Abstract
Oxidative stress and mitochondrial dysfunction are acute consequences of status epilepticus (SE). However, the role of mitochondrial oxidative stress and genomic instability during epileptogenesis remains unknown. Using the kainate animal model of temporal lobe epilepsy, we investigated oxidative mitochondrial DNA (mtDNA) damage and changes in the mitochondrial base excision repair pathway (mtBER) in the rat hippocampus for a period of 3 months after SE. Acute seizure activity caused a time-dependent increase in mitochondrial, but not nuclear 8-hydroxy-2-deoxyguanosine (8-OHdG/2dG) levels and a greater frequency of mtDNA lesions. This was accompanied by increased mitochondrial H2O2 production and a transient decrease in mtDNA repair capacity. The mtBER proteins 8-oxoguanine glycosylase (Ogg1) and DNA polymerase gamma (Pol γ) demonstrated elevated expression at mRNA and protein levels shortly after SE and this was followed by a gradual improvement in mtDNA repair capacity. Recurrent seizures associated with the chronic phase of epilepsy coincided with the accumulation of mtDNA damage, increased mitochondrial H2O2 levels, decreased expression of Ogg1 and Pol γ and impaired mtDNA repair capacity. Together, increased oxidative mtDNA damage, mitochondrial H2O2 production and alterations in the mtBER pathway provide evidence for mitochondrial oxidative stress in epilepsy and suggest that mitochondrial injury may contribute to epileptogenesis.
Keywords: Oxidative stress, Mitochondrial dysfunction, Status epilepticus, Epileptogenesis, mtDNA damage, Base excision repair
Introduction
Epilepsy is a recent addition to the diverse array of acute and chronic neurological disorders in which oxidative stress and mitochondrial dysfunction occurs and may play an important role (Kunz, 2002; Kunz et al., 1999; Patel, 2004).
Status epilepticus (SE) and the subsequent development of epilepsy is associated with several detrimental cellular manifestations (Kann and Kovacs, 2007). The role of mitochondrial genomic instability is particularly intriguing in light of the emerging role of oxidative stress, mitochondrial DNA (mtDNA) damage and mitochondrial abnormalities in epilepsy (Heinemann et al., 2002; Kann et al., 2005; Kovacs et al., 2002; Kudin et al., 2002; Kunz et al., 1999; Liang et al., 2000; Liang and Patel, 2006; Patel et al., 2001; Sleven et al., 2006a). Evidence for the mitochondria being a major site of oxidative damage in SE is supported by the fact that acute seizure activity results in oxidative damage to mitochondrial DNA, lipids and proteins, modulation of the mitochondrial redox status and increased reactive oxygen species (ROS) generation (Liang and Patel, 2004; Liang and Patel, 2006; Patel et al., 2007; Patel et al., 2001). The mitochondrial genome is a particularly vulnerable target for ROS-induced damage (Bohr, 2002). Accordingly, mtDNA damage has been implicated as an important mechanism underlying the cause and/or consequence of epileptic seizures (Cock, 2002; Kunz, 2002; Patel, 2004). However, in contrast to acute seizures, it remains unclear whether, and to what extent, the mitochondrial oxidative stress and mtDNA damage is altered during the development of chronic epilepsy.
Mitochondrial genomic instability and diminished mtDNA repair capacity have been implicated as important factors in several neurodegenerative diseases (Bohr et al., 2002). Epileptic seizures are the presenting sign of several mitochondrial DNA disorders arising from mtDNA mutations and furthermore, stochastic mtDNA injury has been associated with acquired epilepsy (Cock et al., 1999; Deschauer et al., 2003; Horvath et al., 2006; Wallace et al., 1994; Wallace et al., 1992). It should also be emphasized that mutations that impair the mtDNA base excision repair (mtBER) pathway have also been linked with chronic epilepsy (Horvath et al., 2006; Hudson and Chinnery, 2006; Nguyen et al., 2006). Moreover, aging per se is a significant risk factor for developing the epileptic phenotype with both mitochondrial oxidative stress and oxidative mtDNA damage strongly correlated with aging (Bohr, 2002; Bohr et al., 2002; Liang and Patel, 2004).
Previous studies have investigated the critical role of nuclear DNA repair pathways, such as nuclear base excision repair (nBER), nonhomologous end joining repair (NHEJ) and mismatch repair (MMR) after kainate-induced SE (Neema et al., 2005; Quach et al., 2005). However, the importance of mtDNA repair during epileptogenesis still remains relatively unexplored. The predominant pathway for the removal of oxidized bases in the mitochondria is the BER and impairment and/or imbalance of this pathway has been implicated in neuronal dysfunction (Audebert et al., 2002; Beal, 2005; Druzhyna et al., 1998; Fishel et al., 2007; Fishel et al., 2003; Harrison et al., 2005; Neema et al., 2005; Quach et al., 2005). The mtBER pathway involves a highly coordinated process catalyzed by the sequential actions of DNA repair enzymes. Briefly, the mitochondrial variant of 8-oxoguanine DNA glycosylase (Ogg1) is localized to the mitochondrial inner membrane and excises oxidatively damaged bases from mtDNA, prior to incising the sugar 3′ to the lesion (Stuart et al., 2005). The single nucleotide gap is processed and DNA polymerase γ (Pol γ) inserts an undamaged nucleotide and finally the nick is sealed by DNA ligase (Wilson and Bohr, 2007). Although various reports have demonstrated changes in BER during several neuronal disorders such as stroke, Alzheimer’s disease and amyotrophic lateral sclerosis (Copani et al., 2006; Coppede et al., 2007; Kisby et al., 1997; Li et al., 2006), no study has yet examined mtBER during the development of chronic epilepsy.
We used the rat kainate model of temporal lobe epilepsy to characterize mitochondrial oxidative stress, mtDNA damage and mtBER repair in the hippocampus during the process of epileptogenesis. The objectives of this study were two fold; the first was to determine whether acute epileptic seizures result in increased ROS production, damage to mtDNA, and whether this activates mtDNA repair via mtBER. The second was to determine whether, and to what extent, the development of chronic epilepsy involves mitochondrial ROS production, mtDNA damage and changes in the mtBER pathway.
Materials and methods
Kainate administration
Animal housing was conducted in compliance with University of Colorado Health Sciences Center procedures and protocols. Adult male Sprague-Dawley rats were sub-subcutaneously injected with either saline or kainic acid (kainate; 12mg/kg, pH 7.4) purchased from Ocean Products International, Canada; Lot. No. OC-001-1. The dose of 12mg/kg was titrated to produce both acute and chronic seizures as well as comparable levels of oxidative stress observed previously with other sources of kainate (Liang et al., 2000). This resulted in approximately 10–15% mortality. The rats were sacrificed 24h, 48h, 96h, 7d, 21d or 3m after injection and control rats were injected with saline alone. The time points were chosen as they encompass both the acute and chronic epileptic condition (Hellier et al., 1998). In order to reduce the fatality rate of rats after SE, all animals were administered a saline injection (1.5ml per 100g body weight) and the rat chow was moistened to aid recovery. Since the hippocampus has particular significance for the propagation of epileptiform activity that occurs in temporal lobe epilepsy, it was analyzed during this study (Gao et al., 2007; Hellier and Dudek, 1999; Kann et al., 2005; Kunz et al., 1999). Mitochondria were isolated from the hippocampus at the indicated time points and were either used immediately for quantifying H2O2 production, mtDNA repair capacity or snap frozen in liquid nitrogen and stored at −80°C for biochemical analyses.
Monitoring of behavioral seizures
The severity of the behavioral seizures following SE was evaluated after the initial injection, every 30min during the first 4h and hourly for 8h after kainate treatment. The following rating scale was used as previously described (Patel et al., 2001). 0, normal, 1 immobilization, and occasional “wet-dog shakes”, 2, head nodding, unilateral forelimb clonus, frequent “wet-dog shakes”; 3 rearing, salivation, bilateral forelimb clonus; 4, generalized limbic seizures with falling, running and salivation; 5, continuous generalized motor seizures with tonic limbic extension. Only rats that reached a score of ≥4 were used in future studies. Chronic seizures in animals were monitored for at least 6h a week and the spontaneous seizure score and seizure duration was monitored throughout the investigation, as previously described (Hellier and Dudek, 1999; Hellier et al., 1998). It is noteworthy to mention that behavioral monitoring may underestimate the seizure frequency and seizure scores. Rats observed to have at least ≥ 2 spontaneous seizures were defined as suffering from chronic epilepsy (Hellier and Dudek, 1999; Hellier et al., 1998).
