Abstract
Background
Discordance of birth weight has been observed in twin pairs, though little is known about prenatal and early neonatal discordance of head and brain size, and the role that zygosity and chorionicity play in discordances of early brain development in twins.
Aims
To compare prenatal and neonatal discordances of head size in monozygotic –monochorionic (MZ-MC), monozygotic-dichorionic (MZ-DC), and same-sex dizygotic-dichorionic twin pairs (DZ).
Study Design
Subjects prospectively had ultrasounds at 22 and 32 weeks gestational age, and magnetic resonance imaging (MRI) of the brain MRI after birth.
Subjects
88 twin pairs recruited from two university hospital prenatal diagnostic clinics; 22 MZ-MC, 17 MZ-DC, and 49 same sex DZ pairs.
Outcome measures
Discordance of head circumference (HC) and weight at 22 weeks, 32 weeks and birth, as well as intracranial volume (ICV) on neonatal MRI.
Results
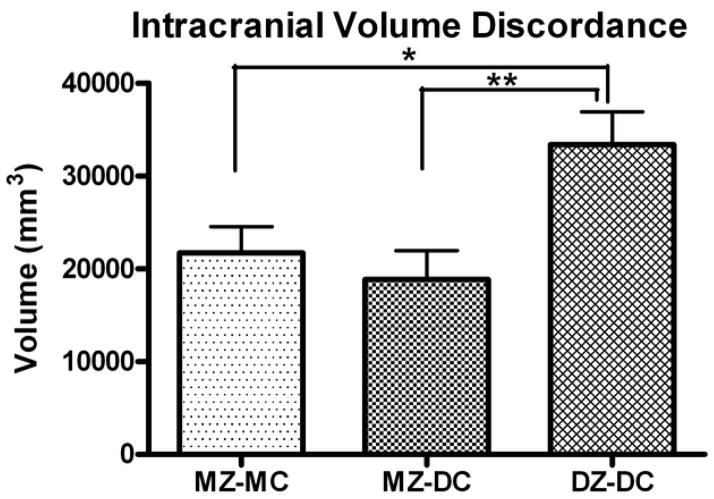
There were no group differences in discordance of head circumference and weight on the 22 or 32 week ultrasounds, or at birth. MZ-MC twins tended to have numerically greater discordances of HC and weight. There was a significant group difference in ICV on neonatal MRI (ANOVA, p = 0.0143), with DZ twins having significantly greater discordance than MZ-MC (p = 0.028) or MZ-DC (p = 0.0131) twins.
Conclusions
This study indicates that zygosity and chorionicity do not contribute to significant discordances of head size in late prenatal development. DZ twins do have significantly greater discordances of ICV on neonatal MRI, suggesting a relatively greater genetic influence on brain growth in the first weeks after birth.
Introduction
Twin studies compare phenotypic concordance in monozygotic (MZ) and dizygotic (DZ) twins to estimate the heritability of and environmental contributions to development of a trait or disease. The twin study methodology has been applied to many neuropsychiatric and neurodevelopmental disorders, yielding heritability estimates of greater than 90% for autism (Freitag 2007), 70–88% for epilepsy (Kjeldsen et al., 2001), 81% for schizophrenia (Sullivan et al., 2003), and 70–80% for ADHD (Martin et al., 2002).
Twin studies are based on the “equal-environment assumption” that twins have identical prenatal environments (Kendler et al., 1993). However, adverse prenatal environments experienced by MZ twins may predispose them to disease and violate this assumption (Phillips 1993). One-third of MZ twin pregnancies are dichorionic (DC) and two-thirds are monochorionic (MC) (Machin 1995); DZ twins are DC. Differences in placental status and thus in intrauterine environment may result in discordant growth. The main difference between MC and DC placentas is the presence of vascular anastomoses in MC placentas that result in unequal blood supply, which in extreme cases can cause twin-twin transfusion syndrome (TTTS).
Estimates of the incidence of discordant growth in twin pregnancies range from 10 to 30% depending on definition (Bagchi and Salihu, 2006). Discordance of birth weight has been associated with increased risk of neurologic morbidity in preterm twins and MC twins are more likely to have neurologic morbidity because of TTTS and cotwin death in utero (Adegbite et al., 2004), increased risk of morbidity and mortality in small for gestational age twins (Blickstein and Keith, 2004; Yinon et al., 2005) and in twins born at term (Hack et al., 2008). Overall, birth weight discordance has been shown to be greater in MC than DC twins (Corey et al., 1979; Gonzalez-Quintero et al., 2003; Race et al., 2006; Hack et al., 2008; but see Chauhan et al., 2004; Blickstein et al., 2006).
