Abstract
Prokineticin 1 (PROK1) is a recently described protein with a wide range of functions including tissue-specific angiogenesis, modulation of inflammatory responses, and regulation of hematopoiesis. The objective of this study was to investigate the role of PROK1 and prokineticin receptor 1 (PROKR1) in human endometrium during early pregnancy. PROK1 and PROKR1 expression is significantly elevated in first-trimester decidua, compared with nonpregnant endometrium. Expression of PROK1 and PROKR1 was localized in glandular epithelial and various cellular compartments within the stroma. To investigate the signaling pathways and target genes activated by PROK1, we generated an endometrial epithelial cell line stably expressing PROKR1 (Ishikawa PROKR1 cells). PROK1-PROKR1 interaction induced inositol phosphate mobilization and sequential phosphorylation of c-Src, epidermal growth factor receptor, and ERK 1/2. Gene microarray analysis on RNA extracted from Ishikawa PROKR1 cells treated with 40 nm PROK1 for 8 h revealed 49 genes to be differentially regulated. A number of these genes, including cyclooxygenase (COX)-2, leukemia inhibitory factor, IL-6, IL-8, and IL-11 are regulated in the endometrium during implantation and early pregnancy. We subsequently investigated the effect of PROK1 on expression of COX-2 in Ishikawa PROKR1 cells and first-trimester decidua. COX-2 mRNA and protein expression, and prostaglandin synthesis, were elevated in response to treatment with PROK1. Moreover, expression of COX-2 by PROK1 was dependent on activation of the Gq-phospholipase C-β-cSrc-epidermal growth factor receptor-MAPK/ERK kinase pathway. These data demonstrate that PROK1 and PROKR1 expression is elevated in human decidua during early pregnancy and that PROK1-PROKR1 interaction regulates expression of a host of implantation-related genes.
Prokineticins are secreted proteins with pleiotropic functions in human and animal tissues. They comprise prokineticin (PROK)-1 or endocrine gland-vascular endothelial growth factor (1, 2) and PROK2 or Bv8 (3). The receptors for prokineticins are two closely related G protein-coupled receptors, prokineticin receptor (PROKR)-1 and PROKR2 (4, 5). Structure-function analysis have highlighted that the prokineticin N-terminal amino acid sequence, AVITGA, is completely conserved and essential to their activity (6, 7).
Expression and regulation of the prokineticins has been described in human endometrium and early pregnancy tissues. PROK1 but not PROK2 displays differential expression across the menstrual cycle with elevation in the secretory phase (8, 9), and expression of PROK1 is known to be regulated by steroid hormones (8, 9). However, PROKR1 and PROKR2 mRNA expression does not change across the menstrual cycle, and little is known about their mode of regulation. PROK1 and PROK2 localize to the glandular and luminal epithelium, endothelial, and stromal cells of the endometrium (8). Similarly, PROKR1 localizes to the glandular and luminal epithelium, endothelial, and stromal cells in the functional layer of the endometrium (8). PROK1 and PROKR1 expression have also been reported to change in placental tissues during early pregnancy with elevation between wk 8 and 9 of gestation (10). PROK1 localizes to the syncytiotrophoblast and cytotrophoblast layers (10), whereas PROKR1 expression remains to be investigated.
We demonstrate elevated expression of PROK1 and PROKR1 in first-trimester decidua, compared with nonpregnant endometrium. Expression of both proteins in first-trimester decidua localized to glandular epithelium and endothelial cells of the microvasculature. Additionally, PROK1 but not PROKR1 was detected in uterine natural killer cells. Treatment of endometrial epithelial Ishikawa cells stably transfected with PROKR1 with PROK1 resulted in inositol phosphate production and phosphorylation of cSrc, epidermal growth factor receptor (EGFR), and ERK1/2. Gene array analysis of these cells treated with PROK1 identified 49 PROK1-PROKR1 regulated genes. These included genes known to function in regulation of implantation, such as cyclooxygenase (COX)-2, leukemia inhibitory factor (LIF), and IL-11. We subsequently established that PROK1 elevates expression of COX-2 via a Gq-phospholipase C (PLC)-β-cSRC-EGFR-ERK1/2 pathway in PROKR1-Ishikawa cells and first-trimester decidua.
Materials and Methods
Reagents
DMEM F-12 Glutamax culture medium was purchased from Invitrogen Life Technologies (Paisley, UK). Phosphotyrosine, c-Myc, phospho-cSrcY418, phospho-EGFR, β-actin, and COX-2 antibodies were purchased from Santa Cruz Biotechnology/Autogen Bioclear (Wiltshire, UK). Phospho-ERK and total-ERK antibodies were purchased from Cell Signaling Technologies (Hertfordshire, UK). Prokineticin 1 antibody was purchased from Phoenix Pharmaceuticals (Belmont, CA). Prokineticin receptor 1 antibody was purchased from Lifespan Biosciences (Atlanta, GA). Alkaline phosphatase secondary antibodies and BSA were purchased from Sigma (Dorset, UK). Superfect transfection reagent was purchased from QIAGEN (Crawley, UK). Tritiated myo-inositol and enhanced chemifluorescence were purchased from Amersham (Little Chalfont, Buckinghamshire, UK). Fluorescent secondary antibodies were purchased from Li-Cor Biosciences UK Ltd. (Cambridge, UK). PLC inhibitor (U73122, final concentration 10 μm), cSrc inhibitor [4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d] pyrimidine (PP2), final concentration 10 μm], EGFR inhibitor (AG1478, final concentration 200 nm), MAPK kinase (MEK) inhibitor (PD98059, final concentration 50 μm), and COX-2 inhibitor (NS-398, final concentration 10 μm) were purchased from Calbiochem (Nottingham, UK). Gq inhibitor (YM254890, final concentration 1 μm) was kindly supplied by Dr. Jun Takasaki (Molecular Medicine Laboratories, Yamanouchi Pharmaceutical Co. Ltd., Tokyo, Japan). Recombinant human PROK1 was purchased from Promokine (Heidelberg, Germany).
Patients and tissue collection
Endometrial biopsies (n = 51) were obtained from women with regular menstrual cycles (25–35 d) who had not received hormonal preparation in the 3 months preceding biopsy collection and dated according to histological assessment by a pathologist. Furthermore, circulating estradiol and progesterone concentrations were consistent for both stated last menstrual period and histological assessment of menstrual cycle stage. First-trimester decidua (7–12 wk, n = 33) was collected from women undergoing elective first-trimester surgical termination of pregnancy. Ethical approval was obtained from Lothian Research Ethics Committee, and written informed consent obtained before tissue collection.
Cell/tissue culture and treatment
Ishikawa endometrial epithelial cells obtained from the European Collection of Cell Culture (Health Protection Agency, Porton Down, Wiltshire, UK) were routinely maintained in DMEM F-12 Glutamax culture medium with 10% fetal bovine serum, 100 IU penicillin, and 100 μg streptomycin at 37 C and 5% CO2 as of recommended, with addition 200 μg/ml G418 to culture medium of stably transfected cells. First-trimester decidua tissue for explant studies (n = 24) was isolated at surgical termination of pregnancy. Tissue explants were chopped finely with scissors and maintained in DMEM. Tissue was divided into equal portions for experimental procedures.
To produce PROKR1-Ishikawa cells, human receptor cDNA was amplified using Easy-A DNA polymerase (Stratagene, La Jolla, CA) and specific primers: forward; 5′-GGA TCC AGG CTT GAT GGA GAC CAC CAT GGG G-3′, reverse; 5′-CTC GAG GAT ATC TTT TAG TCT GAT GCA GTC CAC CT-3′. The PCR product was gel purified, ligated into sequencing vector pCR4 (Invitrogen) and cloned into Escherichia coli TOP10 cells. Cloned plasmid DNA was sequenced before subcloning into pcDNA3.1(+), transfected into Ishikawa cells using electroporation, and G418-resistant clones isolated. A selected clone was characterized for PROKR1 expression by PCR and activation of signaling. Transient transfections were performed using Myc-tagged ERK and dominant-negative (DN) isoforms of cSrc, EGFR, Ras, and MEK (kindly donated by Professor Zvi Naor, Department of Biochemistry, Tel Aviv University, Tel Aviv, Israel).
