-option of FGF signaling to render Hey2 Notch-independent, also liberated pillar cells from the need for direct contact with surrounding hair cells, and enabled evolutionary remodeling of the complex cellular mosaic of the inner ear.
Introduction
The Notch signaling pathway mediates many inductive interactions in vertebrate and invertebrate development (Artavanis-Tsakonas et al., 1999; Gridley, 2007; High and Epstein, 2008; Lai, 2004; Louvi and Artavanis-Tsakonas, 2006; Maillard et al., 2005; Weinmaster and Kopan, 2006). The many circumstances in which Notch signaling is used prompt the question of whether this pathway is sufficient to specify intricate arrangements of differentiated cell types. The development of the organ of Corti, the auditory organ of the inner ear of mammals, is one of the most striking examples of how these multiple roles help choreograph the numerous cell-cell interactions required to form a complex structure (Barald and Kelley, 2004; Kelley, 2006, 2007). The organ of Corti is composed of a rigidly stereotyped array of one row of inner hair cells and three rows of outer hair cells running along the entire length of the cochlear sensory epithelium (Fig. 1A; B). Each hair cell is surrounded by specialized supporting cells, - inner phalangeal cells, which lie beneath each inner hair cell, and three or four Deiters’ cells which lie beneath outer hair cells. In addition, the inner and outer hair cell regions are separated by two specialized supporting cells – inner and outer pillar cells - which form the sides of the tunnel of Corti in the mature organ (Fig. 1A), and which are required for proper biomechanical function.
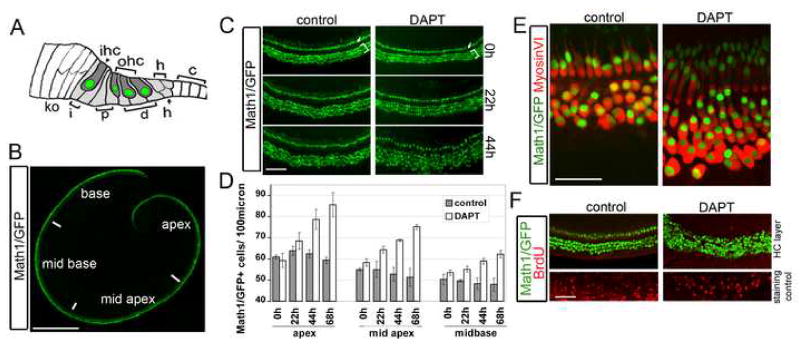
Fig. 1. Treatment of neonatal organ of Corti explants with the gamma-secretase inhibitor DAPT induces ectopic hair cells.
A: Schematic of the organ of Corti at postnatal day 0 (P0). Inner (ihc) and outer (ohc) hair cells (green nuclei) are surrounded by supporting cell subtypes: Inner phalangeal cells (i), inner and outer pillar cells (p), Deiters’ cells (d) and Hensen cells (h). B: Whole mount of a neonatal Math1/GFP transgenic organ of Corti. Math1/GFP expression labels hair cells (green). C: Time course of ectopic hair cell production in neonatal cochlear organ cultures in response to DAPT. Arrow marks inner and bracket marks outer hair cells (Math1/GFP, green). D: Quantification of C. Similar results were obtained in two independent preparations (error bar ± s.e.m.). E: Ectopic Math1/GFP+ cells (green) in DAPT express MyosinVI (red). F: No BrdU incorporation (red) is seen in Math1/GFP+ hair cells (green) in control or DAPT treated cochlear organs. The mesenchymal layer of cochlear organs (taken from a confocal plane below the basal lamina of the sensory epithelium) was used as BrdU staining control. Scale bar: 0.5mm in A; 50μm in C, E, F.
Evidence from birds and mice suggests that one function of Notch signaling is to negatively regulate hair cell fate during organ of Corti development (Adam et al., 1998; Brooker et al., 2006; Eddison et al., 2000; Kiernan et al., 2005; Lanford et al., 1999; Takebayashi et al., 2007). Consistent with this lateral inhibition model, conditional deletion of Notch1 in the inner ear, or deletion of Dll1 and Jag2, two Notch ligands expressed in hair cells, leads to an overproduction of hair cells (Brooker et al., 2006; Kiernan et al., 2006). However, a simple model of Notch-dependent lateral inhibition cannot account for the highly asymmetric pattern of hair cell and supporting cell differentiation, particularly with regard to inner pillar cells which appear to develop without contact from hair cells that express Notch ligands.
We have investigated the relationship between Notch signaling and the stability of the differentiated state of supporting cells. By disrupting Notch signaling with pharmacological inhibitors or in mutant mice lacking the Notch1 receptor or the Notch effector RBPJ, we show that while most types of supporting cells readily convert into hair cells consistent with a lateral inhibition model, pillar cells do not. We show the organ of Corti is divided into compartments on the basis of combinatorial expression of Hes and Hey Notch effectors. In particular, we show that Hey2 is regulated by FGF signaling in a Notch-independent fashion in pillar cells, and that this may account for the stability of inner pillar cell fate in the absence of contact with hair cells. We suggest that the establishment of complex Hes/Hey expression patterns, some of which are regulated by alternative signaling pathways including the FGF pathway, underlies the highly asymmetric cellular pattern of the organ of Corti.
Materials and Methods
Mouse breeding and genotyping
Mouse experiments were approved by the House Ear Institute IACUC committee. The Math1/GFP transgenic line was obtained from Jane Johnson (Lumpkin et al., 2003). The Hey2 mutant line has been described previously (Fischer et al., 2004). Both lines were maintained on a CD1 background. To obtain Hey2−/− Math1/GFP+ and wild type Math1/GFP+ littermates, Hey2−/+ mice were crossed with Math1/GFP+ mice and the Hey2−/+ Math1/GFP+ offspring were intercrossed resulting in 25% Hey2−/− Math1/GFP+and 25% Hey2+/+ Math1/GFP+ pups. Mice were genotyped using PCR. Hey2 mutant and wild type alleles: Hey2-1: (TCGGTGAATTGGACCTCATCACTGAGC), Hey2-2: (GCTGTCTCAAGGCCTCAACAGCATTG), Hey2-3 (ATCGGTGCGGGCCTCTTCGCTATTA).
Conditional inactivation of Notch1 and RBPJ in the inner ear
Mice homozygous for conditional alleles of either Notch1 (Pan et al., 2004) or RBPJ (Han et al., 2002) were crossed with Pax2-Cre mice (Ohyama and Groves, 2004) that were also heterozygous for null mutation in either gene. Primers for genotyping are listed in Supplementary Methods.
Organotypic cochlear culture
Tissue isolation
Cochleas of stage E13.0–E14.5 embryos were collected in PBS (Invitrogen). To free the cochlear duct from surrounding condensed mesenchyme, tissue was incubated in calcium-magnesium free PBS (Invitrogen) containing dispase (1mg/ml; Invitrogen) and collagenase (1mg/ml; Worthington) as previously described (Doetzlhofer et al., 2004). Cochleas of neonatal pups were dissected in Hanks solution (Invitrogen). To obtain a flat cochlear surface preparation the spiral ganglia, Reissner’s membrane and the most basal cochlear segment were removed (average cochlear explant length 4350 +/− 350μm, excluding the basal 1300μm). For Q-PCR experiments both cochlear base and apex were removed and only cochlear mid-turn was used. (average length 3200+/− 250 μm).
Culture
Neonatal and embryonic cochlear explants were cultured on SPI black membranes (Spi supplies) in DMEM-F12 (Invitrogen) with B27 supplement (Invitrogen), 5ng/ml EGF (Sigma) and 2.5ng/ml FGF2 (NIH). For experiments requiring live imaging, explants were plated onto 8 well CC2 Lab-Tek II chamber slides (Nunc) coated with poly-D-lysine (0.5 mg/ml; Sigma) and fibronectin (25 μg/ml; Gibco). All cultures were maintained in a 5% CO2/20% O2 humidified incubator (Forma Scientific).
Electroporation
E13.5 cochlear ducts were placed in a home made electroporation chamber (hollowed acrylic square with a dialysis membrane attached to the bottom) in a modified Petri dish containing a single electrode. A 1–2μg/μl DNA solution in 0.5% Fast Green and 10% sucrose was used to allow easy introduction of DNA onto the explants. 8 to 9 30V square wave pulses of 50 ms were applied. The following expression plasmids were used: Math1: pCBA-Math-1 (1.2μg/ul); Hey2: pCS2-Hey2 (1.8μg/ul); GFP:, pCIG (1μg/ul). Empty pCS2 or pCBA vectors were used to maintain a constant amount of electroporated DNA
In vitro manipulation of Notch and FGF signaling
DAPT (γ-secretase inhibitor IX, Calbiochem-EMD Biosciences) was stored as a 25mM stock in DMSO at −80°C and used at a final concentration of 3μM. Control explants received 0.08% DMSO. DAPT was added the morning after cultures were prepared. To determine if DAPT causes proliferation of neonatal supporting cells, 3μm BrdU was added at the start of the 72h culture period. FGFR inhibitor SU5402 (3-[3-(2-Carboxyethyl)-4-methylpyrrol-2-methylidenyl]-2-indolinone, EMD Biosciences) in DMSO was used at a final concentration of 10μM. Fgf17 (R&D) in PBS/1%BSA (60μg/ml Stock) was used at final concentration of 300ng/ml (Jacques et al., 2007) along with DMSO (final concentration 0.1%) and heparin (final concentration 1μg/μl). If not otherwise stated hair cell and supporting cell phenotype was analyzed after 72h.
