Abstract
Chronic pain is the most difficult type of pain to treat. Previously, the development of analgesics has focused on neuronal targets; however, current analgesics are only modestly effective, have significant side effects and do not provide universal efficacy. New strategies are needed for the development of more effective analgesics. Glial cells have integral roles in CNS homeostasis, and chronic pain etiology and progression. In this review, the role of glia in neuropathic pain and opioid administration is described, as well as the potential superior efficacy and wider therapeutic indices provided by drugs that modulate specific glial function via novel targets.
Keywords: Astrocyte, cannabinoid, microglia, minocycline, neuropathic pain, propentofylline
Introduction
An estimated 100 million individuals in the US suffer from chronic pain [1]. Chronic pain is the most difficult pain to treat, especially neuropathic pain, which follows nerve injury. Chronic pain is debilitating and can cause extreme physical, psychological and social disruption for patients. Although opioid agonists may be modestly effective in the treatment of neuropathic pain, tolerance and physical dependence significantly limit their use. Additionally, newer agents, such as calcium channel α2-δ ligands, tricyclic antidepressants and selective serotonin/norepinephrine reuptake inhibitors, have significant side effects and do not provide universal efficacy [2].
Thus, there is a need for the development of new agents for the treatment and prevention of chronic pain that utilize novel mechanisms, display enhanced effectiveness and possess decreased side-effect profiles. Previous drug development for pain has been predominately focused on altering neuronal function. It is increasingly recognized that glial cells play a major role in maintaining CNS homeostasis, and in disease etiology and progression [3]. Accordingly, attention has shifted to the discovery of glial-modulating agents for the treatment of a range of neurodegenerative diseases, as well as chronic pain. Potential drug targets to modulate glial-neuronal function are shown in Figure 1. This review highlights how glia enhance pain states following nerve injury or opioid administration, and how drugs that modulate specific glial function (including migration, proliferation and neurotransmitter expression) via novel targets may provide superior efficacy and wider therapeutic indices.
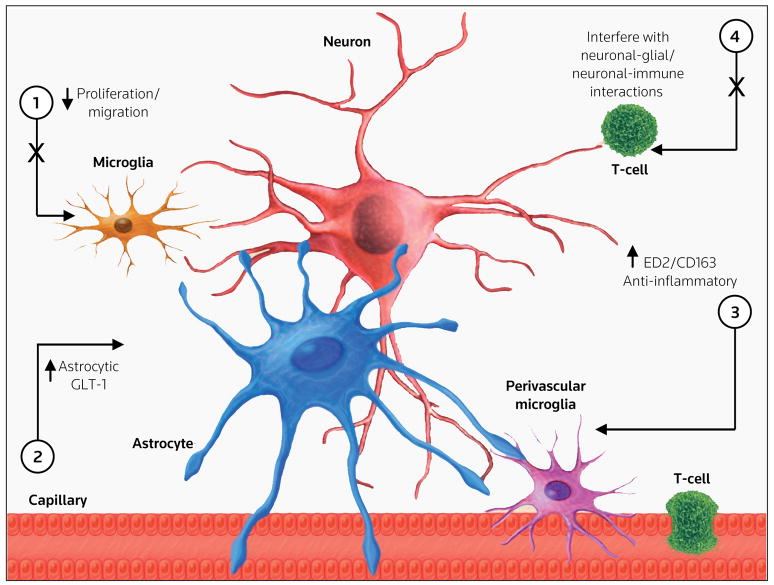
Figure 1. Potential drug targets to modulate glial-neuronal function.
The major cellular contributors of chronic pain states and their interface with the microvasculature, including neurons, microglia, astrocytes, perivascular microglia and T-leukocytes are illustrated. Potential drug targets to modulate glial-neuronal function include: (1) inhibiting microglial proliferation and migration, (2) enhancing astrocytic GLT-1 expression, (3) enhancing perivascular ED2/CD163 expression, and (4) interfering with specific neuronal-glial and/or neuronal-immune interactions.
Glial cells and pain
Glial cells, including parenchymal (resident) microglia, perivascular microglia, astrocytes and oligodendrocytes, constitute > 70% of the total cell population in the brain and spinal cord. Although once considered merely a physical support system for neurons, glial cells have been identified as key neuromodulatory, neurotrophic and neuroimmune elements in the CNS. Microglia (cells of monocytic origin) are the macrophages of the brain, and perform a vast number of immune-related roles [4••]. Microglia are the first cell types to respond to several forms of CNS injury [5••,6••]. The initial signal that triggers microglial reactivity is not fully understood; however, neuronal depolarization and extracellular ion changes following nerve injury may be major stimuli [7]. Alternatively, neuronal signals, such as nitric oxide (NO) or proinflammatory cytokines, may induce this reactivity [8,9]. It has been proposed that TLR4 and CD14 may constitute the ‘receptor complex’ for these activating signals from neurons [10,11•,12].
During autoimmune inflammation of the nervous system, microglia release and respond to several cytokines, including IL-1, IL-6, TNFα and IFNγ, which are instrumental in astrocytic activation, induction of cellular adhesion molecule expression and recruitment of T-leukocytes into the lesion [10,11•,12]. In addition to synthesizing inflammatory cytokines, microglia may also act as cytotoxic effector cells by releasing harmful substances, including proteases, reactive oxygen intermediates and NO. Perivascular microglia are CNS antigen-presenting cells [4••] and immune modulators that possess surveillance functions [13]. These cells play an important role in the communication of the CNS with the peripheral immune system, especially in pathological conditions [14] such as neuropathic pain [15•]. Perivascular microglia shed or express the surface marker ED2/CD163 [16,17] and secrete anti-inflammatory proteins [18]. The production of these classes of molecules is associated with the resolution of inflammation [19]. Of relevance to chronic pain states, it has been reported that spinal ED2/CD163 expression is reduced following peripheral nerve injury [20•]. Perivascular and parenchymal microglial cells are thought to have an important role in antigen recognition and presentation in the CNS, but their different location and surface markers (parenchymal microglia express CR23/CD11b and Iba1, and perivascular microglia express ED2/CD163) may confer different functions. Perivascular microglial cells are integral to the integrity of the blood-brain barrier and are continually replaced by bone-marrow derived cells under normal conditions; this turnover is accelerated in inflammatory processes [4••,21]. Conversely, parenchymal microglia represent a stable cell pool, which in adult animals is only exceptionally replaced by hematogenous cells, even after recovery from severe brain inflammation. In addition, they surveil, migrate and proliferate in a dynamic and plastic manner [4••,21]. It has been demonstrated that parenchymal reactive microglia (surrounding encephalitis lesions) may express ED2/CD163 in chronically simian immunodeficiency virus-infected macaques with encephalitis [22].