There have been a few studies that indicate that twins also have divergent brain growth. Prenatal biparietal diameter is discordant in MZ and DZ twins; this discordance becomes greater at later gestational ages (Persson and Grennert, 1979). We have previously shown in a retrospective study that MZ twins have discordances of head circumference and biparietal diameter on ultrasound in the second trimester that are similar to those observed in DZ twins (Gilmore et al., 1996). This pattern of MZ twins having similar discordances as DZ twins was also seen in subsequent studies of prenatal biparietal diameter (Charlemaine et al., 1997) and head circumference (Charlemaine et al., 2000).
The aim of this study is to better characterize the effects of zygosity and chorionicity on discordance of prenatal and neonatal measures of brain size using prenatal ultrasound and neonatal MRI. Additional aims include the examination of: 1) the relationship of discordance of brain structure and weight; 2) the development of discordance over time; and 3) prenatal challenges and perinatal morbidities as predictors and outcomes of growth discordance.
Methods
Subjects
This study was approved by the Institutional Review Board of the University of North Carolina (UNC) School of Medicine and Duke University Medical Center (DUMC). Mothers with same-sex twin pregnancies were recruited from the outpatient OB-GYN clinics at UNC Hospitals from 2004 until 2007 and from the OB-GYN clinics at DUMC from 2004 to 2006. Exclusion criteria included maternal HIV infection, major congenital abnormality on fetal ultrasound, and chromosomal abnormalities of fetuses. Informed consent was obtained from all subjects. Fetal ultrasounds were performed in the second and/or third trimester, depending on gestational age at the time of recruitment, and MRI scans were performed at term, approximately 40 weeks gestational age. For zygosity testing, PCR-STR analysis of 14 loci was performed on DNA isolated from buccal swab cell collection (BRT Laboratories, Baltimore, MD). Chorionicity of twin pairs was determined by placental pathology (N=63) or ultrasound if placental pathology was not performed or unavailable (N=25).
Birth weight, length and head circumference were obtained from delivery records. Pregnancy and delivery records were reviewed for the presence of pregnancy, birth and neonatal complications.
Image Acquisition
Mothers who were recruited in time for ultrasounds at 20–24 and/or 30–34 weeks gestation age received study ultrasounds in which head circumference (HC), biparietal diameter (BPD), abdominal circumference (AC), and femur length (FL) were measured. Estimated fetal weight (EFW) was calculated by the Hadlock A formula (Hadlock et al., 1984). Ultrasounds were done on an ATL Philips Ultramark HDI 5000 (Philips, Amsterdam, Netherlands) or GE Voluson Expert (General Electric, Fairfield, CT) and were performed at UNC or DUMC by one of two study sonographers at each site. Ultrasounds were clinically reviewed by a maternal-fetal physician (HMW) at UNC or a fetal ultrasound radiology specialist (BH) at DUMC.
MRI scans were done on a Siemens 3T head-only scanner (Allegra, Siemens Medical System, Erlangen, Germany). Neonates were scanned unsedated; subjects were fed before scanning, swaddled, given ear protection, and held in place with a vacuum-fixation device for the head. A nurse was present during all scans, and heart rate and oxygen saturation were monitored with a pulse oximeter. T1-weighted structural pulse sequences were T1w structural pulse sequences were either a 3D magnetization prepared rapid gradient echo (MP-RAGE TR/TI/TE/Flip Angle 1820/400/4.38ms/7°) or a 3D spoiled gradient (FLASH TR/TE/Flip Angle 15/7msec/25°). Proton density and T2 weighted images were obtained with a turbo spin echo sequence (TSE TR/TE1/TE2/Flip Angle 6200/20/119ms/150°). Spatial resolution was 1 × 1 × 1mm voxel for T1 weighted images, 1.25 × 1.25 × 1.5mm voxel with 0.5 mm interslice gap for proton density/T2 weighted images. MRIs were clinically reviewed by a neuroradiologist (JKS).
Image Analysis
Brain tissue was automatically classified as gray matter, non-myelinated white matter, myelinated white matter and cerebrospinal fluid using an atlas-moderated iterative expectation maximization segmentation algorithm as previously described (Gilmore et al., 2007). Intracranial volume (ICV) was the sum of the automatic full brain segmentation results for gray, white and CSF (ventricles and subarachnoid space) volumes.