RNA Extraction and Real Time PCR
For RNA extraction three cochlear cultures were pooled and total RNA was isolated using a QIAGEN RNeasy Micro kit. cDNA was synthesized using TaqmanR Reverse Transcription Reagents (Applied Biosystems). QPCR was performed with a Master SYBR Green kit (Applied Biosystems) and gene-specific primer sets on a 7500 Real time PCR Detection System (Applied Biosystems). Each PCR reaction was carried out in triplicate. Relative gene expression was analysed using the ΔΔCT method (Livak and Schmittgen, 2001). cDNA from neonatal cochlear explants was used as a calibrator and a ribosomal gene (L19) and E-cadherin were used as endogenous references. Gene-specific Primer sets are listed in Supplementary Methods.
In situ hybridization
E14.5, E16.5 or P1 inner ears were fixed in 4% paraformaldehyde in PBS overnight at 4°C, sunk in 30% sucrose in PBS at 4°C, incubated in OCT at room temperature for 1 hour, and frozen in liquid nitrogen. Digoxigenin-labeled antisense ribroprobes to mouse Hey1, Hey2, HeyL and Hes5 were synthesized using standard protocols (Stern, 1998). Plasmids containing full length mouse Hey1, HeyL and Hey2 cDNAs were provided by Manfred Gessler, and a plasmid containing a full length mouse Hes5 cDNA was provided by Ryoichiro Kageyama. The in situ hybridization procedure was modified from a protocol by Domingos Henrique (Henrique et al., 1995). Detailed protocols are available on request.
Immunohistochemistry
Antibodies used in this study were anti-BrdU (RDI), anti-p27Kip1 (NeoMarker), anti-parvalbumin, clone PARV-19 (Sigma), anti-myosin-VI (Proteus), anti-p75NGFR (Chemicon), anti-Prox1 (Chemicon) and anti-Hey2. Hey2 antibody production: A fragment from the mouse Hey2 gene coding for aa 2 - 37 (krpceettsesdldetidvgsennypghatssvmrsn) was expressed in bacteria as a GST fusion protein and injected into New Zealand white rabbits (IMGENEX). Antisera was purified by affinity chromatography and specificity was tested using Hey2−/− tissue as control (details upon request). Fluorescently labeled secondary antibodies were from Jackson ImmunoResearch. For anti-p27Kip1 staining, sections were boiled for 10 minutes in 10mM citric acid pH 6.0. For anti-BrdU staining, cultures were hydrolyzed in 2N HCl for 10 minutes. Cell nuclei were fluorescently labeled using Hoechst-33258 (Sigma).
Cell counts
Inner hair cell (IHC) outer hair cell (OHC) and supporting cell counts were performed on cochlear whole mounts. Hair cells and supporting cells (Deiters’ and pillar cells) were identified with Myosin VI and Prox1 antibodies respectively. High power images of the full length cochlea or cochlear explant cultures were assembled and analyzed in PhotoShop CS2 (Adobe). ImageJ software (NIH) was used to measure the total length of cochlear whole mounts and the length of individual counted segments. For Hey mutants and their wild type littermates, the total number of IHC or OHC was counted in each of four cochlear segment of 1200–1400μm (apical, mid-apical, mid-basal and basal). Density (cells per 100μm) was then calculated for each segment. These numbers were averaged to calculate the hair cell density (IHC/100micron and OHC/100micron) for each cochlea. For Notch1 mutants the mid basal segment was used to calculate hair cell and supporting cell density.
Results
Notch signaling is not required to maintain pillar cell fate
Loss of Notch signaling in the neonatal organ of Corti produces ectopic hair cells (Yamamoto et al., 2006). This was demonstrated by blocking Notch activity through mutation of the Notch effector gene CSL1/RBPJ, or with γ-secretase inhibitors, which block cleavage of Notch and release of the Notch intracellular domain that co-operates with CSL1/RBPJ to activate transcription of Notch-responsive genes (Dovey et al., 2001). We confirmed these experiments using cultured neonatal mouse organ of Corti in the presence or absence of the γ-secretase inhibitor DAPT. We monitored our cultures with Math1/GFP transgenic mice, which express GFP in hair cells (Lumpkin et al., 2003; Fig. 1B - brackets: outer hair cells, arrow: inner hair cells). Addition of DAPT to cochlear organ cultures dramatically increased GFP+ cells compared to controls (Fig. 1C; D), and the appearance of new GFP+ cells continued until at least 68 hours of DAPT treatment. We confirmed the hair cell identity of new Math1-GFP+ cells with the hair cell marker MyosinVI (Fig. 1E). Ectopic hair cells were induced throughout the organ of Corti with a maximum response in the apical region (Fig. 1D). Interestingly, we observed no proliferation (measured by BrdU incorporation) within the sensory epithelium of DAPT-treated or control explants (Fig. 1F).
These results suggest that the supernumerary hair cells that appear following DAPT treatment arise by direct trans-differentiation of postmitotic supporting cells. To further characterize the effects of blocking Notch signaling, we examined the expression of supporting cell markers in our cultures. Pillar cells and Deiters’ cells express the transcription factor Prox1 (Bermingham-McDonogh et al., 2006), which, in control explants labels two rows of pillar cells (Fig. 2A, yellow bracket) and three to four rows of Deiters’ cells (Fig. 2A, white bracket). The number of Prox1+ cells in the Deiters’ cell region was reduced throughout the DAPT-treated organ of Corti explants, in parallel with the increase in Math1/GFP+ hair cells (Fig. 2A–C) and after 72 hours of DAPT treatment only a few Deiters’ cells of the inner most row of Deiters’ cells remained in basal regions of the cochlea (Fig. 2A). The correlation between the increase in hair cells and the decrease in Prox1+ cells in the presence of DAPT suggests that many Prox1+ supporting cells trans-differentiate into hair cells in the absence of Notch signaling.
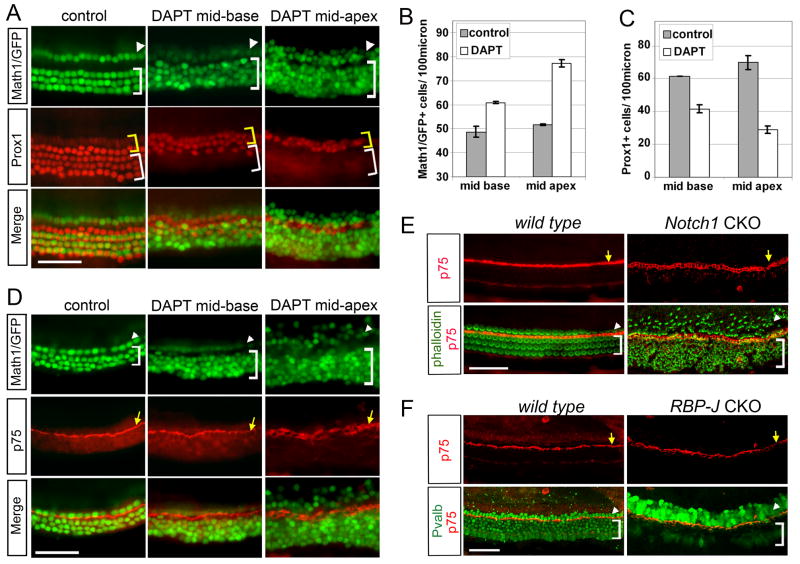
Fig. 2. Deiters’ cells, but not pillar cells, trans-differentiate into hair cells in the absence of Notch signaling.