Astrocytes may have a primary role in nociceptive processing, and in the thermal and mechanical hyperalgesia produced by peripheral nerve injury [23]. Astrocytic changes in response to injury include proliferation, hypertrophy and overexpression of glial fibrillary acidic protein (GFAP). Spinal GFAP increases following chronic nerve constriction [24], nerve crush and axotomy [25,26]. There has been considerable focus on the mechanisms of the initiation versus maintenance phases of persistent pain states and it has been proposed that distinct glial populations may have an integral role. Using real time RT-PCR, it was demonstrated that peripheral nerve injury induces an early microglial activation, as assessed by ITGAM (integrin,αM[complement, complement 3 receptor 3 subunit]), TLR4 and CD14, followed by a delayed but sustained mRNA GFAP expression [12]. Interestingly, in a more recent study, increases in spinal CR3/CD11b protein, a microglial marker, were observed at days 28 and 42 after nerve injury in the absence of transcript changes [27]. Therefore, the concept that microglia are only involved in initiating chronic pain is oversimplified and requires re-evaluation. Superficial reliance on limited activation markers may not relate to glial functioning and their ability to interact with nociceptive neurons. These findings suggest that the therapeutic window for glial-modulating agents may be broader than originally speculated.
Similarly, it has been proposed that astrocytic expression of MAPK plays a key role in maintaining neuropathic pain [28]. Additionally, Kawasaki et al observed a rapid and transient increase in matrix metalloprotease (MMP)-9 in dorsal root ganglia sensory neurons and a delayed increase of MMP-2 in satellite cells and spinal astrocytes after spinal nerve ligation [29]. These studies underscore the value of investigating functional glial properties versus simply observing morphological reactivity.
Opioids – The current gold standard
Opioids are among the most potent analgesic agents available for clinical use and are the gold standard for treating acute, post-surgical and cancer pain; however, their use in neuropathic pain is often limited by the development of analgesic tolerance and unwanted side effects that are unmasked during the resulting dose escalation. Much of the early research on combating opioid tolerance focused on determining potential neuronal mechanisms of tolerance formation and designing opioid analogs with improved tolerance profiles. Beitner-Johnston et al demonstrated that naltrexone, a μ-opioid receptor antagonist, limited the development of tolerance to opioids [30]. This finding led to the synthesis of new μ-opioid receptor agonists, mixed κ-receptor agonist and μ-receptor antagonists, and partial μ-receptor agonists, which have all failed to improve upon morphine and thus have only limited value in the treatment of pain. Opioid receptor desensitization involves the NMDA receptor cascade. Preclinical [31,32] and preliminary clinical studies [33,34] suggest that the blockade of NMDA reduces opioid-induced tolerance; however, large-scale clinical trials using NMDA antagonists in conjunction with opioids to limit tolerance formation have produced disappointing results [35].
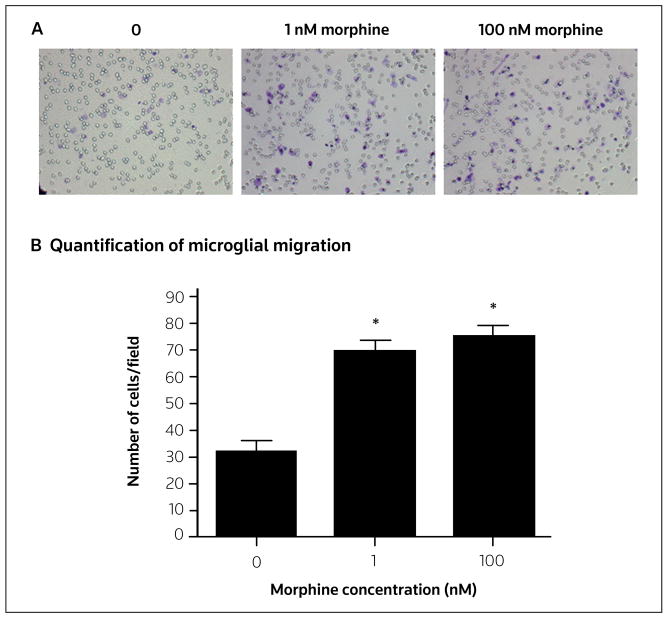
Song and Zhao have identified a casual link between glial activation and morphine tolerance [36•]. In accordance with these findings, it has been demonstrated that spinal CR3/CD11b, GFAP (an astrocyte marker) and expression of the cytokines IL-1α, IL-6 and TNFα increase following chronic morphine administration [37•]. Also, chronic subcutaneous and intrathecal morphine administration induces analgesic tolerance within 6 and 3 days, respectively [38]. The burgeoning body of research implicating glia in opioid tolerance led to the investigation of whether selectively targeting glial cells can provide a potential method for attenuating opioid tolerance. Both inhibiting chronic opioid-induced glial reactivity using propentofylline [39] and inhibiting proinflammatory cytokines [40] attenuate morphine-induced tolerance. Minocycline, which inhibits microglial migration [41], attenuates the development of anti-nociceptive tolerance to chronic morphine through inhibition of p38 MAPK in activated microglia [42]. These studies indicate that microglial migration may have a critical role in morphine tolerance, as has been demonstrated in neuropathic pain states [43,44•]. It was discovered that morphine enhances microglial Iba-1 expression [RJ Horvath, unpublished data] and migration [45] toward ADP in a μ-opioid receptor-dependent manner (Figure 2). It was proposed that chronic opioid administration induces microglial reactivity and migration toward the dorsal horn, which leads to increased proinflammatory/algesic factor production and neuronal sensitization. Microglial migration might thus prove to be an attractive pharmacological target to inhibit the induction of opioid tolerance.
Figure 2. Migration of morphine-treated primary neonatal rat cortical microglia toward ATP.
Primary neonatal rat cortical microglia were harvested, incubated with morphine (0, 1 or 100 nM) for 2 h, then allowed to migrate toward ADP (10 μM) for 2 h. (A) Images of microglia that had migrated through a porous membrane and were then stained with crystal violet. (B) Microglial migration was quantified by counting ten random fields at 40x magnification for each membrane (n = 3 for all treatments). Error bars represent the standard error of the mean. *p < 0.05.
