Abstract
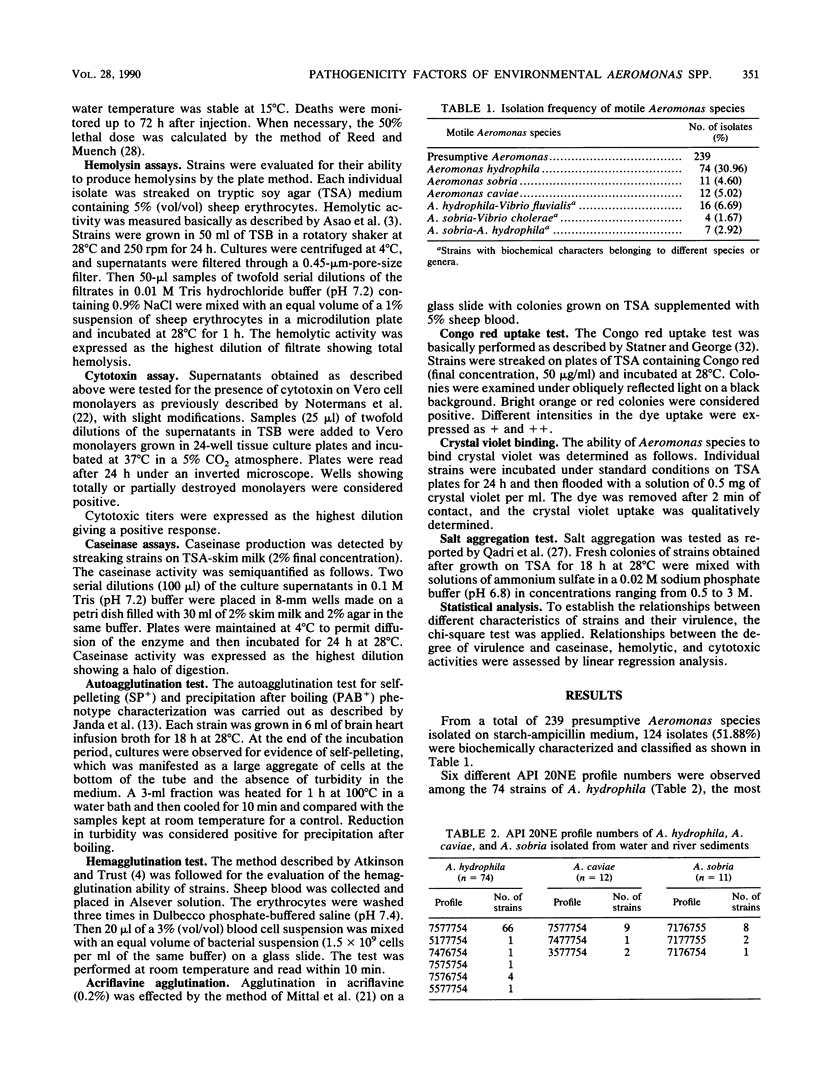
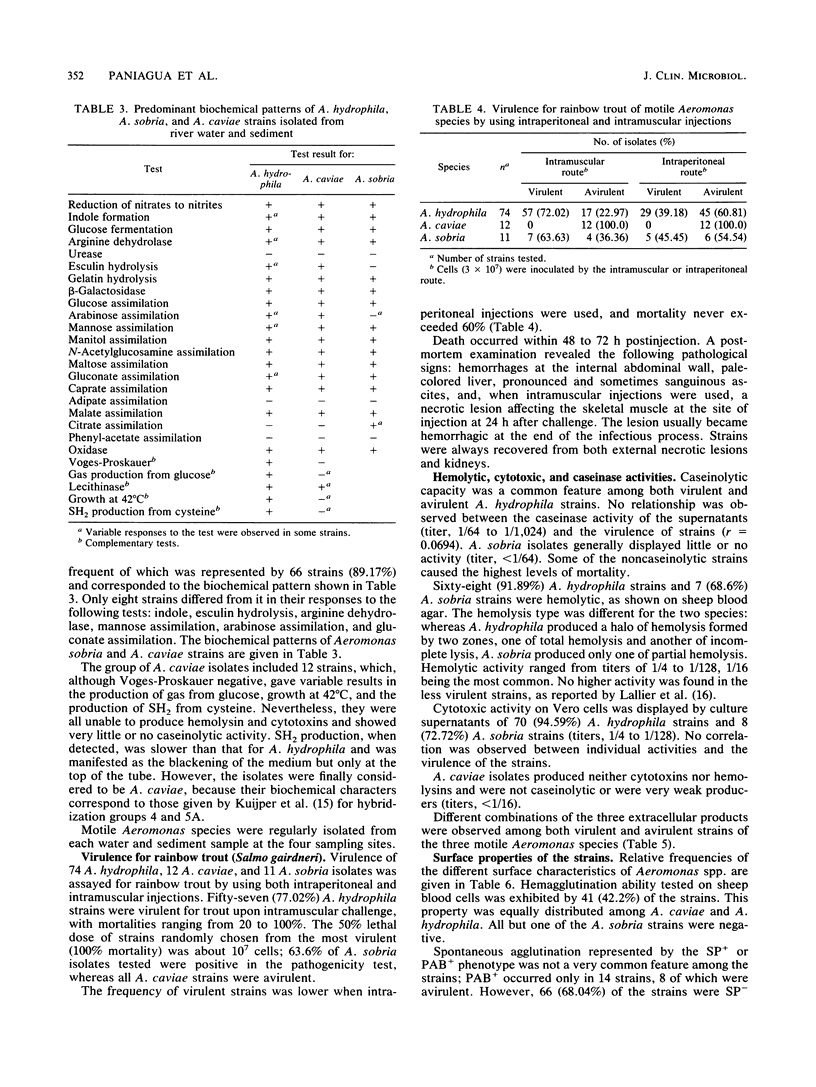
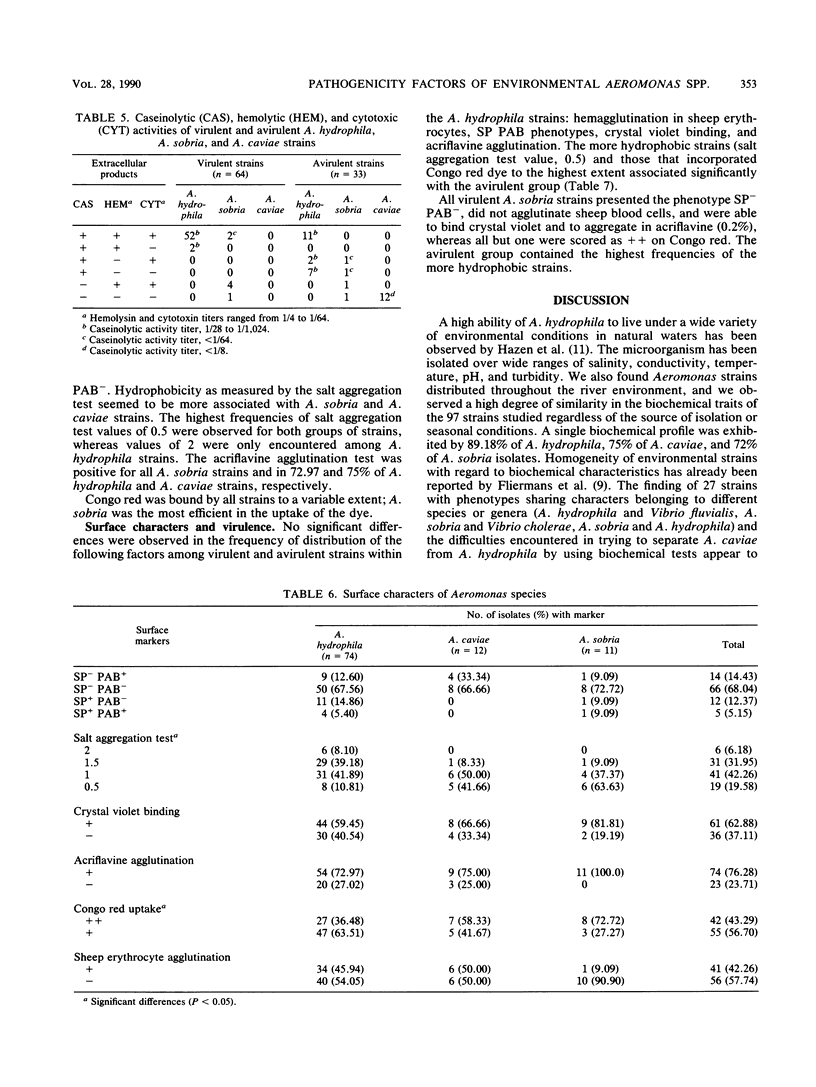
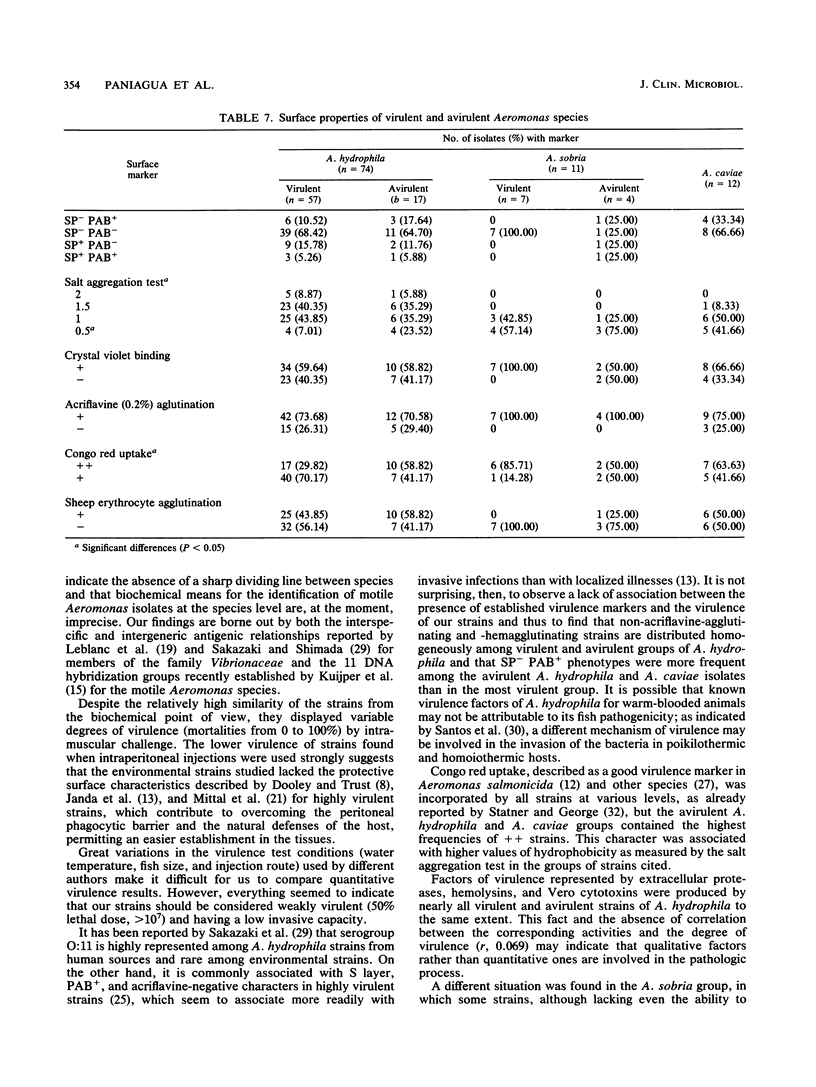
Ninety-seven motile Aeromonas strains were isolated over a period of a year from samples of water and sediment collected at different sites along a river. Strains were regularly recovered from all samples, regardless of the source of isolation or seasonal conditions. Isolates were biochemically characterized by the API 20NE system (Analytab Products, Plainview, N.Y.) and classified as Aeromonas hydrophila (74 strains), Aeromonas sobria (11 strains), and Aeromonas caviae (12 strains). Despite the high level of homogeneity observed in their biochemical patterns, they displayed different degrees of virulence for fish; 72.02% of A. hydrophila isolates and 63% of A. sobria isolates were virulent for fish by intramuscular challenge, but lower frequencies of virulence were observed when intraperitoneal injections were used. All A. caviae strains proved to be avirulent. Caseinases, hemolysins, and Vero cytotoxins were produced by 100, 91, and 94.59%, respectively, of A. hydrophila strains and with lower frequencies and lower caseinase activities by A. sobria isolates. No correlation was found between these activities and the degree of virulence of the strains for fish. Most hydrophobic strains seem to be concentrated in A. caviae, A. sobria, and avirulent A. hydrophila groups. Known virulence markers commonly associated with virulent strains (acriflavine negative and self-pelleting negative and precipitation after boiling positive phenotypes) had a low representation in the total of strains studied and were not associated with virulence.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan B. J., Stevenson R. M. Extracellular virulence factors of Aeromonas hydrophila in fish infections. Can J Microbiol. 1981 Oct;27(10):1114–1122. doi: 10.1139/m81-174. [DOI] [PubMed] [Google Scholar]