A–D: P0 Math1/GFP transgenic cochlear explants cultured for 72 hours in the presence of DAPT or DMSO (control). A: Ectopic hair cells (green) in DAPT are accompanied by a loss of Prox1+ cells (red) in the Deiters’ cell region (white bracket). B–C: Quantification of Math1/GFP+ hair cells (B) and Prox1+ supporting cells (C) after 72 hours with and without DAPT treatment. The number of Math1/GFP+ hair cells and Prox1+ supporting cells of mid-apical and mid-basal regions of cochlear explants was normalized to 100μm (n=5; error bar ± s.e.m.). D: Pillar cells persist in the absence of Notch signaling: p75 antibody staining (red) marks pillar cells in control (DMSO treated) and DAPT treated cochlear explants (yellow arrowhead). E-: Pillar cells are maintained in P0 Notch1 mutant organ of Corti: Phalloidin staining (green) labels the actin rich hair cell bundles (stereocilia). p75 antibody staining (red) labels the apical tips of pillar cells (yellow arrow). F: Pillar cell differentiation is unaffected in the RBP-J mutant organ of Corti. E13 RBP-J mutant and wild type cochlear explants were cultured for 4 days. p75 antibody staining labels pillar cells (red, yellow arrow). Parvalbumin staining labels inner (green, arrowhead) and outer hair cells (green, bracket). Scale bar: 50μm in A, D, E, F.
We noted that Prox1+ cells in the pillar cell region of our explants typically failed to convert to hair cells in the presence of DAPT (Fig. 2A, yellow bracket). To verify that these remaining Prox1+ cells were pillar cells, we used antibodies to the p75 low affinity NGF receptor (p75) which is strongly expressed in the apical projections of neonatal pillar cells (Jacques et al., 2007; Mueller et al., 2002; von Bartheld et al., 1991) and is visualized as staining between the inner hair cell and outer hair cell regions (Fig. 2D, yellow arrow). Neonatal organ of Corti explants cultured in DAPT showed strong p75 staining in the pillar cell region, dividing the organ of Corti into inner and outer hair cell regions (Fig. 2D). To test if Notch signaling is also required for pillar cell differentiation, we cultured embryonic (E14.5) cochlear explants for 24 hours and then blocked Notch with DAPT signaling for a further 48 hours. The persistence of Prox1+ and p75+ cells in the pillar cell region of E14.5 cochlear organs cultured in the presence of DAPT (Supplemental Fig. S1A and B) suggests that pillar cell differentiation in the embryonic cochlea does not require Notch signaling.
Gamma secretase complexes cleave a number of transmembrane proteins in addition to Notch receptors, such as ErbB and insulin receptors, the amyloid precursor protein APP, CD44 and EphrinB2 (Esler and Wolfe, 2001; Georgakopoulos et al., 2006; Lammich et al., 2002; McElroy et al., 2007; Sardi et al., 2006). To confirm that our results with DAPT were due to inhibition of Notch signaling, we examined Notch1 or RBPJ mutant mice. We inactivated Notch1 or RBPJ conditionally in the inner ear with Pax2-Cre mice (Ohyama and Groves, 2004). Notch1 mutants were examined at postnatal day 1, but since RBPJ;Pax2-Cre conditional mice die at E13.5 due to kidney defects (Cheng et al., 2007), cochleas from E13 RBPJ;Pax2-Cre embryos were cultured for four days in vitro. As previously reported, (Kiernan et al., 2005) conditional Notch1 mutants showed significantly more hair cells compared to wild type as shown by phalloidin staining (Fig. 2E). Conditional RBPJ mutants showed excess inner hair cells (Fig. 2F), but the outer hair cells appeared to die during the culture period (data not shown). Nevertheless, as in our DAPT-treated cultures, pillar cells appeared unaffected by either Notch1 or RBPJ mutations, as shown by the persistence of p75 expression in the pillar cell region (Fig. 2E; F).
The organ of Corti is compartmentalized by the expression of Hes and Hey transcription factors that show differential requirements for Notch signaling
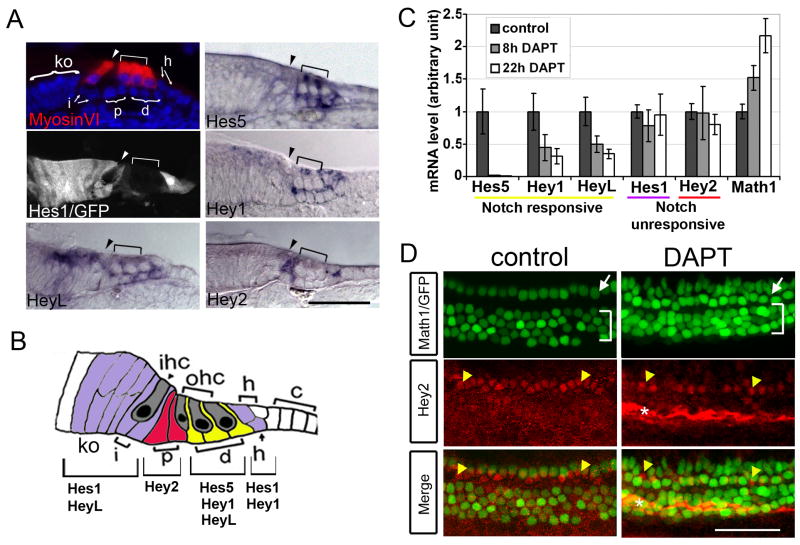
To understand why most supporting cells, but not pillar cells trans-differentiate into hair cells when Notch signaling is blocked, we examined expression of the Hes/Hey family of bHLH repressors, which are known to be targets of the Notch pathway (Fischer and Gessler, 2007; Iso et al., 2003). In agreement with previously published data (Zheng et al., 2000; Zine et al., 2001), Hes1 expression in the organ of Corti is expressed in a region of epithelial cells medial to inner hair cells known as Kölliker’s organ (Ko) (as visualized by a Hes1/GFP BAC transgenic mouse line; Fig. 3A, large bracket). Hes1-GFP was also seen in inner phalangeal cells and in Hensen’s cells, whereas Hes5 is detected in Deiters’ cells. Additionally, we examined the expression of three Hes-related genes, Hey1, Hey2 and HeyL. Prior to the onset of hair cell differentiation, Hey1 and Hey2 are expressed along with p27Kip1, a cyclin-dependent kinase inhibitor, throughout the pro-sensory domain. As hair cell differentiation commences in the organ of Corti between E14.5 and E16.5, Hey1 and Hey2 expression refines to distinct supporting cell populations (Hayashi et al., 2008; Li et al., 2008) (Supplemental Fig. S2A). Following the basal to apical gradient of hair cell differentiation, the initially broad Hey2 protein expression domain is progressively restricted to future pillar cells (Supplemental Fig. S2B, white bracket). In the neonatal organ of Corti Hey1 expression is detected in the outer hair cell region, including Deiters’ cells, and Hensen’s cells, and Hey2 continues to be expressed in pillar cells and is also weakly expressed in Hensen’s cells (Fig. 3A). HeyL is not detected in the organ of Corti prior to hair cell differentiation (data not shown), as has also been observed for Hes1 and Hes5 (Lanford et al., 2000; Zine et al., 2001). At neonatal stages, HeyL is co-expressed in inner phalangeal cells, Kölliker’s organ and Deiters’ cells (Fig. 3A). Together, our data suggest that different supporting cell types in the early postnatal organ of Corti are defined by combinations of Hes and Hey genes, with Hey2 defining pillar cells; Hes5, Hey1 and HeyL defining Deiters’ cells; Hes1 and HeyL defining the inner phalangeal cells and Kölliker’s organ; and Hes1 and Hey1 defining Hensen cells (Fig. 3B).
Fig. 3. Notch signaling is not necessary for Hey2 expression in pillar cells.
A: Expression of Hes5, Hey1, Hey2 and HeyL transcripts and Hes1/GFP transgene in the neonatal (P0-P2) organ of Corti. Hair cells are visualized by MyosinVI staining (red). Arrowheads point to inner hair cells and bracket marks outer hair cells. Two brackets point to nuclei (blue) of pillar cells (p) and Deiters’ cells (d); arrows point to Hensen (h) and inner phalangeal cells (iph). Large bracket marks Köllikers organ (ko). B: Schematic of the organ of Corti indicating the organization of expression patterns of Hes and Hey genes. C: Hey2 and Hes1 mRNA levels are unchanged in the presence of DAPT. Relative expression levels (QPCR) of Math1 and Hey2, Hes1, HeyL, Hey1, Hes5 mRNA in stage P1 cochlear organs exposed for 8h DAPT (gray bar), 22h DAPT (white bar) and 22h DMSO vehicle control (black bar; n=3; error bars ± s.e.m.). D: Hey2 protein expression is maintained in pillar cells in the absence of Notch signaling. Math1/GFP transgenic P1 cochlear organs were cultured for 48 hours in the presence of DAPT or DMSO (control) and stained with Hey2 antibody (red). Math1/GFP expression (green) labels inner (white arrow) and outer hair cells (white bracket). Yellow arrowhead marks pillar cells. * Non-specific binding of Hey2 antibody to extracellular matrix. Scale bar: 50μm in A, D.