Cannabinoids and neuroimmune interactions
The cannabinoid system regulates and modulates both neuronal and immune functions using at least two protein-coupled cannabinoid receptors (CBRs), CBR1 and CBR2. CBR1s are expressed in the brain, spinal cord and peripheral nerves, and are responsible for the psychotropic effects of cannabinoids [46–50]. Neuronal CBR1s are synthesized in cells of the dorsal root ganglia and are inserted by axonal transport onto terminals in the periphery [51]. Additionally, CBR1s are also expressed in microglial cells and may act as immune modulators in the CNS [52–54]. CBR2 activation produces a host of peripheral immune effects, including regulation of cell migration, inhibition of cell proliferation, reduction of cytokine production, downregulation of surface marker expression and impairment of cell functions [55–57]. CBR2s are expressed peripherally in immune cells [58] and keratinocytes [59]. CBR2s also exist in the CNS, mainly in microglia and perivascular microglia cells, in healthy human and rat brains [60]. CBR2s regulate microglial migration [61] and proliferation [62]. CBR1 expression is enhanced in the spinal dorsal horn after peripheral nerve injury, an effect that is at least in part mediated by ERK [48]. The acute spinal CBR1 activation induces anti-nociception, but also causes neurological side effects [20•,63]. In addition, chronic spinal CBR1 activation induces anti-nociceptive tolerance and hypersensitivity [64]. Interestingly, inflammation, nerve injury and other neuronal insults induce increased CBR2 expression in microglia, perivascular microglia and astrocytes [20•,65–68]. CBR2 is expressed in microglia and perivascular microglia (Figure 3), but not in neurons or astrocytes, 4 days after peripheral nerve injury [20•,69]. These findings are interesting because at this time point, microglial cells have an integral role in the development of pain following peripheral nerve injury [70,71]. Furthermore, it has been demonstrated for the first time that perivascular microglial cells seem to be instrumental in the maintenance of pain following L5 nerve transection [20•]. Thus, this cell type provides a potential target for modulating the spinal immune response in neuropathic pain conditions. Others have failed to demonstrate CBR2 expression in spinal microglial cells and have observed CBR2 expression mainly in neurons 14 days after peripheral nerve injury [72]. The discrepancy between these results may be due to non-specific antibodies, peripheral nerve injury models or the time after surgery (4 versus 14 days, respectively) used in both studies. It is possible that CBR2 is expressed in different cells at different time points in neuropathic pain conditions; this differential expression has been observed with ERK following peripheral nerve injury [28].
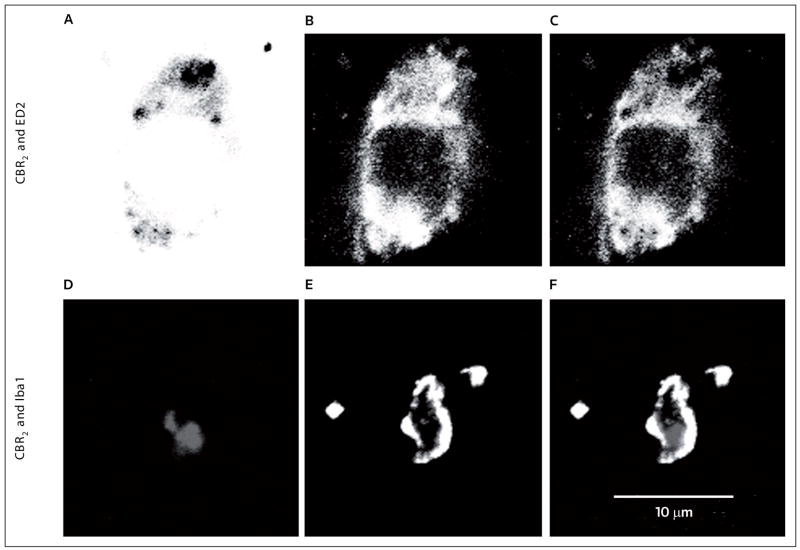
Figure 3. The expression of CBR2 in perivascular and microglia following peripheral nerve injury.
CBR2s are expressed in perivascular microglia (ED2/CD163) and microglia (Iba-1) of dorsal horn L5 spinal cord ipsilateral to L5 nerve transection (4 days after surgery). The top row shows representative confocal images of (A) CBR2 (black), (B) ED2/CD163 (white) and (C) co-regionalization of both markers in perivascular microglia. The bottom row shows representative confocal images of (D) CBR2 (gray), (E) Iba-1 (white) and (F) co-regionalization of both markers in microglia. All images were converted to grayscale and the colors in image ‘A’ were inverted to facilitate the appreciation of the double staining.
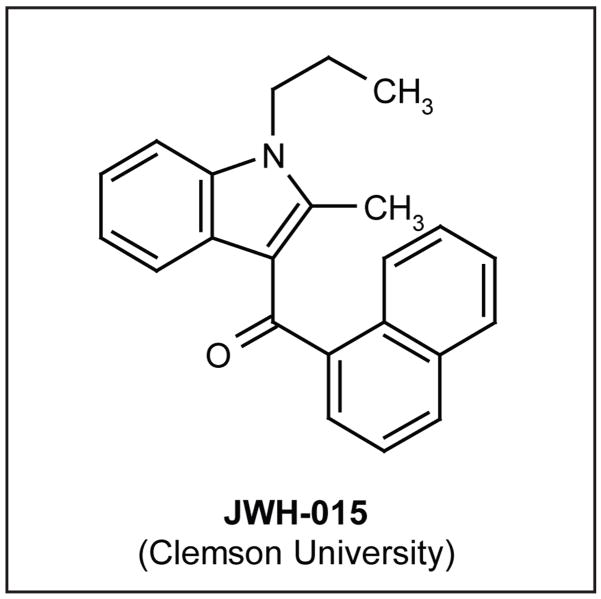
CBR2 activation induces anti-nociception [63,72,73]. Spinal CBR2 activation using the selective CBR2 agonist JWH-015 (Clemson University; Figure 4) induces anti-nociception while producing a concomitant reduction of spinal Iba-1, and CD11b/CR3 and GFAP expression in postoperative and neuropathic pain models. JWH-015 also increases the anti-inflammatory factor ED2/CD163 in spinal perivascular microglia following peripheral nerve injury [20•]. In vitro, JWH-015 inhibits primary microglial migration [EA Romero-Sandoval, unpublished data] and TNFα production [74]. The ability of CBR2 agonists to reduce macrophage migration [75] and the release of microglial proinflammatory factors [76•] relies on the modulation of ERK1/2.
Figure 4.
The structure of JWH-015.
Additionally, CBR2 mediates inhibition of the immune response (including a reduction of microglial reactivity) by reducing the number and regulating the function of infiltrating T-leukocytes into the brain of rodent models of multiple sclerosis [57,62,77,78•]. This CBR2 action is significant because T-leukocyte and macrophage trafficking into the CNS following peripheral nerve injury is instrumental for the maintenance of microglial activation and pain [10,15•]. CBR2 provides an attractive target that may pharmacologically act to induce a spinal microglial anti-inflammatory phenotype, reduce microglial migration to damaged neurons, and possibly modulate the function and number of infiltrating T-cells in pain conditions.