Cells and tissue were incubated in serum-free medium overnight before treatment with PROK1 alone or in the presence of inhibitors, at concentrations indicated above, with pretreatment for 1 h (8). Cells and tissue were harvested and RNA or protein extracted for PCR and Western immunoblot analysis. Cells cotransfected with Myc-tagged ERK and DN were subjected to immunoprecipitation before Western immunoblot analysis.
Total inositol phosphate assay
Accumulation of total inositol phosphates in the presence of Li+ was measured in wild-type (WT) and PROKR1-Ishikawa cells, preloaded with [3H]myo-inositol and subsequently treated with PROK1, according to published protocols (11).
Immunohistochemistry and immunofluorescent microscopy
Five-micrometer paraffin-embedded sections were dewaxed and rehydrated in graded ethanol. Sections were incubated overnight at 4 C with rabbit antihuman PROK1 (1:1000) or rabbit antihuman PROKR1 (1:250). An avidin-biotin peroxidase detection system was applied (Dako Ltd., Cambridge, UK) with 3,3′-diaminobenzidine as the chromagen. Colocalization of PROKR1 with COX-2 or CD31 (endothelial cell marker) and PROK1 with CD56 (natural killer cell marker) were performed by dual-immunofluorescence histochemistry. Sections were prepared and blocked using 5% normal horse serum (PROKR1/COX-2) or 5% normal goat serum (PROK1/CD56 and PROKR1/CD31). Sections were incubated with goat anti-COX-2 antibody (1:50), mouse anti-CD56 (1:250), or mouse anti-CD31 (1:20) overnight at 4 C. Subsequently sections were incubated with biotinylated antibodies, followed by incubation with fluorochromes streptavidin 488 or 546 (1:200 in PBS). Sections were reblocked with 5% normal goat serum and incubated with rabbit anti-human PROK1 (1:1500) or rabbit antihuman PROKR1 (1:500) overnight at 4 C. Negative control sections were incubated with rabbit IgG. Sections were incubated with peroxidase goat antirabbit (1:200 in PBS) followed by fluorochromes TSA-plus fluorescein (PerkinElmer, Applied Biosystems, Warrington, UK) or cyanine-3 (1:50 in substrate). Sections were washed and incubated with nuclear counterstain ToPro (1:2000 in PBS), mounted in Permafluor, coverslipped, visualized, and photographed using a laser-scanning microscope (LSM 510; Carl Zeiss, Jena, Germany) using a 40 × 1.4 aperture oil immersion lens.
Taqman quantitative RT-PCR
RNA was extracted with TRI reagent (Sigma) following the manufacturer's guidelines using phase lock tubes (Eppendorf, Cambridge, UK). RNA samples were reverse transcribed as described (6). PCRs were carried out using an ABI Prism 7700 (Applied Biosystems, Foster City, CA). Primer and FAM (6-carboxyfluorescein)-labeled probe sequences are supplied in Table 1. Gene expression was normalized to RNA loading using primers and VIC (Applied Biosystems)-labeled probe for ribosomal 18s as an internal standard. Results are expressed as relative to a positive RNA standard (cDNA obtained from a single endometrial tissue) included in all reactions.
TABLE 1.
Taqman primer and probe sequences for COX-2, LIF, IL-6, IL-8, IL-11, and 18s
| Gene | Primers and probe (5′–3′) |
|---|---|
| PROK1 forward | GTGCCACCCGGGCAG |
| PROK1 reverse | AGCAAGGACAGGTGTGGTGC |
| PROK1 probe (FAM) | ACAAGGTCCCCTTGTTCAGGAAACGCA |
| PROKR1 forward | TCTTACAATGGCGGTAAGTCCA |
| PROKR1 reverse | CTCTTCGGTGGCAGGCAT |
| PROKR1 probe (FAM) | TGCAGACCTGGACCTCAAGACAATTGG |
| COX-2 forward | CCTTCCTCCTGTGCCTGATG |
| COX-2 reverse | ACAATCTCATTTGAATCAGGAAGCT |
| COX-2 probe (FAM) | TGCCCGACTCCCTTGGGTGTCA |
| LIF forward | TGGTGGAGCTGTACCGCATA |
| LIF reverse | TGGTCCCGGGTGATGTTG |
| LIF probe (FAM) | TCGTGTACCTTGGCACCTCCCTGG |
| IL-6 forward | GCCGCCCCACACAGACA |
| IL-6 reverse | CCGTCGAGGATGTACGGAAT |
| IL-6 probe (FAM) | CCACTCACCTCTTCAGAACGAATTGACAAAC |
| IL-8 forward | CTGGCCGTGGCTCTCTT |
| IL-8 reverse | TTAGCACTCCTTGGCAAAACTG |
| IL-8 probe (FAM) | CCTTCCTGATTTCTGCAGCTCTGTGTGAA |
| IL-11 forward | CCCAGTTACCCAAGCATCCA |
| IL-11 reverse | AGACAGAGAACAGGGAATTAAATGTGT |
| IL-11 probe (FAM) | CCCCAGCTCTCAGACAAATCGCCC |
| 18s forward | CGGCTACCACATCCAAGGAA |
| 18s reverse | GCTGGAATTACCGCGGCT |
| 18s probe (VIC) | TGCTGGCACCAGACTTGCCCTC |
PCR analysis
PROK1 and PROKR1 expression in uterine natural killer (uNK) cells was assessed by conventional RT-PCR. Natural killer cells were isolated from first-trimester decidua according to published protocols (12). RNA was extracted, reverse transcribed, and cDNA subjected to PCR analysis with specific primers for PROK1 and PROKR1: PROK1 forward, 5′-GTG CCA CCC GGG CAG-3′; PROK1 reverse, 5′-AGC AAG GAC AGG TGT GGT GC-3′; PROKR1 forward, 5′-GGA TCC AGG CTT GAT GGA GAC CAC CAT GGG G-3′; PROKR1 reverse, 5′-CTC GAG GAT ATC TTT TAG TCT GAT GCA GTC CAC CT-3′. cDNA prepared from human endometrium with/without reverse transcriptase enzyme was positive and negative controls, respectively. PCR products were run on a 1% agarose gel.
Protein extraction and Western immunoblot analysis
Cells/tissue were harvested in Nonidet P-40 lysis buffer and protein content quantified (Bio-Rad Laboratories, Hemel Hempstead, UK). Proteins (40 μg) were solubilized in Laemmli buffer and boiled for 5 min. For immunoprecipitation experiments, 1 mg of lysate was incubated with antiphosphotyrosine agarose preconjugated slurry or anti-Myc agarose preconjugated slurry overnight at 4 C and solubilized as above. Proteins were resolved, blotted, incubated with specific primary antibodies, visualized by ECF or direct fluorescent system, and quantified by phosphorimager analysis (Typhoon 9400 system; Amersham) or infrared imaging system (Odyssey; LiCor). To account for variability in protein loading, phosphorylation of protein was normalized by dividing the value obtained from the phosphorylated blots by the value obtained from the total ERK (for ERK Westerns) or light-chain IgG blots (for cSrc and EGFR Westerns). Relative density of COX-2 blot was normalized against β-actin.
Gene array
PROKR1-Ishikawa cells were treated with vehicle or 40 nm PROK1 for 8 h. RNA was extracted and hybridized to GeneChip Human Genome U133 plus 2.0 (Affymetrix, High Wycombe, UK) and AB1700 version 2 Applied Biosystems Human Genome Survey microarrays. After hybridization the GeneChip arrays were stained and washed on the fluidics station and scanned.
Gene array data analysis
Cross-mapping between AB1700 probes and Affymetrix probe sets was courtesy provided by Applied Biosystems. Data acquired using ABI technology were preprocessed according to the manufacturers' recommendations. The data for both platforms were normalized using variance stabilized normalization (13). Normalized data were analyzed for differential expression with the LIMMA package as described in the LIMMA user guide (14). The P values were adjusted for multiple testing with Benjamini and Hochberg method (15). The resulting gene list included only the genes that had a fold change value of 1.5 or higher and a P < 0.05. Bioinformatics was performed using the gene set analysis tool kit (16). A hypergeometric test was used to calculate significantly over-represented ontologies from the gene list.