Since Hes and Hey gene family members are frequently targets of Notch signaling, we tested whether their expression in the organ of Corti was affected by treatment with DAPT. DAPT treatment of neonatal explants caused a complete loss of Hes5 and a significant decrease in Hey1 and HeyL mRNA within 22 hours (Fig. 3C). In contrast, Hey2 and Hes1 mRNA levels did not change significantly in DAPT-treated explants [DAPT] (Fig. 3C). Moreover, 48 hours of DAPT treatment had no significant effect on Hey2 protein expression in pillar cells (Fig. 3D). Higher concentrations of DAPT (10μM) or increased duration of DAPT treatment (72 hours) failed to reduce Hes1 or Hey2 expression levels (data not shown), suggesting that Notch signaling is not necessary for the maintenance of Hey2 or Hes1 in the neonatal organ of Corti.
Hey2 is essential for maintaining a pillar cell fate in the absence of Notch signaling, and blocks the hair cell-promoting activity of Math1
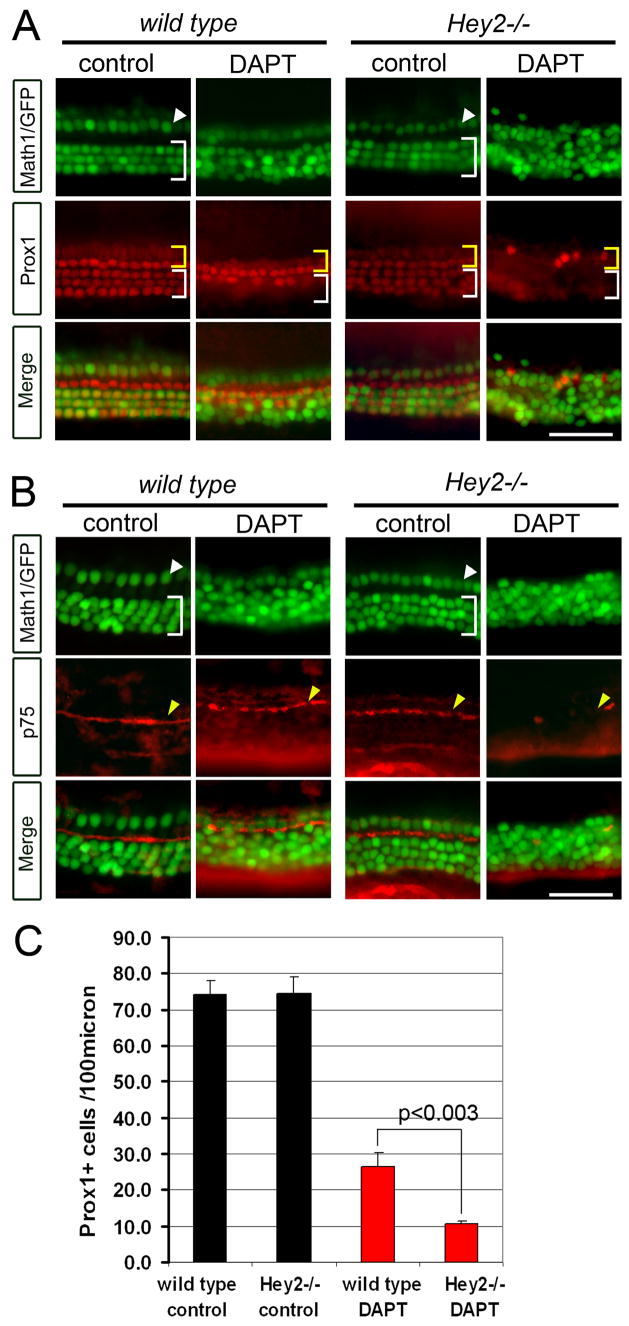
Since Notch signaling is not necessary for the expression of Hey2 (Fig. 3C, D) or pillar cell identity (Fig. 2 and Supplemental Fig. S1), we hypothesized that expression of Hey2 prevents pillar cell trans-differentiation in the absence of Notch signaling. We predicted that blocking Notch signaling in Hey2 mutant mice would allow pillar cells to transform into hair cells. We tested this by treating neonatal Hey2 mutant cochlear explants with DAPT for 72 hours and assaying for the presence of pillar cells. As in our previous experiments, wild type explants cultured in DAPT showed ectopic hair cells, a significant reduction in Prox1+ cells, but a persistence of Prox1+ and p75+ cells in the pillar cell region (Fig. 4A–C). However, Hey2 mutant explants treated with DAPT showed a further reduction in Prox1+ cells and contained virtually no p75+ cells, indicating that Hey2 expression is necessary to maintain pillar cells in the absence of Notch signaling (Fig. 4A–C).
Fig. 4. Hey2 is required to maintain pillar cell fate in the absence of Notch signaling.
A–C: P0 Math1/GFP+ wild type and mutant Hey2 (Hey2−/−) cochlear explants were cultured for 72 hours in DAPT or DMSO (control). A: DAPT treated Hey2−/− cochlear explants have strongly reduced numbers of Prox1+ cells (red) in pillar cell region (yellow bracket) and no Prox1+ cells in Deiters’ cell region (white bracket). B: Pillar cell specific p75 staining (red, yellow arrow) confirms the severe loss of pillar cells in Hey2−/− cochlear explants in DAPT. C: Quantification of Prox1+ cells/100 μm in control wild type and Hey2 mutant cultures (black bars) and DAPT treated cultures (red bars). For each condition a minimum of three cochlear cultures from three independent experiments were analyzed (error bars ± s.e.m.). Scale bar: 50μm in A, B.
Although Hey2 expression is apparently limited to pillar cells and is necessary to maintain pillar cell fate in the absence of Notch signaling, loss of Hey2 results only in a minor change in inner and outer hair cell density (Supplemental Fig. S3A–C) and overall hair cell and pillar cell patterning remains indistinguishable from wild type (Supplemental Fig. S4A). This failure of pillar cells to trans-differentiate into hair cells as a result of Hey2 mutation was somewhat surprising, as Hey2 is the only Hes or Hey gene whose expression is detectable in this cell type in neonatal mice (Fig. 3A). Further examination of Hey2 mutants suggested the existence of cross-inhibitory interactions between Hey2 and other Hes and Hey genes. In particular, Hes5 expression is up-regulated in pillar cells in Hey2 mutants (Supplemental Fig. S4B), suggesting that Hey2 normally represses Hes5 expression in pillar cells.
Our results suggest that Hey2 expression in pillar cells is responsible for blocking their conversion to hair cells when Notch signaling is lost. Earlier studies have indicated that Math1 is both necessary and sufficient in the ear for hair cell differentiation (Bermingham et al., 1999; Zheng and Gao, 2000). In addition, Hes1 is sufficient to block production of hair cells by Math1 (Zheng et al., 2000). Since Hes and Hey genes are structurally and functionally highly conserved (Iso et al., 2003), we tested whether Hey2 is similarly able to suppress the hair cell promoting activity of Math1. As previously done with Hes1 (Zheng et al., 2000), we co-electroporated Math1 and GFP-expressing constructs into embryonic cochlea cultures, in the presence or absence of a Hey2 expression construct. Greater than 80% of cells electroporated with Math1 plasmid expressed ectopic hair cell markers (Supplemental Fig. S5A, B), while in control cultures electroporated with either GFP or Hey2 alone, fewer than 5% of electroporated cells expressed hair cell markers. In contrast, when Math1 was co-electroporated with Hey2, fewer than 20% of electroporated cells expressed ectopic hair cell markers (Supplemental Fig. S5A, B). While not evidence of direct interaction, our results show that Hey2, like Hes1, is able to suppress Math1-induced hair cell differentiation.
Notch and FGF signaling co-operate to maintain Hey2 expression and pillar cell identity
Our results show that Notch signaling is not necessary to maintain Hey2 expression in pillar cells. A good candidate regulator of Hey2 expression in pillar cells is the FGF signaling pathway. FGF8 is expressed in inner hair cells adjacent to pillar cells, and inhibition of FGF receptor activity with the tyrosine kinase inhibitor SU5402 (Mohammadi et al., 1997) or loss of FGFR3 results in arrested pillar cell development (Hayashi et al., 2007; Jacques et al., 2007; Mueller et al., 2002; Puligilla et al., 2007). We therefore hypothesized that FGF signaling might regulate Hey2 expression and maintain pillar cell identity. To test this we treated organ of Corti explants with inhibitors of both the FGF and Notch signaling pathways.
