Abstract
Objective. SSc is characterized by microvascular abnormalities and leucocyte infiltration. Previous studies have suggested a proadhesive phenotype in SSc skin, but the functional consequences of this phenotype are not fully understood. Molecules known to mediate leucocyte adhesion include those present at intracellular junctions, such as junctional adhesion molecule-B (JAM-B), JAM-C and CD99, as well as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). The aim of this study was to examine adhesive interactions in SSc skin.
Methods. The expression of JAM-B, JAM-C, CD99, ICAM-1 and VCAM-1 in SSc skin was determined by immunohistology and cell surface ELISA. Myeloid U937 cell–SSc dermal fibroblast adhesion assays or in situ adhesion assays to SSc skin were performed.
Results. JAM-C and CD99 expression on endothelial cells (ECs) in SSc skin was decreased compared with expression on normal ECs. CD99 was overexpressed on mononuclear cells in SSc skin and on SSc dermal fibroblasts. Neutralizing ICAM-1 inhibited the binding of U937 cells to SSc dermal fibroblasts. In addition, blocking both ICAM-1 and VCAM-1 inhibited U937 cell adhesion to either proximal (less involved) or distal (more involved) SSc skin.
Conclusions. These studies show that JAM-C and CD99 are aberrantly expressed in SSc skin. However, these adhesion molecules do not mediate myeloid cell–SSc skin adhesion. In contrast, we demonstrate an important role for ICAM-1 and VCAM-1 in the retention of myeloid cells in SSc skin, suggesting that targeting these molecules may be useful SSc therapies.
Keywords: Systemic sclerosis, Adhesion molecules, Junctional adhesion molecules, VCAM-1, ICAM-1
Introduction
SSc or scleroderma is a multisystem disorder characterized by RP, vasculopathy and fibrosis of the skin and internal organs. Patients with SSc have decreased numbers of capillaries, capillary haemorrhages and clusters of enlarged, distorted, capillary loops that can be seen in the skin fold of the fingernails and which correlate with sclerodermatous involvement of the skin and internal organs [1, 2]. Endothelial cells (ECs), monocytes and fibroblasts are among key players in the pathogenesis of SSc.
Previous studies have suggested that cellular adhesion molecules may play a role in SSc pathogenesis. Serum levels of several soluble adhesion molecules are increased in SSc compared with healthy controls [3]. Serum levels of soluble E-selectin, vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) correlate with SSc disease severity [4–6]. Moreover, the levels of soluble E-selectin and VCAM-1 in SSc serum correlate with a positive response to some therapies [7, 8].
In addition to increased levels of soluble adhesion molecules in SSc serum, we and others have shown that several adhesion molecules are overexpressed in SSc skin and on SSc dermal fibroblasts. These include VCAM-1, ICAM-1, P-selectin, E-selectin, CD44 and integrins β1, β2 and α6 [9–12]. Aberrant adhesion molecule expression may play multiple roles in SSc pathogenesis. The increased expression of specific adhesion molecules may lead to increased accumulation of leucocytes in SSc skin [13, 14]. Abraham et al. [13] demonstrated that SSc fibroblasts have a greater propensity for binding T lymphocytes compared with normal fibroblasts, in part by their overexpression of ICAM-1. Overexpression of selected adhesion molecules resulting in the accumulation of specific, activated leucocytes may play a role in the induction of fibrosis via cytokine release leading to excess extracellular matrix synthesis [15]. Alternatively, the accumulation of leucocytes caused by increased adhesion molecule expression, and subsequent angiogenic/angiostatic cytokine release, may play a role in the disorganized angiogenesis in SSc skin.
We hypothesized that adhesion molecules that are overexpressed in SSc skin may play a role in SSc pathogenesis by promoting the adhesion of monocytes. We first sought to determine the expression of adhesion molecules in SSc skin. These molecules include junctional adhesion molecule-B (JAM-B), JAM-C and CD99, which have previously been shown to play a role in leucocyte adhesion and trans-endothelial migration [16, 17]. We then determined which adhesion molecules mediate myeloid cell adhesion to SSc skin. Our results demonstrate an important role for ICAM-1 and VCAM-1 in the retention of myeloid cells in SSc skin.
Materials and methods
Patients and controls
Punch biopsy skin samples (4 mm) were obtained from 21 subjects with SSc (all with active, diffuse disease) and 10 control subjects. Two biopsies were taken from SSc patients, one from the proximal arm (less clinically involved) and the other from the distal forearm (more clinically involved). One biopsy was taken from the forearm of healthy control subjects. All SSc patients fulfilled the ACR criteria for classification of SSc and also met the criteria for diffuse SSc [18]. Biopsies were taken with informed consent, and this study was approved by the University of Michigan Institutional Review Board.
Cell culture
Normal and SSc dermal fibroblast cell lines were established as described previously [19]. All SSc fibroblasts were obtained from untreated patients with diffuse cutaneous SSc of recent onset. The cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 supplemented with 10% fetal bovine serum (FBS) at 37°C in a humidified atmosphere with 5% CO2. Cells were used at passages 5–15, at which time they were a homogeneous, 85–95% confluent population of fibroblasts. U937 cells (human monocytic lymphoma cell line) were cultured in RPMI supplemented with 10% FBS at 37°C in a humidified atmosphere with 5% CO2.