HPLC measurement of 8-hydroxy-2-deoxyguanosine (8-OHdG) and 2-deoxyguanosine (2-dG)
DNA was extracted from rat hippocampi by homogenization in buffer containing 1% sodium dodecyl sulphate, 10mM Tris, 1mM EDTA (pH 7.4), and an overnight incubation in 0.5mg/ml proteinase K at 55°C. Homogenates were incubated with RNase (0.1mg/ml) at 50°C for 10 min and extracted with chloroform/isoamyl alcohol. The extracts were mixed with 3M sodium acetate and 2 vols of 100% ethanol to precipitate DNA at −20°C. The samples were washed twice with 70% ethanol, air-dried for 15min and dissolved in 100μl of 10mM Tris/1mM EDTA (pH 7.4). DNA digestion was performed as previously described (Patel et al., 2001). The adduct 8-OHdG was measured with high-performance liquid chromatography (HPLC) equipped with a CoulArray system (Model 5600). Analytes were detected on two coulometric array modules, each containing four electrochemical sensors attached in series, which allows identification targets based on reduction potential. UV detection was set to 260nm. The HPLC was controlled and the data acquired and analyzed using CoulArray software. The mobile phase was composed of 50mM sodium acetate/5% methanol at pH 5.2. Electrochemical detector potentials for 8-OHdG and 2-dG were 120/230/280/420/600/750/840/900mV and the flow rate was 1ml/min.
mtDNA and nDNA damage in vivo
Genomic DNA was extracted using a commercially available Qiagen genomic-tip kit (Qiagen, Inc., CA). The quantitative polymerase chain reaction (QPCR) assay measures the average oxidative lesion frequency. The DNA damage was quantified by comparing the relative efficiency of the amplification of the large mtDNA and nDNA gene fragments (13.4kb for the mtDNA and 12.5 for the nDNA) and normalized to 250bp fragments from saline treated controls (this small fragment has a statistically negligible chance of sustaining oxidative stress-induced base damage). The PCR conditions used in this study were based on previously reported sequences for mtDNA primers, with minor modifications (Ayala-Torres et al., 2000; Jarrett and Boulton, 2005). The primer sequences were as follows for the rat mitochondrial genome 5′-GGC AAT TAA GAG TGG GAT GGA GCG AA-3′ and 5′-AAA ATC CCC GCA AAC AAT GAC CAC CC-3′. For the nuclear gene fragment PCR primers were 5′-AGA CGC GTG AGA CAG CTG CAC CTT TTC-3′ and 5′-CGA GAG CAT CAA GTG CAG GCA TTA GAC-3′. QPCR was carried out on a DNA Engine thermal cycler with all reactions being a total volume of 100μl containing 15ng of total genomic DNA, 1unit of XL rTth polymerase, 3.3 XL PCR buffer II (containing potassium acetate, glycerol and DMSO) and final concentrations of 200μM dNTP’s, 1.2mM Mg(AOC)2 and 0.1μM primers.
The gene fragments were amplified using the following thermo-cycling profile: The PCR was initiated with the addition of the 1unit of XL rTth polymerase when samples had reached a temperature of 75°C. This was followed by an initial denaturation for 1min at 94°C, cycles of denaturation at 94°C for 30s and primer extension at 60°C for 13min. After the PCR cycles had been completed a final extension at 72°C for 10min was performed. The nuclear and mitochondrial gene products underwent 28 cycles and 26 cycles of thermo-cycling, respectively. After the completion of the QPCR, the gene products were resolved on a 1% agarose gel and digitally photographed on a UV transilluminator (UVi Tec, UK). The intensity of the PCR product bands was quantified with Scion Image analysis software (Scion Corporation, Version Beta 4.0.2).
Isolation of mitochondrial fractions
Hippocampi from each rat were pooled and homogenized with a Dounce tissue grinder (Wheaton, Milville, NJ) in a isolation buffer (70mM sucrose, 210mM mannitol, 5mM Tris-HCL, 1mM EDTA; pH 7.4) and diluted 1:1 in 24% Percoll. Homogenates were centrifuged at 30,700g at 4°C for 10min. The sediment was subjected to Percoll gradient (19% on 40%) centrifugation at 30700g at 4°C for 10min. The material located at the interface of the lowest two layers was slowly diluted 1:4 with mitochondrial isolation buffer containing 1mg/ml bovine serum albumin and centrifuged at 6700g at 4°C for 10min to obtain final pellets consisting of respiring mitochondria. The final pellet was either used immediately for H2O2 production studies or frozen in liquid nitrogen and stored at −80°C for biochemical analyses.
Real-Time PCR analyses
An ABI Prism 770 Real-Time PCR System (Applied Biosystems, CA) was used to amplify OGG1 and POL γ gene transcripts from isolated hippocampus (Rn01421382-m1 and Rn01478316-g1; Applied Biosystems, CA). Amplification reactions were performed in a final volume of 50μl containing 8% glycerol, 1X TaqMan buffer A (500mM KCl, 100mM Tris-HCl, 0.1M EDTA, 600nM passive reference dye ROX, pH 8.3 at room temperature), 300μM each of dATP, dGTP, dCTP and 600μM dUTP, 5.5mM MgCl2, 900nM forward primer, 300nM reverse primer, 200nM probe, 1.25U AmpliTaq Gold DNA Polymerase (Perkin-Elmer, CA), 12.5U Moloney Murine leukemia virus reverse transcriptase (Life Technologies, MD), 20U RNAsin ribonuclease inhibitor (Promega, WI) and the template RNA. Thermal cycling conditions were as follows: RT was performed at 48°C for 30min followed by activation of TaqGold at 95°C for 10min. Subsequently 40 cycles of amplification were performed at 95°C for 15s and 60°C for 1min. A standard curve was generated using the fluorescent data from 10-fold serial dilutions of HeLA RNA and quantities of either OGG1 or POL γ at the indicated time points were normalized to the corresponding 18s rRNA.
Immunoblot analyses
Samples (50μg protein) were loaded in a 10% Tris-HCL Ready Gel (Bio-Red, Hercules, CA). The samples were separated by SDS-PAGE and transferred to PVDF membrane (0.45μm). The membrane blots were immunoreacted with 1μg/ml antibodies against DNA Polymerase γ, 8-oxoguanine DNA-glycosylase and β-Actin (Santa Cruz Biotechnology, Santa Cruz, CA). The blots were incubated with either horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (1:2,000) or horseradish peroxidase-conjugated anti-donkey immunoglobulin G (1:25,000) and developed using Enhanced Chemiluminescence Plus reagents (Amersham Biosciences, Buckinghamshire, England). The bands were scanned on a Storm Optical Scanner (Molecular Dynamics, Inc., Sunnyvale, CA) and quantitative analysis of bands performed by ImageQuant software (Amersham Biosciences, UK).
mtDNA repair capacity
To determine the extent of mtDNA repair, hippocampal mitochondria were isolated at various time points (24h, 48h, 96h, 7d, 21d and 3m after kainate treatment or control rats were injected with saline) and re-suspended in 50μM H2O2 dissolved in mitochondrial isolation buffer (70mM sucrose, 210mM mannitol, 5mM Tris-HCL, 1mM EDTA; pH 7.4). Samples were exposed to H2O2 for 30min and either harvested immediately or allowed to repair for 30min. For the mtDNA repair capacity assessment, the oxidant was removed and the mitochondria washed with isolation buffer. The DNA was extracted and QPCR performed as previously reported in Materials and Methods. The data from each sample was normalized to the respective 30min DNA damage levels and designated as 0% repair and the repair efficiency was expressed as % repair compared to 30min damage levels.