Novel glial-modulating drugs and targets
Currently, the total number of specific glial modulators is limited, but includes a broad spectrum of chemical structures and mechanisms, including: (i) fluorocitrate, a non-selective metabolic inhibitor [23]; (ii) minocycline, a tetracycline derivative with selective in vitro microglial inhibition [71]; (iii) ibudilast, a non-selective PDE inhibitor [79,80]; (iv) methionine sulfoximine (MSO), an astrocytic glutamine synthetase inhibitor [81]; and (v) propentofylline, a methylxanthine [82,83]. It is important to consider that some of the CNS glial-modulating properties of these agents may be downstream from their primary mechanism of action.
It was first demonstrated in 1991 by Garrison et al that lumbar spinal GFAP increases following a sciatic nerve constriction injury [24]. The role of spinal astrocytes in nociceptive processing and hyperalgesia was further demonstrated using fluorocitrate in the chronic constriction injury rodent model [23]; however, due to the potential neurotoxicity of fluorocitrate, its use as a selective glial inhibitor is not favored. More recently, a more selective astrocytic metabolic inhibitor, intrathecal MSO was used to demonstrate that an astroglial glutamate-glutamine shuttle induces central sensitization of nociceptive neurons following peripheral inflammation [81].
Minocycline, a semi-synthetic second-generation tetracycline, is an antibiotic that possesses superior penetration through the blood-brain barrier and into the CNS [84]. Minocycline is a potent inhibitor of microglial reactivity and has no direct action on astrocytes or neurons in vitro [85–88]. The anti-inflammatory profile of minocycline is distinct from its antimicrobial action, and minocycline provides effective neuroprotection in experimental brain ischemia [89], in a mouse model of Huntington’s disease [90], in traumatic brain injury [91], and following 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration [92].
In a rat model of neuropathic pain induced by L5 spinal nerve transaction, systemic administration of minocycline: (i) reversed the development of mechanical allodynia and hyperalgesia, but did not produce an effect on existing behavioral hypersensitivity; (ii) prevented increased spinal microglial CR23/CD11b and GFAP expression in the pre-emptive treatment, but inhibited only microglial reactivity when treatment was started on day 5 after nerve injury; and (iii) inhibited the production of proinflammatory cytokines, such as IL-1β, IL-6 and TNFα, in the L5 lumbar spinal cord in association with the reversal of hyperalgesia and allodynia [71]. In a separate in vitro study, minocycline significantly reduced microglial migration to cellular debris, astrocyte-conditioned medium, ADP and algesic mediators, and significantly reduced the expression of CD29 (β1 integrin), but not CD18 (β2 integrin) [41]. Minocycline also reduces the effect of extracellular potassium and later decreases microglial Kv1.3 expression [41]. These results indicate a novel effect of minocycline: it inhibits microglial motility by reducing β1 integrin expression and Kv1.3 channel activity and, later, expression. Reducing microglial trafficking to injured neurons following nerve injury decreases the release of proinflammatory mediators into the synaptic milieu and prevents neuronal sensitization, the pathological counterpart to chronic pain.
Ibudilast is a non-selective PDE inhibitor that decreases CNS CR3/CD11b and GFAP expression following nerve injury or morphine administration. Based on these findings, it has been postulated that the primary mechanism of action of ibudilast is glial modulation, not PDE inhibition as previously thought. The efficacy of ibudilast in rat models of neuropathy has thus far been inconclusive because both transient and sustained effects on behavioral hypersensitivity have been observed [80]; however, ibudilast enhances morphine analgesia while decreasing morphine tolerance and morphine withdrawal symptoms, and thus may have clinical utility as an opioid adjuvant to decrease dose escalation and adverse opioid side effects.
Finally, the contribution of glia to nerve injury-induced behavioral hypersensitivity has been further probed using propentofylline, an atypical methylxanthine, which previously decreased GFAP expression in a rodent model of ischemia [93]. Systemic or intrathecal administration of propentofylline attenuates spinal GFAP, CR23/CD11b, IL-1β, IL-6 and TNFα expression in vivo [27,83,94]. In addition to being neuroprotective and anti-inflammatory, propentofylline reduces glial reactivity, proliferation and migration [27,39,71,81,95•,96,97], [JA DeLeo, unpublished data]. The specific mechanism of its anti-nociceptive effects remains unknown; however, several actions have been proposed. Propentofylline inhibits adenosine transport, as well as the cAMP-specific PDE4 [98–100]. Propentofylline increases cAMP levels, suppresses an activated astrocytic phenotype, enhances a specific glutamate transporter, GLT-1, at the mRNA and protein levels, and increases glutamate uptake in vivo and in vitro [101,102]. Interestingly, other methylxanthines that inhibit PDE have demonstrated limited efficacy in attenuating existing nerve injury-induced behavioral hypersensitivity [103], [JA DeLeo, unpublished data]. Therefore, the role of PDEs may be limited in driving glial activation and as targets for chronic pain agents.
Conclusions
Within minutes to hours following a peripheral nerve injury, spinal microglia may become reactive by using TLR4/CD14 as a receptor complex to sense changes in the glial/neuronal milieu. This signaling leads to the release of cytokines and growth factors from microglia, and promotes microglial migration and proliferation, which in turn alerts astrocytes to a perturbed synaptic milieu. Astrocytes then produce and release specific sensitizing chemokines, such as monocyte chemoattractant protein, which enable T-leukocytes and/or macrophage migration into the parenchyma of the CNS. This cell migration propagates the immune response and further induces microglial and astrocytic immunocompetence (Figure 1). There are several novel drug targets involved in glial function and the immune response, including: (i) inhibition of glial proliferation and migration, (ii) modulation of perivascular ED2/CD163 and astrocytic glutamate transporter GLT-1, and (iii) interference of proinflammatory interactions between populations of immune and glial cells.
Acknowledgments
The authors would like to acknowledge Matthew S Alkaitis for his excellent editorial assistance, Robert Lee for his work on Figure 1, and NIH/NIDA DA11276.