Antibodies and reagents
Recombinant human TNF-α, IFN-γ, IL-1β, IL-17, IL-18, mouse anti-human VCAM-1, mouse anti-human ICAM-1, goat anti-human JAM-B and JAM-C antibodies were purchased from R&D Systems (MN, USA). Mouse anti-human CD99 antibody was purchased from Abcam Inc. (Cambridge, MA, USA). Neutralizing rat anti-mouse/human JAM-C antibody (F26) that was previously shown to recognize human JAM-C was used in fibroblast adhesion assays [20]. IgG controls were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Secondary antibodies were obtained from Molecular Probes Inc. (Eugene, OR, USA).
Immunohistology
Frozen samples obtained from proximal and distal SSc skin and normal skin were cut (∼5 μm) and stained using an immunoperoxidase method. Slides were fixed in cold acetone for 10 min. Following incubation with 3% H2O2 for 5 min to block endogenous peroxidase, the samples were incubated with 10% horse serum in phosphate-buffered saline (PBS) at 37°C for 1 h, and then incubated with primary antibody against JAM-B, JAM-C or CD99 (each 10 μg/ml) or purified non-specific IgG for 1 h at room temperature in blocking buffer. The skin samples were washed with PBS, and a 1 : 100 dilution (in blocking buffer) of biotinylated secondary antibody was added and incubated for an additional 1 h at room temperature. After washing, the antibody binding was detected using Vectastain ABC Elite kit (Vector Labs, Burlingame, CA, USA) and the chromogen 3,3′-diaminobenzidine (DAB) (Vector Labs). Skin samples were counterstained with Gill's haematoxylin. Staining was evaluated under blinded conditions and graded by a pathologist. Slides were examined for cellular immunoreactivity and cell types were distinguished based on their characteristic morphology.
Cell surface ELISA
SSc and normal fibroblasts (1 × 104/well) were seeded in 96-well plates (BD Falcon, Bedford, MA, USA). Upon reaching confluence, the fibroblasts were incubated with or without TNF-α, IL-1β, IFN-γ, IL-17 or IL-18 (each 25 ng/ml) for 24 h in serum-free media. Cells were fixed with 3.7% formalin in PBS and cell surface ELISAs were performed as previously described [21]. Antibodies specific for JAM-B, JAM-C, CD99, ICAM-1 or VCAM-1 (each 10 µg/ml) were used, and the plates were read with an ELISA reader (Bio-Rad, Hercules, CA, USA) at 450 nM.
In vitro cell adhesion assay
Adhesion of U937 cells to SSc fibroblasts grown to confluence in 96-well plates was tested. SSc dermal fibroblasts were serum starved overnight, and 1 h prior to assay, the fibroblasts were pre-incubated with neutralizing antibodies against either JAM-B, JAM-C, CD99, ICAM-1 or irrelevant IgG controls (each 25 μg/ml). The cells were collected and labelled with calcein-AM fluorescent dye (5 μM; Invitrogen, Carlsbad, CA, USA) for 20 min. After washing twice, 1 × 105 cells were added to each well and incubated for 20 min at 37°C. At the end of the assay, non-adherent cells were washed off and fluorescence was measured using a Synergy HT fluorescence plate reader (Bio-Tek Instruments, Winooski, VT, USA). The inhibitory effect of each antibody was given as the percentage of maximal binding, which was defined as the number of adherent cells in the control antibody-treated sections.
Stamper–Woodruff assay
In situ adhesion assays were performed as previously described [22]. Briefly, frozen SSc skin samples were cut (∼5 μm) and incubated with neutralizing antibodies against ICAM-1, VCAM-1, a combination of the two antibodies or irrelevant IgG control. U937 cells were labelled with calcein-AM fluorescent dye (5 μM, Invitrogen) for 20 min. After incubation, medium was removed and 1 × 105 fluorescent-labelled U937 cells were added to all sections and the sections were incubated for 1 h at room temperature. At the end of the experiment, non-adherent cells were washed off. Fluorescent U937 cell adhesion to SSc skin was counted blindly using a BX51 Fluorescence Microscope System and DP Manager imaging software (Olympus America, Melville, NY, USA). The inhibitory effect of each antibody was given as the percentage of maximal binding, which was defined as the number of adherent cells in the control antibody-treated sections.
Statistical analysis
Data were analysed using Student's t-test assuming equal variances. P-values <0.05 were considered statistically significant. Data are represented as the mean ± s.e.m.