Seizure-induced mitochondrial hydrogen peroxide (H2O2) production
H2O2 formation in mitochondrial fractions was measured by using an Amplex Red reagent kit (Molecular Probes) as previously described (Castello et al., 2007). Briefly, fluorometric detection of H2O2 production was achieved using the horseradish peroxidase-linked Amplex Ultra Red fluorometric assay. Cellular fractions (10μg) were added to a 96-well plate containing 100μl of reaction buffer containing 0.1 units/ml horseradish peroxidase, 50M Amplex UltraRed, and 2.5mM malate plus 10mM glutamate. A microplate reader equipped for excitation in the range of 530–560nm and fluorescence emission detection at 590nm was used to determine the production.
Aconitase and Fumurase enzyme activity
Aconitase and fumurase activities were measured spectrophotometrically as previously described (Liang et al., 2000; Liang and Patel, 2004). Immediately prior to the addition of aconitase and fumurase activity determinations, mitochondrial fractions were resuspended in a reaction buffer containing 50mM Tris-HCL (pH 7.4) containing 0.6mM MnCl2. Aconitase activity was immediately measured by monitoring the formation of cis-aconitase from isocitrate at 240nm in reaction buffer. One unit was defined as the amount of enzyme necessary to produce 1μmol cis-aconitase per minute (ε=3.6mM−1). Fumurase activity was measured by monitoring the absorbance at 240nm in a reaction buffer containing 30mM potassium phosphate (pH 7.4)/0.1mM L-malate. One unit was defined as the amount of enzyme necessary to produce 1 μmol fumurate per minute (ε=2.4 mM−1).
Protein determination
Protein levels were measured using a BCA protein assay kit (Pierce Chemical Co., Rockford, USA) according to the manufacturer’s protocol using a BCA standard curve.
Statistical analysis
For comparison between three or more experimental groups, one way ANOVA with the Bonferroni post hoc test was used. A two-tailed t test was used for comparison between two treatments. Values of *P<0.05 or more were considered statistically significant.
Results
Development of the epileptic phenotype
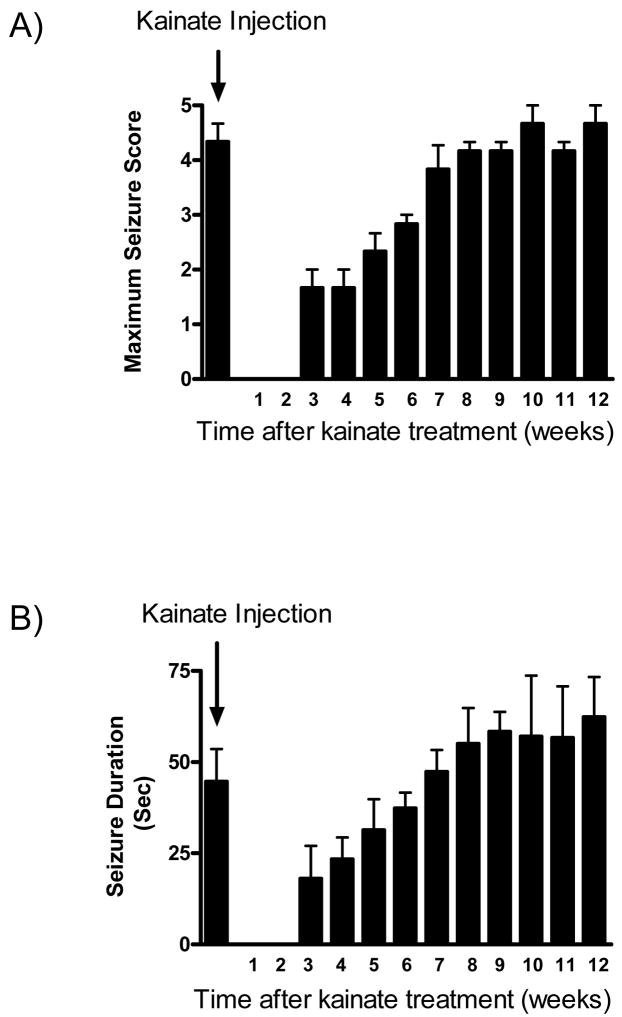
We determined the epileptogenic effect of a single high dose of kainate (12mg/kg; s.c) as measured by spontaneous motor seizures (approximately 80% of the surviving rats injected with kainate became epileptic). A time-dependent increase in the severity of spontaneous behavioral seizures and seizure duration following kainate administration was observed (Fig. 1). By 3m after kainate administration, the mean seizure score was 4.3 ± 0.3 and the mean seizure duration was 62 ± 19s. Saline treated controls did not show any seizure activity or modulation of oxidative stress indices throughout the time points of the study (data not shown).
Fig. 1.
The development of spontaneous seizures in kainate-treated rats. Rats were treated with kainate (12mg/kg; s.c) to initiate SE and seizures scored as described in Materials and Methods. Behavioral observations of seizure activity were monitored for 6hr a week and rats that suffered ≥ 2 spontaneous seizures were considered to be epileptic. Saline treated controls did not show any seizure activity or modulation of oxidative stress indices throughout the study. For each animal A) the most severe motor seizure score and B) length of each seizure was determined at the indicated time point after kainate administration. Bars represent mean ± SEM, n=3–4 per time point.
Acute seizures induce mitochondrial but not nuclear 8-hydroxy-2-deoxyguanosine (8-OHdG) generation
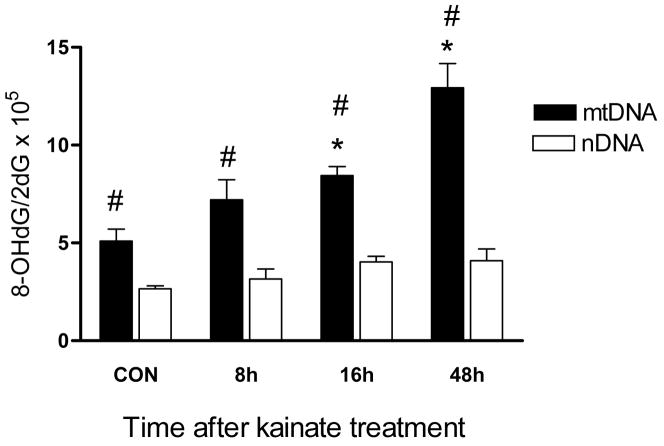
The mitochondrial genome is known to be vulnerable to the deleterious consequences of oxidative stress (Ballinger et al., 2000; Bohr, 2002). We and others have previously shown that kainate-induced SE produces oxidative DNA damage using the 8-OHdG as a surrogate oxidative stress marker (Lan et al., 2000; Liang and Patel, 2004; Penkowa et al., 2005). However, the relative contribution of the mitochondrial vs. the nuclear genome in the accumulation of this lesion is unknown. Thus, we determined whether acute SE activity caused differential oxidative damage to the mitochondrial and nuclear genomes (Fig. 2). Kainate-induced SE generated a 1.8-and 2.8-fold increase in the hippocampal 8-OHdG/2-dG ratio in mtDNA 16h and 48h post-treatment, respectively (P<0.05; one-way ANOVA). By contrast, no change in the levels of the nDNA 8-OHdG/2-dG ratio was detected during the experimental time course. This suggests that SE produces a greater amount of mtDNA damage in comparison to the nDNA.
Fig. 2.