Prostaglandin (PG)-E2 and PGF2α measurement
PROKR1-Ishikawa cells were treated with 40 nm PROK1 alone or in the presence of COX-2 inhibitor NS398 with the addition of 3 μg/ml arachidonic acid. Medium was removed and assayed by ELISA for PGE2 and PGF2α (17). Data are presented as fold above vehicle-treated control.
Statistics
Data were subjected to statistical analysis with ANOVA and Fisher's protected least significant difference tests (Statview 5.0; Abacus Concepts Inc., Berkeley, CA).
Results
Expression of PROK1 and PROKR1 in cycling endometrium and first-trimester decidua
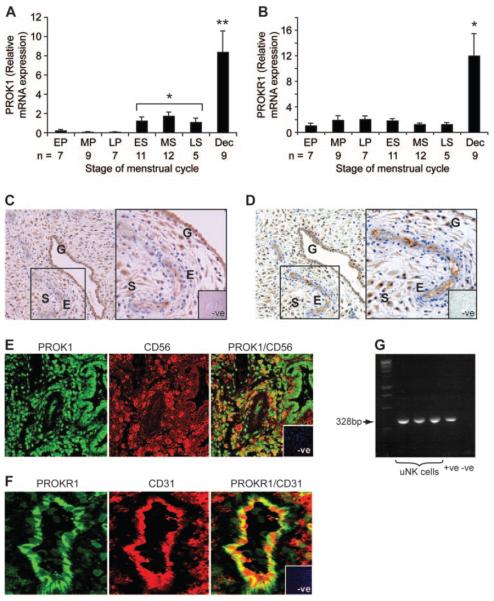
PROK1 mRNA expression was significantly elevated in the secretory phase of the menstrual cycle, compared with the proliferative phase (P < 0.05, Fig. 1A), whereas PROKR1 expression did not vary (Fig. 1B). mRNA expression of both PROK1 and PROKR1 in first-trimester decidua was elevated, compared with nonpregnant endometrium (P < 0.001, Fig. 1, A and B, respectively). PROK1 and PROKR1 protein localize to glandular epithelium, stroma, and vascular endothelial cells of first-trimester decidua (Fig. 1, C and D, PROK1 and PROKR1, respectively), similar to the localization reported for PROK1 and PROKR1 in nonpregnant endometrium (8). Expression of PROK1 and CD56 (a marker of natural killer cells) or PROKR1 and CD31 (a marker of endothelial cells) were colocalized (Fig. 1, E and F, respectively) in the decidualized endometrium. Expression of PROK1 in uNK cells within the pregnant endometrium was confirmed by RT-PCR analysis. A PCR band of the expected size for PROK1 (328 bp) was amplified from cDNA prepared using RNA from uNK cells isolated from three individual first-trimester decidual tissues (Fig. 1G). PROKR1 expression was not detected in uNK cells by PCR analysis or immunohistochemical colocalization (data not shown).
FIG. 1.
Temporal expression and localization of PROK1 and PROKR1 in human endometrium and first-trimester decidua. PROK1 (A) and PROKR1 (B) mRNA expression in endometrium and first-trimester decidua are shown. EP, Early proliferative, MP, mid proliferative, LP, Late proliferative; ES, early secretory; MS, midsecretory; LS, late secretory (sample size from each stage of the cycle is depicted in the figure). In first-trimester deciduas, PROK1 and PROKR1 expression (n = 7; representative sections shown in C and D, respectively) localize to the glandular epithelium (G), stromal cells (S), and vascular endothelial cells (E) of the microvasculature (×10 magnification, inset, black box, ×20 magnification). E, A population of PROK1-expressing stromal cells were identified as natural killer cells by immunofluorescent histochemistry and confocal microscopy for CD56 (natural killer cell marker, red panel) and PROK1 (green panel) by co-localization (yellow channel). This was confirmed by conventional PCR (G) conducted using natural killer cell RNA isolated from individual decidua tissue (n = 3). PROKR1 localization to endothelial cells was confirmed by double-immunofluorescent histochemistry (F) for CD31 (endothelial cell marker, red panel) and PROKR1 (green panel) by colocalization (yellow channel). Data are shown as mean ± sem (*, P < 0.05; **, P < 0.001). Negative control (−ve) is indicated in the figure.
PROK1 via PROKR1 induces ERK 1/2 phosphorylation via a cSrc and EGFR dependent pathway
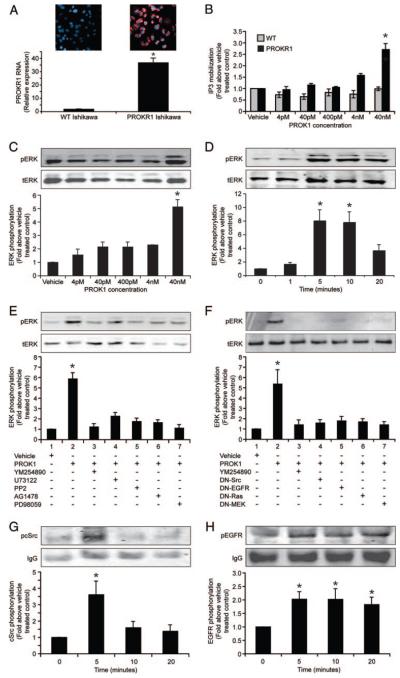
To investigate signaling and gene activation upon PROKR1 activation, Ishikawa cells were stably transfected with PROKR1 cDNA. Real-time PCR revealed elevation (~35-fold) of PROKR1 mRNA in these cells, compared with wild-type Ishikawa cells (Fig. 2A). Immunofluorescent histochemistry demonstrates differential expression of PROKR1 in PROKR1 Ishikawa cells, compared with WT Ishikawa cells (Fig. 2A). PROKR1 is thought to couple to Gq and therefore activate PLC (4). We therefore measured total inositol phosphate (IP3) accumulation in WT and PROKR1-Ishikawa cells after PROK1 treatment. There was no increase in IP3 production in WT Ishikawa cells; however, IP3 production in PROKR1-Ishikawa cells was significantly elevated after treatment with 40 nm PROK1 (2.71 ± 0.25-fold above vehicle-treated control, P < 0.05, Fig. 2B). We subsequently assessed the effect of PROK1 on the activation of ERK 1/2. Phosphorylation of ERK 1/2 in PROKR1-Ishikawa cells was observed after treatment with 40 nm PROK1 (5.21 ± 0.52-fold above vehicle-treated control, P < 0.001, Fig. 2C). Phosphorylation of ERK 1/2 in response to 40 nm PROK1 was maximal within 5–10 min and declined by 20 min (Fig. 2D).
FIG. 2.
Expression of PROKR1 in PR OKR1-Ishikawa cells and signaling induced by PROKR1 via PROK1. A, PROKR1 RNA expression is significantly elevated in PROKR1-Ishikawa cells, compared with WT Ishikawa cells as assessed by real-time PCR analysis. Similarly, immunofluorescent histochemistry demonstrates expression of PROKR1 protein (red staining) in PROKR1-Ishikawa cells, which is absent in WT Ishikawa cells. B, Inositol phosphate mobilization is elevated in PROKR1, compared with WT Ishikawa cells after treatment with 40 nM PROK1. C, ERK1/2 phosphorylation was determined by Western blot analysis; phosphorylation is maximal in PROKR1-Ishikawa cells after treatment with 40 nM PROK1. D, ERK1/2 phosphorylation in PROKR1-Ishikawa cells incubated with 40 nM PROK1 is maximal at 5 min. E, ERK 1/2 phosphorylation was measured in PROKR1-Ishikawa cells in response to the administration of vehicle or 40 nM PROK1 for 5 min in the presence or absence of pretreatment with YM254890 (Gq inhibitor, lane 3), U73122 (PLC inhibitor, lane 4), PP2 (cSrc inhibitor, lane 5), AG1478 (EGFR inhibitor, lane 6), or PD98059 (MEK inhibitor, lane 7). The PROK1-induced ERK 1/2 response was significantly inhibited in the presence of these inhibitors. F, PROKR1-Ishikawa cells were transiently cotransfected with Myc-tagged ERK and either empty vector (pcDNA3) or cDNA constructs encoding DN isoforms of cSrc, EGFR, Ras, or MEK. Transfected cells were subjected to stimulation with vehicle or 40 nM PROK1 for 5 min. PROK1-induced ERK response was significantly inhibited by the DN cDNA constructs. G and H, cSrc and EGFR phosphorylation is increased in PROKR1-Ishikawa cells after treatment with 40 nM PROK1. The PROK1-induced cSrc and EGFR phosphorylation peaked at 5 min. ERK1/2, cSrc and EGFR phosphorylation was calculated as fold above vehicle-treated controls. For each, a representative Western immunoblot is shown with semiquantitative analysis determined as described in Materials and Methods. In all panels each bar represents the mean ± sem of at least three individual experiments (*, P < 0.05). −, Absence of agent; +, presence of agent.