Results
Immunohistochemical analysis of JAM-B, JAM-C and CD99 in SSc skin
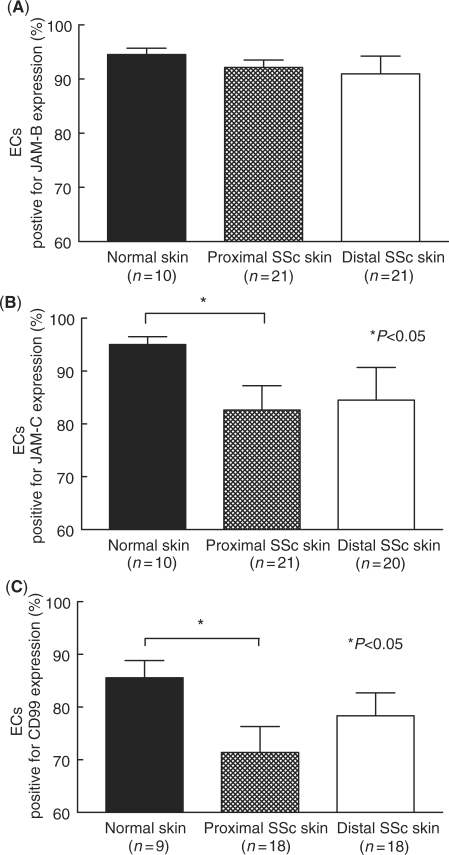
Previous studies have suggested a proadhesive phenotype in SSc skin. We hypothesized that adhesion molecules that are overexpressed in SSc skin may play a role in SSc pathogenesis by promoting the adhesion of monocytes. We first sought to determine the expression of intracellular adhesion molecules, such as JAM-B, JAM-C and CD99, in SSc skin by immunohistochemistry. SSc adhesion molecule expression was determined in both proximal (clinically less involved) and distal (clinically more involved) skin [23]. The expression of each adhesion molecule on specific cell types, including ECs, mononuclear cells, and in the epidermis, was determined by a pathologist in a blinded manner. Our results indicated that JAM-B and JAM-C were mainly expressed on dermal ECs in normal and SSc skin (Fig. 1). However, while JAM-B expression on dermal ECs was conserved between normal, proximal and distal SSc skin, JAM-C expression on dermal ECs was decreased on proximal and distal SSc skin compared with normal skin (Fig. 1B).
Fig. 1.
Immunohistochemical analysis of JAM-B, JAM-C and CD99 on SSc and normal skin ECs. Frozen sections of proximal and distal SSc and normal skin were stained for JAM-B, JAM-C and CD99. (A) JAM-B was highly expressed on normal (95%), proximal (92%) and distal SSc (91%) ECs. (B) JAM-C expression was decreased on proximal SSc (83%) and distal SSc (85%) ECs compared with normal ECs (95%). (C) CD99 expression was decreased on proximal SSc (71%) and distal SSc (78%) ECs compared with normal ECs (86%). Means are given with the SEM. n = number of patients. P< 0.05 was considered significant.
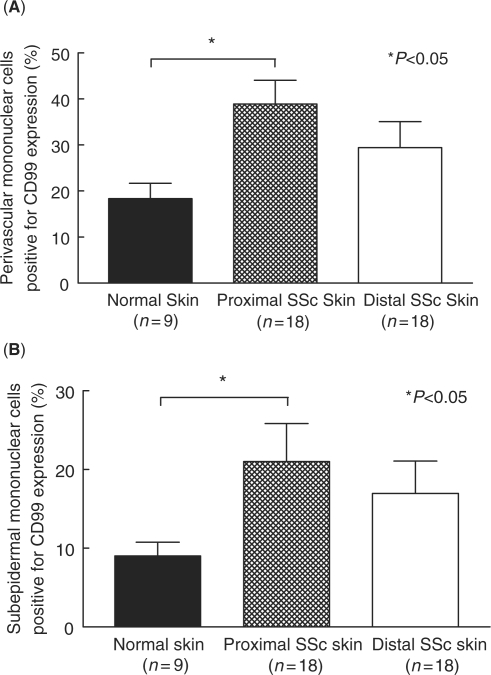
Similarly, CD99 expression on proximal and distal SSc skin was decreased compared with normal skin (Fig. 1C). However, CD99 expression was found on dermal ECs, on perivascular and subepidermal mononuclear cells, and in the stratum spinosum of the epidermis. CD99 expression in the stratum spinosum was unchanged between normal, proximal and distal SSc skin (data not shown). In contrast, the expression of CD99 was increased on both perivascular and subepidermal mononuclear cells in proximal and distal SSc skin compared with normal skin (Fig. 2).
Fig. 2.
Immunohistochemical analysis of CD99 on SSc and normal skin mononuclear cells. Frozen sections of proximal and distal SSc and normal skin were immunostained for CD99. (A) CD99 expression was increased on perivascular mononuclear cells in proximal (39%) and distal (29%) SSc skin compared with normal skin (18%). (B) CD99 expression was increased on subepidermal mononuclear cells in proximal (21%) and distal (17%) SSc skin compared with normal skin (9%). Means are given with the SEM. n = number of patients. P< 0.05 was considered significant.