A comparison of 8-OHdG generation in the mitochondrial and nuclear genomes following SE. Hippocampal tissue was harvested at the indicated time points, DNA extracted and HPLC performed as described in Materials and Methods. DNA damage was expressed as the ratio of oxidized DNA base (8-OHdG) to non-oxidized base (2-dG) in mtDNA and nDNA. Bars represent mean ± SEM. *P<0.05 mtDNA vs. saline treated controls, #P<0.05 mtDNA vs. nDNA, one way ANOVA, n= 6 per group.
mtDNA lesions but not nDNA lesions are altered during epileptogenesis
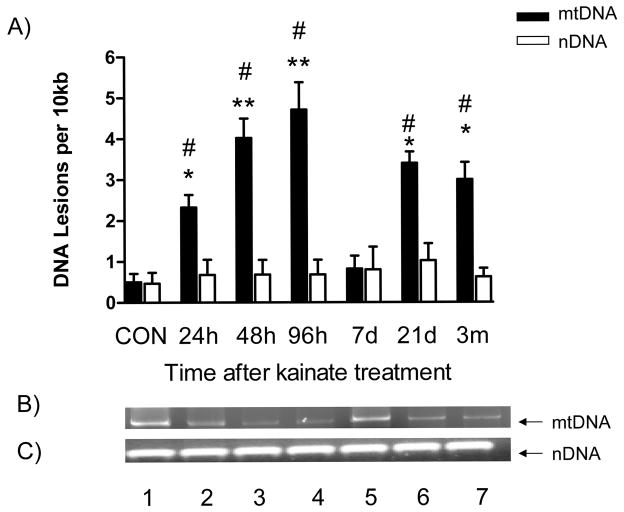
Damage to the mitochondrial genome and mtDNA lesions are one consequence of the 8-OHdG/2-dG ratio accumulation. To determine if this occurred, we employed a well-validated, sensitive QPCR assay specific to a 13.4kb mitochondrial and 12.5kb nuclear fragment to investigate whether mtDNA and nDNA damage occurred in the hippocampus during the development of the epileptic phenotype (Fig. 3). Kainate-induced SE generated a 5.5-fold increase in mtDNA damage in hippocampal tissue 24hr after injection and remained elevated for 96h thereafter (P<0.01; one-way ANOVA). By 7d mtDNA lesions returned to levels that did not differ from saline treated controls. However, at the 21d and 3m time points, mtDNA lesions were elevated by 6.5-fold and 7.5-fold, respectively (P<0.05; one way ANOVA). By contrast, no change in nDNA lesions were detected at any of the time points studied.
Fig. 3.
A comparison of mitochondrial and nuclear DNA damage during epileptogenesis. Hippocampal tissue was harvested at the indicated time points, DNA extracted and QPCR performed. The graph represents the number of DNA lesions per 10kb in a mitochondrial or nuclear gene fragment. Data was normalized to 250bp fragments amplified from saline treated controls. A) mtDNA and nDNA lesions per 10kb at the indicated time points after kainate treatment. B and C) Agarose gels derived from QPCR analysis of hippocampal tissue after kainate treatment. Lane 1, CON; lane 2, 24h; lane 3; Lane 4, 48h; Lane 5, 96h; Lane 6, 7d; Lane 7, 21d; Lane 8, 3m. Bars represent mean ± SEM. *P<0.05; **P<0.01, vs. saline treated controls; #P<0.05 mtDNA vs. nDNA, one way ANOVA, n= 6 per group.
The BER proteins 8-oxoguanine DNA-glycosylase and DNA polymerase γ demonstrate adaptive behavior during epileptogenesis
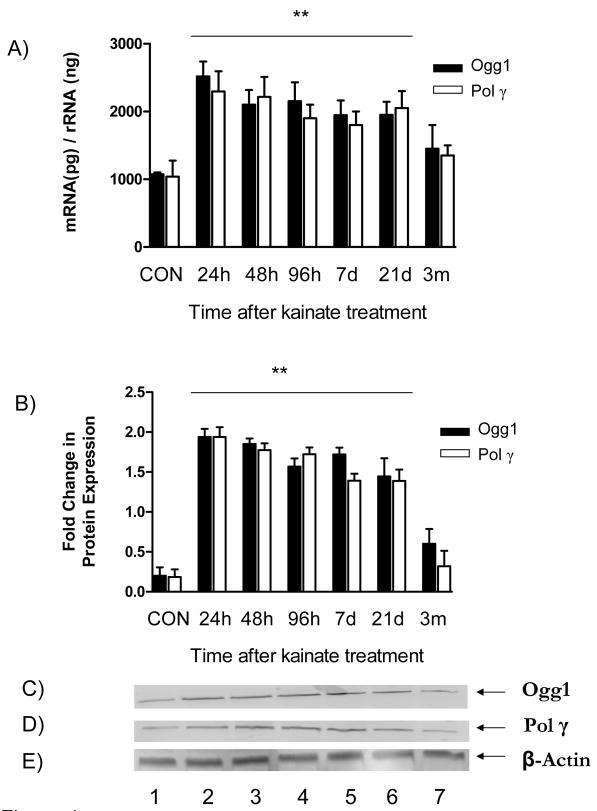
The predominant pathway for repairing mitochondrial genomic damage is the BER (Chen et al., 2000). Therefore, during this study components of BER were examined to determine their role in the repair of mtDNA lesions. We chose to characterize enzymes Ogg1 and Pol γ at mRNA and protein levels, as they have a fundamental role in BER efficiency and cellular oxidative stress sensitivity (Fig. 4) (Bohr, 2002; Bohr et al., 2002; Harrison et al., 2007). An increase in Ogg1 mRNA (2.4-fold) and protein (2.2-fold) levels, as well as Pol γ mRNA (3.8-fold) and protein (3.1-fold) levels occurred 24hr following kainate administration and remained elevated thereafter for 21d (P<0.01; one way ANOVA). However, 3m post-treatment the mRNA and protein levels of Ogg1 and Pol γ subsided to levels not significantly different from the respective saline treated controls.
Fig. 4.
Expression of Ogg1 and Pol γ at mRNA and protein levels during epileptogenesis. To assess the induction of the BER pathway during epileptogenesis, the levels of both Ogg1 and Pol γ at an mRNA and protein level were monitored as described in Materials and Methods. A) mRNA expression at the indicated time points was determined by real-time PCR performed on a primer/probe set specific to Ogg1 and Pol γ. B) Protein expression of Ogg1 and Pol γ at the indicated time points was determined via immunoblot analysis. C and D) Immunoblots of Ogg1 and Pol γ, respectively from hippocampal tissue after kainate treatment. Lane 1, CON; lane 2, 24h; lane 3; Lane 4, 48h; Lane 5, 96h; Lane 6, 7d; Lane 7, 21d; Lane 8, 3m. Bars represent mean ± SEM. **P<0.01, vs. saline treated controls, one way ANOVA, n= 3–5 per group.
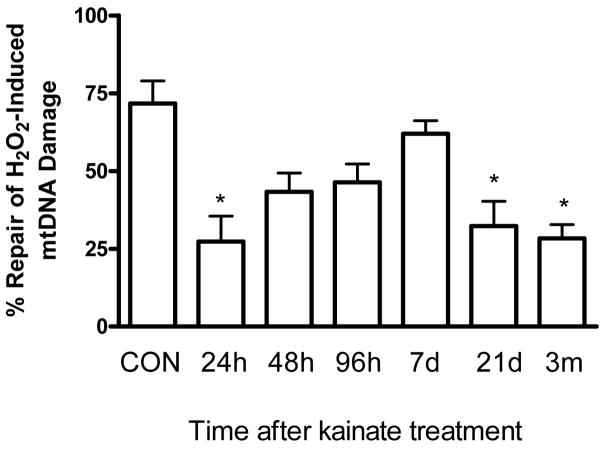
mtDNA repair capacity is altered during epileptogenesis and severely impaired in epileptic rats
A failure in DNA repair has been strongly implicated with various neurological disorders (Bohr, 2002; Dogru-Abbasoglu et al., 2007; Harrison et al., 2005; Lovell et al., 2000; Weissman et al., 2007). It should be stressed that despite the well documented role of the BER in the nucleus, little is known about the mitochondrial BER capacity in neuronal disorders, such as epilepsy. Since the mRNA and protein levels of Ogg1 and Pol γ are increased we asked whether mtDNA capacity was altered during the progression of SE to chronic epilepsy (Fig. 5). The repair capacity was defined as the ability of isolated mitochondria to repair exogenously added H2O2-induced mtDNA damage. A 3-fold decrease in the mtDNA repair capacity occurred 24h after kainate treatment (P<0.05; one way ANOVA). However at the 48h time-point, the repair returned to levels that did not significantly differ from control levels up to 7d. At the 21d and 3m post-kainate time points, mtDNA repair returned to decreased levels (P<0.05; one way ANOVA) which coincided with the decline in mtDNA repair enzymes.
Fig. 5.
A comparison of mtDNA repair capacity in isolated mitochondria during epileptogenesis. Hippocampal mitochondria were freshly isolated at the indicated time points after kainate administration and treated with H2O2 (50μM) for 30min and either harvested immediately or allowed to repair for 30min. The data was normalized to the respective 30min DNA damage levels and repair efficiency expressed as percentage repair. Bars represent mean ± SEM. *P<0.05, vs. saline treated controls, one way ANOVA, n= 4 per group.
