Abstract
It was recently found that nociceptive sensations (stinging, pricking, or burning) can be evoked by cooling or heating the skin to innocuous temperatures (e.g., 29°, 37°C). Here we show that this low-threshold thermal nociception (LTN) can be traced to sensitive ‘spots’ in the skin equivalent to classically defined warm spots and cold spots. Because earlier work had shown that LTN is inhibited by simply touching a thermode to the skin, a spatial search procedure was devised that minimized tactile stimulation by sliding small thermodes (16 mm2 and 1 mm2) set to 28° or 36°C slowly across the lubricated skin of the forearm. The procedure uncovered three types of temperature-sensitive sites (thermal, bimodal and nociceptive) that contained one or more thermal, nociceptive or (rarely) bimodal spots. Repeated testing indicated that bimodal and nociceptive sites were less stable over time than thermal sites, and that mechanical contact differentially inhibited nociceptive sensations. Intensity ratings collected over a range of temperatures showed that LTN increased monotonically on heat-sensitive sites but not on cold-sensitive sites. These results provide psychophysical evidence that stimulation from primary afferent fibers with thresholds in the range of warm fibers and cold fibers is relayed to the pain pathway. However, the labile nature of LTN implies that these low-threshold nociceptive inputs are subject to inhibitory controls. The implications of these findings for the roles of putative temperature receptors and nociceptors in innocuous thermoreception and thermal pain are discussed.
Keywords: warmth, cold, psychophysics, pain, cold receptors, warm receptors, nociceptors
Introduction
Proposed by von Frey over a century ago, the specificity theory of somesthesis grew out of the discovery that the sensitivities to touch, temperature and pain were distributed separately throughout the skin in a spot-like manner (Boring 1942;Norrsell et al. 1999). The basic tenets of the theory as it pertains to temperature sensitivity subsequently received support from a variety of electrophysiological and psychophysical findings. The discovery of putative ‘warm fibers’ and ‘cold fibers’ that had thermal thresholds in the nonpainful temperature range (Hensel and Zotterman 1951;Hensel and Boman 1960;Hensel and Iggo 1971) and punctate receptive fields (Kenshalo and Gallegos 1967;Hensel and Iggo 1971;Darian-Smith et al. 1979) provided a physiological basis for warm spots and cold spots, and evidence that stimulation of the spots evoked only sensations of warmth or cold (Dallenbach 1927;Jarvilehto 1973) gave psychophysical credence to the assumption that warm fibers and cold fibers encode only thermal sensations. Further support for the sensory specificity of temperature spots came from the phenomena of ‘paradoxical’ cold and warmth, which sometimes occur when warm spots and cold spots are stimulated by temperatures of opposite pole (Pavlicek and Jenkins 1933;Boring 1942;Jenkins and Karr 1957).
However, recent studies have shown that cooling the skin of the forearm to temperatures as mild as 28°–31°C, which are assumed to be sensed by cold fibers (Dubner et al. 1975;Hensel 1982), can evoke nociceptive sensations of stinging, pricking or burning (Green and Pope 2003;Green and Schoen 2005). A signal characteristic of these sensations is their susceptibility to inhibition by contact, e.g., by simply touching a thermode to the skin. A study of individual differences in temperature perception recently revealed a similar phenomenon for heat stimulation in which a majority of subjects reported nociceptive sensations during static contact heating of the skin to just 37°C (Green & Akirav, in press).
The absence of prior evidence that nociceptive sensations can be evoked by stimulation of warm spots or cold spots raises questions about the neural mechanisms that are responsible for what we refer to hereafter as low-threshold thermal nociception (LTN). One possibility is that LTN requires spatial summation in a class of sensitive thermal nociceptors that when stimulated individually fail to evoke sensation. However, data from the first study of cold LTN showed that increasing stimulus area from 2.56 cm2 to 10.84 cm2 led to only a small increase in intensity of nociceptive sensations (Green and Pope 2003). It seemed more likely that LTN was missed in earlier studies of temperature-sensitive spots because of its high susceptibility to inhibition by contact. The common practice of searching for spots by touching a punctate thermode to the skin would have worked against detection of LTN spots. This possibility was tested using a novel search method that minimized tactile stimulation by lubricating the skin and sliding a small thermode across the surface. Two experiments confirmed the existence of nociceptive sites (16 mm2) and spots (1.0 mm2) that are sensitive to mild warming or cooling and which are inhibited by contact.