Adhesion molecule expression on SSc dermal fibroblasts
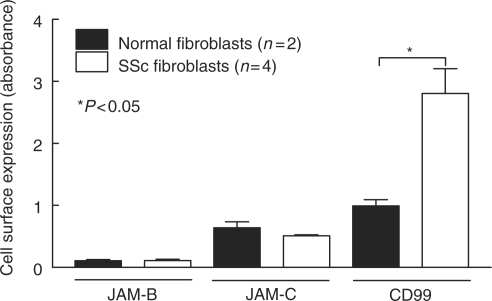
Previous studies have demonstrated that SSc dermal fibroblasts have an increased expression of select adhesion molecules. Cell surface ELISAs were performed to determine the expression of JAM-B, JAM-C, CD99, ICAM-1 and VCAM-1 on normal and SSc dermal fibroblasts. In addition, we tested whether cytokines that are overexpressed in SSc skin and serum are able to regulate the expression of these adhesion molecules on SSc dermal fibroblasts. Our findings indicate that JAM-B and JAM-C were expressed on both normal and SSc dermal fibroblasts (Fig. 3). However, their expression was not inducible by TNF-α, IL-1β or IFN-γ (data not shown). In contrast to these adhesion molecules, we found that CD99 expression was significantly increased on SSc dermal fibroblasts compared with normal dermal fibroblasts (Fig. 3). However, as with JAM-B and JAM-C, CD99 expression was not inducible by stimulation with TNF-α, IL-1β or IFN-γ.
Fig. 3.
JAM-B, JAM-C and CD99 are expressed on SSc dermal fibroblasts. Cell surface ELISAs were performed to determine if JAM-B, JAM-C and CD99 are expressed on the surface of normal or SSc dermal fibroblasts. JAM-B, JAM-C and CD99 are present on the surface of both normal and SSc dermal fibroblasts. CD99 expression was significantly greater on SSc dermal fibroblasts compared with normal. Means ± SEM are shown. n = number of patient-derived fibroblast lines.
Previous studies have shown that ICAM-1 and VCAM-1 are overexpressed in SSc skin and serum [4–6,9, 11]. We found that the basal level of VCAM-1 expressed was low on SSc dermal fibroblasts (Fig. 4). However, its expression was highly inducible by TNF-α, in a dose-dependent fashion. In addition, we found that ICAM-1 was expressed on SSc dermal fibroblasts and that its expression was inducible by stimulation with TNF-α, IL-1β or IFN-γ, consistent with previous findings (Fig. 4) [9]. Our results also showed that ICAM-1 expression on SSc dermal fibroblasts was inducible by IL-17 in a dose-dependent manner.
Fig. 4.
The expression of VCAM-1 and ICAM-1 on SSc dermal fibroblasts is highly inducible. Cell surface ELISAs were performed to determine if VCAM-1 and ICAM-1 expression on the surface of SSc dermal fibroblasts was inducible by cytokine stimulation. (A) VCAM-1 expression on SSc dermal fibroblasts was induced by TNF-α (25 ng/ml) stimulation. (B) TNF-α-induced VCAM-1 expression on SSc dermal fibroblasts in a dose-dependent manner. (C) ICAM-1 expression on SSc dermal fibroblasts was induced by TNF-α, IFN-γ, IL-1β, IL-17, but not IL-18 (each 25 ng/ml). (D) IL-17-induced ICAM-1 expression on SSc dermal fibroblasts in a dose-dependent manner. Means ± SEM are shown. n = number of patient-derived fibroblast lines.
Adhesion molecule-mediated myeloid–SSc skin adhesion
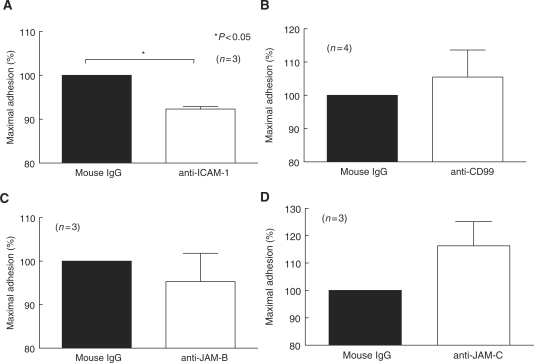
The functional consequence of adhesion molecule expression in SSc skin has not been thoroughly investigated. We performed myeloid cell–SSc dermal fibroblast adhesion assays to determine whether JAM-B, JAM-C or CD99 play a role in myeloid cell adhesion to SSc dermal fibroblasts. Our results indicate that these adhesion molecules do not mediate myeloid cell adhesion to SSc dermal fibroblasts (Fig. 5). However, neutralizing anti-ICAM-1 antibody significantly inhibited U937 cell binding to SSc dermal fibroblasts (Fig. 5). These results suggest that ICAM-1 is important in myeloid cell–SSc dermal fibroblast adhesion.
Fig. 5.
ICAM-1 mediates adhesion of U937 cells to SSc dermal fibroblasts. Adhesion assays were performed using U937 cells and SSc dermal fibroblasts in the presence of neutralizing antibodies against ICAM-1 (A), CD99 (B), JAM-B (C) and JAM-C (D). The percent of maximal binding was defined as the number of cells adhering to the fibroblasts in the presence of the test antibody divided by the number of adherent cells on the control-treated fibroblasts. U937 cell adhesion to SSc dermal fibroblasts was significantly inhibited by a neutralizing antibody against ICAM-1 (92% of maximal binding). Means ± SEM are shown. n = the number of SSc patient-derived fibroblast lines. P< 0.05 was considered significant.