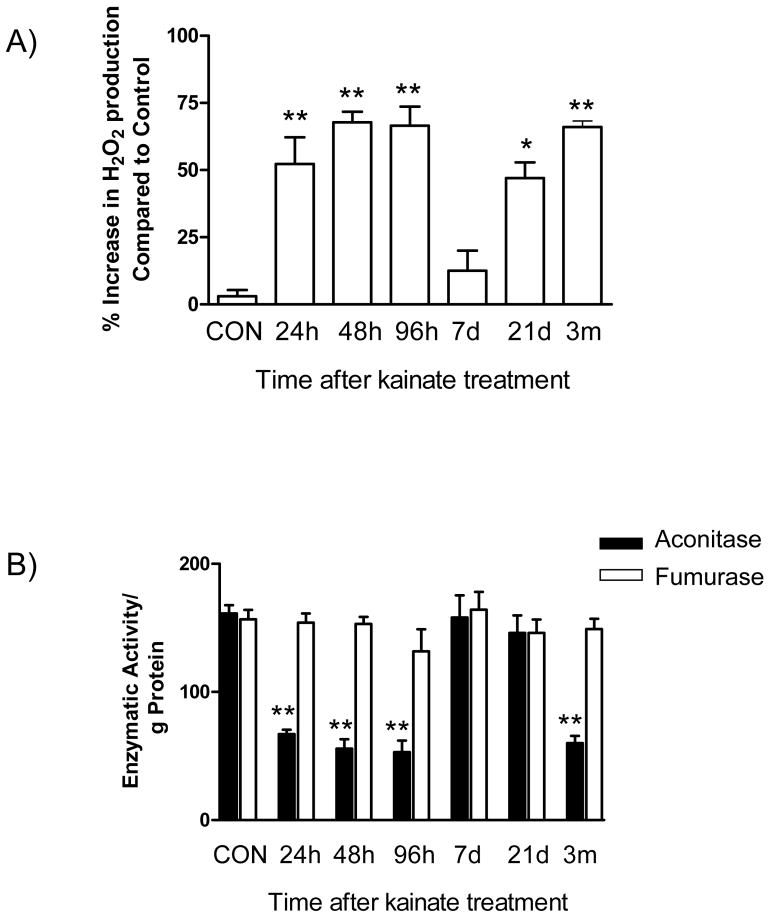
Mitochondrial oxidative stress during epileptogenesis
To substantiate the mechanism underlying the mitochondrial genomic injury during epileptogenesis, we measured two indices of mitochondrial oxidative stress (Fig. 6). First, we asked whether steady-state mitochondrial H2O2 levels were altered throughout epileptogenesis. Kainate-induced SE triggered an 5-fold increase in H2O2 production from hippocampal mitochondria at the 24h time point and this continued until 96h (P<0.01; one way ANOVA); however H2O2 returned to levels of saline treated saline treated controls by day 7. Remarkably, coincident with the development of spontaneous seizures (associated with the chronic phase of epileptogenesis), mitochondrial H2O2 production was elevated 2-fold at the 3m time point after kainate administration (P<0.01; one way ANOVA).
Fig. 6.
Mitochondrial oxidative stress during epileptogenesis. To assess the state of mitochondrial oxidative stress in the hippocampus during epileptogenesis the levels of A) H2O2 production and B) mitochondrial aconitase activity were monitored as described in Materials and Methods. H2O2 production was expressed as percentage mitochondrial H2O2 change compared to saline treated controls. Aconitase activity was expressed as activity/g of protein. Bars represent mean ± SEM. *P<0.05; **P<0.01, vs. saline treated controls, one way ANOVA, n= 3–4 per group.
Secondly, to further provide evidence of mitochondrial oxidative stress, we measured the enzymatic activities of mitochondrial aconitase and fumarase. The selective inactivation of mitochondrial aconitase, an oxidatively labile enzyme can be used as a index of mitochondrial oxidative stress (Liang et al., 2007; Liang et al., 2000; Liang and Patel, 2004). Hippocampal mitochondrial aconitase levels decreased 2.4-fold 24hr following kainate administration and remained depressed thereafter, recovering on day 7 (P<0.01; one way ANOVA). However, at the 3m time point, the occurrence of chronic seizures coincided with decreased aconitase activity 2.6-fold to levels lower than slaine treated controls (P<0.05; one way ANOVA). Furthermore, fumarase (an oxidatively stable mitochondrial enzyme) did not demonstrate any significant change in activity at any of the time points studied.
Discussion
Three major findings from this investigation provide novel information regarding the role of mitochondrial oxidative stress, mtDNA damage and mtBER in epileptogenesis. First, acute SE caused increased oxidative mtDNA damage which coincided with mitochondrial H2O2 production, activation of the mtBER pathway and a transient decrease in mtDNA repair capacity. Secondly, Ogg1 and Pol γ demonstrated elevated expression at mRNA and protein levels and this coincided with reduced mtDNA damage and a gradual improvement in mtDNA repair capacity. Finally, the chronic phase of epileptogenesis coincided with mitochondrial ROS production, accumulation of mtDNA damage, a failure of mtBER response and impaired mtDNA repair capacity. Together, these studies demonstrate a novel association of mitochondrial dysfunction and oxidative stress in epileptogenesis.
Oxidative DNA damage has been mechanistically associated with acute and chronic neuronal disorders (Beal, 2005). Previous studies have demonstrated that seizure activity increases total cellular oxidative DNA damage (Lan et al., 2000; Liang et al., 2000; Neema et al., 2005); however the role of oxidative stress-induced mtDNA injury in the etiology of acute and chronic epilepsy is unknown. This study demonstrates increased mtDNA damage immediately after SE, a recovery during the period of low seizure probability, and a recrudescence of mtDNA damage during the chronic phase of epilepsy, during which frequent seizures occur (Hellier et al., 1998). Acute seizure activity caused extensive 8-OHdG accumulation in the mitochondrial genome, by contrast, no measurable damage to the nuclear DNA was observed.
This present study supports a disproportionately greater role of mitochondrial genomic injury following SE. The increased susceptibility of the mitochondrial genome compared to the nuclear genome to oxidative insults has been observed in a wide array of neurodegenerative diseases (Ballinger et al., 2000; Beal, 2005; Bohr, 2002; Jarrett and Boulton, 2005; Liang and Godley, 2003). A number of factors could explain the increased genomic sensitivity of the mitochondria during epileptogenesis. First, oxidative stress can be both a cause and consequence of epileptic seizures (Patel, 2004). It is also worth noting that the epileptic condition is associated with defects in complex I of the electron transport chain, which is also associated with increased ROS leakage (Kunz et al., 2000); Second, SE causes a disproportionately greater decrease in mitochondrial GSH/GSSG ratios, thus depleting the mitochondrial antioxidant capacity (Liang and Patel, 2006); Third, Pol γ is the rate limiting enzyme in the mtBER and has a high sensitivity to oxidative-inactivation, which may hinder successful mtDNA repair (Graziewicz et al., 2002; Harrison et al., 2005; Kemeleva et al., 2006); Finally, mtDNA is deficient in histones which provide protection against ‘oxidative attack’ to the nDNA (Ballinger et al., 2000). In addition, the present study suggests that oxidative stress may have an important role in mtDNA damage following SE. This is evidenced by increased levels of the 8-OHdG/2-dG ratio in conjunction with increased mitochondrial H2O2 production. Furthermore, the activity of the oxidative-sensitive aconitase was markedly decreased during SE, whereas the oxidative-insensitive fumurase was not affected.
The mtBER proteins Ogg1 and Pol γ were up-regulated immediately after SE. Presumably, the increased expression of DNA repair proteins is a protective mechanism due to increased levels of mitochondrial oxidative stress, 8-OHdG accumulation and an attempt to repair this lesion. Oxidative stress has been previously shown to modulate DNA repair proteins in a variety of pathologies (Hollensworth et al., 2000; LeDoux et al., 1999; Stuart et al., 2004). However, the characterization of the mtBER following SE remains relatively unexplored. It is noteworthy to mention, that after the decline of acute SE associated oxidative stress, the levels of Ogg1 and Pol γ remained elevated followed by a gradual improvement in mtDNA repair capacity. This mtDNA repair response is reminiscent of an adaptive response (Ballinger et al., 1999; Jarrett and Boulton, 2005) and the up-regulation of BER proteins may be an endogenous protective factor against pathophysiological events arising from seizure activity.