We investigated the intracellular signaling mechanism of ERK 1/2 phosphorylation by PROK1-PROKR1 activation. PROK1-induced ERK 1/2 phosphorylation at 5 min (Fig. 2E, lane 2) was abolished by cotreatment with Gq inhibitor (Fig. 2E, lane 3), PLC-β inhibitor (Fig. 2E, lane 4), cSrc inhibitor (Fig. 2E, lane 5), EGFR inhibitor (Fig. 2E, lane 6), or MEK inhibitor (Fig. 2E, lane 7). PROK1-induced ERK 1/2 phosphorylation was not inhibited on cotreatment with pertussis toxin (data not shown). Transient cotransfection of PROKR1-Ishikawa cells with a Myc-tagged ERK cDNA construct and empty vector (pcDNA3) or cDNA encoding DN isoforms of cSrc, EGFR, Ras, or MEK also significantly reduced PROK1-induced elevation of Myc-tagged ERK phosphorylation at 5 min (Fig. 2F, P < 0.001).
Treatment of PROKR1-Ishikawa cells with 40 nm PROK1 induced rapid phosphorylation of both cSrc (Fig. 2G) and EGFR (Fig. 2H), which peaked at 5 min (3.61 ± 0.8- and 2.02 ± 0.27-fold above vehicle-treated control for cSrc and EGFR phosphorylation respectively, P < 0.05).
PROK1 induces expression of genes with established roles in implantation
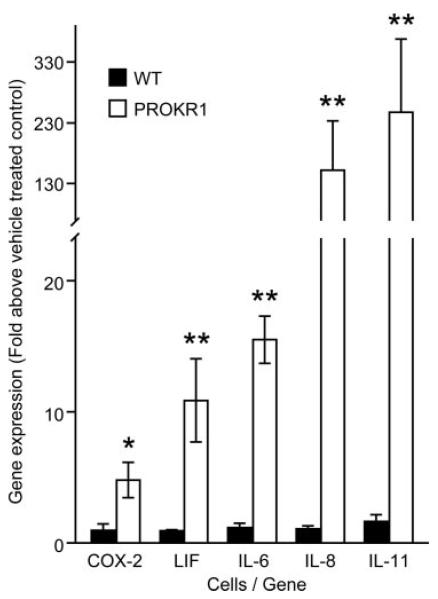
To examine gene expression regulated by PROK1, PROKR1-Ishikawa cells were treated with 40 nm PROK1 or vehicle for 8 h (three independent experiments). RNA was extracted, hybridized to AB1700 and Affymetrix gene chips, and subjected to gene array analysis. We identified 46 up- and three down-regulated genes yielding fold changes of more than 1.5-fold on both platforms with an adjusted P ≤ 0.05 on at least one of the platforms (Table 2). Five genes were selected to verify gene array data by real-time PCR. Basal level of expression of these genes was not different in WT, compared with PROKR1 Ishikawa cells. However, PROK1 treatment of PROKR1-Ishikawa cells for 8 h confirmed the elevation observed in the arrays for COX-2 (4.8 ± 1.2-fold), LIF (12.4 ± 3.5-fold), IL-6 (15.55 ± 1.8-fold), IL-8 (152 ± 81.1-fold), and IL-11 (249.6 ± 88.3-fold). No increase in gene expression was detected in WT Ishikawa cells (Fig. 3). It has been demonstrated that gene array analysis has a dynamic range of around 3 orders of magnitude, compared with a dynamic range of around 7 orders of magnitude for Taqman real-time PCR based analysis (18). This confers a higher detection sensitivity on Taqman PCR-based analysis. Additionally, it is well recognized that due to technical limitations associated with the amount of bound DNA, array analysis can result in an underestimation of differential gene expression (19). The difference in fold change observed with IL-8 and IL-11 between array vs. PCR analysis is therefore likely due to the smaller dynamic range of the gene array at large fold changes, compared with high dynamic range of PCR-based analysis.
TABLE 2.
Regulation of gene expression by PROK1 on activation of PROKR1
| Gene symbol | Gene product | Mean fold change |
|---|---|---|
| ACSL4 | Acyl-CoA synthetase long-chain family member 4 isoform 2 | 1.5 |
| AKAP12 | A-kinase anchor protein 12 isoform 2 | 2.8 |
| AREG | Amphiregulin preproprotein | 5.3 |
| BNC1 | Basonuclin 1 | 2.4 |
| CD44 | CD44 antigen isoform 4 precursor | 1.9 |
| CORO1C | Coronin | 2.3 |
| DAF | Decay accelerating factor for complement | 9.8 |
| DCAMKL1 | Doublecortin and CaM kinase-like 1 | 2.6 |
| DKK1 | Dickkopf homolog 1 | 8.5 |
| DNAJB9 | DnaJ (Hsp40) homolog, subfamily B, member 9 | 1.9 |
| DSCR1 | Calcipressin 1 isoform c | 5.2 |
| DTR | Diphtheria toxin receptor/heparin binding EGF like growth factor | 3.6 |
| DUSP1 | Dual-specificity phosphatase 1 | 4.4 |
| DUSP14 | Dual-specificity phosphatase 14 | 1.9 |
| DUSP4 | Dual-specificity phosphatase 4 isoform 2 | 9.4 |
| DUSP5 | Dual-specificity phosphatase 5 | 7.2 |
| EGR1 | Early growth response 1 | 2.1 |
| EIF2AK3 | Eukaryotic translation initiation factor 2-α kinase 3 | 1.7 |
| ENAH | Enabled homolog | 1.7 |
| FGD6 | RhoGEF and PH domain containing 6 | 1.8 |
| GEM | GTP-binding mitogen-induced T-cell protein | 5.3 |
| GREM1 | Cysteine knot superfamily 1 | 12.4 |
| HERC4 | Hect domain and RLD 4 | 1.9 |
| HMGB2 | High-mobility group box 2 | −1.6 |
| IER3 | Immediate early response 3 | 9.2 |
| IL11 | IL-11 precursor | 19.8 |
| IL6 | IL-6 | 17.2 |
| IL8 | IL-8 precursor | 9.7 |
| KRTHA4 | Type I hair keratin 4 | 5.8 |
| LAMA3 | Laminin-α3 subunit isoform 2 | 2.0 |
| LIF | Leukemia inhibitory factor | 2.6 |
| Molecule possessing ankyrin repeats induced by lipopolysaccharide | 3.6 | |
| NR3C1 | Nuclear receptor subfamily 3 | 1.6 |
| NR4A1 | Nuclear receptor subfamily 4, group A, member 1 isoform | 7.0 |
| NR4A2 | Nuclear receptor subfamily 4, group A, member 2 isoform | 4.3 |
| PBEF1 | Pre-B-cell colony enhancing factor 1 isoform | 1.8 |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 | 6.3 |
| PTPRR | Protein tyrosine phosphatase, receptor type, R | 2.0 |
| SCHIP1 | Schwannomin interacting protein 1 | 2.0 |
| SERPINE1 | Plasminogen activator inhibitor-1 | 3.9 |
| SLC20A2 | Solute carrier family 20, member 2 | 1.9 |
| STK38 liter | Serine/threonine kinase 38 like | 1.9 |
| TMEM22 | Transmembrane protein 22 | 1.8 |
| TMPRSS2 | Transmembrane protease, serine 2 | − 2.1 |
| TNFAIP1 | TNF, α-induced protein 1 | 2.3 |
| TRIB1 | G-protein-coupled receptor induced protein | 2.7 |
| TRIB3 | Tribbles 3 | 2.0 |
| TXNIP | Thioredoxin interacting protein | − 3.0 |
| ZNF165 | Zinc finger protein 165 | 1.6 |
PROKR1-Ishikawa cells were treated with vehicle or 40 nM PROK1 for 8 h and subjected to gene array analysis on AB1700 and Affymetrix arrays (n = 3 individual experiments). With application of adjusted P ≤ 0.05 on at least one platform, 49 genes were found to be differentially regulated with 46 genes up-regulated and three genes down-regulated.