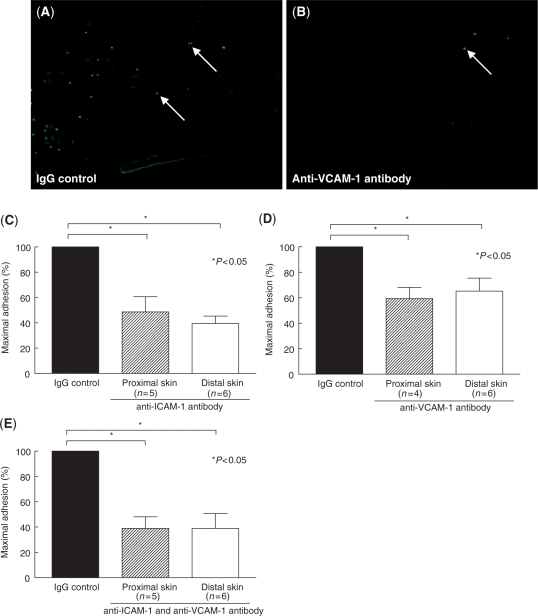
We also sought to determine whether, in addition to mediating myeloid–fibroblast adhesion, ICAM-1 mediates myeloid cell adhesion to SSc skin. In situ adhesion assays were performed using frozen proximal and distal SSc skin sections and U937 cells. Our results indicate that a neutralizing anti-ICAM-1 antibody significantly inhibits U937 cell binging to both proximal and distal SSc skin (Fig. 6). In addition, we found that a neutralizing anti-VCAM-1 and the combination of ICAM-1 and VCAM-1 significantly inhibits U937 cell binding to both proximal and distal SSc skin. These results demonstrate that ICAM-1 and VCAM-1 play a role in myeloid cell adhesion to SSc skin.
Fig. 6.
ICAM-1 and VCAM-1 mediate adhesion of U937 cells to proximal and distal SSc skin. Stamper–Woodruff in situ assays were performed using frozen skin sections and fluorescent-labelled U937 cells. The percent of maximal binding was defined as the number of adherent cells on the test sections divided by the number of adherent cells on the control sections. A representative image of U937 cell binding to proximal SSc skin in the presence of an IgG isotype control (A) or anti-VCAM-1 antibody (B) is shown. Arrows indicate U937 cells bound to SSc skin. (C) Anti-ICAM-1 antibody-inhibited U937 binding to proximal SSc skin (49% of maximal binding) and distal SSc skin (39% of maximal binding). (D) Anti-VCAM-1 antibody-inhibited U937 binding to proximal SSc skin (59% of maximal binding) and distal SSc skin (65% of maximal binding). (E) The combination of anti-ICAM-1 and anti-VCAM-1 antibody-inhibited U937 binding to proximal SSc skin (39% of maximal binding) and distal SSc skin (39% of maximal binding). Magnification was × 100. Means ± SEM are given. n = the number of patients. P< 0.05 was considered significant.
Discussion
SSc is characterized by excessive fibrosis of the skin and internal organs. Leucocyte infiltration occurs early in the disease process and may contribute to both fibrosis and the vascular components of the disease. Studies have pointed to a proadhesive phenotype in SSc, and in particular SSc skin. We have previously shown differences in adhesion molecule expression between normal and SSc skin [11]. P-selectin, VCAM-1 and CD44 are overexpressed on the stratum granulosum in the SSc epidermis, while VCAM-1 is also overexpressed on SSc dermal ECs [11]. CD44 is overexpressed on ECs and leucocytes [11]. In addition, by demonstrating that the expression of ICAM-1, E-selectin and integrins β1 and β2 is elevated in SSc skin, Sollberg et al. [12] contributed to the idea of a proadhesive phenotype. Moreover, the serum levels of many soluble adhesion molecules in SSc serum are increased compared with normal serum, possibly as a result of EC injury [15]. Collectively, these studies suggest a proadhesive phenotype in SSc skin.
Adhesion molecules at intercellular junctions have many roles that may be important in SSc skin pathogenesis. These adhesion molecules regulate vascular permeability, leucocyte adhesion and transmigration and, in some cases, angiogenesis. JAM-B is an adhesion molecule expressed at the intercellular junction of ECs [24]. It has been shown to be a key factor in a number of inflammatory states, as its expression is up-regulated on ECs in models of asthma, bronchitis, interstitial nephritis and autoimmune hepatitis [25]. In addition, JAM-B plays a role in leucocyte adhesion and trans-endothelial migration [17]. We found that JAM-B is highly expressed by ECs from both proximal and distal SSc skin, at levels similar to those of ECs from normal skin. In addition, we observed that JAM-B is expressed on both normal and SSc dermal fibroblasts at similar levels.