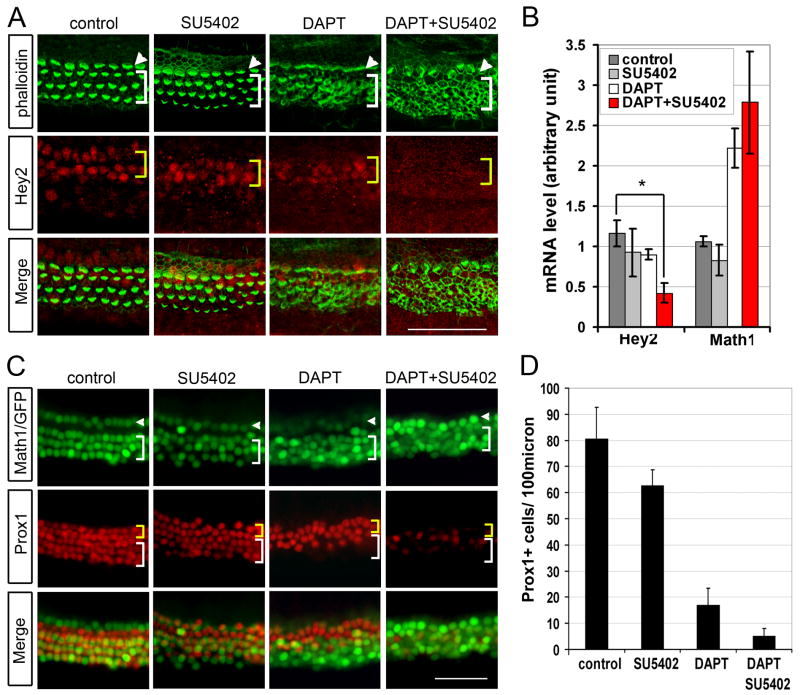
Blocking FGF signaling in cochlear explants with SU5402 alone did not significantly decrease Hey2 transcript or protein levels (Fig. 5A, B), or increase Math1 expression (Fig. 5B). SU5402 treatment also did not affect expression of Hey1, HeyL, Hes1, or Hes5 (data not shown). Moreover, blocking FGF signaling did not lead to a significant conversion of pillar cells to hair cells, as observed by the lack of increase in Math1-GFP+ hair cells or significant decrease in Prox1+ cells (Fig. 5C, D). However, simultaneous inhibition of both FGF and Notch signaling in neonatal cochlear explants with SU5402 and DAPT significantly reduced Hey2 transcript levels (p<0.005; Fig. 5B), and abolished Hey2 expression in pillar cells (Fig. 5A), resulting in a virtually complete loss of Prox1+ cells (Fig. 5C, D). The loss of Prox1+ cells in the pillar cell region, and the appearance of ectopic Math1-GFP+ cells in the space between the inner and outer hair cell region in the presence of SU5402 and DAPT (see Fig. 5C DAPT+SU5402, yellow bracket), suggests that pillar cells converted into hair cells. Thus, although FGF alone is sufficient to maintain Hey2 expression in pillar cells, in the absence of FGF signaling, the Notch signaling pathway acts redundantly to maintain expression of Hey2 as well as a pillar cell fate, while inactivation of both pathways leads to loss of pillar cells.
Fig. 5. FGF and Notch signaling prevent pillar cells from trans-differentiating into hair cells.
A: Inhibition of Notch and FGF signaling results in a loss of pillar cell specific Hey2 protein expression (red) and an increase in phalloidin+ hair cells (green). P0 cochlear explants were stained with Hey2 antibody (red) and phalloidin (green) after being cultured for 48 hours in DMSO (control) or in the presence of FGF inhibitor SU5402, DAPT or both. Note: As previously reported (Kiernan et al., 2005), loss of Notch signaling results in disorganization of phalloidin labeled hair cell bundles (DAPT, DAPT+SU5402, green) (see Fig. 2E). B: Culture of cochlear explants in DAPT and SU5402 (red bar) for 22 hours results in a significant decrease in Hey2 mRNA levels. Bars represent mean of three independent experiments performed (error bars ± s.e.m.) (*<0.01). C: P0 Math1/GFP transgenic cochlear explants were stained with Prox1 antibody (red) after being cultured for 72 hours in the presence or absence of DAPT and SU5402. D: Quantification of Prox1+cells as in C. A minimum of three cochlear cultures were analyzed for each condition (error bars ± s.e.m.). Scale bar: 50μm in A, C.
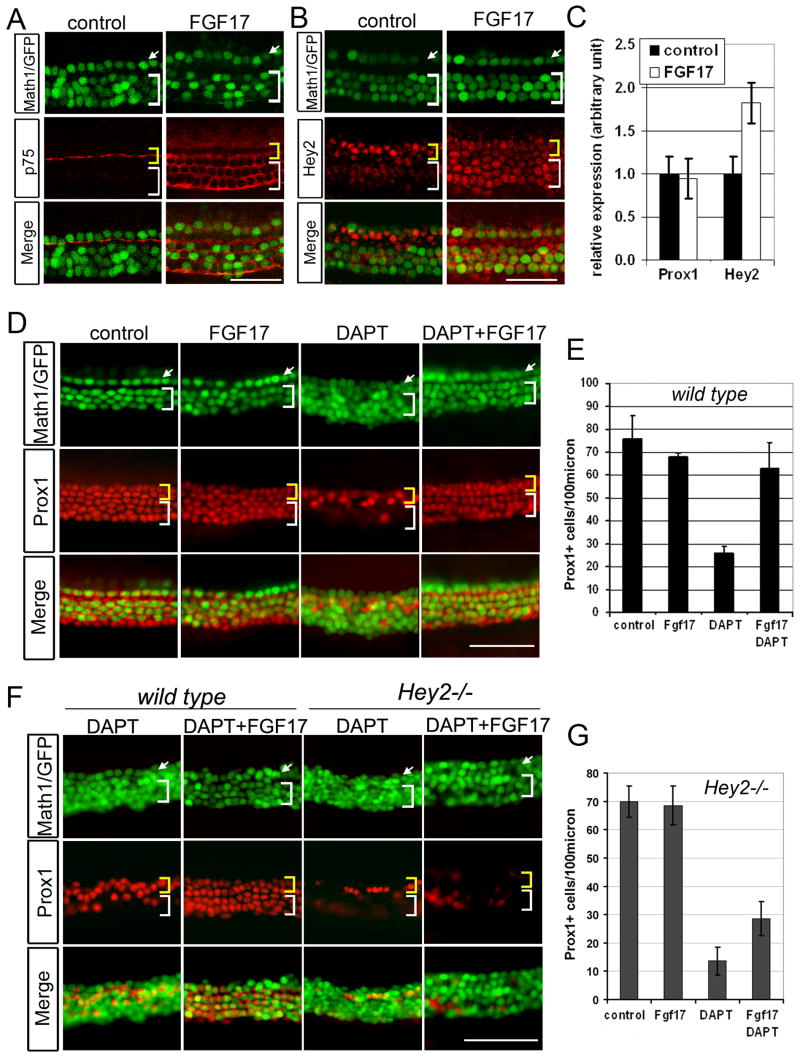
Over-activation of FGFR signaling in embryonic cochlear cultures, either with high concentrations of FGFR3 ligands, or by inactivating negative regulators of FGF signaling such as Sprouty2, can induce ectopic pillar cells and inhibit the development of Deiters’ cell and outer hair cells (Mueller et al., 2002; Shim et al., 2005). To further test if Hey2 expression is regulated by FGF signaling, we cultured postnatal organ cultures with FGF17, which has been shown to efficiently up-regulate p75 in the organ of Corti (Jacques et al., 2007). FGF17 treatment increased Hey2 levels by almost 2-fold (Fig. 6C), and expanded the domain of Hey2 and p75 expression into the Deiters’ cell region (Fig. 6A,B, white brackets).
Fig. 6. Up-regulation of Hey2 by FGF17 prevents Deiters’ cell conversion in the absence of Notch signaling.
A–C: P0 cochlear explants were cultured for 48 hours in presence of FGF17. A–B: Expansion of pillar cell specific p75 and Hey2 expression in the presence of FGF17. C: Up-regulation of Hey2 mRNA expression in the presence of FGF17. Bars represent mean of three independent experiments performed (error bars ± s.e.m.). D: FGF17 prevents Deiters’ cell trans-differentiation in the absence of Notch signaling. E: Quantification of Prox1+ cells in D. Three cochlear cultures were analyzed for each condition and black bar represents mean of Prox1+ cell/100μm (error bars ± s.e.m.). F: FGF17 does not block the effect of DAPT in Hey2 mutant cochlear explants. G: Quantification of Prox1+ cells in F. A minimum of three cochlear cultures were analyzed for each condition (error bars ± s.e.m.). Top panel (A, B, D, F): Math1/GFP (green) labels inner (arrow) and outer (bracket) hair cells. Middle panel shows p75 (A), Hey2 (B) and Prox1 (D, F) antibody staining in red. Yellow bracket marks pillar cell domain, white bracket marks Deiters’ cell domain. Scale bar: 50μm in A, B, D, F.