Methods
Subjects
A total of 86 subjects (53 females and 33 males) were recruited from Yale University and its immediate environs to participate in the two experiments of the study. Seventy-four were recruited for experiment 1 and 38 for experiment 2. Twenty-six of the latter subjects had served in experiment 1. None of the subjects were aware of the hypotheses being tested and all were told that the purpose of the experiment was to test the sensitivity of the forearm to temperature. Informed consent was obtained and subjects were paid for their participation.
Equipment and Stimuli
Searches for temperature-sensitive sites in experiment 1 were conducted using a 4 mm × 4 mm (0.16 cm2) Peltier thermoelectric module (Melcor™) that was powered by a system designed and built in the John B. Pierce Laboratory electronics shop and controlled by LabView™ software. The ceramic surface of the module was covered with a machined copper plate and temperature at the skin-thermode interface was monitored by a 40-gauge copper constantan thermocouple epoxied into a .5-mm deep groove in the center of the plate. The backside of the module was bonded to a water-circulated heat sink. Tubing and wire connections to the thermode extended from the opposite end of the device and were attached to a counterbalanced pulley system that suspended the thermode just above the subject’s arm. This arrangement gave the experimenter unencumbered manual control of the thermode and allowed it to be touched to the forearm with minimal force. The thermode was set to regulate at 28°C to search for cold-sensitive sites and to 36°C to search for heat-sensitive sites. These temperatures were tightly controlled via a PID loop, and the output of the thermocouple was displayed in real time on a computer monitor that was constantly visible to the experimenter. Brief deviations from the regulated temperatures were observed on initial contact with the skin, with smaller deviations (< ±0.5°C) occurring as the thermode was drawn slowly across the skin. The same thermode system was used to measure psychophysical functions for cold- and heat-sensitive sites in experiment 2.
Sensitive spots were located within sensitive sites using an aluminum cylinder tapered at one end to a rounded, 1-mm2 stimulating area. The temperature of this device, hereafter referred to as the ‘spot stimulator’, was controlled by submerging it in a circulated constant temperature water bath set to 28°C (±0.5°) for cold-sensitive spots or 36°C (±0.5°) for heat-sensitive spots. The cylinder remained in the water bath for at least 10 min prior to use, then was quickly dried and used to search a single site before being replaced in the bath.
All testing was conducted in an environmental chamber with air temperature and relative humidity regulated at 24°C and 33%, respectively. Skin temperature on the volar forearm was measured with an infrared sensor at the beginning of each session. If skin temperature was less than 33°C a thermostatically controlled heating pad 34°C (±1.0°) was placed on the left forearm for 5 min, after which and the temperature retested and recorded. Average skin temperature at the time testing began was 33.4°C.
Experiment 1: Sensitive Sites and Sensitive Spots
Practice Procedure
Subjects who had not served previously in thermal psychophysical experiments in our laboratory participated in a separate practice session that included instructions about how to use the general version of the Labeled Magnitude Scale (gLMS; (Green et al. 1993;Bartoshuk et al. 2003) to rate sensation intensity. The gLMS is bounded at the bottom by “no sensation” and at the top by “strongest imaginable sensation of any kind”, and is displayed vertically on a computer monitor. Subjects made their ratings by using a computer mouse to move a cursor to the appropriate place on the scale. Practice was given by asking subjects to use the scale to rate the intensity of 16 commonly experienced thermal sensations (e.g. washing hands in cold tap water; briefly touching a hot light bulb).
Before data collection began all subjects were given practice feeling thermal sensations produced by the Peltier thermode and the spot thermode. The experimenter explained that both thermodes would be used to identify sites on the forearm that were sensitive to cold or heat. Subjects were allowed to slide the thermodes along their own arm with instructions to attend to variations in sensation intensity and quality. During this practice, the subjects were also familiarized with a list of thermal (cool, cold, icy, warm, or hot) and nociceptive (burning, stinging, pricking, aching, or painful) qualities and their definitions. The instructions were to describe sensations they experienced on sensitive sites and spots by choosing one or more words from the list. It was emphasized that people vary in the kinds of sensations they perceive, and that they should feel free to use as many or as few of the descriptors as necessary to describe whatever sensations they felt. The list of qualities and definitions was posted on the wall of the environmental chamber where it was visible throughout testing.
Experimental Procedure
Each testing session had three parts: (1) A search for sensitive sites on the forearm, (2) a search for and characterization of sensitive spots within identified sensitive sites, and (3) measurement of the intensity and quality of sensations produced by thermal stimulation of sensitive sites during normal or sliding contact. To maintain the subjects’ attention and concentration, testing sessions were limited to approximately 1 hr, which accommodated locating and testing up to three sites. Most subjects for whom sensitive sites could be identified served in a total of three sessions.