Like JAM-B, JAM-C is also expressed on SSc dermal fibroblasts. However, in contrast to JAM-B, JAM-C expression on dermal ECs in proximal and distal SSc skin is decreased compared with ECs in normal skin. JAM-C is a multifunctional adhesion molecule that mediates leucocyte adhesion and transmigration, controls vascular permeability and plays a role in many inflammatory conditions [17]. However, in addition to these properties, JAM-C has been shown to play a role in angiogenesis. Administration of a neutralizing anti-JAM-C antibody abolishes vessel outgrowth ex vivo [26]. Moreover, anti-JAM-C antibody treatment reduces tumour growth and vascularization in vivo [26]. As EC-expressed JAM-C has been shown to be involved in both leucocyte migration and angiogenesis, it's down-regulation in SSc skin could interfere with these events. However, future studies are needed to investigate the role of JAM-C in SSc trans-endothelial migration and angiogenesis.
In addition to these JAMs, we also evaluated the expression of CD99. CD99 is a small, highly O-glycosylated cell surface protein found on leucocytes and at cellular junctions between ECs [27]. This JAM also plays a critical role in monocyte trans-EC migration, and has been shown to be crucial in a number of inflammatory models [16, 27, 28]. Our results are in accordance with previous findings that indicated that CD99 is expressed on numerous cell types [27]. However, we found that the expression of CD99 is decreased on SSc dermal ECs, particularly in those of proximal SSc skin. Reduced CD99 expression on SSc dermal ECs may affect the migration of leucocytes through the endothelium. However, functional studies of CD99 EC expression are needed to elucidate the effect of diminished CD99 expression on SSc ECs. In contrast to the expression of CD99 on SSc ECs, CD99 expression was increased on perivascular and subepidermal mononuclear cells and on SSc dermal fibroblasts. However, our results indicate that while CD99 is overexpressed on SSc dermal fibroblasts, it is not a contributing factor to the binding of myeloid cells to SSc dermal fibroblasts. Further studies are needed to determine the role of altered CD99 expression in SSc skin.
Previous analysis of adhesion molecule expression in SSc skin has demonstrated that the adhesion molecules ICAM-1 and VCAM-1 are up-regulated in SSc skin and on SSc dermal fibroblasts [9, 11]. Moreover, Marlor et al. [29] demonstrated that VCAM-1 expression is up-regulated in response to TNF-α on normal synovial and dermal fibroblasts. TNF-α is a prominent pro-inflammatory, pro-fibrotic cytokine that is elevated in SSc serum [30]. Our results indicate that SSc dermal fibroblast VCAM-1 expression is highly inducible by TNF-α in a dose-dependent manner. Moreover, we observed that ICAM-1 is inducible by TNF-α, IFN-γ and IL-1β, as previously described [9]. In addition, we also determined whether ICAM-1 expression was inducible by IL-17. IL-17 has previously been shown to enhance the surface expression of ICAM-1 in normal human dermal fibroblasts, and to be increased in the serum of patients with SSc [31, 32]. We found that IL-17 stimulation enhanced ICAM-1 expression on SSc dermal fibroblasts in a dose-dependent manner. These results show that key cytokines in the pathogenesis of SSc are able to induce the expression of VCAM-1 and ICAM-1 on SSc dermal fibroblasts.
Next, we sought to determine if the adhesion molecules expressed in SSc skin mediate myeloid cell adhesion. Previous studies have shown that JAM-B and JAM-C mediate leucocyte adhesion through their interactions with the leucocyte expressed integrins VLA-4 and Mac-1, respectively [33, 34]. In addition, EC-expressed CD99 interacts with CD99 on the surface of neutrophils and monocytes to promote leucocyte adhesion [35]. However, we found that neutralizing antibodies against either JAM-B, JAM-C or CD99 did not inhibit myeloid cell binding to SSc dermal fibroblasts. In contrast, we observed that ICAM-1 mediates myeloid cell adhesion to SSc dermal fibroblasts. Previously, ICAM-1 has been shown to mediate T-cell binding to SSc dermal fibroblasts [13]. In our study, we found that ICAM-1 not only mediates myeloid cell adhesion to SSc dermal fibroblasts, but also myeloid cell adhesion to proximal and distal SSc skin. Similar results were observed using a neutralizing anti-VCAM-1 antibody. A combination of neutralizing anti-ICAM-1 and anti-VCAM-1 antibodies resulted in augmented inhibition of binding. Therefore, while we demonstrated a novel role for ICAM-1 and VCAM-1 in mediating myeloid cell adhesion in SSc skin, we did not observe a role for JAM-B, JAM-C or CD99 in myeloid cell adhesion to SSc dermal fibroblasts or SSc skin. It is possible that these adhesion molecules may play a role in the adhesion of other cell types to SSc dermal fibroblasts, or that they may facilitate adhesion between adjacent fibroblasts.
Our study was designed to determine the expression of adhesion molecules in SSc skin and on SSc dermal fibroblasts, and to determine if they mediate myeloid cell adhesion to SSc skin. We have shown that JAM-C and CD99 are aberrantly expressed in SSc skin; however, we found that they do not mediate myeloid cell adhesion to SSc dermal fibroblasts. Importantly, we demonstrated that ICAM-1 and VCAM-1 functionally mediate myeloid cell adhesion to SSc skin and thus potentially contribute to the binding and retention of leucocytes in sclerodermatous skin. Targeting ICAM-1 and VCAM-1 may be useful in SSc.