References
•• of outstanding interest
• of special interest
- 1.Research America: An Alliance for Discoveries in Health: Investment in research saves lives and money: Facts about. Pain. 2008;10:1 . http://www.researchamerica.org/
- 2.Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, Bushnell MC, Farrar JT, Galer BS, Haythornthwaite JA, Hewitt DJ, et al. Advances in neuropathic pain: Diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60(11):1524–1534. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 3.Scholz J, Woolf CJ. The neuropathic pain triad: Neurons, immune cells and glia. Nat Neurosci. 2007;10(11):1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 4.••.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239(4837):290–292. doi: 10.1126/science.3276004. Rat bone marrow chimeras and encephalitogenic MHC-restricted T-helper lymphocytes were used to demonstrate that perivascular microglial cells are bone-marrow derived and fully competent to present antigen to lymphocytes in an appropriately restricted manner. These findings are important for bone marrow transplantation and for neuroimmunological diseases. [DOI] [PubMed] [Google Scholar]
- 5.••.Hanisch UK, Kettenmann H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. A comprehensive review that focuses on several key observations that illustrate the multifaceted activities of microglia in the normal and pathological brain, and explains why engagement of microglia can be either neuroprotective or neurotoxic resulting in containment or aggravation of disease progression. [DOI] [PubMed] [Google Scholar]
- 6.••.Kreutzberg GW. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. The transformation of microglia into potentially cytotoxic cells is under strict control and occurs mainly in response to neuronal or terminal degeneration. [DOI] [PubMed] [Google Scholar]
- 7.Caggiano AO, Breder CD, Kraig RP. Long-term elevation of cyclooxygenase-2, but not lipoxygenase, in regions synaptically distant from spreading depression. J Comp Neurol. 1996;376(3):447–462. doi: 10.1002/(SICI)1096-9861(19961216)376:3<447::AID-CNE7>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauser CJ. Regional macrophage activation after injury and the compartmentalization of inflammation in trauma. New Horiz. 1996;4(2):235–251. [PubMed] [Google Scholar]
- 9.Merrill JE, Benveniste EN. Cytokines in inflammatory brain lesions: Helpful and harmful. Trends Neurosci. 1996;19(8):331–338. doi: 10.1016/0166-2236(96)10047-3. [DOI] [PubMed] [Google Scholar]
- 10.Cao L, Deleo JA. CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur J Immunol. 2008;38(2):448–458. doi: 10.1002/eji.200737485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.•.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA. 2005;102(16):5856–5861. doi: 10.1073/pnas.0501634102. For the first time, the critical role for CNS innate immunity by means of microglial TLR4 is demonstrated in the induction phase of behavioral hypersensitivity in neuropathic pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int. 2004;45(2–3):397–407. doi: 10.1016/j.neuint.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: Implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193(8):905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer J, Huitinga I, Zhao W, Lassmann H, Hickey WF, Dijkstra CD. The role of macrophages, perivascular cells, and microglial cells in the pathogenesis of experimental autoimmune encephalomyelitis. Glia. 1995;15(4):437–446. doi: 10.1002/glia.440150407. [DOI] [PubMed] [Google Scholar]
- 15.•.Sweitzer SM, Hickey WF, Rutkowski MD, Pahl JL, DeLeo JA. Focal peripheral nerve injury induces leukocyte trafficking into the central nervous system: Potential relationship to neuropathic pain. Pain. 2002;100(1–2):163–170. doi: 10.1016/s0304-3959(02)00257-9. This paper describes the trafficking of leukocytes into the lumbar spinal cord at time points that correlate with mechanical allodynia, suggesting a role for central neuroinflammation in persistent neuropathic pain. [DOI] [PubMed] [Google Scholar]
- 16.Droste A, Sorg C, Högger P. Shedding of CD163, a novel regulatory mechanism for a member of the scavenger receptor cysteine-rich family. Biochem Biophys Res Commun. 1999;256(1):110–113. doi: 10.1006/bbrc.1999.0294. [DOI] [PubMed] [Google Scholar]
- 17.Graeber MB, Streit WJ, Kreutzberg GW. Identity of ED2-positive perivascular cells in rat brain. J Neurosci Res. 1989;22(1):103–106. doi: 10.1002/jnr.490220114. [DOI] [PubMed] [Google Scholar]
- 18.Zwadlo-Klarwasser G, Vogts M, Hamann W, Belke K, Baron J, Schmutzler W. Generation and subcellular distribution of histamine in human blood monocytes and monocyte subsets. Inflamm Res. 1998;47(11):434–439. doi: 10.1007/s000110050357. [DOI] [PubMed] [Google Scholar]
- 19.Zwadlo G, Voegeli R, Osthoff KS, Sorg C. A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the down-regulatory phase of the inflammatory process. Exp Cell Biol. 1987;55(6):295–304. doi: 10.1159/000163432. [DOI] [PubMed] [Google Scholar]
- 20.•.Romero-Sandoval A, Nutile-McMenemy N, DeLeo JA. Spinal microglial and perivascular cell cannabinoid receptor type 2 activation reduces behavioral hypersensitivity without tolerance after peripheral nerve injury. Anesthesiology. 2008;108(4):722–734. doi: 10.1097/ALN.0b013e318167af74. CBR2 proteins are observed for the first time in spinal microglia and perivascular cells, and their selective activation induces analgesia and reduces microglial reactivity in neuropathic pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lassmann H, Schmied M, Vass K, Hickey WF. Bone marrow derived elements and resident microglia in brain inflammation. Glia. 1993;7(1):19–24. doi: 10.1002/glia.440070106. [DOI] [PubMed] [Google Scholar]
- 22.Borda JT, Alvarez X, Mohan M, Hasegawa A, Bernardino A, Jean S, Aye P, Lackner AA. CD163, a marker of perivascular macrophages, is up-regulated by microglia in simian immunodeficiency virus encephalitis after haptoglobin-hemoglobin complex stimulation and is suggestive of breakdown of the blood-brain barrier. Am J Pathol. 2008;172(3):725–737. doi: 10.2353/ajpath.2008.070848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meller ST, Dykstra C, Grzybycki D, Murphy S, Gebhart GF. The possible role of glia in nociceptive processing and hyperalgesia in the spinal cord of the rat. Neuropharmacology. 1994;33(11):1471–1478. doi: 10.1016/0028-3908(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 24.Garrison CJ, Dougherty PM, Kajander KC, Carlton SM. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain Res. 1991;565(1):1–7. doi: 10.1016/0006-8993(91)91729-k. [DOI] [PubMed] [Google Scholar]