Statistical Analysis
Intra-twin pair discordance of measures of brain and body size was represented as both absolute difference and as relative discordance (defined as |intrapair difference|/measurement in larger twin). Relative discordance was included to correct for baseline differences in size at the time of scanning. One-way analysis of variance (ANOVA) was used to detect differences in intrapair discordance of biometrics from the second and third trimester ultrasounds, birth, and neonatal MRI between the MZ-MC, MZ-DC, and DZ groups. Pearson’s correlation coefficients were calculated to examine the relationship between intrapair discordance of weight and intrapair discordance of other biometrics. Linear regression on weight and head circumference discordance over time was used to look for differences in development of discordance between the three zygosity/chorionity groups. For analysis of incidence of prenatal and postnatal complications in the three groups, Fisher’s exact test was used for categorical variables and one-way ANOVA for continuous variables. Additional analysis of prenatal and postnatal complications was performed by the same method with twin pairs divided into birth weight discordance groups based on relative birth weight discordance <15% or ≥15%. All statistical hypothesis tests were conducted at a significance level of 0.05.
Results
The final study sample consisted of 88 twin pairs with successful T1 scans, including 22 MZ-MC, 17 MZ-DC, and 49 DZ twin pairs. Within this sample, abnormalities noted on review of ultrasounds included polyhydramnios (5 subjects), oligohydramnios (6 subjects), twin-twin transfusion syndrome (1 subject), left ventricular echogenic focus (3 subjects), choroid plexus cyst (2 subjects), dangling/droopy choroid (1 subject), mild pelviectasis (8 subjects), hydronephrosis (2 subjects), and multicystic kidney (1 subject). Abnormalities noted on review of MRIs included mild ventriculomegaly (2 MZ-MC, 2 DZ subjects), small subdural hemorrhages (3 MZ-MC subjects), calcifications (1 MZ-MC, 1 MZ-DC, 1 DZ subjects), prominent CSF spaces (1 MZ-DC, 1 DZ subject, periventricular leukomalacia (grade 2, with small cysts; 1 DZ subject), and dilated periventricular spaces (1 DZ subject). All MRI abnormalities were considered minor and one of these subjects were excluded from this analysis. There were no significant differences in maternal age or ethnicity, gender, gestational age at birth, or gestational age at second trimester ultrasound, third trimester ultrasound, or neonatal MRI between groups. The MZ-MC group had significantly lower average birth weight and birth length than the MZ-DC and DZ groups (Table 1).
Table 1.
Sample Demographics
| MZ-MC | MZ-DC | DZ(-DC) | Overall | p-value* | ||
|---|---|---|---|---|---|---|
| Number of pairs | 22 | 17 | 49 | 88 | ||
|
Maternal ethnicity N (%) |
White | 17 (77.27%) | 11 (64.71%) | 35 (71.43%) | 63 (71.59%) | |
| African- American or Black | 4 (18.18%) | 5 (29.41%) | 14 (28.57%) | 23 (26.14%) | ||
| Asian | 1 (4.55%) | 1 (5.88%) | 0 (0%) | 2 (2.27%) | 0.3452 | |
|
Gender N (%) |
Male-male | 9 (40.91%) | 9 (52.94%) | 27 (55.10%) | 45 (51.14%) | |
| Female-female | 13 (59.09%) | 8 (47.06%) | 22 (44.90%) | 43 (48.86%) | 0.5456 | |
|
Maternal age (years) Mean (SD) |
31.41 (6.28) | 27.71 (4.13) | 30.45 (6.67) | 30.16 (6.23) | 0.1639 | |
|
Gestational age at 2nd tr ultrasound (weeks) Mean (SD) |
27.21 (6.09) | 28.10 (7.91) | 27.05 (6.64) | 29.30 (6.70) | 0.8581 | |
|
Gestational age at 3rd tr ultrasound (weeks) Mean (SD) |
31.70 (1.15) | 32.18 (0.89) | 31.81 (1.00) | 31.84 (1.02) | 0.4525 | |
|
Gestational age at birth (weeks) Mean(SD) |
34.68 (2.57) | 35.67 (2.79) | 35.83 (2.31) | 35.51 (2.50) | 0.1924 | |
|
Gestational age at MRI (weeks) Mean (SD) |
40.59 (1.79) | 40.61 (1.73) | 41.48 (2.27) | 41.09 (2.09) | 0.1491 | |
|
Days from birth to MRI (days) Mean (SD) |
41.41 (19.03) | 34.59 (22.36) | 39.71 (22.85) | 39.15 (21.75) | 0.6064 | |
|
Average birth weight (g) Mean (SD) |
2152.11 (540.32) | 2423.35 (542.8) | 2461.46 (516.24) | 2375.79 (540.72) | 0.0055** | |
|
Average birth length (cm) Mean (SD) |
44.37 (3.68) | 47.26 (2.88) | 46.28 (3.33) | 45.98 (3.48) | 0.0006*** |
Demographic factors were tested with three-group ANOVA except for maternal ethnicity and gender, which were tested with Fisher’s exact test. Overall p-values shown.