- Amy P. S., Morita R. Y. Starvation-survival patterns of sixteen freshly isolated open-ocean bacteria. Appl Environ Microbiol. 1983 Mar;45(3):1109–1115. doi: 10.1128/aem.45.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asao T., Kozaki S., Kato K., Kinoshita Y., Otsu K., Uemura T., Sakaguchi G. Purification and characterization of an Aeromonas hydrophila hemolysin. J Clin Microbiol. 1986 Aug;24(2):228–232. doi: 10.1128/jcm.24.2.228-232.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson H. M., Trust T. J. Hemagglutination properties and adherence ability of Aeromonas hydrophila. Infect Immun. 1980 Mar;27(3):938–946. doi: 10.1128/iai.27.3.938-946.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke V., Robinson J., Gracey M., Peterson D., Partridge K. Isolation of Aeromonas hydrophila from a metropolitan water supply: seasonal correlation with clinical isolates. Appl Environ Microbiol. 1984 Aug;48(2):361–366. doi: 10.1128/aem.48.2.361-366.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily O. P., Joseph S. W., Coolbaugh J. C., Walker R. I., Merrell B. R., Rollins D. M., Seidler R. J., Colwell R. R., Lissner C. R. Association of Aeromonas sobria with human infection. J Clin Microbiol. 1981 Apr;13(4):769–777. doi: 10.1128/jcm.13.4.769-777.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley J. S., Trust T. J. Surface protein composition of Aeromonas hydrophila strains virulent for fish: identification of a surface array protein. J Bacteriol. 1988 Feb;170(2):499–506. doi: 10.1128/jb.170.2.499-506.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliermans C. B., Gorden R. W., Hazen T. C., Esch G. W. Aeromonas distribution and survival in a thermally altered lake. Appl Environ Microbiol. 