Abstract
Feeding is critical for survival. Yet, patients with chronic pain often lose their appetite and eat less. We previously showed that ad libitum fed male rats continue to feed rather than withdraw from a brief noxious stimulus. This study examined the effects of a sustained noxious stimulus on feeding by testing ad libitum fed male rats for five eating behaviors –latency to eat, time taken to eat each chip, pauses and scanning during eating, and the number of chocolate chips eaten - during the hour following a sham injection or an injection of a low (0.5%) or moderate (1.5%) dose of formalin into the hind paw. Sham-injected rats showed no pain-related behaviors, rats injected with 0.5% formalin showed very few pain-related behaviors, and rats injected with 1.5% formalin showed favoring, lifting and licking of the injured paw with a characteristic biphasic time course. Besides taking less time to commence eating during the first phase of formalin pain, rats injected with either dose of formalin did not differ from sham-injected rats on any of the other eating measures. Rats injected with 0.5% formalin showed no pain behaviors during eating, whereas those given 1.5% formalin typically ate while not exhibiting any pain behaviors but occasionally ate while favoring the paw, rarely while lifting the paw, and never while licking the paw. These results show that eating is a protected activity even in the presence of persistent pain in male rats.
Keywords: eating behavior, formalin, chocolate
A decrease in appetite and eating problems are common complaints associated with acute and chronic pain. In a sample of older adult outpatients with non-malignant chronic pain, approximately 40% reported eating problems [1]. Even among a non-clinical population of children and adolescents surveyed for acute and chronic pain, over 50% attributed appetite problems to their pain experience [2]. Anorexia is extremely prevalent in migraine sufferers, occurring in > 80% of sufferers during attacks [3]. The timing of appetite loss coincides well with the appearance of head pain and precedes the onset of symptoms more directly associated with loss of appetite such as nausea and vomiting [3].
In an animal model of migraine pain, male rats that received noxious chemical stimulation of the dura ate less than those given vehicle stimulation in the first four hours following stimulation [3]. However, dural stimulation produces cutaneous facial allodynia [4, 5] and possible hypersensitivity in the intraoral and maxillary regions required for feeding, raising the possibility that rats ate less simply because it was painful to move their mouths. Indeed, a painful stimulus distant from the mouth has no effect on food intake in food-restricted male rats [6].
The present study examines the impact of persistent pain elicited by formalin injection into a hind paw on eating behaviors in freely fed male rats. Freely fed rats were used, thereby eliminating any effects of hunger, food deprivation, or glucoprivation upon pain responsiveness [7–9]. Eating behaviors were studied during both the first and second phases of formalin pain. Only the first phase of formalin pain is opioid-sensitive [10], and may therefore be more likely to be associated with changes in feeding, which is also influenced by opioids, than is the second phase [11, 12].
Three groups of ad libitum fed rats were sham-injected or injected with a low (0.5%) or moderate (1.5%) dose of formalin to the hind paw and were then tested for the latency to eat, the time taken to eat each chip, the number and duration of pauses and scanning during each eating bout, and the number of chocolate chips eaten. Chocolate chips were selected as the test food because they are highly palatable and rats with free access to chow readily eat them [13].
Materials and methods
Subjects
All procedures were reviewed and approved by the University of Chicago Institutional Animal Care and Use Committee. Subjects were 30 male Sprague-Dawley rats (Charles River Laboratories, Portage, MI) weighing 380 – 560 g at the start of the experiment. They were housed in pairs and were kept in a 12:12 h light-dark controlled vivarium maintained at a temperature of ≈ 23°C. Laboratory chow and water were available ad libitum during housing and throughout all phases of the experiment. Apparatus. The apparatus consisted of a Plexiglas box (32 × 32 cm, 100 cm height) with a wire mesh floor located 40 cm above the base of the box. Three cameras were located in the front, on the right side, and underneath the box to record the rat’s behavior. A fourth camera was used to record the display of a timer (1 s resolution). All four images were acquired simultaneously with a Quad processor (Everfocus, Duarte, CA) connected to a video-recorder.