Pairwise comparisons: MZ-MC vs. MZ-DC: p=0.0256; MZ-MC vs. DZ: p=0.0015; MZ-DC vs. DZ: p=0.7179.
Pairwise comparisons: MZ-MC vs. MZ-DC: p=0.0003; MZ-MC vs. DZ: p=0.0022; MZ-DC vs. DZ: p=0.1520.
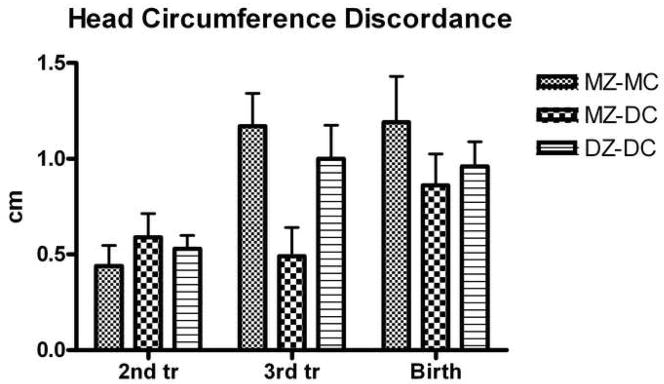
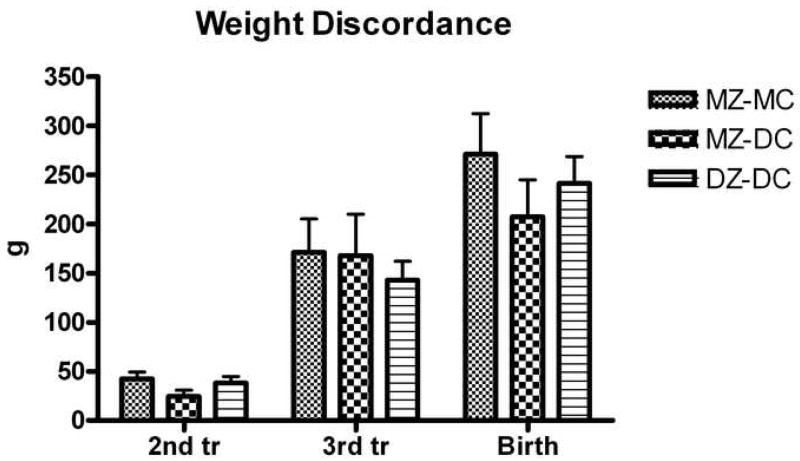
There were no significant differences in absolute or relative discordance of EFW or HC at second or third trimester ultrasounds between groups (Figures 1, 2). Similarly, there were no significant differences in absolute or relative discordance of BPD, FL, and AC at second or third trimester ultrasound between groups (data not shown).
Figure 1.
Discordance of head circumference. There were no significant differences in absolute intrapair discordance of head circumference at 2nd trimester, 3rd trimester, or birth between groups. Data shown in Tables 2–4.
Figure 2.
Discordance of estimated fetal weight and birth weight. There were no significant differences in absolute intrapair discordance of weight at 2nd trimester, 3rd trimester, or birth between groups. Data shown in Tables 2–4.
At birth, there were no significant differences in absolute or relative discordance of birth weight, birth length, or birth head circumference between groups (Table 2). The DZ group had significantly greater discordance of head circumference at the MRI visit than the MZ-MC group (Table 2), as well as significantly higher intracranial volume discordance overall (Figure 3).
Table 2.