(1) Sensitive Site Search
Because previous studies had shown that LTN is readily inhibited by merely touching a thermode to the skin (Green and Pope 2003;Green and Schoen 2005), we sought an alternative procedure to search for thermally-sensitive sites that minimized mechanical stimulation. An initial attempt to slide the 0.16 cm2 thermode lightly across the skin produced distracting sensations of touch and failed to evoke any nociceptive sensations. A literature search for “spot-mapping” techniques revealed that Dallenbach (1927) and others had tried the same method and concluded that it gave unreliable results. However, we found that lubricating the skin with mineral oil greatly reduced tactile sensations produced by sliding the thermode, and that after this treatment nociceptive as well as thermal sensations were readily detectable. Although it is probable that lubricating the skin did not eliminate all tactile inhibition of LTN, the ability to identify nociceptive sites that were undetectable by normal contact (see below) confirmed the utility of the sliding procedure as a search and stimulation method.
The subject sat at a table with his or her left forearm resting volar side up on a padded armrest. The experimenter sat across the table from the subject and used a pen to draw two parallel 10-cm lines (separated by 4 mm) along the midline of the forearm. A small amount of light mineral oil was then rubbed lightly into the same area of skin. To begin a search the experimenter instructed the subject to look away from the arm and to close his or her eyes. The experimenter then touched the thermode to the skin at the proximal ends of the two lines and began to slide it slowly between the lines at a rate of approximately 2 mm/sec. Subjects were instructed to say “there” the moment they felt a thermal or nociceptive sensation (as previously defined) and to indicate the sensation or sensations they experienced. The experimenter marked the distal edge of the site with a pen and lifted the thermode from the skin. After recording the subject’s response the experimenter touched the thermode back down onto the same site before the search was resumed. Subjects were told to ignore any sensations experienced at the time of re-contact, but to report any new sensations once the thermode began moving again. This procedure was repeated each time a new sensation was reported.
After reaching the end of the 10-cm track subjects were given a 3-min break to allow for recovery of adaptation before a second search of the same area was conducted. The second search, which also began at the proximal end of the track, served to (1) determine which sensitive sites could be verified by replication, and (2) make certain that no sensation was perceived in the region immediately adjacent to verified sites, i.e., that the site was spatially isolated. The latter criterion was intended to insure that the sensitivity of sites could be attributed to sensitive spots beneath the thermode rather than to spots in nearby skin. Seventy-four subjects participated in this part of the study, with 47 tested for cold-sensitive sites and 63 tested for heat-sensitive sites. Study of cold-sensitive sites began first, with some subjects tested for both cold and heat-sensitive sites. Thirty-four subjects (72.3% of those tested) yielded one or more isolated and replicated cold-sensitive sites, and 47 (74.6%) yielded one or more isolated and replicated heat-sensitive sites.
(2) Sensitive Spot Search
Searches for sensitive spots within replicated sensitive sites were conducted in the testing session in which the sites were identified. It was possible to study up to three sites within each session. The procedure was to slide the 1-mm spot stimulator slowly back and forth across each 4mm × 4mm site. When the subject reported a sensation the location was marked by a dot of ink and the sensation or sensations were recorded. After each site had been thoroughly surveyed for sensitive spots, a plastic template with an open area equal to that of the thermode was centered over the site. When a spot was found on the perimeter of the site, the template was moved slightly to insure the spot would be contacted during later testing with the Peltier thermode. The template was then used to trace a 4mm × 4mm square in ink on the skin, which served as a clear target for thermode placement during subsequent testing of the site.
(3) Normal versus Sliding Contact Stimulation of Sensitive Sites
After a 5-min break measurements were made of the perceived intensity and quality of sensations produced on sensitive sites by thermal stimulation under two conditions: sliding contact, which was expected to allow both thermal and nociceptive sensations to be perceived, and normal (perpendicular) contact, which was expected to differentially inhibit nociceptive sensations. In the sliding contact condition the experimenter manually slid the thermode onto the site in a manner similar to that used to search for sites. The thermode was placed adjacent to the proximal edge of the target site and the subjects were asked if they felt any temperature-related sensations. If so, the thermode was moved to another side of the target site until no sensation was reported. This was rarely required, however, because of the initial criterion that target sites be spatially isolated. The experimenter then instructed the subject to “attend now” as the she slid the thermode slowly onto the marked site. Three seconds after the thermode was centered on the site the experimenter said “rate” and lifted the thermode from the skin. Subjects were told their intensity ratings should reflect the maximum intensity of sensations felt between the time the experimenter said “attend now” and “rate”. Subjects moved a mouse with their right (free) hand to make separate ratings of thermal intensity and nociceptive intensity on the gLMS. Lists of thermal (“nothing”, “cool”, “cold”, “icy”, “warm”, and “hot”) and nociceptive (“nothing”, “burning”, “stinging”, “pricking”, “aching”, and “painful”) descriptors then appeared on the computer screen and subjects used the mouse to click on as many or as few terms as necessary to fully describe the sensation they had perceived.