Procedure
All rats were familiarized to handling and to the apparatus and were trained to eat Nestle® Milk Chocolate Morsels (chocolate chips) before the start of the experiment proper. The experiment was conducted across 5 days during the light part of the rat’s diurnal cycle in a room maintained at a temperature of ≈ 23°C. On each of days 1 – 3, rats were assessed for their baseline chocolate chip consumption during a 60 min exposure to the test apparatus. Rats (n = 2) that ate < 3 chips per session were excluded from further testing. The remaining 28 rats were assigned to one of three groups in a manner which equated the groups on overall mean baseline chip consumption. On day 5, rats in two of the groups were given a subcutaneous injection of formalin (0.5%, n = 9 or 1.5%, n = 10, 50 μl) into the ventral surface of the right hind paw. Formalin was diluted to these concentrations from a stock solution of 100% (formaldehyde solution 37% w/w, Fisher Scientific Company, Fairlawn, NJ) and was injected using a single-use 1 ml syringe and a 30-gauge needle. Since we wanted to compare formalin-injected rats with rats without edema and persistent pain and since saline injection into the hind paw produces edema [14], we chose to give control rats a sham injection: a 30-gauge needle was inserted subcutaneously and then withdrawn (n = 9). This procedure adequately controlled for the stress due to handling and needle insertion without causing frank edema. Immediately after the formalin or sham injection, the rat was placed into the test cage for 60 min and given a chocolate chip. On all baseline and test sessions, chocolate chips were given one at a time. All rats were sacrificed immediately after testing.
Behavioral scoring and analyses
Pain and eating behaviors were scored from analyses of video-recordings. The time and duration that the injected paw was normal, favored, lifted, or licked/bitten were scored [15]. The eating behaviors scored were: latency to eat, time taken to eat each chip, eating pauses and scanning during each eating bout, and the number of chips eaten. Latency to eat was defined as the time between the ultimate retrieval of a chip till the commencement of eating. The number and time taken to eat the chips were chosen because they have been shown to be affected by a number of experimental manipulations such as exposure to intense noise (80 – 110 dB), a non-noxious stressor [16–18].
Statistical analyses
The rats were allocated to groups to equate for baseline chip intake. Statistical analysis confirmed that there were no significant differences in the mean number of chocolates eaten on baseline days (Means ± S.E.M.s = 7.6 ± 0.5, 7.6 ± 0.7, 7.6 ±0.7, F0.5(2,25) < 1.0). The proportion of time rats spent favoring, lifting, and licking the injected paw was calculated. The average latency to eat, time taken to eat each chip, number and duration of eating pauses, proportion of time spent scanning during each eating bout, and number of scans made per eating bout, and the number of chips eaten by each of the three groups were also calculated. Mixed design ANOVAs, with significance level set at 0.05, were used to test for significant differences between groups and across time.
Results
All rats had ad libitum access to water and chow throughout the experiment including during testing.
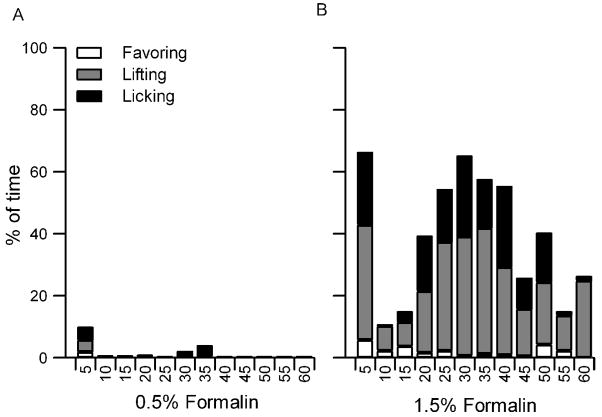
Sham injected rats showed no pain behaviors at any stage during testing. Rats injected with 0.5% formalin spent only 1% of the total time displaying pain-related behaviors. They showed few pain behaviors during either the first (0–10 min) or second (10–60 min) phases of formalin pain, and were not statistically different from sham-injected rats in this respect (Fig. 1A). Rats injected with 0.5% formalin never displayed any pain behaviors during eating. They showed a significantly shorter latency to eat than sham-injected rats during the first phase of formalin pain (Fig. 2A). Yet, they did not differ from sham-injected rats on the proportion of eating trials interrupted by pauses, the mean duration of eating pauses, the proportion of time spent scanning during each eating bout, or the number of scans made per eating bout throughout testing (data not shown). Moreover, both groups of rats took the same amount of time to consume each chip and ate the same number of chocolate chips during both phases of formalin pain (Fig. 2B & C).
Figure 1.