During the chronic phase of epilepsy, recurrent spontaneous seizures and longer seizure durations were observed in conjunction with increased levels of mtDNA damage together with a complete failure of the mtBER response. Spontaneous motor seizures occurred concurrently with the re-emergence of mtDNA damage. It is unclear whether mtDNA damage may be an epileptogenic event per se or the spontaneous seizures themselves cause the re-emergence of the mtDNA damage. However, this study supports previous observations implicating mitochondrial dysfunction during chronic epilepsy (Kann et al., 2005; Kunz et al., 2000). Of further note, the increased mtDNA damage occurred concurrently with down-regulation of mtBER proteins and impaired mtDNA repair capacity. It is noteworthy to emphasize that although the up-regulated mtDNA repair mechanisms are capable of counteracting the damage during the less severe interictal and ictal discharges that occur during the early stages of epileptogenesis, as spontaneous seizures re-emerge, mitochondrial repair mechanisms become incapable of keeping up with damage to the mitochondrial genome. It is likely that the failure of the mtDNA repair mechanism(s) could, in part, contribute to the high vulnerability of mtDNA to oxidative stress and exacerbate the progressive mitochondrial genomic decline that occurs in the epileptic condition (Kunz, 2002; Liang and Patel, 2004; Neema et al., 2005). This is further evidenced by the fact that chronic epilepsy in our model was accompanied by elevated mitochondrial oxidative stress, as demonstrated by increased levels of H2O2 production and aconitase inactivation. The specific mechanisms that result in the malfunction of the mtBER in the chronic epileptic state remain to be elucidated. It is well documented that the components of DNA repair pathways are sensitive to oxidative inactivation and furthermore, enzymatic inactivation/imbalance of DNA repair proteins may be correlated with DNA repair capacity (Ballinger et al., 1999; Graziewicz et al., 2002; Harrison et al., 2005). It is also plausible that the increased mitochondrial oxidative stress sustained during chronic epilepsy may further diminish the mtDNA repair capacity by preventing localization of the DNA repair proteins in the mitochondria. This is supported by the fact that oxidative stress can inhibit the import of mtDNA repair proteins into the mitochondria which is especially relevant to the mtBER as all the protein components of this pathway are nuclear encoded (Nakabeppu, 2001). However, other factors may also have contributed to the late decrease in mtDNA repair capacity such as cell loss, reduced mitochondrial density, or a general deterioration of nuclear and mitochondrial maintenance/repair mechanisms (Neema et al., 2005; Sleven et al., 2006b).
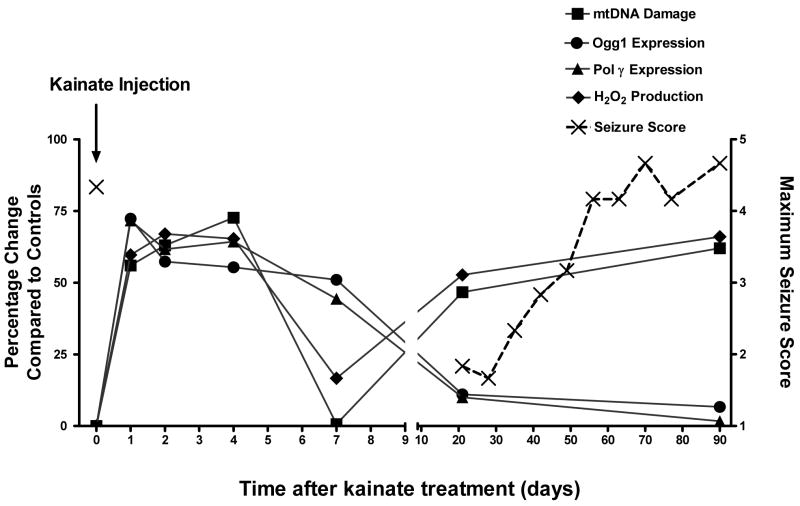
In summary, our findings strongly implicate a role for oxidative mitochondrial genomic instability in epileptogenesis. This present study has uncovered a unique relationship between seizures, mitochondrial oxidative stress and mtDNA damage (Fig. 7). Acute SE seizure activity causes preferential oxidative damage to the mtDNA and the mtBER is induced to attempt to repair mtDNA lesions. However, there is a failure in the mtBER during the chronic phase of epilepsy, which may hinder the repair of mtDNA damage and may contribute to increased mtDNA instability and mitochondrial oxidative stress. Together, increased oxidative mtDNA damage, mitochondrial H2O2 production and alterations in the mtBER pathway provide evidence that mitochondrial injury may contribute to epileptogenesis.
Fig. 7.
The relationship betweens seizures, mtDNA damage, mtBER expression and H2O2 production. Indices of the mitochondrial oxidative burden measured during this study illustrate how mitochondrial oxidative stress and mtDNA damage could play a role in the progression from acute seizure activity to chronic epilepsy. Acute seizure activity causes preferential oxidative damage to the mtDNA and the mtBER is induced to attempt to repair mtDNA lesions. However, there is a failure in the mtBER during the chronic phase of epilepsy, which may hinder the repair of mtDNA damage and contribute to increased mitochondrial oxidative stress. The increased mitochondrial oxidative stress burden and genomic instability may render the brain more susceptible to subsequent chronic epileptic seizures. The (X) represents the mean seizure score and the other data points represent the mean percentage change of mtDNA Damage, Ogg1, Pol γ and H2O2 production compared to controls.
Acknowledgments
This work was supported by an Epilepsy Foundation Postdoctoral Research Fellowship (S.J) and by NIH R01NS39587 (M.P). The authors would like to thank Dr Simon Waldbaum for useful discussions and suggestions on the manuscript and Umarani Pugazhenthi at the University of Colorado Cancer Center RT-PCR Core facility for the excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Audebert M, Charbonnier JB, Boiteux S, Radicella JP. Mitochondrial targeting of human 8-oxoguanine DNA glycosylase hOGG1 is impaired by a somatic mutation found in kidney cancer. DNA Repair (Amst) 2002;1:497–505. doi: 10.1016/s1568-7864(02)00034-4. [DOI] [PubMed] [Google Scholar]
- Ayala-Torres S, Chen Y, Svoboda T, Rosenblatt J, Van Houten B. Analysis of gene-specific DNA damage and repair using quantitative polymerase chain reaction. Methods. 2000;22:135–147. doi: 10.1006/meth.2000.1054. [DOI] [PubMed] [Google Scholar]
- Ballinger SW, Patterson C, Yan CN, Doan R, Burow DL, Young CG, Yakes FM, Van Houten B, Ballinger CA, Freeman BA, Runge MS. Hydrogen peroxide- and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res. 2000;86:960–966. doi: 10.1161/01.res.86.9.960. [DOI] [PubMed] [Google Scholar]
- Ballinger SW, Van Houten B, Jin GF, Conklin CA, Godley BF. Hydrogen peroxide causes significant mitochondrial DNA damage in human RPE cells. Exp Eye Res. 1999;68:765–772. doi: 10.1006/exer.1998.0661. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Bohr VA. Repair of oxidative DNA damage in nuclear and mitochondrial DNA, and some changes with aging in mammalian cells. Free Radic Biol Med. 2002;32:804–812. doi: 10.1016/s0891-5849(02)00787-6. [DOI] [PubMed] [Google Scholar]
- Bohr VA, Stevnsner T, de Souza-Pinto NC. Mitochondrial DNA repair of oxidative damage in mammalian cells. Gene. 2002;286:127–134. doi: 10.1016/s0378-1119(01)00813-7. [DOI] [PubMed] [Google Scholar]
- Castello PR, Drechsel DA, Patel M. Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. J Biol Chem. 2007;282:14186–14193. doi: 10.1074/jbc.M700827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Lan J, Pei W, Chen J. Detection of DNA base-excision repair activity for oxidative lesions in adult rat brain mitochondria. J Neurosci Res. 2000;61:225–236. doi: 10.1002/1097-4547(20000715)61:2<225::AID-JNR13>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Chuang YC, Chang AY, Lin JW, Hsu SP, Chan SH. Mitochondrial dysfunction and ultrastructural damage in the hippocampus during kainic acid-induced status epilepticus in the rat. Epilepsia. 2004;45:1202–1209. doi: 10.1111/j.0013-9580.2004.18204.x. [DOI] [PubMed] [Google Scholar]