In the normal contact condition the thermode was touched to the skin for 5 sec instead of 3 sec. The longer duration took into account that in the sliding condition, the thermode usually contacted one or more spots within each site before it covered the entire site and the 3-sec duration was initiated. This conservative strategy helped to rule out the possibility that any differences in outcome between conditions might be attributable to briefer stimulation of spots during normal contact. The subject was instructed to “attend now” immediately before the thermode was touched to the skin and to “rate” as the thermode was lifted from the skin. Ratings were made in the same manner as in the sliding condition. The order of testing of the two conditions was counterbalanced across subjects, and there was a 3-min break between measurements in the two conditions.
Experiment 2: Psychophysical Functions on Sensitive Sites
Practice Procedure
Subjects who had not previously served in a thermal perception experiment in our laboratory participated a practice procedure similar to that used in experiment 1.
Experimental Procedure
Sensitive sites were located using the search method of experiment 1. Because of the need for repeated thermal testing on each site, a maximum of two verified sites were tested per session.
There was a 3-min break after the site or sites had been replicated before testing was resumed. At each site subjects received a series of 5 trials, one for each target temperature. Cooling temperatures were 31°, 29°, 26°, 23° and 20°C, and heating temperatures were 35°, 37°, 39°, 41° and 43°C. Stimuli were presented in ascending order (descending temperature for cooling stimuli) with an interstimulus interval of 2 min. To enable intensity scaling of nociceptive as well as thermal sensations, stimulation always occurred via the sliding contact method of experiment 1. The duration of stimulation and instructions for intensity ratings and quality judgments were as before. Subjects participated in two sessions, one for cold-sensitive sites and one for warm-sensitive sites, with sessions conducted on separate days. The order of testing was counterbalanced so that half of the subjects received cold stimuli and half received warm stimuli in session 1.
Results
Types and Frequencies of Sensitive Sites
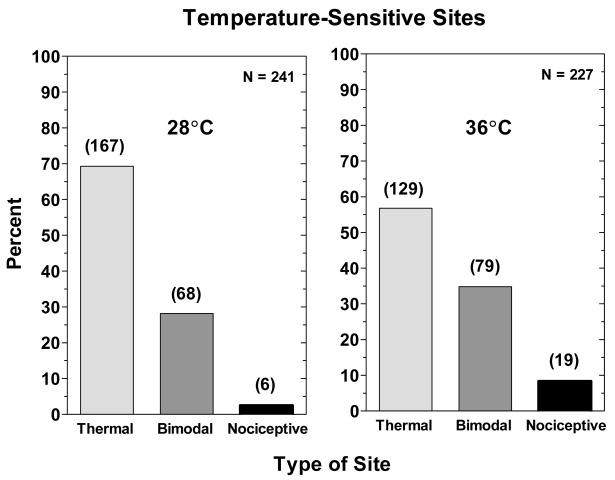
A total of 468 temperature-sensitive sites were located and replicated in sliding searches with the 16-mm2 Peltier thermode set to 28° or 36°C. All of the sites were one of three types: thermal sites, on which subjects reported only temperature sensations; bimodal sites, on which subjects reported both temperature sensations and nociceptive sensations; and nociceptive sites, on which only nociceptive sensations were reported. Fig. 1 shows the percentages of the three types of sites based on sensations reported during the intensity rating task (part 3) of Exp. 1. Sensations rated less than ‘barely detectable’ were disregarded when classifying sensitivity sites. As expected, thermal sites were by far the most common, accounting for 69.3% of cold-sensitive sites and 58.6% of heat-sensitive sites. However, 28.2% and 34.2% of cold- and heat-sensitive sites were bimodal. Purely nociceptive sites were rare, equaling only 8.4% of all heat-sensitive sites and 2.5% of all cold-sensitive sites.
Figure 1.