- 25.Hajós F, Csillik B, Knyihár-Csillik E. Alterations in glial fibrillary acidic protein immunoreactivity in the upper dorsal horn of the rat spinal cord in the course of transganglionic degenerative atrophy and regenerative proliferation. Neurosci Lett. 1990;117(1–2):8–13. doi: 10.1016/0304-3940(90)90111-l. [DOI] [PubMed] [Google Scholar]
- 26.Tetzlaff W, Graeber MB, Bisby MA, Kreutzberg GW. Increased glial fibrillary acidic protein synthesis in astrocytes during retrograde reaction of the rat facial nucleus. Glia. 1988;1(1):90–95. doi: 10.1002/glia.440010110. [DOI] [PubMed] [Google Scholar]
- 27.Tawfik VL, Nutile-McMenemy N, Lacroix-Fralish ML, DeLeo JA. Efficacy of propentofylline, a glial modulating agent, on existing mechanical allodynia following peripheral nerve injury. Brain Behav Immun. 2007;21(2):238–246. doi: 10.1016/j.bbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114(1–2):149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 29.Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, Ji RR. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14(3):331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beitner-Johnson D, Guitart X, Nestler EJ. Glial fibrillary acidic protein and the mesolimbic dopamine system: Regulation by chronic morphine and Lewis-Fischer strain differences in the rat ventral tegmental area. J Neurochem. 1993;61(5):1766–1773. doi: 10.1111/j.1471-4159.1993.tb09814.x. [DOI] [PubMed] [Google Scholar]
- 31.Mao J, Price DD, Caruso FS, Mayer DJ. Oral administration of dextromethorphan prevents the development of morphine tolerance and dependence in rats. Pain. 1996;67(2–3):361–368. doi: 10.1016/0304-3959(96)03120-x. [DOI] [PubMed] [Google Scholar]
- 32.Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: A current view of their possible interactions. Pain. 1995;62(3):259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- 33.Caruso FS. MorphiDex pharmacokinetic studies and single-dose analgesic efficacy studies in patients with postoperative pain. J Pain Symptom Manage. 2000;19(1 Suppl):S31–S36. doi: 10.1016/s0885-3924(99)00128-1. [DOI] [PubMed] [Google Scholar]
- 34.Katz NP. MorphiDex (MS:DM) double-blind, multiple-dose studies in chronic pain patients. J Pain Symptom Manage. 2000;19(1 Suppl):S37–S41. doi: 10.1016/s0885-3924(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 35.Galer BS, Lee D, Ma T, Nagle B, Schlagheck TG. MorphiDex (morphine sulfate/dextromethorphan hydrobromide combination) in the treatment of chronic pain: Three multicenter, randomized, double-blind, controlled clinical trials fail to demonstrate enhanced opioid analgesia or reduction in tolerance. Pain. 2005;115(3):284–295. doi: 10.1016/j.pain.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 36.•.Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39(3):281–286. doi: 10.1016/s0168-0102(00)00226-1. This paper provides the first evidence for the role of glial cells in the development of morphine tolerance in vivo. The researchers injected morphine for 9 consecutive days, which led to a significant increase in GFAP immunostaining in the spinal cord, posterior cingulate cortex and hippocampus. Glial inhibition by fluorocitrate attenuated the spinal tolerance to morphine analgesia. The increase in spinal GFAP immunostaining was also blocked. [DOI] [PubMed] [Google Scholar]
- 37.•.Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002;22(22):9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. Spinal inhibition of proinflammatory cytokines restored acute morphine anti-nociception in neuropathic pain, and also significantly reversed the development of morphine tolerance and withdrawal-induced hyperalgesia and allodynia in neuropathic pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tawfik VL, LaCroix-Fralish ML, Nutile-McMenemy N, DeLeo JA. Transcriptional and translational regulation of glial activation by morphine in a rodent model of neuropathic pain. J Pharmacol Exp Ther. 2005;313(3):1239–1247. doi: 10.1124/jpet.104.082420. [DOI] [PubMed] [Google Scholar]
- 39.Raghavendra V, Tanga FY, DeLeo JA. Attenuation of morphine tolerance, withdrawal-induced hyperalgesia, and associated spinal inflammatory immune responses by propentofylline in rats. Neuropsychopharmacology. 2004;29(2):327–334. doi: 10.1038/sj.npp.1300315. [DOI] [PubMed] [Google Scholar]
- 40.Raghavendra V, DeLeo JA. Cytokine modulation of opioid action. In: Schmidt RF, Willis WD, editors. Encyclopedia of Pain. Springer-Verlag Berlin and Heidelberg GmbH & Co; New York, NY, USA: 2006. pp. 227–235. [Google Scholar]
- 41.Nutile-McMenemy N, Elfenbein A, DeLeo JA. Minocycline decreases in vitro microglial motility, β1-integrin, and Kv1.3 channel expression. J Neurochem. 2007;103(5):2035–2046. doi: 10.1111/j.1471-4159.2007.04889.x. [DOI] [PubMed] [Google Scholar]
- 42.Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, Chen PX, Feng JQ. A novel role of minocycline: Attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun. 2008;22(1):114–123. doi: 10.1016/j.bbi.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 43.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: A big problem from molecules in “small” glia. Trends Neurosci. 2005;28(2):101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 44.•.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424(6950):778–783. doi: 10.1038/nature01786. P2X4 receptors, a subtype of the ionotropic ATP receptor, are increased in the spinal cord microglia (but not in neurons or astrocytes) in neuropathic pain and their blockade reversed tactile allodynia. [DOI] [PubMed] [Google Scholar]
- 45.Horvath RJ, Tawfik VL, LaCroix-Fralish ML, Nutile-McMenemy N, DeLeo JA. Morphine induced activation of spinal microglial cells: Implications for neuroimmune activation and tolerance development in neuropathic pain. Abstr Soc Neurosci. 2007;37 Abs 287.12. [Google Scholar]
- 46.Bridges D, Rice AS, Egertová M, Elphick MR, Winter J, Michael GJ. Localisation of cannabinoid receptor 1 in rat dorsal root ganglion using in situ hybridisation and immunohistochemistry. Neuroscience. 2003;119(3):803–812. doi: 10.1016/s0306-4522(03)00200-8. [DOI] [PubMed] [Google Scholar]
- 47.Farquhar-Smith WP, Egertová M, Bradbury EJ, McMahon SB, Rice AS, Elphick MR. Cannabinoid CB1 receptor expression in rat spinal cord. Mol Cell Neurosci. 2000;15(6):510–521. doi: 10.1006/mcne.2000.0844. [DOI] [PubMed] [Google Scholar]
- 48.Lim G, Sung B, Ji RR, Mao J. Upregulation of spinal cannabinoid-1-receptors following nerve injury enhances the effects of Win 55,212-2 on neuropathic pain behaviors in rats. Pain. 2003;105(1–2):275–283. doi: 10.1016/s0304-3959(03)00242-2. [DOI] [PubMed] [Google Scholar]