FIG. 3.
Validation of gene array analysis by real-time PCR. Expression of COX-2 (PTGS2), LIF, IL-6, IL-8, and IL-11 is significantly elevated in PROKR1-Ishikawa cells stimulated with 40 nM PROK1 for 8 h. No elevation was observed in WT Ishikawa cells. Each bar represents the mean ± sem of at least three individual experiments (*, P < 0.05; **, P < 0.01).
PROK1 induces COX-2 expression and prostaglandin production in PROKR1-Ishikawa cells and first-trimester decidua
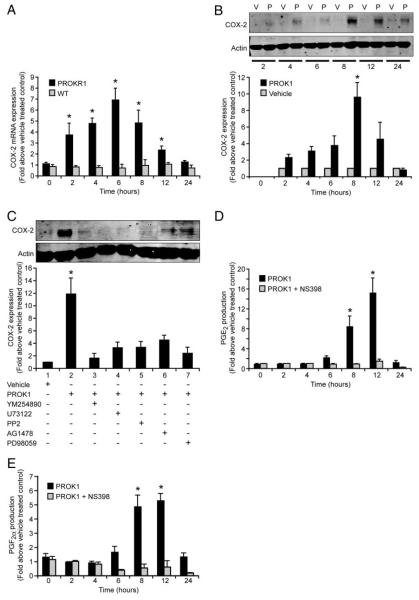
COX-2 plays a role in mouse decidualization and is regulated during the human menstrual cycle. We therefore investigated the temporal regulation and mechanism of COX-2 expression induced by PROK1. Treatment of PROKR1-Ishikawa cells with 40 nm PROK1 resulted in a time-dependent increase in COX-2 mRNA and protein expression with a peak observed at 6 h (6.9 ± 1.2-fold increase above vehicle treated control, P < 0.01, Fig. 4A) and 8 h, respectively (9.59 ± 2.1-fold increase above vehicle treated control, P < 0.01, Fig. 4B). No increase in COX-2 mRNA expression was observed in the WT Ishikawa cells in response to PROK1 (Fig. 4A). PROK1-induced COX-2 protein expression at 8 h (Fig. 4C, lane 2) was significantly inhibited (P < 0.001) on cotreatment with inhibitors of Gq (Fig. 4C, lane 3), PLC-β (Fig. 4C, lane 4), cSrc (Fig. 4C, lane 5), EGFR (Fig. 4C, lane 6), or MEK (Fig. 4C, lane 7).
FIG. 4.
PROK1-induced COX-2 mRNA expression is mediated via ERK 1/2 and results in de novo synthesis of prostanoids. A, COX-2 expression in WT and PROKR1-Ishikawa cells after treatment with 40 nM PROK1. No elevation in COX-2 mRNA expression was observed in WT Ishikawa cells, whereas COX-2 expression peaked at 6 h in PROKR1-Ishikawa cells. B, COX-2 protein expression, as determined by Western blot analysis, was maximal in PROKR1-Ishikawa cells after treatment with 40 nM PROK1 (p) for 8 h. No elevation in COX-2 protein expression was observed in vehicle (v)-treated cells. C, COX-2 protein expression was measured in PROKR1-Ishikawa cells in response to the administration of vehicle or 40 nM PROK1 for 8 h in the presence or absence of pretreatment with YM254890 (Gq inhibitor, lane 3), U73122 (PLC inhibitor, lane 4), PP2 (cSrc inhibitor, lane 5), AG1478 (EGFR inhibitor, lane 6), or PD98059 (MEK inhibitor, lane 7). The PROK1-induced COX-2 expression was significantly inhibited in the presence of these inhibitors. PROK1-induced de novo synthesis of PGF2α (D) and PGE2 (E) is COX-2 dependent, indicated by abrogation of prostaglandin production in presence of specific COX-2 inhibitor NS-398. For B and C, a representative Western immunoblot is shown with semiquantitative analysis determined as described in Materials and Methods. In all panels each bar represents the mean ± sem of at least three independent experiments (*, P < 0.05). −, Absence of agent; +, presence of agent.
Downstream of PROK1-mediated COX-2 expression, de novo PGE2 and PGF2α production were significantly elevated in response to PROK1 with maximal concentration of both prostaglandins detected 12 h after treatment with 40 nm PROK1, compared with vehicle-treated cells (15.3 ± 2.9- and 5.3 ± 0.5-fold increase for PGE2 and PGF2α respectively, Fig. 4, D and E, P < 0.001). Cotreatment of PROKR1-Ishikawa cells with PROK1 and NS-398, a specific COX-2 inhibitor, abolished synthesis of both prostaglandins.
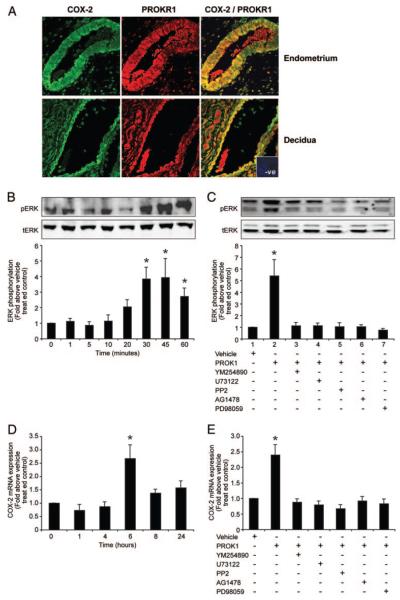
Potential regulation of COX-2 by PROK1 in the human endometrium and first-trimester decidua was subsequently investigated. COX-2 (green channel) and PROKR1 (red channel) expression were colocalized (PROKR1/COX-2, yellow channel) to glandular epithelium and some stromal cells (Fig. 5A) within human endometrium and first-trimester decidua. To correlate our findings in the PROKR1-Ishikawa cell line, we investigated PROK1 signaling in first-trimester decidua tissue explants. Upon treatment with 40 nm PROK1, maximal phosphorylation of ERK 1/2 in decidua explants was observed at 30–45 min (3.8 ± 0.7- and 3.9 ± 1.2-fold above vehicle treated controls, respectively, P < 0.01, Fig. 5B). Elevation of PROK1-induced ERK 1/2 in decidua explants at 30 min (Fig. 5C, lane 2) was significantly inhibited (P < 0.001) on cotreatment with inhibitors of Gq (Fig. 5C, lane 3), PLC-β (Fig. 5C, lane 4), cSrc (Fig. 5C, lane 5), EGFR (Fig. 5C, lane 6), or MEK (Fig. 5C, lane 7).
Fig. 5.