Acknowledgements
Funding: This work was supported by the National Institute of Health (grants AI-40987 and AR-48267 to A.E.K. and AR-19616 to S.A.J.), the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, the Frederick G. L. Huetwell and William D. Robinson, MD, Professorship in Rheumatology, Scleroderma Research Foundation, NIH General Clinical Research Center grant M01-RR-00042, NIH Center for Translational Science Activities grant UL1-RR-024986, the clinical research unit at the University of Michigan, and by funding from the Scleroderma Center of the University of Michigan.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Maricq HR, Spencer-Green G, LeRoy EC. Skin capillary abnormalities as indicators of organ involvement in scleroderma (systemic sclerosis), Raynaud's syndrome and dermatomyositis. Am J Med. 1976;61:862–70. doi: 10.1016/0002-9343(76)90410-1. [DOI] [PubMed] [Google Scholar]
- 2.Maricq HR. Wide-field capillary microscopy. Arthritis Rheum. 1981;24:1159–65. doi: 10.1002/art.1780240907. [DOI] [PubMed] [Google Scholar]
- 3.Kuryliszyn-Moskal A, Klimiuk PA, Sierakowski S. Soluble adhesion molecules (sVCAM-1, sE-selectin), vascular endothelial growth factor (VEGF) and endothelin-1 in patients with systemic sclerosis: relationship to organ systemic involvement. Clin Rheumatol. 2005;24:111–16. doi: 10.1007/s10067-004-0987-3. [DOI] [PubMed] [Google Scholar]
- 4.Denton CP, Bickerstaff MC, Shiwen X, et al. Serial circulating adhesion molecule levels reflect disease severity in systemic sclerosis. Br J Rheumatol. 1995;34:1048–54. doi: 10.1093/rheumatology/34.11.1048. [DOI] [PubMed] [Google Scholar]
- 5.Ihn H, Sato S, Fujimoto M, et al. Circulating intercellular adhesion molecule-1 in the sera of patients with systemic sclerosis: enhancement by inflammatory cytokines. Br J Rheumatol. 1997;36:1270–5. doi: 10.1093/rheumatology/36.12.1270. [DOI] [PubMed] [Google Scholar]
- 6.Sfikakis PP, Tesar J, Baraf H, Lipnick R, Klipple G, Tsokos GC. Circulating intercellular adhesion molecule-1 in patients with systemic sclerosis. Clin Immunol Immunopathol. 1993;68:88–92. doi: 10.1006/clin.1993.1100. [DOI] [PubMed] [Google Scholar]
- 7.Allanore Y, Borderie D, Lemarechal H, Ekindjian OG, Kahan A. Nifedipine decreases sVCAM-1 concentrations and oxidative stress in systemic sclerosis but does not affect the concentrations of vascular endothelial growth factor or its soluble receptor 1. Arthritis Res Ther. 2004;6:R309–14. doi: 10.1186/ar1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apras S, Ertenli I, Ozbalkan Z, et al. Effects of oral cyclophosphamide and prednisolone therapy on the endothelial functions and clinical findings in patients with early diffuse systemic sclerosis. Arthritis Rheum. 2003;48:2256–61. doi: 10.1002/art.11081. [DOI] [PubMed] [Google Scholar]
- 9.Cho M, Jimenez SA, Johnson B, Harlow LA, Burrows JC, Koch AE. Cytokine regulation of intercellular adhesion molecule-1 expression in scleroderma fibroblasts. Pathobiology. 1994;62:73–81. doi: 10.1159/000163881. [DOI] [PubMed] [Google Scholar]
- 10.Iannone F, Matucci-Cerinic M, Falappone PC, et al. Distinct expression of adhesion molecules on skin fibroblasts from patients with diffuse and limited systemic sclerosis. A pilot study. J Rheumatol. 2005;32:1893–8. [PubMed] [Google Scholar]
- 11.Koch AE, Kronfeld-Harrington LB, Szekanecz Z, et al. In situ expression of cytokines and cellular adhesion molecules in the skin of patients with systemic sclerosis. Their role in early and late disease. Pathobiology. 1993;61:239–46. doi: 10.1159/000163802. [DOI] [PubMed] [Google Scholar]
- 12.Sollberg S, Peltonen J, Uitto J, Jimenez SA. Elevated expression of beta 1 and beta 2 integrins, intercellular adhesion molecule 1, and endothelial leukocyte adhesion molecule 1 in the skin of patients with systemic sclerosis of recent onset. Arthritis Rheum. 1992;35:290–98. doi: 10.1002/art.1780350307. [DOI] [PubMed] [Google Scholar]
- 13.Abraham D, Lupoli S, McWhirter A, et al. Expression and function of surface antigens on scleroderma fibroblasts. Arthritis Rheum. 1991;34:1164–72. doi: 10.1002/art.1780340913. [DOI] [PubMed] [Google Scholar]
- 14.Rudnicka L, Majewski S, Blaszczyk M, et al. Adhesion of peripheral blood mononuclear cells to vascular endothelium in patients with systemic sclerosis (scleroderma) Arthritis Rheum. 1992;35:771–5. doi: 10.1002/art.1780350710. [DOI] [PubMed] [Google Scholar]