- 49.Pettit DA, Harrison MP, Olson JM, Spencer RF, Cabral GA. Immunohistochemical localization of the neural cannabinoid receptor in rat brain. J Neurosci Res. 1998;51(3):391–402. doi: 10.1002/(SICI)1097-4547(19980201)51:3<391::AID-JNR12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 50.Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83(2):393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 51.Hohmann AG, Herkenham M. Cannabinoid receptors undergo axonal flow in sensory nerves. Neuroscience. 1999;92(4):1171–1175. doi: 10.1016/s0306-4522(99)00220-1. [DOI] [PubMed] [Google Scholar]
- 52.Cabral GA, Harmon KN, Carlisle SJ. Cannabinoid-mediated inhibition of inducible nitric oxide production by rat microglial cells: Evidence for CB1 receptor participation. Adv Exp Med Biol. 2001;493:207–214. doi: 10.1007/0-306-47611-8_24. [DOI] [PubMed] [Google Scholar]
- 53.Sinha D, Bonner TI, Bhat NR, Matsuda LA. Expression of the CB1 cannabinoid receptor in macrophage-like cells from brain tissue: Immunochemical characterization by fusion protein antibodies. J Neuroimmunol. 1998;82(1):13–21. doi: 10.1016/S0165-5728(97)00181-1. [DOI] [PubMed] [Google Scholar]
- 54.Waksman Y, Olson JM, Carlisle SJ, Cabral GA. The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. J Pharmacol Exp Ther. 1999;288(3):1357–1366. [PubMed] [Google Scholar]
- 55.Miller AM, Stella N. CB2 receptor-mediated migration of immune cells: It can go either way. Br J Pharmacol. 2008;153(2):299–308. doi: 10.1038/sj.bjp.0707523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cabral GA, Raborn ES, Griffin L, Dennis J, Marciano-Cabral F. CB2 receptors in the brain: Role in central immune function. Br J Pharmacol. 2008;153(2):240–251. doi: 10.1038/sj.bjp.0707584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dittel BN. Direct suppression of autoreactive lymphocytes in the central nervous system via the CB2 receptor. Br J Pharmacol. 2008;153(2):271–276. doi: 10.1038/sj.bjp.0707493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, Friedman H. The cannabinoid system and immune modulation. J Leukoc Biol. 2003;74(4):486–496. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- 59.Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, Malan TP., Jr CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci USA. 2005;102(8):3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310(5746):329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 61.Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, Stella N. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23(4):1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carrier EJ, Kearn CS, Barkmeier AJ, Breese NM, Yang W, Nithipatikom K, Pfister SL, Campbell WB, Hillard CJ. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol Pharmacol. 2004;65(4):999–1007. doi: 10.1124/mol.65.4.999. [DOI] [PubMed] [Google Scholar]
- 63.Romero-Sandoval A, Eisenach JC. Spinal cannabinoid receptor type 2 activation reduces hypersensitivity and spinal cord glial activation after paw incision. Anesthesiology. 2007;106(4):787–794. doi: 10.1097/01.anes.0000264765.33673.6c. [DOI] [PubMed] [Google Scholar]
- 64.Gardell LR, Burgess SE, Dogrul A, Ossipov MH, Malan TP, Lai J, Porreca F. Pronociceptive effects of spinal dynorphin promote cannabinoid-induced pain and antinociceptive tolerance. Pain. 2002;98(1–2):79–88. doi: 10.1016/s0304-3959(01)00475-4. [DOI] [PubMed] [Google Scholar]
- 65.Ashton JC, Friberg D, Darlington CL, Smith PF. Expression of the cannabinoid CB2 receptor in the rat cerebellum: An immunohistochemical study. Neurosci Lett. 2006;396(2):113–116. doi: 10.1016/j.neulet.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 66.Ashton JC, Rahman RM, Nair SM, Sutherland BA, Glass M, Appleton I. Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the brain. Neurosci Lett. 2007;412(2):114–117. doi: 10.1016/j.neulet.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 67.Núñez E, Benito C, Pazos MR, Barbachano A, Fajardo O, González S, Tolón RM, Romero J. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: An immunohistochemical study. Synapse. 2004;53(4):208–213. doi: 10.1002/syn.20050. [DOI] [PubMed] [Google Scholar]
- 68.Sheng WS, Hu S, Min X, Cabral GA, Lokensgard JR, Peterson PK. Synthetic cannabinoid WIN55,212-2 inhibits generation of inflammatory mediators by IL-1β-stimulated human astrocytes. Glia. 2005;49(2):211–219. doi: 10.1002/glia.20108. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O’Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 2003;17(12):2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- 70.Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115(1–2):71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 71.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306(2):624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 72.Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, Reggiani A. CB2 receptor-mediated antihyperalgesia: Possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23(6):1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- 73.Scott DA, Wright CE, Angus JA. Evidence that CB-1 and CB-2 cannabinoid receptors mediate antinociception in neuropathic pain in the rat. Pain. 2004;109(1–2):124–131. doi: 10.1016/j.pain.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 74.Romero-Sandoval EA, Nutile-McMenemy N, DeLeo JA. Microglia cannabinoid receptor type 2 activation reduces cytokine and nitric oxide production by modulating mitogen-activated protein kinase-phosphatase (MKP) and extracellular signal-regulated kinase (ERK) Neuropharmacology Conference, Cannabinoid Signaling in the Nervous System, San Diego, CA, USA. 2007;17 Abs NEUR2007 53. [Google Scholar]
- 75.Montecucco F, Burger F, Mach F, Steffens S. The CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am J Physiol Heart Circ Physiol. 2008;294(3):H1145–H1155. doi: 10.1152/ajpheart.01328.2007. [DOI] [PubMed] [Google Scholar]
- 76.•.Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, Hoertnagl H, Raine CS, Schneider-Stock R, Nitsch R, Ullrich O. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49(1):67–79. doi: 10.1016/j.neuron.2005.11.027. CBR2 activation through the ERK pathway, as a new mechanism of neuroimmune communication following CNS injury, is reported. This response controls and limits the immune response after primary CNS damage. [DOI] [PubMed] [Google Scholar]