Shown are the percentages of temperature-sensitive sites classified as thermal, bimodal and nociceptive found using the 0.16 cm2 Peltier thermode in the sliding search procedure of Exp. 1. The graphs on the left and right side of the figure contain the results obtained with the 28° and 36°C stimuli, respectively. The number of each type of site that was found is indicated in the parentheses above each bar.
Types and Frequencies of Sensitive Spots
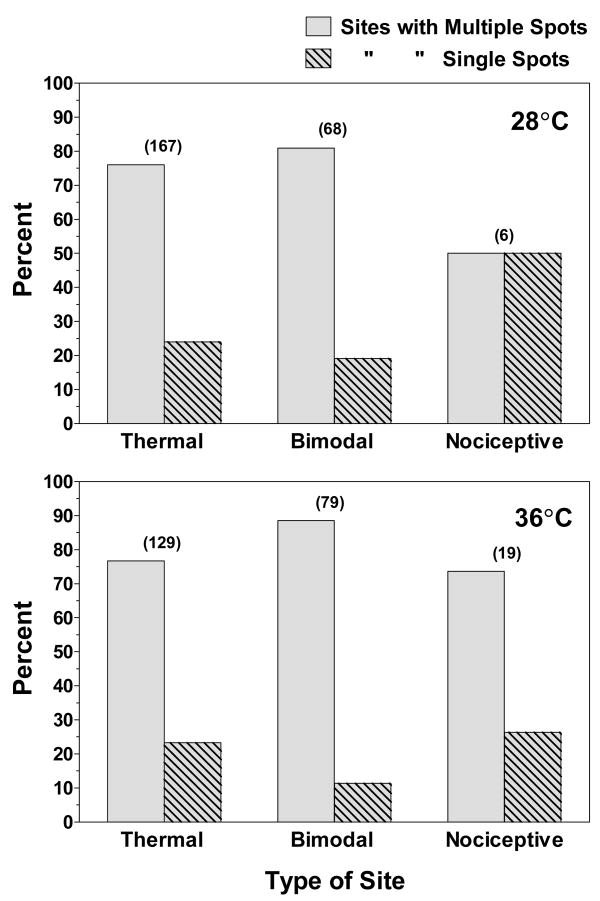
Fig. 2 shows the percentages of each category of site in which either single or multiple spots were found using the 1-mm aluminum thermode cooled or heated to 28° or 36°C. Most sites contained multiple spots. The only exception appeared to be cold-sensitive nociceptive sites, but too few were studied (n = 6) to be confident that this is a reliable trend. Overall, cold-sensitive sites contained 1.9 spots and heat-sensitive sites contained 2.3 spots.
Figure 2.
The percentage of temperature-sensitive sites in which multiple (gray bars) or single (hatched bars) temperature-sensitive spots were found during sliding searches with the 1.0 mm2 thermode adjusted to 28° (top) or 36°C (bottom). The number of each type of site that was studied is indicated in the parentheses above each pair of bars.
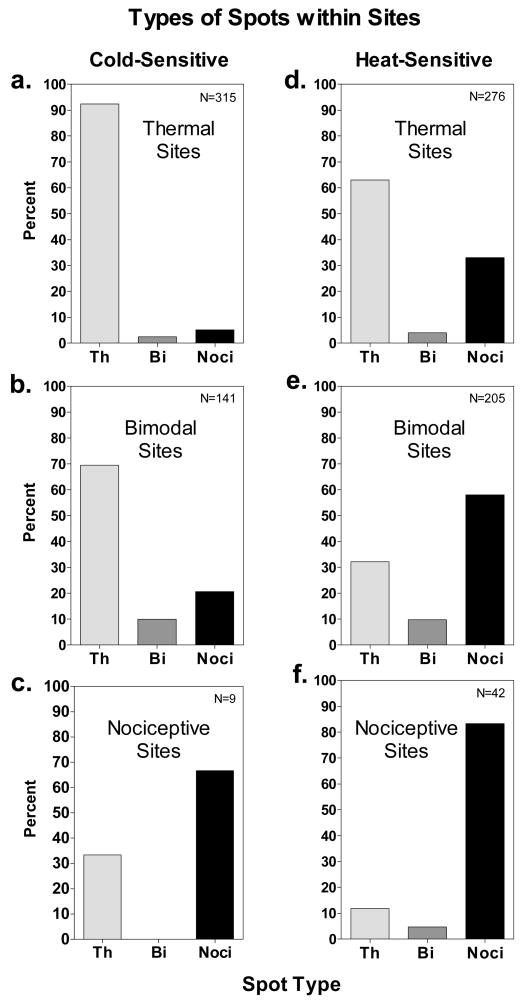
Fig. 3 shows that sensitive spots found within sensitive sites were of the same three qualitative types as sensitive sites, i.e., thermal, bimodal and nociceptive. Classically defined cold spots and warm spots were most common within thermal sites (Fig. 3a,d). However, nociceptive spots were also found on some thermal sites, particularly heat-sensitive sites, where they accounted for 1/3 of all sensitive spots. Bimodal spots were detected only rarely within thermal sites.