- Cock HR. The role of mitochondria and oxidative stress in neuronal damage after brief and prolonged seizures. Prog Brain Res. 2002;135:187–196. doi: 10.1016/S0079-6123(02)35018-0. [DOI] [PubMed] [Google Scholar]
- Cock HR, Cooper JM, Schapira AH. Functional consequences of the 3460-bp mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. J Neurol Sci. 1999;165:10–17. doi: 10.1016/s0022-510x(99)00088-x. [DOI] [PubMed] [Google Scholar]
- Cock HR, Tong X, Hargreaves IP, Heales SJ, Clark JB, Patsalos PN, Thom M, Groves M, Schapira AH, Shorvon SD, Walker MC. Mitochondrial dysfunction associated with neuronal death following status epilepticus in rat. Epilepsy Res. 2002;48:157–168. doi: 10.1016/s0920-1211(01)00334-5. [DOI] [PubMed] [Google Scholar]
- Copani A, Hoozemans JJ, Caraci F, Calafiore M, Van Haastert ES, Veerhuis R, Rozemuller AJ, Aronica E, Sortino MA, Nicoletti F. DNA polymerase-beta is expressed early in neurons of Alzheimer’s disease brain and is loaded into DNA replication forks in neurons challenged with beta-amyloid. J Neurosci. 2006;26:10949–10957. doi: 10.1523/JNEUROSCI.2793-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppede F, Mancuso M, Lo Gerfo A, Manca ML, Petrozzi L, Migliore L, Siciliano G, Murri L. A Ser326Cys polymorphism in the DNA repair gene hOGG1 is not associated with sporadic Alzheimer’s disease. Neurosci Lett. 2007;414:282–285. doi: 10.1016/j.neulet.2006.12.035. [DOI] [PubMed] [Google Scholar]
- Deschauer M, Bamberg C, Claus D, Zierz S, Turnbull DM, Taylor RW. Late-onset encephalopathy associated with a C11777A mutation of mitochondrial DNA. Neurology. 2003;60:1357–1359. doi: 10.1212/01.wnl.0000055869.99975.4b. [DOI] [PubMed] [Google Scholar]
- Dogru-Abbasoglu S, Aykac-Toker G, Hanagasi HA, Gurvit H, Emre M, Uysal M. The Arg194Trp polymorphism in DNA repair gene XRCC1 and the risk for sporadic late-onset Alzheimer’s disease. Neurol Sci. 2007;28:31–34. doi: 10.1007/s10072-007-0744-x. [DOI] [PubMed] [Google Scholar]
- Druzhyna N, Nair RG, LeDoux SP, Wilson GL. Defective repair of oxidative damage in mitochondrial DNA in Down’s syndrome. Mutat Res. 1998;409:81–89. doi: 10.1016/s0921-8777(98)00042-1. [DOI] [PubMed] [Google Scholar]
- Fishel ML, He Y, Smith ML, Kelley MR. Manipulation of base excision repair to sensitize ovarian cancer cells to alkylating agent temozolomide. Clin Cancer Res. 2007;13:260–267. doi: 10.1158/1078-0432.CCR-06-1920. [DOI] [PubMed] [Google Scholar]
- Fishel ML, Seo YR, Smith ML, Kelley MR. Imbalancing the DNA base excision repair pathway in the mitochondria; targeting and overexpressing N-methylpurine DNA glycosylase in mitochondria leads to enhanced cell killing. Cancer Res. 2003;63:608–615. [PubMed] [Google Scholar]
- Gao J, Chi ZF, Liu XW, Shan PY, Wang R. Mitochondrial dysfunction and ultrastructural damage in the hippocampus of pilocarpine-induced epileptic rat. Neurosci Lett. 2007;411:152–157. doi: 10.1016/j.neulet.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Graziewicz MA, Day BJ, Copeland WC. The mitochondrial DNA polymerase as a target of oxidative damage. Nucleic Acids Res. 2002;30:2817–2824. doi: 10.1093/nar/gkf392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JF, Hollensworth SB, Spitz DR, Copeland WC, Wilson GL, LeDoux SP. Oxidative stress-induced apoptosis in neurons correlates with mitochondrial DNA base excision repair pathway imbalance. Nucleic Acids Res. 2005;33:4660–4671. doi: 10.1093/nar/gki759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JF, Rinne ML, Kelley MR, Druzhyna NM, Wilson GL, Ledoux SP. Altering DNA base excision repair: Use of nuclear and mitochondrial-targeted N-methylpurine DNA glycosylase to sensitize astroglia to chemotherapeutic agents. Glia. 2007 doi: 10.1002/glia.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann U, Buchheim K, Gabriel S, Kann O, Kovacs R, Schuchmann S. Cell death and metabolic activity during epileptiform discharges and status epilepticus in the hippocampus. Prog Brain Res. 2002;135:197–210. doi: 10.1016/S0079-6123(02)35019-2. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Dudek FE. Spontaneous motor seizures of rats with kainate-induced epilepsy: effect of time of day and activity state. Epilepsy Res. 1999;35:47–57. doi: 10.1016/s0920-1211(98)00127-2. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Hollensworth SB, Shen C, Sim JE, Spitz DR, Wilson GL, LeDoux SP. Glial cell type-specific responses to menadione-induced oxidative stress. Free Radic Biol Med. 2000;28:1161–1174. doi: 10.1016/s0891-5849(00)00214-8. [DOI] [PubMed] [Google Scholar]
- Horvath R, Hudson G, Ferrari G, Futterer N, Ahola S, Lamantea E, Prokisch H, Lochmuller H, McFarland R, Ramesh V, Klopstock T, Freisinger P, Salvi F, Mayr JA, Santer R, Tesarova M, Zeman J, Udd B, Taylor RW, Turnbull D, Hanna M, Fialho D, Suomalainen A, Zeviani M, Chinnery PF. Phenotypic spectrum associated with mutations of the mitochondrial polymerase gamma gene. Brain. 2006;129:1674–1684. doi: 10.1093/brain/awl088. [DOI] [PubMed] [Google Scholar]
- Hudson G, Chinnery PF. Mitochondrial DNA polymerase-gamma and human disease. Hum Mol Genet. 2006;15(Spec No 2):R244–252. doi: 10.1093/hmg/ddl233. [DOI] [PubMed] [Google Scholar]
- Jarrett SG, Boulton ME. Antioxidant up-regulation and increased nuclear DNA protection play key roles in adaptation to oxidative stress in epithelial cells. Free Radic Biol Med. 2005;38:1382–1391. doi: 10.1016/j.freeradbiomed.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Kann O, Kovacs R. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 2007;292:C641–657. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- Kann O, Kovacs R, Njunting M, Behrens CJ, Otahal J, Lehmann TN, Gabriel S, Heinemann U. Metabolic dysfunction during neuronal activation in the ex vivo hippocampus from chronic epileptic rats and humans. Brain. 2005;128:2396–2407. doi: 10.1093/brain/awh568. [DOI] [PubMed] [Google Scholar]
- Kemeleva EA, Sinitsyna OI, Kolosova NG, Vasyunina EA, Zharkov DO, Conlon KA, Berrios M, Nevinsky GA. Immunofluorescent detection of 8-oxoguanine DNA lesions in liver cells from aging OXYS rats, a strain prone to overproduction of free radicals. Mutat Res. 2006;599:88–97. doi: 10.1016/j.mrfmmm.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Kisby GE, Milne J, Sweatt C. Evidence of reduced DNA repair in amyotrophic lateral sclerosis brain tissue. Neuroreport. 1997;8:1337–1340. doi: 10.1097/00001756-199704140-00004. [DOI] [PubMed] [Google Scholar]
- Kovacs R, Schuchmann S, Gabriel S, Kann O, Kardos J, Heinemann U. Free radical-mediated cell damage after experimental status epilepticus in hippocampal slice cultures. J Neurophysiol. 2002;88:2909–2918. doi: 10.1152/jn.00149.2002. [DOI] [PubMed] [Google Scholar]