- 15.Sato S. Abnormalities of adhesion molecules and chemokines in scleroderma. Curr Opin Rheumatol. 1999;11:503–7. [PubMed] [Google Scholar]
- 16.Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3:143–50. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- 17.Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7:467–77. doi: 10.1038/nri2096. [DOI] [PubMed] [Google Scholar]
- 18.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- 19.Louneva N, Huaman G, Fertala J, Jimenez SA. Inhibition of systemic sclerosis dermal fibroblast type I collagen production and gene expression by simvastatin. Arthritis Rheum. 2006;54:1298–308. doi: 10.1002/art.21723. [DOI] [PubMed] [Google Scholar]
- 20.Aurrand-Lions M, Duncan L, Ballestrem C, Imhof BA. JAM-2, a novel immunoglobulin superfamily molecule, expressed by endothelial and lymphatic cells. J Biol Chem. 2001;276:2733–41. doi: 10.1074/jbc.M005458200. [DOI] [PubMed] [Google Scholar]
- 21.Amin MA, Haas CS, Zhu K, et al. Migration inhibitory factor up-regulates vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 via Src, PI3 kinase, and NFkappaB. Blood. 2006;107:2252–61. doi: 10.1182/blood-2005-05-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamagna C, Meda P, Mandicourt G, et al. Dual interaction of JAM-C with JAM-B and alpha(M)beta2 integrin: function in junctional complexes and leukocyte adhesion. Mol Biol Cell. 2005;16:4992–5003. doi: 10.1091/mbc.E05-04-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claman HN, Giorno RC, Seibold JR. Endothelial and fibroblastic activation in scleroderma. The myth of the “uninvolved skin”. Arthritis Rheum. 1991;34:1495–501. doi: 10.1002/art.1780341204. [DOI] [PubMed] [Google Scholar]
- 24.Mandell KJ, Parkos CA. The JAM family of proteins. Adv Drug Deliv Rev. 2005;57:857–67. doi: 10.1016/j.addr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Liang TW, Chiu HH, Gurney A, et al. Vascular endothelial-junctional adhesion molecule (VE-JAM)/JAM 2 interacts with T, NK, and dendritic cells through JAM 3. J Immunol. 2002;168:1618–26. doi: 10.4049/jimmunol.168.4.1618. [DOI] [PubMed] [Google Scholar]
- 26.Lamagna C, Hodivala-Dilke KM, Imhof BA, Aurrand-Lions M. Antibody against junctional adhesion molecule-C inhibits angiogenesis and tumor growth. Cancer Res. 2005;65:5703–10. doi: 10.1158/0008-5472.CAN-04-4012. [DOI] [PubMed] [Google Scholar]
- 27.Petri B, Bixel MG. Molecular events during leukocyte diapedesis. FEBS J. 2006;273:4399–407. doi: 10.1111/j.1742-4658.2006.05439.x. [DOI] [PubMed] [Google Scholar]
- 28.Bixel G, Kloep S, Butz S, Petri B, Engelhardt B, Vestweber D. Mouse CD99 participates in T-cell recruitment into inflamed skin. Blood. 2004;104:3205–13. doi: 10.1182/blood-2004-03-1184. [DOI] [PubMed] [Google Scholar]
- 29.Marlor CW, Webb DL, Bombara MP, Greve JM, Blue ML. Expression of vascular cell adhesion molecule-1 in fibroblastlike synoviocytes after stimulation with tumor necrosis factor. Am J Pathol. 1992;140:1055–60. [PMC free article] [PubMed] [Google Scholar]
- 30.Scala E, Pallotta S, Frezzolini A, et al. Cytokine and chemokine levels in systemic sclerosis: relationship with cutaneous and internal organ involvement. Clin Exp Immunol. 2004;138:540–6. doi: 10.1111/j.1365-2249.2004.02642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurasawa K, Hirose K, Sano H, et al. Increased interleukin-17 production in patients with systemic sclerosis. Arthritis Rheum. 2000;43:2455–63. doi: 10.1002/1529-0131(200011)43:11<2455::AID-ANR12>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 32.Yao Z, Painter SL, Fanslow WC, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–6. [PubMed] [Google Scholar]
- 33.Cunningham SA, Rodriguez JM, Arrate MP, Tran TM, Brock TA. JAM2 interacts with alpha4beta1. Facilitation by JAM3. J Biol Chem. 2002;277:27589–92. doi: 10.1074/jbc.C200331200. [DOI] [PubMed] [Google Scholar]
- 34.Santoso S, Sachs UJ, Kroll H, et al. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med. 2002;196:679–91. doi: 10.1084/jem.20020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lou O, Alcaide P, Luscinskas FW, Muller WA. CD99 is a key mediator of the transendothelial migration of neutrophils. J Immunol. 2007;178:1136–43. doi: 10.4049/jimmunol.178.2.1136. [DOI] [PubMed] [Google Scholar]