1977 Jan;33(1):114–122. doi: 10.1128/aem.33.1.114-122.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen T. C., Esch G. W. Effect of effluent from a nitrogen fertilizer factory and a pulp mill on the distribution and abundance of Aeromonas hydrophila in Albemarle Sound, North Carolina. Appl Environ Microbiol. 1983 Jan;45(1):31–42. doi: 10.1128/aem.45.1.31-42.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen T. C., Fliermans C. B., Hirsch R. P., Esch G. W. Prevalence and distribution of Aeromonas hydrophila in the United States. Appl Environ Microbiol. 1978 Nov;36(5):731–738. doi: 10.1128/aem.36.5.731-738.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro E. E., Ainsworth T., Trust T. J., Kay W. W. Congo red agar, a differential medium for Aeromonas salmonicida, detects the presence of the cell surface protein array involved in virulence. J Bacteriol. 1985 Dec;164(3):1233–1237. doi: 10.1128/jb.164.3.1233-1237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda J. M., Oshiro L. S., Abbott S. L., Duffey P. S. Virulence markers of mesophilic aeromonads: association of the autoagglutination phenomenon with mouse pathogenicity and the presence of a peripheral cell-associated layer. Infect Immun. 1987 Dec;55(12):3070–3077. doi: 10.1128/iai.55.12.3070-3077.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J., Seidler R. J., Lockman H., Colwell R. R. Medium for the presumptive identification of Aeromonas hydrophila and Enterobacteriaceae. Appl Environ Microbiol. 1979 Nov;38(5):1023–1026. doi: 10.1128/aem.38.5.1023-1026.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijper E. J., Steigerwalt A. G., Schoenmakers B. S., Peeters M. F., Zanen H. C., Brenner D. J. Phenotypic characterization and DNA relatedness in human fecal isolates of Aeromonas spp. J Clin Microbiol. 