- Kudin AP, Kudina TA, Seyfried J, Vielhaber S, Beck H, Elger CE, Kunz WS. Seizure-dependent modulation of mitochondrial oxidative phosphorylation in rat hippocampus. Eur J Neurosci. 2002;15:1105–1114. doi: 10.1046/j.1460-9568.2002.01947.x. [DOI] [PubMed] [Google Scholar]
- Kunz WS. The role of mitochondria in epileptogenesis. Curr Opin Neurol. 2002;15:179–184. doi: 10.1097/00019052-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Kunz WS, Goussakov IV, Beck H, Elger CE. Altered mitochondrial oxidative phosphorylation in hippocampal slices of kainate-treated rats. Brain Res. 1999;826:236–242. doi: 10.1016/s0006-8993(99)01279-2. [DOI] [PubMed] [Google Scholar]
- Kunz WS, Kudin AP, Vielhaber S, Blumcke I, Zuschratter W, Schramm J, Beck H, Elger CE. Mitochondrial complex I deficiency in the epileptic focus of patients with temporal lobe epilepsy. Ann Neurol. 2000;48:766–773. [PubMed] [Google Scholar]
- Lan J, Henshall DC, Simon RP, Chen J. Formation of the base modification 8-hydroxyl-2′-deoxyguanosine and DNA fragmentation following seizures induced by systemic kainic acid in the rat. J Neurochem. 2000;74:302–309. doi: 10.1046/j.1471-4159.2000.0740302.x. [DOI] [PubMed] [Google Scholar]
- LeDoux SP, Driggers WJ, Hollensworth BS, Wilson GL. Repair of alkylation and oxidative damage in mitochondrial DNA. Mutat Res. 1999;434:149–159. doi: 10.1016/s0921-8777(99)00026-9. [DOI] [PubMed] [Google Scholar]
- Li W, Luo Y, Zhang F, Signore AP, Gobbel GT, Simon RP, Chen J. Ischemic preconditioning in the rat brain enhances the repair of endogenous oxidative DNA damage by activating the base-excision repair pathway. J Cereb Blood Flow Metab. 2006;26:181–198. doi: 10.1038/sj.jcbfm.9600180. [DOI] [PubMed] [Google Scholar]
- Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003;76:397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- Liang LP, Beaudoin ME, Fritz MJ, Fulton R, Patel M. Kainate-induced seizures, oxidative stress and neuronal loss in aging rats. Neuroscience. 2007;147:1114–1118. doi: 10.1016/j.neuroscience.2007.03.026. [DOI] [PubMed] [Google Scholar]
- Liang LP, Ho YS, Patel M. Mitochondrial superoxide production in kainate-induced hippocampal damage. Neuroscience. 2000;101:563–570. doi: 10.1016/s0306-4522(00)00397-3. [DOI] [PubMed] [Google Scholar]
- Liang LP, Patel M. Mitochondrial oxidative stress and increased seizure susceptibility in Sod2(−/+) mice. Free Radic Biol Med. 2004;36:542–554. doi: 10.1016/j.freeradbiomed.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Liang LP, Patel M. Seizure-induced changes in mitochondrial redox status. Free Radic Biol Med. 2006;40:316–322. doi: 10.1016/j.freeradbiomed.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Markesbery WR. Decreased base excision repair and increased helicase activity in Alzheimer’s disease brain. Brain Res. 2000;855:116–123. doi: 10.1016/s0006-8993(99)02335-5. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y. Regulation of intracellular localization of human MTH1, OGG1, and MYH proteins for repair of oxidative DNA damage. Prog Nucleic Acid Res Mol Biol. 2001;68:75–94. doi: 10.1016/s0079-6603(01)68091-7. [DOI] [PubMed] [Google Scholar]
- Neema M, Navarro-Quiroga I, Chechlacz M, Gilliams-Francis K, Liu J, Lamonica K, Lin SL, Naegele JR. DNA damage and nonhomologous end joining in excitotoxicity: neuroprotective role of DNA-PKcs in kainic acid-induced seizures. Hippocampus. 2005;15:1057–1071. doi: 10.1002/hipo.20123. [DOI] [PubMed] [Google Scholar]
- Nguyen KV, Sharief FS, Chan SS, Copeland WC, Naviaux RK. Molecular diagnosis of Alpers syndrome. J Hepatol. 2006;45:108–116. doi: 10.1016/j.jhep.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Patel M. Mitochondrial dysfunction and oxidative stress: cause and consequence of epileptic seizures. Free Radic Biol Med. 2004;37:1951–1962. doi: 10.1016/j.freeradbiomed.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Patel M, Liang LP, Hou H, Williams BB, Kmiec M, Swartz HM, Fessel JP, Roberts LJ., 2nd Seizure-induced formation of isofurans: novel products of lipid peroxidation whose formation is positively modulated by oxygen tension. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2007.04974.x. [DOI] [PubMed] [Google Scholar]
- Patel M, Liang LP, Roberts LJ., 2nd Enhanced hippocampal F2-isoprostane formation following kainate-induced seizures. J Neurochem. 2001;79:1065–1069. doi: 10.1046/j.1471-4159.2001.00659.x. [DOI] [PubMed] [Google Scholar]
- Penkowa M, Florit S, Giralt M, Quintana A, Molinero A, Carrasco J, Hidalgo J. Metallothionein reduces central nervous system inflammation, neurodegeneration, and cell death following kainic acid-induced epileptic seizures. J Neurosci Res. 2005;79:522–534. doi: 10.1002/jnr.20387. [DOI] [PubMed] [Google Scholar]
- Quach N, Chan T, Lu TA, Schreiber SS, Tan Z. Induction of DNA repair proteins, Ref-1 and XRCC1, in adult rat brain following kainic acid-induced seizures. Brain Res. 2005;1042:236–240. doi: 10.1016/j.brainres.2005.02.053. [DOI] [PubMed] [Google Scholar]
- Sleven H, Gibbs JE, Heales S, Thom M, Cock HR. Depletion of reduced glutathione precedes inactivation of mitochondrial enzymes following limbic status epilepticus in the rat hippocampus. Neurochem Int. 2006a;48:75–82. doi: 10.1016/j.neuint.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Sleven HJ, Gibbs JE, Cock HR. The antioxidant N-acetyl-L-cysteine does not prevent hippocampal glutathione loss or mitochondrial dysfunction associated with status epilepticus. Epilepsy Res. 2006b;69:165–169. doi: 10.1016/j.eplepsyres.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Stuart JA, Hashiguchi K, Wilson DM, 3rd, Copeland WC, Souza-Pinto NC, Bohr VA. DNA base excision repair activities and pathway function in mitochondrial and cellular lysates from cells lacking mitochondrial DNA. Nucleic Acids Res. 2004;32:2181–2192. doi: 10.1093/nar/gkh533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart JA, Mayard S, Hashiguchi K, Souza-Pinto NC, Bohr VA. Localization of mitochondrial DNA base excision repair to an inner membrane-associated particulate fraction. Nucleic Acids Res. 2005;33:3722–3732. doi: 10.1093/nar/gki683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC, Lott MT, Shoffner JM, Ballinger S. Mitochondrial DNA mutations in epilepsy and neurological disease. Epilepsia. 1994;35(Suppl 1):S43–50. doi: 10.1111/j.1528-1157.1994.tb05928.x. [DOI] [PubMed] [Google Scholar]
- Wallace DC, Lott MT, Shoffner JM, Brown MD. Diseases resulting from mitochondrial DNA point mutations. J Inherit Metab Dis. 1992;15:472–479. doi: 10.1007/BF01799605. [DOI] [PubMed] [Google Scholar]
- Weissman L, de Souza-Pinto NC, Stevnsner T, Bohr VA. DNA repair, mitochondria, and neurodegeneration. Neuroscience. 2007;145:1318–1329. doi: 10.1016/j.neuroscience.2006.08.061. [DOI] [PubMed] [Google Scholar]
- Wilson DM, 3rd, Bohr VA. The mechanics of base excision repair, and its relationship to aging and disease. DNA Repair (Amst) 2007;6:544–559. doi: 10.1016/j.dnarep.2006.10.017. [DOI] [PubMed] [Google Scholar]