Absolute and relative discordance of biometrics at birth and at MRI visit
| Discordance type | MZ-MC | MZ-DC | DZ(-DC) | Overall | p-value (ANOVA) | |
|---|---|---|---|---|---|---|
| Number of pairs | 22* | 17** | 49*** | 88 | ||
|
Birth weight Mean (SD) |
Absolute (g) | 271.14 (193.57) | 207.29 (156.57) | 241.46 (188.32) | 242.29 (183.16) | 0.5633 |
| Relative | 0.12 (0.08) | 0.08 (0.06) | 0.09 (0.07) | 0.10 (0.07) | 0.2880 | |
|
Birth length Mean (SD) |
Absolute (cm) | 1.85 (1.60) | 2.09 (1.97) | 1.51 (1.30) | 1.70 (1.51) | 0.3835 |
| Relative | 0.04 (0.03) | 0.04 (0.04) | 0.03 (0.03) | 0.04 (0.03) | 0.4509 | |
|
Birth HC Mean (SD) |
Absolute (cm) | 1.19 (1.10) | 0.86 (0.66) | 0.96 (0.89) | 1.00 (0.91) | 0.5051 |
| Relative | 0.04 (0.03) | 0.03 (0.02) | 0.03 (0.03) | 0.03 (0.03) | 0.3692 | |
|
HC at MRI Mean (SD) |
Absolute (cm) | 0.45 (0.43) | 0.57 (0.58) | 0.87 (0.75) | 0.71 (0.67) | 0.03101 |
| Relative | 0.01 (0.01) | 0.02 (0.02) | 0.02 (0.02) | 0.02 (0.02) | 0.04212 |
21 MZ-MC pairs with birth length and birth head circumference
15 MZ-DC pairs with birth length; 16 MZ-DC pairs with birth head circumference
48 DZ pairs with birth weight; 47 with birth length and birth head circumference
Pairwise: MZ-MC vs. MZ-DC: 0.5658; MZ-MC vs. DZ: 0.0138; MZ-DC vs. DZ: 0.1068
Pairwise: MZ-MC vs. MZ-DC: 0.5638; MZ-MC vs. DZ: 0.0183; MZ-DC vs. DZ: 0.1298
Figure 3.
Discordance of intracranial volume at MRI visit. Overall ANOVA: p = 0.0143; *DZ vs. MZ-MC: p = 0.0281; **DZ vs. MZ-DC: p = 0.0131.
When placed in zygosity groups (without the additional factor of chorionicity), MZ twins had birth weight discordance comparable to that of DZ twins (MZ: 243.31g ± 179.04; DZ: 241.46 g ± 188.32; p = 0.9630) while having lower intracranial volume discordance (MZ: 20473.91 mm3 ± 12838.63; DZ: 33364.16 mm3 ± 24541.19; p = 0.0039).
We sought to determine if relative discordance in head size was related to relative discordance in body size. There were no significant correlations between discordance of EFW and discordance of head size metrics (BPD, HC) in the second or third trimester, although there were significant correlations between discordance of EFW and discordance of other measures of body size (AC, FL) in some or all three zygosity-chorionicity groups (data not shown). Postnatally, there was no significant correlation between birth weight discordance and birth head circumference discordance in any zygosity/chorionicity group (data not shown).
Longitudinal analysis of discordance of weight and head circumference at second trimester ultrasound, third trimester ultrasound, and birth was performed for the subset of twins with scans at all three visits (N=41 pairs). There were no significant differences in the changing pattern of weight discordance (heterogeneity of slopes = 0.50; p = 0.7383) or head circumference discordance (heterogeneity of slopes = 1.29; p = 0.2826) over time between the MZ-MC, MZ-DC, and DZ groups.
There were no significant differences in the rates of prenatal complications, including preterm labor, preterm premature rupture of membranes, pregnancy hypertension, perinatal diabetes, bleeding, placenta previa, infection during pregnancy, and advanced maternal age, between the MZ-MC, MZ-DC, and DZ groups (data not shown). There was also no significant difference in the distribution of pairs with birth weight discordance <15% and ≥15% between the three groups. (%≥15% MZ-MC: 68.18%; MZ-DC: 88.24%; DZ: 81.63%; p = 0.3244). The rates of the same prenatal complications were then compared in groups of <15% and ≥15% birth weight discordant pairs rather than zygosity-chorionicity groups. The rate of pregnancy hypertension differed between groups (BWD <15%: 8 with pregnancy hypertension (11.43%); BWD ≥15%: 6 (33.33%); p=0.0342). When 20% discordance was used, pregnancy hypertension remained significantly more frequent. There were no significant differences in rates of perinatal complications and proxies for prematurity, including total number of perinatal complications for pair, gestational age at birth, total days in NICU, total days of supplemental oxygen, and total days of intubation, between the three zygosity-chorionicity groups.
Discussion
The primary goal of our study was to examine the effects of zygosity and chorionicity on discordance of brain size in twins before and in the first weeks after birth. We found that there were no significant differences between MZ-MC, MZ-DC, and DZ twins in discordance of brain and body size measures in the second or third trimester or at birth. There were no significant correlations between discordance of brain and body size measures before or at birth in any zygosity-chorionicity group. Longitudinally, there were no differences in the changing pattern of weight or head circumference discordance over time between the MZ-MC, MZ-DC, and DZ groups. Rates of most prenatal and perinatal complications were similar for zygosity-chorionicity groups and birth weight discordance groups. Finally, we found head circumference and intracranial volume were significantly more discordant in DZ twins at the term MRI visit.