Based on the observations that i) FGF signaling up-regulates Hey2 expression ectopically in Deiters’ cells, and ii) Notch signaling is not necessary for Hey2 expression, we hypothesized that up-regulation of Hey2 in Deiters’ cells by FGF17 would prevent trans-differentiation of these cells into hair cells when Notch signaling is blocked with DAPT. We therefore treated cochlear explants with FGF17, DAPT or both factors together. FGF17 treatment did not affect the numbers of Prox1+ supporting cells of which pillar cells are a subset, whereas DAPT treatment significantly reduced Prox1+ cells and increased hair cell numbers (Fig. 6D, E, see also Fig. 2). Treatment with FGF17 blocked the reduction of Prox1+ cells otherwise observed in explants treated with DAPT alone (Fig. 6D, E). To confirm that the resistance of Deiters’ cells to loss of Notch signaling in the presence of FGF17 was due to the up-regulation of Hey2 expression in Deiters’ cells (see Supplemental Fig. S6) Hey2 mutant explants were treated with both FGF17 and DAPT. In the absence of Hey2, FGF17 failed to protect Prox1+ cells from the effects of blocking Notch signaling with DAPT, leading to a commensurate increase in hair cells (Fig. 6F, G).
Discussion
The involvement of Notch-dependent lateral inhibition in the development of the inner ear is well established (Adam et al., 1998; Daudet et al., 2007; Daudet and Lewis, 2005; Eddison et al., 2000; Haddon et al., 1998; Kelley, 2006; Kiernan et al., 2005; Lanford et al., 1999). However, different sensory epithelia harbor a variety of mosaic patterns of hair cells and supporting cells, suggesting that in each case, adaptation of the simple model of Notch-dependent lateral inhibition is required to ensure proper patterning during development. This is exemplified by the unique and highly asymmetric placement of hair cell and supporting cell types within the mammalian organ of Corti (Fig. 1). We now describe a mechanism by which the alternating pattern of Notch-dependent hair and supporting cell differentiation is broken in the organ of Corti. We show that in the case of pillar cells, Notch signaling is not necessary for the expression of Hey2. As a consequence pillar cells are resistant to loss of Notch signaling and do not convert into hair cells. We also show that Hey2 expression is regulated by the FGF signaling pathway and that Hey2 is able to block Math1-induced hair cell differentiation. Based on these observations, we suggest that FGF released from inner hair cells maintains Hey2 expression and thus contributes to the establishment of the pillar cell region between inner and outer hair cells.
The role of Notch signaling in maintaining cell identity in the organ of Corti
Our data suggest that early postnatal supporting cells have the plasticity to trans-differentiate into hair cells and that Notch signaling is one of the key pathways to maintain the differentiated state of supporting cells in the postnatal organ of Corti. Confirming previous reports, we show that treatment of embryonic or neonatal organ cultures with gamma secretase inhibitor blocks Notch signaling and leads to a dramatic increase in hair cell number (Fig. 1; Supplemental Fig. S1 (Takebayashi et al., 2007; Yamamoto et al., 2006). Using the transcription factor Prox1, which marks Deiters’ cells and pillar cells, we show that the increase in hair cell number occurs at the expense of Prox1+ supporting cells (Fig. 2A–C) and, significantly, in the absence of cell proliferation (Fig. 1F). These data suggest that blocking the Notch signaling pathway causes supporting cells to trans-differentiate into hair cells. However, since only a small number of hair cell and supporting cell markers have been analyzed in our experiments, it is possible that supernumerary Math1+, Myosin VI+ cells exhibit a hybrid mixture of hair cell and supporting cell phenotypes, and further analysis is required to clarify this issue. At present, we do not know why apical regions of the neonatal cochlea appear more sensitive to DAPT treatment than basal regions (Figure 1D; 2B, C). This may be due to the fact that cochlear differentiation proceeds in a basal to apical direction, and that supporting cells require less Notch signaling to stabilize their differentiated state as they mature. Experiments investigating the role of Notch signaling in the mature organ of Corti are in progress.
It is becoming clear that gamma secretase complexes also process many other transmembrane proteins (Kopan and Ilagan, 2004; Rio et al., 2000; Sardi et al., 2006). It is therefore important to confirm data obtained with these inhibitors with alternate approaches that more specifically inhibit the Notch pathway. We confirmed our gamma secretase inhibitor (DAPT) results using conditional mutants of either Notch1, or RBPJ which should lack all Notch signaling (Fig. 2E, F), It is therefore likely that the excess hair cells seen in our DAPT treated explants represent a specific loss of Notch function.
In contrast to other supporting cell types, pillar cells are strikingly resistant to the effects of blocking Notch signaling. We observed Prox1+, p75+ pillar cells remaining in our DAPT-treated organ cultures at all concentrations and exposure times tested, and also observed p75+ pillar cells remaining in Notch1 and RBPJ mutant mice (Fig. 2E, F). This persistence of pillar cells has been previously observed in other ear-specific mutations of the Notch1 gene and in compound mutants of the Notch ligands Dll1 and Jag2 (Kiernan et al., 2005). Kiernan and colleagues attributed this persistence of pillar cells to the possibility of their possessing stem cell-like properties. Here, we offer an alternative explanation – that pillar cell identity is maintained by the expression of the bHLH transcription factor, Hey2, whose expression does not require Notch signaling.
A combinatorial code of Hes and Hey genes define supporting cell types in the organ of Corti, and have differential requirements for Notch signaling
We show that the postnatal organ of Corti can be divided into four regions based on the expression of different combinations of Hes and Hey genes (Fig. 3B). Hes1 and HeyL define the neural region of the organ of Corti, being expressed in Kölliker’s organ and inner phalangeal cells, while the abneural region is defined by the expression of Hes1 and Hey1 in Hensen’s cells. Hes5, in combination with Hey1 and HeyL, define the Deiters’ cells that lie beneath outer hair cells, while Hey2 defines the pillar cell region. This combinatorial expression may have functional consequences, as Hes and Hey genes can form heterodimers that are often more stable than homodimers of each family member (Fischer and Gessler, 2007). Our data also suggests a basis for the relatively mild cochlear phenotypes seen in single or double mutants of Hes1 and Hes5 (Zheng et al., 2000; Zine et al., 2001), since both Hes1 and Hes5 are expressed in supporting cells with an accompanying Hey gene family member (HeyL and Hey1 respectively), which might act redundantly with Hes1 or Hes5. Similarly, we observed no hair cell phenotypes in Hey1 or HeyL mutant mice, and only very minor changes in hair cell density in Hey2 mutants (Supplemental Fig. S3). Future studies will address whether at embryonic stages, signals initiating hair cell differentiation are responsible for the up-regulation of Hes1, Hes5 and HeyL, and/or for the restriction of Hey1 and Hey2 to specific cell types.
Our data reveals the existence of regulatory hierarchies between different Hes and Hey gene family members. In the absence of Hey2, the domain of Hes5 expression expanded laterally into the pillar cell region (Supplemental Fig. S4B), suggesting that Hey2 can repress Hes5 expression. Such cross-regulation may help to establish asymmetry in the organ of Corti, whereby inner hair cells are separated from outer hair cells by a hair cell-free region of Hey2-expressing pillar cells.
It is interesting that in contrast to the more recently derived cochlea, the mammalian vestibular system lacks pillar-like supporting cells, does not express Hey2 (Hayashi et al., 2008); and contains no supporting cells that are resistant to DAPT (data not shown). Based on the observation that extant basal monotreme mammals, such as the duck-billed platypus and echidna have three to four rows of pillar cells separating inner from outer hair cells (Ladhams and Pickles, 1996), we speculate that co-option of Hey2 and its regulation by FGF signaling rather than the Notch pathway resulted in a lack of lateral inhibition between the multiple rows of pillar cells and their hair cell counterparts. In this evolutionary context, it would be interesting to determine whether Hey2 is expressed in the expanded pillar cell domain of monotremes and whether it plays a similar Notch-independent role in pattern formation in the monotreme inner ear.
Regulation of Hey2 by FGF signaling maintains pillar cell identity
Our results with Notch inhibitors reveal an unexpected complexity in the regulation of Hes and Hey genes. Some family members, such as Hes5, appear to be tightly regulated by Notch signaling, with Hes5 levels falling to undetectable levels within 8 hours after treatment with DAPT (Fig. 3C). We see similar, albeit less dramatic patterns of regulation of Hey1 and HeyL. In contrast, Hey2 and Hes1 levels remain unchanged after exposure to DAPT. We believe that the persistence of pillar cell specific Hey2 expression after blocking Notch signaling in DAPT-treated organ cultures (Fig. 3D, Fig. 5A, Supplemental Fig. S6) is the reason for the persistence of pillar cells in these conditions. This is confirmed by the observation that pillar cells in Hey2 mutant mice readily convert to hair cells when treated with DAPT (Fig. 4).