PROK1 induces ERK 1/2 and COX-2 expression in first-trimester decidua. A, PROKR1 (red) and COX-2 (green) colocalize (yellow) to the glandular epithelium and a subset of stromal cells in human endometrium (n = 5) and first-trimester decidua (n = 5; representative sections shown, inset, negative control incubated with control IgG). B, Treatment of first-trimester decidua explants with 40 nM PROK1 resulted in ERK1/2 phosphorylation (pERK), which was maximal at 30–45 min. C, ERK 1/2 phosphorylation was measured in first-trimester decidua in response to the administration of vehicle or 40 nM PROK1 for 30 min in the presence or absence of pretreatment with YM254890 (Gq inhibitor, lane 3), U73122 (PLC inhibitor, lane 4), PP2 (cSrc inhibitor, lane 5), AG1478 (EGFR inhibitor, lane 6), or PD98059 (MEK inhibitor, lane 7). PROK1-induced ERK 1/2 response was significantly inhibited in the presence of the above inhibitors. D, Treatment of first-trimester decidua explants with 40 nM PROK1 resulted in increased expression of COX-2, which was maximal at 6 h. E, PROK1-induced COX-2 mRNA expression was inhibited by preincubation of first-trimester decidua with YM254890 (Gq inhibitor, lane 3), U73122 (PLC inhibitor, lane 4), PP2 (cSrc inhibitor, lane 5), AG1478 (EGFR kinase inhibitor, lane 6), or PD98059 (MEK inhibitor, lane 7). For B and C, a representative Western immunoblot is shown, with semiquantitative analysis determined as described in Materials and Methods. In all panels each bar represents mean ± sem of at least five experiments (*, P < 0.05). −, Absence of agent; +, presence of agent.
Treatment of first-trimester decidua with 40 nm PROK1 revealed time-dependent regulation of COX-2 mRNA expression in decidua with a peak at 6 h (2.7 ± 0.5-fold above vehicle-treated control, P < 0.05, Fig. 5D). PROK1-induced expression of COX-2 at 6 h (Fig. 5E, lane 2) was significantly reduced (P < 0.001) by cotreatment of tissue explants with Gq inhibitor (Fig. 5E, lane 3), PLC-β inhibitor (Fig. 5E, lane 4), cSrc inhibitor (Fig. 5E, lane 5), EGFR inhibitor (Fig. 5E, lane 6), or MEK inhibitor (Fig. 5E, lane 7).
Discussion
Our data confirm previous reports that PROK1 mRNA expression peaks in the secretory phase of the menstrual cycle, whereas PROKR1 expression does not alter significantly across the cycle (8). Expression of PROK1 and PROKR1 in first-trimester decidua samples taken from 7–12 wk of gestation is significantly elevated, compared with all phases of the menstrual cycle. Elevation of PROK1 and PROKR1 in early pregnancy has been highlighted previously in which expression of both factors has been observed in early placental tissues (10). Both PROK1 and PROKR1 localized to glandular epithelial cells and stromal cells. Within the stroma, PROKR1 was localized to endothelial cells, suggesting a role for PROK1/PROKR1 in regulation of vascular function. Indeed, delivery of PROK1 has been shown to induce neovascularization in the mouse ovary (2). A subset of stromal cells expressing PROK1 were characterized as natural killer cells by CD56 staining. These cells are also known to cluster around the blood vessels in the endometrium and have been suggested to regulate vascular function (20).
To elucidate intracellular signal transduction pathways mediating the effects of PROK1 via PROKR1 in the endometrium, an endometrial epithelial model cell line stably expressing human PROKR1 was used. Prokineticin receptors are reported to couple to Gi or Gq (4, 5, 21). We demonstrated elevation of inositol phosphate mobilization on stimulation of PROKR1 cells with PROK1, indicating Gq coupling in this system. PLC activation leads to activation of downstream phosphorylation cascades, and MAPK phosphorylation has been implicated in signaling downstream of PROKR1 activation (21). Transient phosphorylation of ERK 1/2 was demonstrated on PROKR1 activation in PROKR1-Ishikawa cells and first-trimester decidua tissue, which peaked at 5 and 30 min, respectively. These differences are possibly due to different levels of receptor expression, the presence of heterogeneous cells, and the thickness of the tissue pieces into which the ligand must penetrate in the explant culture, compared with the homogenous cell population of PROKR1-Ishikawa cells. Signaling to ERK1/2 upon G protein-coupled receptor activation involves intracellular phosphorylation cascades (22). PROK1-induced ERK 1/2 phosphorylation was demonstrated, by using chemical inhibitors, to be dependent on activation of Gq, PLC-β, cSrc, EGFR, and MEK phosphorylation, but not Gi activation, in both PROKR1-Ishikawa cells and decidua tissue. Furthermore, phosphorylation of cSrc and EGFR in response to PROK1 peaks within the time frame of maximal ERK 1/2 phosphorylation. Similarly, DN-cSrc, DN-EGFR, DN-Ras, and DN-MEK inhibited PROK1 mediated ERK 1/2 phosphorylation, confirming the role of these molecules in signaling to ERK 1/2.
Global gene profiling was conducted on two different array platforms to elucidate potential functions of PROKR1. Analysis of the combined array platforms revealed differential expression of 49 genes after PROK1 stimulation. Five genes up-regulated in the arrays were selected to verify the changes by real-time PCR. IL-11, IL-8, IL-6, LIF, and COX-2 (PG-endoperoxidase synthase 2) confirmed gene array fold changes after PROK1 stimulation. Analysis of the expressed sequence tag tissue library database (16) indicated that genes whose expression is regulated by PROK1 are significantly overexpressed in uterine tissue, compared with other tissue libraries. These data may suggest that, although the data are obtained from in vitro studies using an immortalized cell line, the response is likely to be representative of a uterine cell. Indeed, analysis of gene expression induced by PROK1 in first-trimester decidua tissue demonstrates similarity in regulation of target genes by PROK1 in Ishikawa cells and first-trimester deciduas. Analysis of the gene list for Gene Ontology annotations indicated roles for PROK1 in regulating genes involved in responses to wounding (inflammation and angiogenesis) and cellular proliferation. These processes are of importance in the endometrial response to implantation. Little is known about the direct role for PROK1 in implantation; however, a host of genes identified in this study to be regulated by PROK1 include those with known roles in endometrial receptivity. For example, PROK1 regulated genes independently validated by Taqman quantitative PCR; LIF, COX-2, IL-6, and IL-11, have suggested roles in implantation and decidualization (23-26).
Other PROK1-regulated genes with known roles in implantation include members of the epidermal growth factor (EGF) family, namely amphiregulin and heparin binding-EGF (diptheria toxin receptor). Mice null for uterine heparin binding-EGF display reduced numbers of implantation sites and reduced litter sizes; however, it was observed that amphiregulin displayed compensatory up-regulation in this model (27). Amphiregulin is expressed in the luminal epithelium of the mouse at the onset of blastocyst attachment (28) and is expressed in the decidual area in the hamster (29). Other genes with suggested roles in implantation include Dickkopf-1, CD44, and CD55. Expression of these factors has been reported in the human endometrium during the window of implantation and has been highlighted in array studies of human endometrium during the window of implantation (30-37). CD55, also known as decay accelerating factor, has also been shown to be down-regulated in antiphospholipid syndrome, a condition associated with recurrent miscarriage (38). CD44 has a proposed role in angiogenesis (39), whereas CD55 is proposed to exert endothelial cytoprotection during inflammatory angiogenesis (40, 41).
We subsequently elucidated the mechanism by which PROK1-PROKR1 regulates COX-2 expression in PROKR1 Ishikawa cells and first-trimester decidua tissue. COX-2 is important in regulation of the decidual response (42), and COX-2 localizes to luminal and glandular epithelial cells in human endometrium (43). Differentiation of endometrial stromal cells into decidua is mediated by PGE2 synthesis through elevation of COX-2. PROK1 action may be important in PGE2-driven changes in decidualized endometrium. We demonstrate that PROKR1 and COX-2 colocalize to glandular epithelial and stromal cells of midsecretory endometrium and first-trimester decidua. This suggests potential regulation of COX-2 expression by PROK1 in both compartments during endometrial receptivity and early pregnancy. PROK1-PROKR1 rapidly induces COX-2 expression via a cascade of signaling molecules: Gq, PLC, Ca2+, cSrc, EGFR, and MEK. Induction of COX-2 appears to be dependent on EGFR transactivation. EGF-mediated signaling is proposed to regulate COX-2 expression in mouse models (44).