1989 Jan;27(1):132–138. doi: 10.1128/jcm.27.1.132-138.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallier R., Bernard F., Lalonde G. Difference in the extracellular products of two strains of Aeromonas hydrophila virulent and weakly virulent for fish. Can J Microbiol. 1984 Jul;30(7):900–904. doi: 10.1139/m84-141. [DOI] [PubMed] [Google Scholar]
- Larsen J. L., Jensen N. J. An Aeromonas species implicated in ulcer-disease of the cod (Gadus morhua). Nord Vet Med. 1977 Apr-May;29(4-5):199–211. [PubMed] [Google Scholar]
- Leblanc D., Mittal K. R., Olivier G., Lallier R. Serogrouping of motile Aeromonas species isolated from healthy and moribund fish. Appl Environ Microbiol. 1981 Jul;42(1):56–60. doi: 10.1128/aem.42.1.56-60.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungh A., Wadström T. Aeromonas toxins. Pharmacol Ther. 1981;15(3):339–354. doi: 10.1016/0163-7258(81)90049-8. [DOI] [PubMed] [Google Scholar]
- Mittal K. R., Lalonde G., Leblanc D., Olivier G., Lallier R. Aeromonas hydrophila in rainbow trout: relation between virulence and surface characteristics. Can J Microbiol. 1980 Dec;26(12):1501–1503. doi: 10.1139/m80-248. [DOI] [PubMed] [Google Scholar]
- Notermans S., Havelaar A., Jansen W., Kozaki S., Guinée P. Production of "Asao toxin" by Aeromonas strains isolated from feces and drinking water. J Clin Microbiol. 1986 Jun;23(6):1140–1142. doi: 10.1128/jcm.23.6.1140-1142.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier G., Lallier R., Larivière S. A toxigenic profile of Aeromonas hydrophila and Aeromonas sobria isolated from fish. Can J Microbiol. 1981 Mar;27(3):330–333. doi: 10.1139/m81-050. [DOI] [PubMed] [Google Scholar]