Our data suggest that either FGF or Notch signaling are sufficient to maintain Hey2 expression, since Hey2 levels are maintained in pillar cells in the presence of only one or the other pathway. In contrast, treatment with both the Notch inhibitor DAPT and the FGFR inhibitor SU5402 reduces Hey2 levels, and causes pillar cells to trans-differentiate into hair cells (Fig. 5). We have further shown that high levels of FGF17 are able to induce Hey2 throughout the supporting cells of the organ of Corti, and that FGF17 treatment prevents these other, normally responsive supporting cells from differentiating into hair cells when Notch signaling is blocked by DAPT Fig. 6A–E). As expected, this protective effect of FGF17 is lost in Hey2 mutant mice (Fig. 6F, G). We hypothesize that the acquisition of Notch sensitivity by pillar cells in Hey2 mutant mice is mediated by the observed up-regulation of Hes5 in the mutant pillar cells. We summarize these signaling and genetic interactions in Fig. 7.
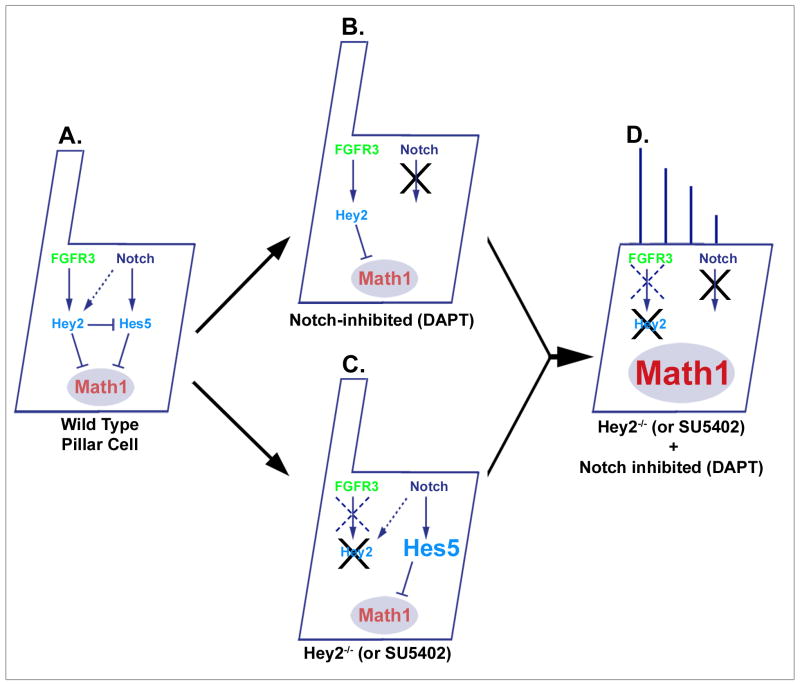
Fig. 7. FGF and Notch signaling act redundantly to prevent pillar cells from transdifferentiating into hair cells.
A: Signaling diagram representing FGF and Notch-mediated effects on Math1 expression and maintenance of the pillar cell phenotype. Math1 is both necessary and sufficient for hair cell differentiation in the context of the inner ear (Bermingham et al., 1999; Zheng and Gao, 2000), and either Hey2 or Hes5 can inhibit Math1 expression and thus prevent pillar cell transdifferentiation into hair cells. B: Hey2 is expressed under the control of FGF signaling and is largely independent of changes in Notch signaling. C: In the absence of Hey2, Hes5 is upregulated in pillar cells (see Fig. S5), leading to a continued Notch-dependent block to transdifferentiation, and suggesting that Hey2 normally inhibits Hes5 expression. D: If both FGF and Notch signaling are blocked, neither Hey2 nor Hes5 is expressed leading to Math1 de-repression and trans-differentiation of pillar cells into hair cells.
Recent studies suggest that Notch signaling is not required for Hey2 expression in certain tissues. (Kokubo et al., 2005; Leimeister et al., 2000; Rutenberg et al., 2006; Watanabe et al., 2006). Recently the expression of Hes7, a Hey2 related HES family member, has also been shown to be alternately regulated by Notch and FGF signaling pathways in different phases of the segmentation clock (Kawamura et al., 2005), demonstrating the important role of Notch-independent regulation of HES/HEY factors. As far as we are aware, this is the first demonstration of a role for FGF signaling in the regulation of Hey2. The likely source of FGF signaling for pillar cells is inner hair cells. Kelley and colleagues have shown that FGF8 is present in inner hair cells, and that FGF17, a close relative of FGF8, stimulates the production of excess pillar cells at the expense of outer hair cells in organ of Corti culture (Jacques et al., 2007).
Our results suggest a rudimentary model for how different supporting cell types arise in the organ of Corti. Initially, a prosensory zone of non-proliferating cells is established along the length of the cochlea, characterized by expression of both p27Kip1 (Chen and Segil, 1999; Lee et al., 2006) as well as Hey2 and Hey1 (Supplemental Fig. S2). Currently unknown signals induce the differentiation of inner hair cells from within this non-proliferating sensory domain. As hair cell differentiation proceeds from the base of the cochlea to the apex, Hey1 and Hey2 are down-regulated within this domain, becoming restricted to Deiters’ and pillar cells respectively (Supplemental Fig. S2). Hey2 expression is maintained in pillar cells (Fig. 3A) by FGF signals, presumably from the nearby inner hair cells. Negative regulation of FGF signaling in Deiters’ cells by factors such as Sprouty2 (Shim et al., 2005), and hierarchical inhibitory interactions between Hey2 and Hes5 (Supplemental Fig. S4B) create a clear division between pillar cells and Deiters’ cells. Other Hes and Hey genes are induced in differentiating supporting cells, possibly as a direct result of signaling from Notch ligands expressed in inner and outer hair cells. At present, the signals that cause the differentiation of inner versus outer hair cells and inner versus outer phalangeal (Deiters’) cells remain unknown. However, our results illuminate new aspects of the complex regulatory mechanisms that lead to pattern formation and cell type specification in the organ of Corti.
Supplementary Material
Acknowledgments
We thank Juan Llamas, Welly Makmura, Francesca Della Ripa and Sheri Juntilla for animal care, genotyping and expert technical assistance, and Greta Segil for production of the Hey2-specific antibody. We thank Raphael Kopan for the Notch1 conditional mouse mutants, Tasuku Honjo for the RBPJ conditional mouse mutants and Jane Johnson for Math1/GFP transgenic mice. We also thank Doug Cotanche for bringing the Ladhams and Pickles (1996) paper to our attention. This work was supported by NIH grants DC04189 (N.S.), DC008689 (A.D.) and DC06185 (A.K.G. and N.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development (Cambridge, England) 1998;125:4645–4654. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Barald KF, Kelley MW. From placode to polarization: new tunes in inner ear development. Development (Cambridge, England) 2004;131:4119–4130. doi: 10.1242/dev.01339. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Oesterle EC, Stone JS, Hume CR, Huynh HM, Hayashi T. Expression of Prox1 during mouse cochlear development. The Journal of comparative neurology. 2006;496:172–186. doi: 10.1002/cne.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development (Cambridge, England) 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development (Cambridge, England) 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Cheng HT, Kim M, Valerius MT, Surendran K, Schuster-Gossler K, Gossler A, McMahon AP, Kopan R. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development (Cambridge, England) 2007;134:801–811. doi: 10.1242/dev.02773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudet N, Ariza-McNaughton L, Lewis J. Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development (Cambridge, England) 2007;134:2369–2378. doi: 10.1242/dev.001842. [DOI] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development (Cambridge, England) 2005;132:541–551. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- Doetzlhofer A, White PM, Johnson JE, Segil N, Groves AK. In vitro growth and differentiation of mammalian sensory hair cell progenitors: a requirement for EGF and periotic mesenchyme. Developmental biology. 2004;272:432–447. doi: 10.1016/j.ydbio.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, Fang LY, Freedman SB, Folmer B, Goldbach E, Holsztynska EJ, et al. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem. 2001;76:173–181. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- Eddison M, Le Roux I, Lewis J. Notch signaling in the development of the inner ear: lessons from Drosophila. Proc Natl Acad Sci U S A. 2000;97:11692–11699. doi: 10.1073/pnas.97.22.11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler WP, Wolfe MS. A portrait of Alzheimer secretases--new features and familiar faces. Science. 2001;293:1449–1454. doi: 10.1126/science.1064638. [DOI] [PubMed] [Google Scholar]
- Fischer A, Gessler M. Delta-Notch--and then? Protein interactions and proposed modes of repression by Hes and Hey bHLH factors. Nucleic Acids Res. 2007;35:4583–4596. doi: 10.1093/nar/gkm477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G, Robakis NK. Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. Embo J. 2006;25:1242–1252. doi: 10.1038/sj.emboj.7601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridley T. Notch signaling in vascular development and physiology. Development (Cambridge, England) 2007;134:2709–2718. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- Haddon C, Jiang YJ, Smithers L, Lewis J. Delta-Notch signalling and the patterning of sensory cell differentiation in the zebrafish ear: evidence from the mind bomb mutant. Development (Cambridge, England) 1998;125:4637–4644. doi: 10.1242/dev.125.23.4637. [DOI] [PubMed] [Google Scholar]
- Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, Ikuta K, Honjo T. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. International immunology. 2002;14:637–645. doi: 10.1093/intimm/dxf030. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Cunningham D, Bermingham-McDonogh O. Loss of Fgfr3 leads to excess hair cell development in the mouse organ of Corti. Dev Dyn. 2007;236:525–533. doi: 10.1002/dvdy.21026. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Kokubo H, Hartman BH, Ray CA, Reh TA, Bermingham-McDonogh O. Hesr1 and Hesr2 may act as early effectors of Notch signaling in the developing cochlea. Developmental biology. 2008;316:87–99. doi: 10.1016/j.ydbio.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- High FA, Epstein JA. The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet. 2008;9:49–61. doi: 10.1038/nrg2279. [DOI] [PubMed] [Google Scholar]
- Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- Jacques BE, Montcouquiol ME, Layman EM, Lewandoski M, Kelley MW. Fgf8 induces pillar cell fate and regulates cellular patterning in the mammalian cochlea. Development (Cambridge, England) 2007;134:3021–3029. doi: 10.1242/dev.02874. [DOI] [PubMed] [Google Scholar]
- Kawamura A, Koshida S, Hijikata H, Sakaguchi T, Kondoh H, Takada S. Zebrafish hairy/enhancer of split protein links FGF signaling to cyclic gene expression in the periodic segmentation of somites. Genes Dev. 2005;19:1156–1161. doi: 10.1101/gad.1291205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7:837–849. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- Kelley MW. Cellular commitment and differentiation in the organ of Corti. Int J Dev Biol. 2007;51:571–583. doi: 10.1387/ijdb.072388mk. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Cordes R, Kopan R, Gossler A, Gridley T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development (Cambridge, England) 2005;132:4353–4362. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubo H, Miyagawa-Tomita S, Nakazawa M, Saga Y, Johnson RL. Mouse hesr1 and hesr2 genes are redundantly required to mediate Notch signaling in the developing cardiovascular system. Developmental biology. 2005;278:301–309. doi: 10.1016/j.ydbio.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. Gamma-secretase: proteasome of the membrane? Nat Rev Mol Cell Biol. 2004;5:499–504. doi: 10.1038/nrm1406. [DOI] [PubMed] [Google Scholar]
- Ladhams A, Pickles JO. Morphology of the monotreme organ of Corti and macula lagena. The Journal of comparative neurology. 1996;366:335–347. doi: 10.1002/(SICI)1096-9861(19960304)366:2<335::AID-CNE11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Lai EC. Notch signaling: control of cell communication and cell fate. Development (Cambridge, England) 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- Lammich S, Okochi M, Takeda M, Kaether C, Capell A, Zimmer AK, Edbauer D, Walter J, Steiner H, Haass C. Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an Abeta-like peptide. J Biol Chem. 2002;277:44754–44759. doi: 10.1074/jbc.M206872200. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21:289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Shailam R, Norton CR, Gridley T, Kelley MW. Expression of Math1 and HES5 in the cochleae of wildtype and Jag2 mutant mice. J Assoc Res Otolaryngol. 2000;1:161–171. doi: 10.1007/s101620010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Liu F, Segil N. A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development (Cambridge, England) 2006;133:2817–2826. doi: 10.1242/dev.02453. [DOI] [PubMed] [Google Scholar]
- Leimeister C, Dale K, Fischer A, Klamt B, Hrabe de Angelis M, Radtke F, McGrew MJ, Pourquie O, Gessler M. Oscillating expression of c-Hey2 in the presomitic mesoderm suggests that the segmentation clock may use combinatorial signaling through multiple interacting bHLH factors. Developmental biology. 2000;227:91–103. doi: 10.1006/dbio.2000.9884. [DOI] [PubMed] [Google Scholar]
- Li S, Mark S, Radde-Gallwitz K, Schlisner R, Chin MT, Chen P. Hey2 functions in parallel with Hes1 and Hes5 for mammalian auditory sensory organ development. BMC developmental biology. 2008;8:20. doi: 10.1186/1471-213X-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Collisson T, Parab P, Omer-Abdalla A, Haeberle H, Chen P, Doetzlhofer A, White P, Groves A, Segil N, et al. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr Patterns. 2003;3:389–395. doi: 10.1016/s1567-133x(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–974. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- McElroy B, Powell JC, McCarthy JV. The insulin-like growth factor 1 (IGF-1) receptor is a substrate for gamma-secretase-mediated intramembrane proteolysis. Biochem Biophys Res Commun. 2007;358:1136–1141. doi: 10.1016/j.bbrc.2007.05.062. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, Hubbard SR, Schlessinger J. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- Mueller KL, Jacques BE, Kelley MW. Fibroblast growth factor signaling regulates pillar cell development in the organ of corti. J Neurosci. 2002;22:9368–9377. doi: 10.1523/JNEUROSCI.22-21-09368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Groves AK. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis. 2004;38:195–199. doi: 10.1002/gene.20017. [DOI] [PubMed] [Google Scholar]
- Pan Y, Lin MH, Tian X, Cheng HT, Gridley T, Shen J, Kopan R. gamma-secretase functions through Notch signaling to maintain skin appendages but is not required for their patterning or initial morphogenesis. Developmental cell. 2004;7:731–743. doi: 10.1016/j.devcel.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Puligilla C, Feng F, Ishikawa K, Bertuzzi S, Dabdoub A, Griffith AJ, Fritzsch B, Kelley MW. Disruption of fibroblast growth factor receptor 3 signaling results in defects in cellular differentiation, neuronal patterning, and hearing impairment. Dev Dyn. 2007;236:1905–1917. doi: 10.1002/dvdy.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio C, Buxbaum JD, Peschon JJ, Corfas G. Tumor necrosis factor-alpha-converting enzyme is required for cleavage of erbB4/HER4. J Biol Chem. 2000;275:10379–10387. doi: 10.1074/jbc.275.14.10379. [DOI] [PubMed] [Google Scholar]
- Rutenberg JB, Fischer A, Jia H, Gessler M, Zhong TP, Mercola M. Developmental patterning of the cardiac atrioventricular canal by Notch and Hairy-related transcription factors. Development (Cambridge, England) 2006;133:4381–4390. doi: 10.1242/dev.02607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell. 2006;127:185–197. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Shim K, Minowada G, Coling DE, Martin GR. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Developmental cell. 2005;8:553–564. doi: 10.1016/j.devcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Stern CD. Detection of multiple gene products simultaneously by in situ hybridization and immunohistochemistry in whole mounts of avian embryos. Curr Top Dev Biol. 1998;36:223–243. doi: 10.1016/s0070-2153(08)60505-0. [DOI] [PubMed] [Google Scholar]
- Takebayashi S, Yamamoto N, Yabe D, Fukuda H, Kojima K, Ito J, Honjo T. Multiple roles of Notch signaling in cochlear development. Developmental biology. 2007;307:165–178. doi: 10.1016/j.ydbio.2007.04.035. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS, Patterson SL, Heuer JG, Wheeler EF, Bothwell M, Rubel EW. Expression of nerve growth factor (NGF) receptors in the developing inner ear of chick and rat. Development (Cambridge, England) 1991;113:455–470. doi: 10.1242/dev.113.2.455. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Kokubo H, Miyagawa-Tomita S, Endo M, Igarashi K, Aisaki K, Kanno J, Saga Y. Activation of Notch1 signaling in cardiogenic mesoderm induces abnormal heart morphogenesis in mouse. Development (Cambridge, England) 2006;133:1625–1634. doi: 10.1242/dev.02344. [DOI] [PubMed] [Google Scholar]
- Weinmaster G, Kopan R. A garden of Notch-ly delights. Development (Cambridge, England) 2006;133:3277–3282. doi: 10.1242/dev.02515. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Tanigaki K, Tsuji M, Yabe D, Ito J, Honjo T. Inhibition of Notch/RBP-J signaling induces hair cell formation in neonate mouse cochleas. J Mol Med. 2006;84:37–45. doi: 10.1007/s00109-005-0706-9. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Shou J, Guillemot F, Kageyama R, Gao WQ. Hes1 is a negative regulator of inner ear hair cell differentiation. Development (Cambridge, England) 2000;127:4551–4560. doi: 10.1242/dev.127.21.4551. [DOI] [PubMed] [Google Scholar]
- Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, de Ribaupierre F. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neurosci. 2001;21:4712–4720. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.