Figure 3.
The percentages of thermal (Th), bimodal (Bi) and nociceptive (Noci) spots found within cold-sensitive (a,b,c) and heat-sensitive (d,e,f) thermal, bimodal and nociceptive sites. The spots were located using the 1.0 mm2 thermode and the sliding search procedure. The N indicated in each graph refers to the total number of spots studied for each category of site.
Within bimodal sites, the most common type of spot differed for cooling and heating. Thermal spots were most frequent within cold-sensitive bimodal sites, whereas nociceptive spots were most frequent within heat-sensitive bimodal sites (Fig. 3b,e). The differences in relative frequencies were pronounced: thermal spots accounted for 69.5% of all spots within cold-sensitive sites compared to only 32.2% within heat-sensitive sites, and nociceptive spots totaled 58% of all spots within heat-sensitive sites compared to 20.6% within cold-sensitive sites. Not surprisingly, nociceptive spots were encountered most often within nociceptive sites, where they accounted for 83.3% and 66.7% of the spots found during heating and cooling, respectively. Bimodal spots were by far the rarest, and accounted for less than 10% of spots within bimodal sites (Fig. 3b,e). Thus bimodal sites most often resulted from thermal and nociceptive spots located close to one another. Although 33.3% of spots within cold-sensitive nociceptive sites were thermal spots, the small number of such sites once again prevents conclusions from being drawn about their relative frequencies. It is nevertheless evident that nociceptive spots were sometimes found with thermal spots within both cold- and heat-sensitive sites.
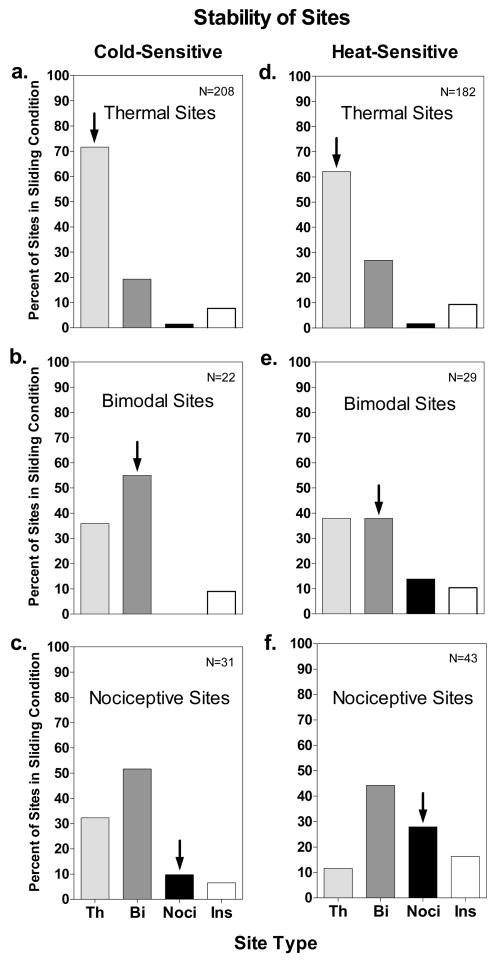
Stability of Sensitive Sites
Not all identified and replicated sensitive sites were consistent throughout testing. After completing the searches for sensitive spots, the sites were tested again with the 0.16 cm2 thermode to compare the perceived intensity of thermal and nociceptive sensations during sliding and normal contact. During this third phase of testing some sites gave rise to different sensations than they had when they were identified and replicated, and a small percentage became insensitive (i.e., no sensations were rated as more than ‘barely detectable’ in the sliding contact condition). Fig. 4 provides data on the stability/instability of cold- and heat-sensitive sites for each type of site. The data reflect changes in the reported qualities of sensation between the initial replication of the sites at the end of the search procedure (part 1) and their testing in the intensity scaling task (part 3). Thus stimulation of spots within the sites as well as the mere passage of time may have contributed to the measured instability. Calculated across all three types of sites and both temperatures, 96.7% of replicated sites remained sensitive during intensity testing, but only 68.2% remained within the same qualitative category. Thermal sites were the most stable and nociceptive sites the least stable. Seventy-one percent of cold-sensitive thermal sites and 62.1% of heat-sensitive thermal sites remained unchanged in the third phase of testing, compared to only 9.7% of cold-sensitive nociceptive sites and 27.9% of heat-sensitive nociceptive sites. When changes in quality occurred, thermal sites and nociceptive sites most often became bimodal sites (Fig. 4a,d and 4c,f), and bimodal sites most often became thermal sites (Fig. 4b,e). These trends indicate that nociceptive sensations were reported less consistently than thermal sensations. However, conversion of 19.2% of cold-sensitive thermal sites (Fig. 4a) and 26.9% of heat-sensitive thermal sites (Fig. 4d) into bimodal sites also indicates that nociceptive sensations sometimes appeared where only thermal sensations had initially been reported.