- 77.Arevalo-Martin A, Vela JM, Molina-Holgado E, Borrell J, Guaza C. Therapeutic action of cannabinoids in a murine model of multiple sclerosis. J Neurosci. 2003;23(7):2511–2516. doi: 10.1523/JNEUROSCI.23-07-02511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.•.Maresz K, Pryce G, Ponomarev ED, Marsicano G, Croxford JL, Shriver LP, Ledent C, Cheng X, Carrier EJ, Mann MK, Giovannoni G, et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med. 2007;13(4):492–497. doi: 10.1038/nm1561. This research demonstrated that the cannabinoid system within the CNS plays a critical role in regulating autoimmune inflammation, with the CNS directly suppressing T-cell effector function via the CBR2. [DOI] [PubMed] [Google Scholar]
- 79.Ledeboer A, Hutchinson MR, Watkins LR, Johnson KW. Ibudilast (AV-411). A new class therapeutic candidate for neuropathic pain and opioid withdrawal syndromes. Expert Opin Investig Drugs. 2007;16(7):935–950. doi: 10.1517/13543784.16.7.935. [DOI] [PubMed] [Google Scholar]
- 80.Ledeboer A, Liu T, Shumilla JA, Mahoney JH, Vijay S, Gross MI, Vargas JA, Sultzbaugh L, Claypool MD, Sanftner LM, Watkins LR, et al. The glial modulatory drug AV411 attenuates mechanical allodynia in rat models of neuropathic pain. Neuron Glia Biol. 2007;2(4):279–291. doi: 10.1017/S1740925X0700035X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chiang CY, Wang J, Xie YF, Zhang S, Hu JW, Dostrovsky JO, Sessle BJ. Astroglial glutamate-glutamine shuttle is involved in central sensitization of nociceptive neurons in rat medullary dorsal horn. J Neurosci. 2007;27(34):9068–9076. doi: 10.1523/JNEUROSCI.2260-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DeLeo J, Schubert P, Kreutzberg GW. Propentofylline (HWA 285) protects hippocampal neurons of Mongolian gerbils against ischemic damage in the presence of an adenosine antagonist. Neurosci Lett. 1988;84(3):307–311. doi: 10.1016/0304-3940(88)90526-5. [DOI] [PubMed] [Google Scholar]
- 83.Sweitzer SM, Schubert P, DeLeo JA. Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. J Pharmacol Exp Ther. 2001;297(3):1210–1217. [PubMed] [Google Scholar]
- 84.Aronson AL. Pharmacotherapeutics of the newer tetracyclines. J Am Vet Med Assoc. 1980;176(10 Spec):1061–1068. [PubMed] [Google Scholar]
- 85.Amin AR, Attur MG, Thakker GD, Patel PD, Vyas PR, Patel RN, Patel IR, Abramson SB. A novel mechanism of action of tetracyclines: Effects on nitric oxide synthases. Proc Natl Acad Sci USA. 1996;93(24):14014–14019. doi: 10.1073/pnas.93.24.14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21(8):2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tikka T, Usenius T, Tenhunen M, Keinanen R, Koistinaho J. Tetracycline derivatives and ceftriaxone, a cephalosporin antibiotic, protect neurons against apoptosis induced by ionizing radiation. J Neurochem. 2001;78(6):1409–1414. doi: 10.1046/j.1471-4159.2001.00543.x. [DOI] [PubMed] [Google Scholar]
- 88.Tikka TM, Koistinaho JE. Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J Immunol. 2001;166(12):7527–7533. doi: 10.4049/jimmunol.166.12.7527. [DOI] [PubMed] [Google Scholar]
- 89.Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA. 1998;95(26):15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med. 2000;6(7):797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 91.Sanchez Mejia RO, Ona VO, Li M, Friedlander RM. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001;48(6):1393–1399. doi: 10.1097/00006123-200106000-00051. [DOI] [PubMed] [Google Scholar]
- 92.Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22(5):1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.DeLeo J, Toth L, Schubert P, Rudolphi K, Kreutzberg GW. Ischemia-induced neuronal cell death, calcium accumulation, and glial response in the hippocampus of the Mongolian gerbil and protection by propentofylline (HWA 285) J Cereb Blood Flow Metab. 1987;7(6):745–751. doi: 10.1038/jcbfm.1987.129. [DOI] [PubMed] [Google Scholar]
- 94.Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA. Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: Mechanistic implications of spinal glia and proinflammatory cytokines. Pain. 2003;104(3):655–664. doi: 10.1016/S0304-3959(03)00138-6. [DOI] [PubMed] [Google Scholar]
- 95.•.Schubert P, Ogata T, Rudolphi K, Marchini C, McRae A, Ferroni S. Support of homeostatic glial cell signaling: A novel therapeutic approach by propentofylline. Ann NY Acad Sci. 1997;826:337–347. doi: 10.1111/j.1749-6632.1997.tb48484.x. This paper describes how propentofylline can modulate microglial cells by restoring homeostatic functions. This is a unique action different from glial inhibition. [DOI] [PubMed] [Google Scholar]
- 96.Si Q, Nakamura Y, Ogata T, Kataoka K, Schubert P. Differential regulation of microglial activation by propentofylline via cAMP signaling. Brain Res. 1998;812(1–2):97–104. doi: 10.1016/s0006-8993(98)00954-8. [DOI] [PubMed] [Google Scholar]
- 97.Si QS, Nakamura Y, Schubert P, Rudolphi K, Kataoka K. Adenosine and propentofylline inhibit the proliferation of cultured microglial cells. Exp Neurol. 1996;137(2):345–349. doi: 10.1006/exnr.1996.0035. [DOI] [PubMed] [Google Scholar]
- 98.Meskini N, Némoz G, Okyayuz-Baklouti I, Lagarde M, Prigent AF. Phosphodiesterase inhibitory profile of some related xanthine derivatives pharmacologically active on the peripheral microcirculation. Biochem Pharmacol. 1994;47(5):781–788. doi: 10.1016/0006-2952(94)90477-4. [DOI] [PubMed] [Google Scholar]
- 99.Nagata K, Ogawa T, Omosu M, Fujimoto K, Hayashi S. In vitro and in vivo inhibitory effects of propentofylline on cyclic AMP phosphodiesterase activity. Arzneimittelforschung. 1985;35(7):1034–1036. [PubMed] [Google Scholar]
- 100.Parkinson FE, Fredholm BB. Effects of propentofylline on adenosine A1 and A2 receptors and nitrobenzylthioinosine-sensitive nucleoside transporters: Quantitative autoradiographic analysis. Eur J Pharmacol. 1991;202(3):361–366. doi: 10.1016/0014-2999(91)90279-y. [DOI] [PubMed] [Google Scholar]
- 101.Tawfik VL, Lacroix-Fralish ML, Bercury KK, Nutile-McMenemy N, Harris BT, DeLeo JA. Induction of astrocyte differentiation by propentofylline increases glutamate transporter expression in vitro: Heterogeneity of the quiescent phenotype. Glia. 2006;54(3):193–203. doi: 10.1002/glia.20365. [DOI] [PubMed] [Google Scholar]
- 102.Tawfik VL, Regan M, Haenggeli C, LaCroix-Fralish ML, Nutile-McMenemy N, Perez N, Rothstein JD, DeLeo JA. Propentofylline-induced astrocyte modulation leads to alterations in glial glutamate promoter activation following spinal nerve transection. Neuroscience. 2008;152(4):1086–1092. doi: 10.1016/j.neuroscience.2008.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu J, Li W, Zhu J, Zhang J, Feng X, Guan R, Xu J. The effect of pentoxifylline on existing hypersensitivity in a rat model of neuropathy. Anesth Analg. 2008;106(2):650–653. doi: 10.1213/ane.0b013e31815efaba. [DOI] [PubMed] [Google Scholar]