PROK1-PROKR1 activation induced secretion of prostaglandins via COX-2 because the inhibitor NS-398 abolished prostaglandin production. Blastocyst attachment in mice coincides with a highly localized increase in vascular permeability associated with increased adhesiveness of the endometrium. This has been associated with expression of COX-2 (45), PGE2 synthase, PGE2 secretion, and E-prostanoid (EP) receptors at the implantation site (46-48). Edema and increased vascular permeability is also evident in the human endometrium at the time of implantation (49). In COX-2-deficient mice, there is impaired vascular permeability at the site of blastocyst apposition (50), possibly due to a deficiency in prostaglandin-influenced uterine angiogenesis (51). The defects in the COX-2-deficient mouse are rescued on administration of stable analogs of PGI2 (24), which is the most abundant prostaglandin in the mouse uterus at the time of implantation (24). However, the human endometrium produces little PGI2 (52), and it is suggested that PGE2-mediated action may be more important in the human (53, 54). PROK1 may therefore play a role in the prostaglandin-directed increase in endometrial vascular permeability. The trophoblast also strongly expresses PROK1 (10), indicating that the trophoblast may promote enhanced PGE2 synthesis in the decidualized endometrium, creating a favorable environment for implantation. Uterine natural killer cells also may cooperate with trophoblast cells to modulate remodeling of spiral arterioles during implantation. This is supported by the demonstration of expression of PROK1 in uNK cells and PROKR1 in trophoblast cells (10).
In conclusion, we propose that PROK1-PROKR1 regulates the expression of a host of genes that are involved in the establishment and maintenance of pregnancy. PROK1-PROKR1 signaling induces the expression of target genes, such as COX-2, via cross talk with the EGFR and downstream phosphorylation of ERK 1/2.
Acknowledgments
We thank Dr. Nicole Kane for uterine natural killer cell RNA and Mrs. Sharon Donaldson and Mrs. Catherine Murray for consenting patients and tissue collection. We also thank Geneservice for their help with microarray processing and analysis.
H.N.J. consults and has received grant support from Ardana Biosciences (2006–2007).
Abbreviations
- COX
Cyclooxygenase
- DN
dominant negative
- EGF
epidermal growth factor
- EGFR
EGF receptor
- IP3
inositol phosphate
- LIF
leukemia inhibitory factor
- MEK
MAPK kinase
- PG
prostaglandin
- PLC
phospholipase C
- PP2
4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d] pyrimidine
- PROK
prokineticin
- PROKR
PROK receptor
- uNK
uterine natural killer
- WT
wild type
Footnotes
Disclosure Statement: J.E., R.D.C., K.M., H.O.D.C., and R.P.M. have no nothing to disclose.
References
- 1.Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- 2.LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L, Frantz G, Rangell L, DeGuzman L, Keller GA, Peale F, Gurney A, Hillan KJ, Ferrara N. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:877–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- 3.Mollay C, Wechselberger C, Mignogna G, Negri L, Melchiorri P, Barra D, Kreil G. Bv8, a small protein from frog skin and its homologue from snake venom induce hyperalgesia in rats. Eur J Pharmacol. 1999;374:189–196. doi: 10.1016/s0014-2999(99)00229-0. [DOI] [PubMed] [Google Scholar]
- 4.Lin DC, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou QY. Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J Biol Chem. 2002;277:19276–19280. doi: 10.1074/jbc.M202139200. [DOI] [PubMed] [Google Scholar]
- 5.Soga T, Matsumoto S, Oda T, Saito T, Hiyama H, Takasaki J, Kamohara M, Ohishi T, Matsushime H, Furuichi K. Molecular cloning and characterization of prokineticin receptors. Biochim Biophys Acta. 2002;11579:173–179. doi: 10.1016/s0167-4781(02)00546-8. [DOI] [PubMed] [Google Scholar]
- 6.Bullock CM, Li JD, Zhou QY. Structural determinants required for the bioactivities of prokineticins and identification of prokineticin receptor antagonists. Mol Pharmacol. 2004;65:582–588. doi: 10.1124/mol.65.3.582. [DOI] [PubMed] [Google Scholar]
- 7.Negri L, Lattanzi R, Giannini E, Colucci MA, Mignogna G, Barra D, Grohovaz F, Codazzi F, Kaiser A, Kreil G, Melchiorri P. Biological activities of Bv8 analogues. Br J Pharmacol. 2005;146:625–632. doi: 10.1038/sj.bjp.0706376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battersby S, Critchley HO, Morgan K, Millar RP, Jabbour HN. Expression and regulation of the prokineticins (endocrine gland-derived vascular endothelial growth factor and Bv8) and their receptors in the human endometrium across the menstrual cycle. J Clin Endocrinol Metab. 2004;89:2463–2469. doi: 10.1210/jc.2003-032012. [DOI] [PubMed] [Google Scholar]
- 9.Ngan ES, Lee KY, Yeung WS, Ngan HY, Ng EH, Ho PC. Endocrine gland-derived vascular endothelial growth factor is expressed in human peri-implantation endometrium, but not in endometrial carcinoma. Endocrinology. 2006;147:88–95. doi: 10.1210/en.2005-0543. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann P, Feige JJ, Alfaidy N. Expression and oxygen regulation of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 and its receptors in human placenta during early pregnancy. Endocrinology. 2006;147:1675–1684. doi: 10.1210/en.2005-0912. [DOI] [PubMed] [Google Scholar]
- 11.Sales KJ, Milne SA, Williams AR, Anderson RA, Jabbour HN. Expression, localization, and signaling of prostaglandin F2α receptor in human endometrial adenocarcinoma: regulation of proliferation by activation of the epidermal growth factor receptor and mitogen-activated protein kinase signaling pathways. J Clin Endocrinol Metab. 2004;89:986–993. doi: 10.1210/jc.2003-031434. [DOI] [PubMed] [Google Scholar]
- 12.Trundley A, Gardner L, Northfield J, Chang C, Moffett A. Methods for isolation of cells from the human fetal-maternal interface. Methods Mol Med. 2006;122:109–122. doi: 10.1385/1-59259-989-3:109. [DOI] [PubMed] [Google Scholar]
- 13.Huber W, von Heydebreck A, Sültmann H, Poustka A, Vingron M. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics. 2002;18(Suppl 1):S96–S104. doi: 10.1093/bioinformatics/18.suppl_1.s96. [DOI] [PubMed] [Google Scholar]
- 14.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article 3. [DOI] [PubMed] [Google Scholar]
- 15.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 16.Zhang B, Kirov S, Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005;33:W741–W748. doi: 10.1093/nar/gki475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denison FC, Grant VE, Calder AA, Kelly RW. Seminal plasma components stimulate interleukin-8 and interleukin-10 release. Mol Hum Reprod. 1999;5:220–226. doi: 10.1093/molehr/5.3.220. [DOI] [PubMed] [Google Scholar]
- 18.Barbacioru CC, Wang Y, Canales RD, Sun YA, Keys DN, Chan F, Poulter KA, Samaha RR. Effect of various normalization methods on Applied Bio-systems expression array system data. BMC Bioinform. 2006;7:533. doi: 10.1186/1471-2105-7-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yue H, Eastman PS, Wang BB, Minor J, Doctolero MH, Nuttall RL, Stack R, Becker JW, Montgomery JR, Vainer M, Johnston R. An evaluation of the performance of cDNA microarrays for detecting changes in global mRNA expression. Nucleic Acids Res. 2001;29:E41–1. doi: 10.1093/nar/29.8.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lash GE, Schiessl B, Kirkley M, Innes BA, Cooper A, Searle RF, Robson SC, Bulmer JN. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J Leukoc Biol. 2006;80:572–580. doi: 10.1189/jlb.0406250. [DOI] [PubMed] [Google Scholar]