Proportion of time that rats favored, lifted, and licked their injected paw during the hour following an injection of formalin. (A). Rats injected with 0.5% formalin showed very few pain-related behaviors. (B). Rats given 1.5% formalin displayed a biphasic pain response, with the first phase occurring within 5 minutes and the second phase peaking 30 minutes after injection. Rats injected with 1.5% formalin spent significantly more time favoring, lifting, and licking their paw than rats injected with 0.5% formalin.
Figure 2.

Eating behaviors in sham- and formalin-injected rats during the first 10 minutes (0 – 10) and the next 50 minutes (10 – 60) after a sham or formalin injection to the hind paw. (A). Mean latency to eat. The time taken from the ultimate retrieval of a chocolate chip to the start of eating was significantly faster in formalin-injected rats than in sham-injected rats during the first phase of formalin pain (*). (B). Average time taken to eat each chip. Formalin-injected rats took the same amount of time to eat each chip as sham-injected rats during either phase of formalin pain. (C). Mean number of chocolate chips eaten. Rats injected with formalin ate the same number of chips as sham-injected rats during the first and second phases of formalin pain.
Rats injected with 1.5% formalin spent significantly more time paw-favoring (2%), paw-lifting (24.5%), and paw-licking (13.5%) than rats injected with the lower dose (F0.5(1,12) = 40.2). They displayed the characteristic biphasic pain response to formalin, with an acute phase that peaked within the first 5 min and a second phase that peaked approximately 30 min post-injection (Fig 1B). While eating, rats injected with 1.5% formalin displayed no pain behaviors most of the time (74%), favoring of the injected paw a small proportion of the time (23%), and paw lifting the remainder of the time (3%). Although it is possible to briefly interrupt feeding to paw-lick or to stop eating mid-chip, none of the 1.5% formalin-injected rats behaved in either of these ways.
Like rats injected with the lower formalin dose, rats injected with 1.5% formalin displayed a significantly shorter latency to eat than sham-injected rats during the first phase (Fig 2A) (F0.5(2,25) = 5.4). It must be noted that eating latency was defined as the time between the ultimate retrieval to the start of eating. Both sham- and formalin-injected rats occasionally retrieved a chocolate chip, dropped it, and then retrieved the chip again some time later. Fewer sham-injected rats (2 of 8) displayed this behavior than rats injected with 0.5% (5 of 9) or 1.5% (5 of 10) formalin. Retrieval latencies from the time of first retrieval to the start of eating were the same between the groups. Although rats injected with 1.5% formalin displayed some pain behaviors during eating, they took the same time to eat each chip (Fig. 2B), showed similar interruptions and scanning during eating bouts (data not shown), and ate the same number of chips (Fig. 2C) as rats in the other two groups.
Discussion
Our results show that eating persists even in the presence of persistent pain in ad lib fed male rats. Rats injected with 1.5% formalin that exhibited pain behaviors during 40% of the testing time ate the same number of chips and took the same time to do so as rats that displayed pain behaviors for only 1% of the test time or those that showed no pain behaviors at all. The similar eating patterns in animals with very different expressions of pain held despite the fact that a quarter of the time that the 1.5% formalin-injected rats were eating, they also behaved as though they were feeling pain. The most prevalent pain behavior occurring concurrent with eating was favoring the injected paw. Rats were less likely to eat when the pain level was presumably higher as indicated by their lifting of the injected paw. Because it is impossible for a rat to paw lick and eat at the same time, it is not surprising that there were no instances of such concurrent behaviors.
Although the present results show that rats continue to eat even in the presence of moderate and persistent pain, rats are likely to stop eating when pain intensity is severe enough. In a pilot study (n = 2), we observed that rats injected with 2.5% formalin licked and bit their paws nearly continuously during the first phase and consequently, ate very few chocolate chips during that time. Thus, it is likely that feeding overrides pain below a certain sensory threshold whereas attending to the injured paw must take priority over feeding above that threshold. In light of our previous finding that withdrawals are delayed or suppressed entirely during eating [13], it appears that pain behaviors are modulated during eating by the active suppression of nociceptive input, a suppression that is only discontinued when conditions pose a serious threat or injury to the animal.
We found that rats injected with formalin began eating faster during the first phase of formalin than did sham-injected rats. Since the first phase of formalin pain is sensitive to opioid receptor antagonists [11, 12], the presence of opioids which are well known to increase palatability [19–21] may function to expedite eating during the initial phase of formalin pain.