Figure 4.
The stability of cold- and heat-sensitive sites is expressed as the percentage of each type of site that was found when the originally identified sites were retested to obtain ratings of perceived intensity during sliding contact. Complete stability would be indicated by 100% of the sites remaining in the original category (indicated by the arrows). Th = thermal sites; Bi = bimodal sites; Noci = nociceptive sites; Ins = Insensitive sites. Note that bimodal and nociceptive sites, which were more unstable than thermal sites, more often became thermal or bimodal sites than becoming insensitive.
Perceived Intensity during Sliding and Normal Contact
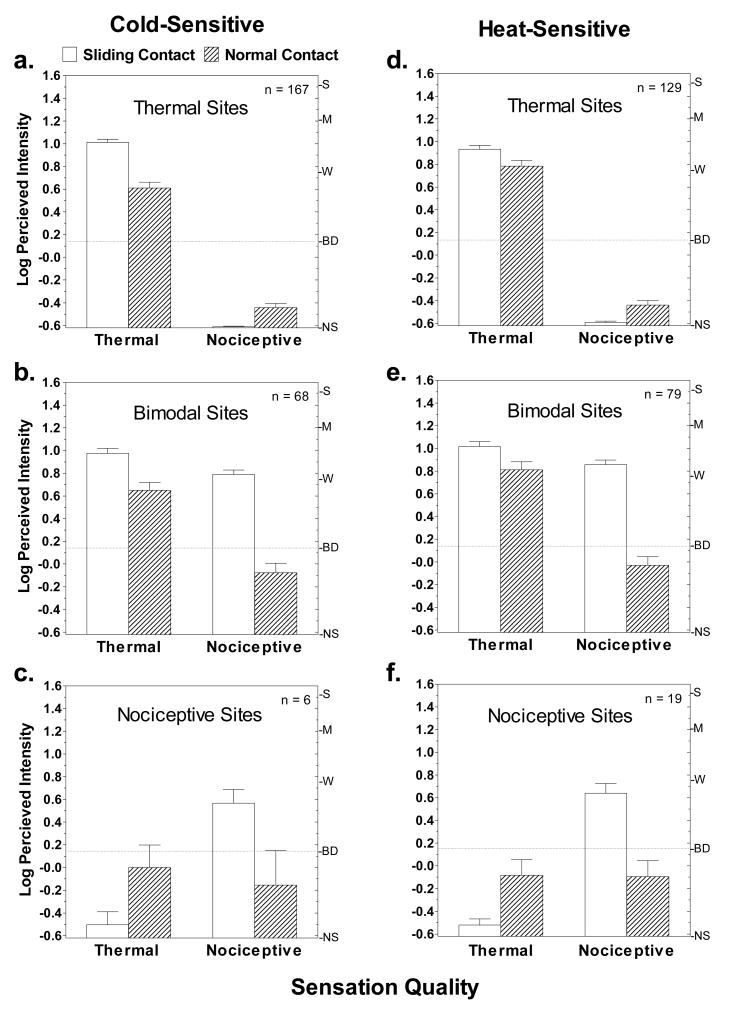
Fig. 5 compares the perceived intensities of thermal and nociceptive sensations reported under conditions of sliding versus normal contact for the three types of sites. Only sites that yielded sensations rated above ‘barely detectable’ were included in the analysis. Sliding contact evoked stronger sensations than normal contact for both thermal and nociceptive sensations, but the differences in perceived intensity were much greater for nociceptive sensations. Compared to sliding contact, during normal contact nociceptive sensations on bimodal sites were reduced by 85.9% and 86.5% (Fig. 5b,e) for the 28° and 36° stimuli, respectively, with mean intensity ratings dropping below ‘barely detectable’ for both temperatures. Nociceptive sensations were similarly attenuated on the few nociceptive sites that were studied (Fig. 5c,f). Thus under conditions of normal contact, nociceptive sensations were virtually undetectable. This result is consistent with prior evidence that tactile stimulation produced by dynamic (normal) contact inhibits LTN (Green and Schoen 2005). In contrast, thermal intensity ratings for the 28° and 36° stimuli were only 40.0% and 29.2% lower during normal contact (Fig. 5a,d). Nevertheless, t-tests for dependent means confirmed these differences were significant (29°: t166=8.7, p<0.0001; 36°: t128=3.7, p<0.001).