- 21.Lin R, LeCouter J, Kowalski J, Ferrara N. Characterization of endocrine gland-derived vascular endothelial growth factor signaling in adrenal cortex capillary endothelial cells. J Biol Chem. 2002;277:8724–8729. doi: 10.1074/jbc.M110594200. [DOI] [PubMed] [Google Scholar]
- 22.Werry TD, Sexton PM, Christopoulos A. “Ins and outs” of seven-transmembrane receptor signalling to ERK. Trends Endocrinol Metab. 2005;16:26–33. doi: 10.1016/j.tem.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Köntgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 24.Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 25.Tabibzadeh S, Kong QF, Babaknia A, May LT. Progressive rise in the expression of interleukin-6 in human endometrium during menstrual cycle is initiated during the implantation window. Hum Reprod. 1995;10:2793–2799. doi: 10.1093/oxfordjournals.humrep.a135793. [DOI] [PubMed] [Google Scholar]
- 26.Robb L, Li R, Hartley L, Nandurkar HH, Koentgen F, Begley CG. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med. 1998;4:303–308. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- 27.Xie H, Wang H, Tranguch S, Iwamoto R, Mekada E, Demayo FJ, Lydon JP, Das SK, Dey SK. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci USA. 2007;104:18315–18320. doi: 10.1073/pnas.0707909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das SK, Chakraborty I, Paria BC, Wang XN, Plowman G, Dey SK. Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol. 1995;9:691–705. doi: 10.1210/mend.9.6.8592515. [DOI] [PubMed] [Google Scholar]
- 29.Khatua A, Wang X, Ding T, Zhang Q, Reese J, DeMayo FJ, Paria BC. Indian hedgehog, but not histidine decarboxylase or amphiregulin, is a progesterone-regulated uterine gene in hamsters. Endocrinology. 2006;147:4079–4092. doi: 10.1210/en.2006-0231. [DOI] [PubMed] [Google Scholar]
- 30.Yaegashi N, Fujita N, Yajima A, Nakamura M. Menstrual cycle dependent expression of CD44 in normal human endometrium. Hum Pathol. 1995;26:862–865. doi: 10.1016/0046-8177(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 31.Albers A, Thie M, Hohn HP, Denker HW. Differential expression and localization of integrins and CD44 in the membrane domains of human uterine epithelial cells during the menstrual cycle. Acta Anat (Basel) 1995;153:12–19. doi: 10.1159/000147710. [DOI] [PubMed] [Google Scholar]
- 32.Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143:2119–2138. doi: 10.1210/endo.143.6.8885. [DOI] [PubMed] [Google Scholar]
- 33.Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, Yuan L, Fritz MA, Lessey B. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod. 2002;8:871–879. doi: 10.1093/molehr/8.9.871. [DOI] [PubMed] [Google Scholar]
- 34.Riesewijk A, Martín J, van Os R, Horcajadas JA, Polman J, Pellicer A, Mosselman S, Simón C. Gene expression profiling of human endometrial receptivity on days LH+2 versus LH+7 by microarray technology. Mol Hum Reprod. 2003;9:253–264. doi: 10.1093/molehr/gag037. [DOI] [PubMed] [Google Scholar]
- 35.Borthwick JM, Charnock-Jones DS, Tom BD, Hull ML, Teirney R, Phillips SC, Smith SK. Determination of the transcript profile of human endometrium. Mol Hum Reprod. 2003;9:19–33. doi: 10.1093/molehr/gag004. [DOI] [PubMed] [Google Scholar]
- 36.Mirkin S, Arslan M, Churikov D, Corica A, Diaz JI, Williams S, Bocca S, Oehninger S. In search of candidate genes critically expressed in the human endometrium during the window of implantation. Hum Reprod. 2005;20:2104–2117. doi: 10.1093/humrep/dei051. [DOI] [PubMed] [Google Scholar]
- 37.Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- 38.Francis J, Rai R, Sebire NJ, El-Gaddal S, Fernandes MS, Jindal P, Lokugamage A, Regan L, Brosens JJ. Impaired expression of endometrial differentiation markers and complement regulatory proteins in patients with recurrent pregnancy loss associated with antiphospholipid syndrome. Mol Hum Reprod. 2006;12:435–442. doi: 10.1093/molehr/gal048. [DOI] [PubMed] [Google Scholar]
- 39.Cao G, Savani RC, Fehrenbach M, Lyons C, Zhang L, Coukos G, Delisser HM. Involvement of endothelial CD44 during in vivo angiogenesis. Am J Pathol. 2006;169:325–336. doi: 10.2353/ajpath.2006.060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mason JC, Lidington EA, Yarwood H, Lublin DM, Haskard DO. Induction of endothelial cell decay-accelerating factor by vascular endothelial growth factor: a mechanism for cytoprotection against complement-mediated injury during inflammatory angiogenesis. Arthritis Rheum. 2001;44:138–150. doi: 10.1002/1529-0131(200101)44:1<138::AID-ANR18>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 41.Mason JC, Lidington EA, Ahmad SR, Haskard DO. bFGF and VEGF synergistically enhance endothelial cytoprotection via decay-accelerating factor induction. Am J Physiol Cell Physiol. 2002;282:C578–C587. doi: 10.1152/ajpcell.00339.2001. [DOI] [PubMed] [Google Scholar]
- 42.Cheng JG, Stewart CL. Loss of cyclooxygenase-2 retards decidual growth but does not inhibit embryo implantation or development to term. Biol Reprod. 2003;68:401–404. doi: 10.1095/biolreprod.102.009589. [DOI] [PubMed] [Google Scholar]
- 43.Jones RL, Kelly RW, Critchley HO. Chemokine and cyclooxygenase-2 expression in human endometrium coincides with leukocyte accumulation. Hum Reprod. 1997;12:1300–1306. doi: 10.1093/humrep/12.6.1300. [DOI] [PubMed] [Google Scholar]
- 44.Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- 45.Chakraborty I, Das SK, Wang J, Dey SK. Developmental expression of the cyclo-oxygenase-1 and cyclo-oxygenase-2 genes in the peri-implantation mouse uterus and their differential regulation by the blastocyst and ovarian steroids. J Mol Endocrinol. 1996;16:107–122. doi: 10.1677/jme.0.0160107. [DOI] [PubMed] [Google Scholar]
- 46.Shi JJ, Ma XH, Diao HL, Ni H, Xu LB, Zhu H, Yang ZM. Differential expression of prostaglandin E receptor subtype EP2 in rat uterus during early pregnancy. Histol Histopathol. 2005;20:1021–1028. doi: 10.14670/HH-20.1021. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Su Y, Deb K, Raposo M, Morrow JD, Reese J, Paria BC. Prostaglandin E2 is a product of induced prostaglandin-endoperoxide synthase 2 and microsomal-type prostaglandin E synthase at the implantation site of the hamster. J Biol Chem. 2004;279:30579–30587. doi: 10.1074/jbc.M400573200. [DOI] [PubMed] [Google Scholar]
- 48.Ni H, Sun T, Ding NZ, Ma XH, Yang ZM. Differential expression of microsomal prostaglandin E synthase at implantation sites and in decidual cells of mouse uterus. Biol Reprod. 2002;67:351–358. doi: 10.1095/biolreprod67.1.351. [DOI] [PubMed] [Google Scholar]
- 49.Okada Y, Asahina T, Kobayashi T, Goto J, Terao T. Studies on the mechanism of edematous changes at the endometrial stroma for implantation. Semin Thromb Hemost. 2001;27:67–77. doi: 10.1055/s-2001-14063. [DOI] [PubMed] [Google Scholar]
- 50.Matsumoto H, Ma WG, Daikoku T, Zhao X, Paria BC, Das SK, Trzaskos JM, Dey SK. Cyclooxygenase-2 differentially directs uterine angiogenesis during implantation in mice. J Biol Chem. 2002;277:29260–29267. doi: 10.1074/jbc.M203996200. [DOI] [PubMed] [Google Scholar]
- 51.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 52.Abel MH, Kelly RW. Differential production of prostaglandins within the human uterus. Prostaglandins. 1979;18:821–828. doi: 10.1016/0090-6980(79)90101-1. [DOI] [PubMed] [Google Scholar]
- 53.Dimitriadis E, Stoikos C, Baca M, Fairlie WD, McCoubrie JE, Salamonsen LA. Relaxin and prostaglandin E(2) regulate interleukin 11 during human endometrial stromal cell decidualization. J Clin Endocrinol Metab. 2005;90:3458–3465. doi: 10.1210/jc.2004-1014. [DOI] [PubMed] [Google Scholar]
- 54.Frank GR, Brar AK, Cedars MI, Handwerger S. Prostaglandin E2 enhances human endometrial stromal cell differentiation. Endocrinology. 1994;134:258–263. doi: 10.1210/endo.134.1.7506205. [DOI] [PubMed] [Google Scholar]