The similarity of discordance of HC and BPD across twin groups is consistent with previous studies (Persson and Grennert, 1979; Gilmore et al., 1996; Charlemaine et al., 1997, Charlemaine et al., 2000). MZ-MC twins had greater relative discordance of birth weight compared to the other groups; while not statistically significant in our study, this is consistent with previous studies (Corey et al., 1979; Dube et al., 2002; Gonzalez-Quintero et al., 2003; Race et al., 2006; Hack et al., 2008).
Head circumference and intracranial volume were significantly more discordant in DZ twins at the term MRI visit, which occurred on average 5.5 weeks after birth, while there were no significant differences in head circumference between groups observed at birth. This is consistent with a previous study that found correlations of head circumference at birth to be similar between MZ and DZ twins, while correlations increased in the MZ twins at one month of age and thereafter, but did not change for DZ twins (Chen et al., 1990). A subsequent study found correlations of head circumference at birth to be greater in MZ twins compared to DZ twins (Livshits et al., 2000). Brain growth in the early postnatal period is very rapid, especially growth of cortical gray matter (Gilmore et al., 2007). In the weeks between birth and MRI, outside of the uterine environment, genetic factors may begin to outweigh environmental factors in the developmental trajectory, with the brain size of DZ twins becoming more discordant, and that of MZ twins becoming more concordant.
In the prenatal period and at birth, MZ twins have discordance of brain and body size that is similar to that observed in DZ twins, in spite of being genetically identical. This may be due to placental factors that make MZ twins more discordant than one might expect given their identical genetic makeup. MC twins face additional challenges in utero. Twin-twin transfusion syndrome complicates 10–15% of MC twin pregnancies (Duncan et al., 1997). MC twins are more likely to have neurologic injury and this may also contribute to discordance of brain growth (Adegbite et al., 2004). Placental factors, such as unequal placental sharing and “placental crowding,” have been associated with discordance and small for gestational age (SGA) size in MC twins (Fick et al., 2006; Blickstein et al., 2006; Lewi et al., 2007). Even DC twins may be affected by placental factors as peripheral cord insertion (suggestive of a spatially limited intrauterine compartment) has been associated with SGA infants and discordant growth in both MC and DC twins (Redline et al., 2001).
Discordant growth of the brain may also arise for other reasons. Though presumed to be genetically identical, MZ twins may have true genetic differences related to epigenetic dissimilarity, including DNA methylation (Singh et al., 2002) or differences in DNA copy number (Bruder et al., 2008).
Our finding of a significantly higher incidence of pregnancy hypertension in twins with ≥15% birth weight discordance concurs with a previous study that found an association between twin birth weight discordance and maternal hypertensive disorders, as well as smoking and increased maternal age (Sannoh et al., 2003). Monochorionicity has been associated with adverse outcomes such as preterm delivery, increased rates of NICU admission and longer NICU stays, neurological and respiratory morbidities, and perinatal mortality Victoria et al., 2001; Acosta-Rojas et al., 2007; Hack et al., 2008). Birth weight discordance has similarly been associated with a greater incidence of adverse perinatal outcomes (Amaru et al., 2004). We found no differences in rates of perinatal morbidities between zygosity-chorionicity groups or birth weight discordance groups, but our ability to detect differences was limited by our sample size.
In summary, we found that MZ twins had discordance of brain size in the late prenatal at birth similar to those observed in DZ twins while after several weeks of postnatal development MZ twin brain size was significantly less discordant than DZ twins. Prenatal discordances of brain size in MZ twins may be the result of variations in placental blood flow that would tend to violate the equal environment assumption of twin studies. However, discordances of brain size after even a few weeks of postnatal brain development appear to reflect “normalization” of developmental trajectories, with MZ twins becoming more concordant. We plan to follow this cohort into childhood to better understand the developmental trajectories of brain development in twins.
Acknowledgments
This research is supported by MH070890 (JHG) and the Doris Duke Charitable Foundation (NM).