Unlike previous research [22], 0.5% formalin did not evoke a biphasic pain response. It is unlikely that ingestion of sucrose in the chocolate during formalin testing produced an analgesic effect because sucrose-induced analgesia is present only in infant rats [23–25]. However, it is possible that daily consumption of chocolate prior to testing may have altered responses to formalin since chronic consumption of sucrose alters morphine-induced analgesia [26–34]. Further, it is also possible that either the availability of food or eating itself produces analgesia. The latter possibility is supported by the analgesic effects of eating observed in food-deprived [35, 36] and freely fed [13] animals tested for pain responses to acute noxious heat as well as by the analgesic effects of non-nutritive suckling in human babies [37, 38]. Thus, both dietary changes and feeding-associated analgesia may account for the greatly reduced effect of 0.5% formalin in the present study compared to previous reports [22].
The present results stand out against reports of decreased eating in chronic pain patients and in freely-fed rats with a model of migraine pain [1–3]. Our findings may differ from widely reported hypophagia in chronic pain patients since we tested rats only once. It is possible, even likely, that chronic pain of longer duration (days, weeks, months) may affect feeding as it does among humans with chronic pain [1, 2]. Differences between our findings and those reported in a rat model of migraine pain are likely due to a heightened oral sensitivity produced by dural stimulation.
The present study is part of an on-going project that examines the interaction between eating and pain and the role of raphe magnus cells in modulating ingestion-induced analgesia [13]. Since raphe magnus cells have only been physiologically characterized in male rats [39], we focused on studying feeding behavior during persistent formalin pain in male rats. Because there are sexual differences in pain responses to formalin and in feeding behaviors [40, 41], it should be noted that the present findings do not necessarily generalize to female rats.
In conclusion, the present results show that eating is a protected behavior in the presence of persistent pain to the hind limb in ad lib fed male rats. The persistence of feeding even in the face of moderate pain underscores the immense need, and thus value of, ingesting food. Under natural conditions, a scarce and/or unpredictable food supply may have driven natural selection to favor mechanisms that ensure that animals feed whenever possible. Feeding analgesia is likely one mechanism that aids in guaranteeing that animals will eat when food is available.
Acknowledgments
This work was supported by grants from NIDA, The Women’s Council of the Brain Research Foundation, and The University of Chicago Young Scientist Training Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bosley BN, Weiner DK, Rudy TE, Granieri E. Is chronic nonmalignant pain associated with decreased appetite in older adults? Preliminary evidence. J Am Geriatr Soc. 2004;52:247–51. doi: 10.1111/j.1532-5415.2004.52063.x. [DOI] [PubMed] [Google Scholar]
- 2.Roth-Isigkeit A, Thyen U, Stoven H, Schwarzenberger J, Schmucker P. Pain among children and adolescents: restrictions in daily living and triggering factors.[erratum appears in Pediatrics. 2005 Apr;115(4):1118] Pediatrics. 2005;115:e152–62. doi: 10.1542/peds.2004-0682. [DOI] [PubMed] [Google Scholar]
- 3.Malick A, Jakubowski M, Elmquist JK, Saper CB, Burstein R. A neurohistochemical blueprint for pain-induced loss of appetite.[erratum appears in Proc Natl Acad Sci U S A 2001 Nov 20;98(24):14186] Proc Natl Acad Sci US A. 2001;98:9930–5. doi: 10.1073/pnas.171616898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–82. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 5.Yamamura H, Malick A, Chamberlin NL, Burstein R. Cardiovascular and neuronal responses to head stimulation reflect central sensitization and cutaneous allodynia in a rat model of migraine. J Neurophysiol. 1999;81:479–93. doi: 10.1152/jn.1999.81.2.479. [DOI] [PubMed] [Google Scholar]