- Palumbo S. A., Maxino F., Williams A. C., Buchanan R. L., Thayer D. W. Starch-Ampicillin Agar for the Quantitative Detection of Aeromonas hydrophila. Appl Environ Microbiol. 1985 Oct;50(4):1027–1030. doi: 10.1128/aem.50.4.1027-1030.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula S. J., Duffey P. S., Abbott S. L., Kokka R. P., Oshiro L. S., Janda J. M., Shimada T., Sakazaki R. Surface properties of autoagglutinating mesophilic aeromonads. Infect Immun. 1988 Oct;56(10):2658–2665. doi: 10.1128/iai.56.10.2658-2665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri F., Hossain S. A., Ciznár I., Haider K., Ljungh A., Wadstrom T., Sack D. A. Congo red binding and salt aggregation as indicators of virulence in Shigella species. J Clin Microbiol. 1988 Jul;26(7):1343–1348. doi: 10.1128/jcm.26.7.1343-1348.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakazaki R., Shimada T. O-serogrouping scheme for mesophilic Aeromonas strains. Jpn J Med Sci Biol. 1984 Oct-Dec;37(5-6):247–255. doi: 10.7883/yoken1952.37.247. [DOI] [PubMed] [Google Scholar]
- Santos Y., Toranzo A. E., Barja J. L., Nieto T. P., Villa T. G. Virulence properties and enterotoxin production of Aeromonas strains isolated from fish. Infect Immun. 1988 Dec;56(12):3285–3293. doi: 10.1128/iai.56.12.3285-3293.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R. J., Allen D. A., Lockman H., Colwell R. R., Joseph S. W., Daily O. P. Isolation, enumeration, and characterization of Aeromonas from polluted waters encountered in diving operations. Appl Environ Microbiol. 1980 May;39(5):1010–1018. doi: 10.1128/aem.39.5.1010-1018.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statner B., George W. L. Congo red uptake by motile Aeromonas species. J Clin Microbiol. 1987 May;25(5):876–878. doi: 10.1128/jcm.25.5.876-878.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