Footnotes
Conflict of Interest Statement
We have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Freitag CM. The genetics of autistic disorders and its clinical relevance: a review of the literature. Mol Psychiatry. 2007;12:2–22. doi: 10.1038/sj.mp.4001896. [DOI] [PubMed] [Google Scholar]
- 2.Kjeldsen MJ, Kyvik KO, Christensen K, Friis ML. Genetic and environmental factors in epilepsy: a population-based study of 11900 Danish twin pairs. Epilepsy Res. 2001;44:167–78. doi: 10.1016/s0920-1211(01)00196-6. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence of from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–92. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 4.Martin N, Scourfield J, McGuffin P. Observer effects and heritability of childhood attention-deficit hyperactivity disorder. Br J Psychiatry. 2002;180:260–5. doi: 10.1192/bjp.180.3.260. [DOI] [PubMed] [Google Scholar]
- 5.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A test of the equal-environment assumption in twin studies of psychiatric illness. Behav Genet. 1993;23:21–7. doi: 10.1007/BF01067551. [DOI] [PubMed] [Google Scholar]
- 6.Phillips DIW. Twin studies in medical research: can they tell us whether diseases are genetically determined? Lancet. 1993;341:1008–9. doi: 10.1016/0140-6736(93)91086-2. [DOI] [PubMed] [Google Scholar]
- 7.Machin GA. Twins and their disorders. In: Reed GB, Claireaux AE, Cockburn F, editors. Diseases of the fetus and newborn. London: Chapman & Hall; 1995. pp. 201–25. [Google Scholar]
- 8.Bagchi S, Salihu MH. Birth weight discordance in multiple gestations: Occurance and outcome. J Obstet Gynaecol. 2006;26:291–296. doi: 10.1080/01443610600594724. [DOI] [PubMed] [Google Scholar]
- 9.Adegbite AL, Castille S, Ward S, Bajoria R. Neuromorbidity in preterm twins in relation to chorionicity and discordant birth weight. Am J Obstet Gynecol. 2004;190:156–63. doi: 10.1016/j.ajog.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Blickstein I, Keith LG. Neonatal mortality rates among growth-discordant twins, classified according to the birth weight of the smaller twin. Am J Obstet Gynecol. 2004;190:170–4. doi: 10.1016/j.ajog.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 11.Hack KEA, Derks JB, Elias SG, Franx A, Roos EJ, Voerman SK, Bode CL, et al. Increased perinatal mortality and morbidity in monochorionic versus dichorionic twin pregnancies: clinical implications of a large Dutch cohort study. Br J Obstet Gynaecol. 2008;115:58–67. doi: 10.1111/j.1471-0528.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 12.Corey LA, Nance WE, Kang KW, Christian JC. Effects of type of placentation on birthweight and its variability in monozygotic and dizygotic twins. Acta Genet Med Gemellol. 1979;28:41–50. doi: 10.1017/s0001566000009326. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Quintero VH, Luke B, O’Sullivan MJ, Misiunas R, Anderson E, Nugent C, et al. Antenatal factors associated with significant birth weight discordance in twin gestations. Am J Obstet Gynecol. 2003;189:813–7. doi: 10.1067/s0002-9378(03)00658-6. [DOI] [PubMed] [Google Scholar]
- 14.Race JP, Townsend GC, Hughes TE. Chorion type, birthweight discordance and tooth-size variability in Australian monozygotic twins. Twin Res Hum Genet. 2006;9:285–91. doi: 10.1375/183242706776382392. [DOI] [PubMed] [Google Scholar]
- 15.Chauhan SP, Shields D, Parker D, Sanderson M, Scardo JA, Magann EF. Detecting fetal growth restriction or discordant growth in twin gestations stratified by placental chorionicity. J Reprod Med. 2004;49:279–84. [PubMed] [Google Scholar]
- 16.Blickstein I, Mincha S, Goldman RD, Machin GA, Keith LG. The Northwestern twin chorionicity study: testing the “placental crowding” hypothesis. J Perinat Med. 2006;34:158–61. doi: 10.1515/JPM.2006.028. [DOI] [PubMed] [Google Scholar]
- 17.Persson PH, Grennert L. The intrauterine growth of the biparietal diameter of twins. Acta Genet Med Gemellol. 1979;28:271–277. doi: 10.1017/s0001566000008771. [DOI] [PubMed] [Google Scholar]
- 18.Gilmore JH, Perkins DO, Kleiwer MA, Hage ML, Silva SG, Chescheir NC, et al. Fetal brain development of twins assessed in utero by ultrasound: implications for schizophrenia. Schizophr Res. 1996;19:141–9. doi: 10.1016/0920-9964(95)00099-2. [DOI] [PubMed] [Google Scholar]