- 6.Aloisi AM, Carli G. Formalin pain does not modify food-hoarding behaviour in male rats. Behav Processes. 1996;36:125–33. doi: 10.1016/0376-6357(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 7.Bodnar RJ, Kelly DD, Brutus M, Mansour A, Glusman M. 2-Deoxy-D-glucose-induced decrements in operant and reflex pain thresholds. Pharmacol Biochem Behav. 1978;9:543–9. doi: 10.1016/0091-3057(78)90056-4. [DOI] [PubMed] [Google Scholar]
- 8.Bodnar RJ, Kelly DD, Steiner SS, Glusman M. Stress-produced analgesia and morphine-produced analgesia: lack of cross-tolerance. Pharmacol Biochem Behav. 1978;8:661–6. doi: 10.1016/0091-3057(78)90263-0. [DOI] [PubMed] [Google Scholar]
- 9.Gillette R, Huang RC, Hatcher N, Moroz LL. Cost-benefit analysis potential in feeding behavior of a predatory snail by integration of hunger, taste, and pain. Proc Natl Acad Sci US A. 2000;97:3585–90. doi: 10.1073/pnas.97.7.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbadie C, Taylor BK, Peterson MA, Basbaum AI. Differential contribution of the two phases of the formalin test to the pattern of c-fos expression in the rat spinal cord: studies with remifentanil and lidocaine. Pain. 1997;69:101–10. doi: 10.1016/s0304-3959(96)03285-x. [DOI] [PubMed] [Google Scholar]
- 11.Holtzman SG. Suppression of appetitive behavior in the rat by naloxone: lack of effect of prior morphine dependence. Life Sci. 1979;24:219–26. doi: 10.1016/0024-3205(79)90222-4. [DOI] [PubMed] [Google Scholar]
- 12.McGivern RF, Berntson GG. Mediation of diurnal fluctuations in pain sensitivity in the rat by food intake patterns: reversal by naloxone. Science. 1980;210:210–1. doi: 10.1126/science.7191143. [DOI] [PubMed] [Google Scholar]
- 13.Foo H, Mason P. Sensory suppression during feeding. Proc Natl Acad Sci US A. 2005;102:16865–9. doi: 10.1073/pnas.0506226102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee IO, Jeong YS. Effects of different concentrations of formalin on paw edema and pain behaviors in rats. J Korean Med Sci. 2002;17:81–5. doi: 10.3346/jkms.2002.17.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–74. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- 16.Krebs H, Weyers P, Macht M, Weijers HG, Janke W. Scanning behavior of rats during eating under stressful noise. Physiol Behav. 1997;62:151–4. doi: 10.1016/s0031-9384(97)00026-7. [DOI] [PubMed] [Google Scholar]
- 17.Kupfermann I. Eating behaviour induced by sounds. Nature. 1964;201:324. doi: 10.1038/201324a0. [DOI] [PubMed] [Google Scholar]
- 18.Rasbury W, Shemberg K. The effects of aversive levels of white noise on consummatory behavior. Psychon Sci. 1971;22:166–7. [Google Scholar]
- 19.Doyle TG, Berridge KC, Gosnell BA. Morphine enhances hedonic taste palatability in rats. Pharmacol Biochem Behav. 1993;46:745–9. doi: 10.1016/0091-3057(93)90572-b. [DOI] [PubMed] [Google Scholar]
- 20.Parker LA, Maier S, Rennie M, Crebolder J. Morphine- and naltrexone-induced modification of palatability: analysis by the taste reactivity test. Behav Neurosci. 1992;106:999–1010. doi: 10.1037//0735-7044.106.6.999. [DOI] [PubMed] [Google Scholar]
- 21.Rideout HJ, Parker LA. Morphine enhancement of sucrose palatability: analysis by the taste reactivity test. Pharmacol Biochem Behav. 1996;53:731–4. doi: 10.1016/0091-3057(95)02077-2. [DOI] [PubMed] [Google Scholar]
- 22.Abbott FV, Franklin KB, Westbrook RF. The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain. 1995;60:91–102. doi: 10.1016/0304-3959(94)00095-V. [DOI] [PubMed] [Google Scholar]
- 23.Anseloni VC, Ren K, Dubner R, Ennis M. A brainstem substrate for analgesia elicited by intraoral sucrose. Neuroscience. 2005;133:231–43. doi: 10.1016/j.neuroscience.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 24.Anseloni VC, Weng HR, Terayama R, Letizia D, Davis BJ, Ren K, Dubner R, Ennis M. Age-dependency of analgesia elicited by intraoral sucrose in acute and persistent pain models. Pain. 2002;97:93–103. doi: 10.1016/s0304-3959(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 25.Ren K, Blass EM, Zhou Q, Dubner R. Suckling and sucrose ingestion suppress persistent hyperalgesia and spinal Fos expression after forepaw inflammation in infant rats. Proc Natl Acad Sci US A. 1997;94:1471–5. doi: 10.1073/pnas.94.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coy RT, Kanarek RB. Chronic sucrose intake reduces the antagonist effect of beta-funaltrexamine on morphine-induced antinociception in female but not in male rats. Nutr Neurosci. 2006;9:131–9. doi: 10.1080/10284150600895881. [DOI] [PubMed] [Google Scholar]