- 19.Charlemaine C, Duyme M, Dubreuil E, Clauzel JP, Brossard Y, Aurengo A, et al. Biparietal diameter in twins at gestational weeks 18–32 – differences and similarities. J Reprod Med. 1997;42:725–30. [PubMed] [Google Scholar]
- 20.Charlemaine C, Duyme M, Ville Y, Aurengo A, Tremblay R, Frydman R, et al. Fetal biometric parameters, twin type and birth weight difference: a longitudinal study. Eur J Obstet Gynaecol Reprod Biol. 2000;93:27–32. doi: 10.1016/s0301-2115(00)00239-6. [DOI] [PubMed] [Google Scholar]
- 21.Hadlock FP, Harrist RB, Carpenter RJ, Deter RL, Park SK. Sonographic estimation of fetal weight: the value of femur length in addition to head and abdomen measurements. Radiology. 1984;150:535–40. doi: 10.1148/radiology.150.2.6691115. [DOI] [PubMed] [Google Scholar]
- 22.Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YS, Knickmeyer RC, et al. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 2007;27:1255–60. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CJ, Yu MW, Wang CH, Tong SL, Tein M, Lee TY, et al. Chronological changes in genetic variance and heritability of anthropometric characteristics among Chinese twin infants. Acta Genet Med Gemellol. 1990;6:726–36. doi: 10.1017/s0001566000003706. [DOI] [PubMed] [Google Scholar]
- 24.Livshits G, Peter I, Vainder M, Hauspie R. Genetic analysis of growth curve parameters of body weight, height and head circumference. Ann Hum Biol. 2000;27:299–312. doi: 10.1080/030144600282181. [DOI] [PubMed] [Google Scholar]
- 25.Duncan KR, Denbow ML, Fisk NM. The aetiology and management of twin-twin transfusion syndrome. Prenat Diagn. 1997;17:1227–36. doi: 10.1002/(sici)1097-0223(199712)17:13<1227::aid-pd328>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Fick AL, Feldstein VA, Norton ME, Wassel Fyr C, Caughey AB, Machin GA. Unequal placental sharing and birth weight discordance in monochorionic diamniotic twins. Am J Obstet Gynecol. 2006;195:178–83. doi: 10.1016/j.ajog.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Lewi L, Cannie M, Blickstein I, Jani J, Huber A, Hecher K, Dymarkowski S, Gratacos E, Lewi P, Deprest J. Placental sharing, birtweight discordance, and vascular anastomoses in monochorionic diamnionic twin placentas. Am J Obstet Gynecol. 2007;197:587.e1–587.e8. doi: 10.1016/j.ajog.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Redline RW, Shah D, Sakar H, Schluchter M, Salvator A. Placental lesions associated with abnormal growth in twins. Pediatr Dev Pathol. 2001;4:473–81. doi: 10.1007/s10024001-0044-z. [DOI] [PubMed] [Google Scholar]
- 29.Singh SM, Murphy B, O’Reilly R. Epigenetic contributors to the discordance of monozygotic twins. Clin Genet. 2002;62:97–103. doi: 10.1034/j.1399-0004.2002.620201.x. [DOI] [PubMed] [Google Scholar]
- 30.Bruder CEG, Piotrowski A, Gijsbers AACJ, Andersson R, Erickson S, Diaz de Stahl T, Menzel U, Sandgren J, von Tell D, Poplawski A, Crowley M, Crasto C, Partridge EC, Tiwari H, Allison DB, Komorowshi J, van Ommen GJB, Boomsma DI, Pedersen NL, den Dunnen JT, Wirdefeldt K, Dumanski JP. Phenotypically concordant and discordant monozygotic twins display different DNA copy-number-variation profiles. Am J Hum Genet. 2008;82:1–9. doi: 10.1016/j.ajhg.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sannoh S, Demissie K, Balasubramanian B, Rhoads GG. Risk factors for intrapair birth weight discordance in twins. J Matern Fetal Neonatal Med. 2003;13:230–6. doi: 10.1080/jmf.13.4.230.236. [DOI] [PubMed] [Google Scholar]
- 32.Victoria A, Mora G, Arias F. Perinatal outcome, placental pathology, and severity of discordance in monochorionic and dichorionic twins. Obstet Gynecol. 2001;97:310–5. doi: 10.1016/s0029-7844(00)01111-x. [DOI] [PubMed] [Google Scholar]
- 33.Acosta-Rojas R, Becker J, Munoz-Abellana B, Ruiz C, Carreras E, Gratacos E. Twin chorionicity and the risk of adverse perinatal outcome. Int J Gynecol Obstet. 2007;96:98–102. doi: 10.1016/j.ijgo.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Amaru RC, Bush MC, Berkowitz RL, Lapinski RH, Gaddipati S. Is discordant growth in twins an independent risk factor for adverse neonatal outcome? Obstet Gynecol. 2004;103:71–6. doi: 10.1097/01.AOG.0000104060.37475.29. [DOI] [PubMed] [Google Scholar]