- 27.D’Anci KE, Kanarek RB. Naltrexone antagonism of morphine antinociception in sucrose-and chow-fed rats. Nutr Neurosci. 2004;7:57–61. doi: 10.1080/1028415042000198807. [DOI] [PubMed] [Google Scholar]
- 28.d’Anci KE, Kanarek RB, Marks-Kaufman R. Duration of sucrose availability differentially alters morphine-induced analgesia in rats. Pharmacol Biochem Behav. 1996;54:693–7. doi: 10.1016/0091-3057(96)00016-0. [DOI] [PubMed] [Google Scholar]
- 29.D’Anci KE, Kanarek RB, Marks-Kaufman R. Beyond sweet taste: saccharin, sucrose, and polycose differ in their effects upon morphine-induced analgesia. Pharmacol Biochem Behav. 1997;56:341–5. doi: 10.1016/s0091-3057(96)00227-4. [DOI] [PubMed] [Google Scholar]
- 30.Kanarek RB, Homoleski B. Modulation of morphine-induced antinociception by palatable solutions in male and female rats. Pharmacol Biochem Behav. 2000;66:653–9. doi: 10.1016/s0091-3057(00)00251-3. [DOI] [PubMed] [Google Scholar]
- 31.Kanarek RB, Homoleski BA, Wiatr C. Intake of a palatable sucrose solution modifies the actions of spiradoline, a kappa opioid receptor agonist, on analgesia and feeding behavior in male and female rats. Pharmacol Biochem Behav. 2000;65:97–104. doi: 10.1016/s0091-3057(99)00181-1. [DOI] [PubMed] [Google Scholar]
- 32.Kanarek RB, Mandillo S, Wiatr C. Chronic sucrose intake augments antinociception induced by injections of mu but not kappa opioid receptor agonists into the periaqueductal gray matter in male and female rats. Brain Res. 2001;920:97–105. doi: 10.1016/s0006-8993(01)03039-6. [DOI] [PubMed] [Google Scholar]
- 33.Kanarek RB, Przypek J, D’Anci KE, Marks-Kaufman R. Dietary modulation of mu and kappa opioid receptor-mediated analgesia. Pharmacol Biochem Behav. 1997;58:43–9. doi: 10.1016/s0091-3057(96)00470-4. [DOI] [PubMed] [Google Scholar]
- 34.Kanarek RB, White ES, Biegen MT, Marks-Kaufman R. Dietary influences on morphine-induced analgesia in rats. Pharmacol Biochem Behav. 1991;38:681–4. doi: 10.1016/0091-3057(91)90034-y. [DOI] [PubMed] [Google Scholar]
- 35.Casey KL, Morrow TJ. Nocifensive responses to cutaneous thermal stimuli in the cat: stimulus-response profiles, latencies, and afferent activity. J Neurophysiol. 1983;50:1497–515. doi: 10.1152/jn.1983.50.6.1497. [DOI] [PubMed] [Google Scholar]
- 36.Wylie LM, Gentle MJ. Feeding-induced tonic pain suppression in the chicken: reversal by naloxone. Physiol Behav. 1998;64:27–30. doi: 10.1016/s0031-9384(98)00020-1. [DOI] [PubMed] [Google Scholar]
- 37.Boyle EM, Freer Y, Khan-Orakzai Z, Watkinson M, Wright E, Ainsworth JR, McIntosh N. Sucrose and non-nutritive sucking for the relief of pain in screening for retinopathy of prematurity: a randomised controlled trial. Archives of Disease in Childhood Fetal & Neonatal Edition. 2006;91:F166–8. doi: 10.1136/adc.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell A, Stevens B, Mungan N, Johnson W, Lobert S, Boss B. Analgesic effects of oral sucrose and pacifier during eye examinations for retinopathy of prematurity. Pain Manag Nurs. 2004;5:160–8. doi: 10.1016/j.pmn.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci. 1983;3:2545–52. doi: 10.1523/JNEUROSCI.03-12-02545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aloisi AM, Albonetti ME, Carli G. Sex differences in the behavioural response to persistent pain in rats. Neurosci Lett. 1994;179:79–82. doi: 10.1016/0304-3940(94)90939-3. [DOI] [PubMed] [Google Scholar]
- 41.Young JK, Nance DM, Gorski RA. Sexual dimorphism in the regulation of caloric intake and body weight of rats fed different diets. Physiol Behav. 1979;23:577–82. doi: 10.1016/0031-9384(79)90058-1. [DOI] [PubMed] [Google Scholar]