Figure 5.
Shown are the log means of perceived intensity ratings given in response to the 28° and 36° stimuli on cold- and heat-sensitive sites under conditions of sliding (open bars) and normal (hatched bars) contact. Vertical bars indicate standard errors of the means. Letters on the right y-axis of each graph represent descriptors on the psychophysical scale of perceived intensity (gLMS) that was used: NS = no sensation; BD = barely detectable; W = weak; M = moderate; S = Strong. The dashed lines highlight the finding that during normal contact, nociceptive sensations were on average rated below ‘barely detectable’.
It is noteworthy that thermal sensations were rated about equally intense under both conditions of stimulation on thermal sites and bimodal sites. The only quantitative difference between these two types of sites was the added perception of nociceptive sensations on bimodal sites.
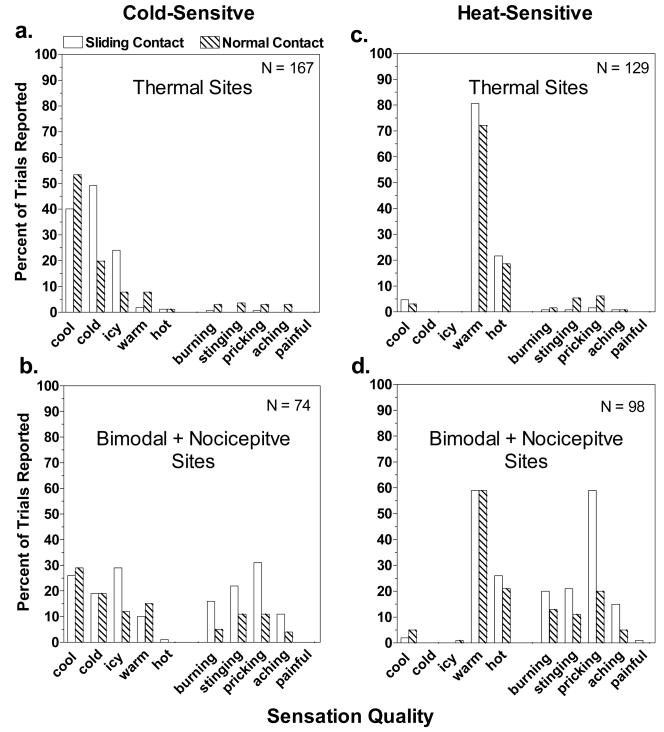
Fig. 6 displays the percentage of trials on which different sensation qualities were reported for the two contact conditions. Because the small number of nociceptive sites (particularly for cold) made it difficult to interpret differences in percentages of ratings across multiple sensation qualities, the data from nociceptive sites have been combined with the data from bimodal sites (Fig. 6b,d). On thermal sites, normal contact reduced reports of ‘cold’ and ‘icy’ sensations at 28° by more than half (Fig. 6a), and reports of ‘cool’ became more frequent. These differences were consistent with the slightly lower intensity ratings for thermal sensations during normal contact compared to sliding contact. In contrast, 36° stimulation was most often described as ‘warm’ in both conditions, with only slight decreases in frequencies of ‘warm’ and ‘hot’ ratings in the normal contact condition (Fig. 6c). Normal contact had its greatest effect on the incidence of nociceptive sensations, which were consistently reported less often than during sliding contact (Fig. 6b,d). For both the 28° and 36° stimuli, ‘pricking’ was the most frequently reported nociceptive sensation during sliding contact, being reported on 31% of trials during cooling and 59% of trials during warming. For both temperatures, normal contact reduced the incidences of these sensations by approximately 2/3, to only 11% and 20%, respectively.
Figure 6.
The qualities of sensation reported in the sliding (open bars) and normal contact (hatched bars) conditions of experiment 1 for cold- and heat-sensitive sites expressed as percentages of the total number of trials on which thermal stimulation was delivered. The data for bimodal and nociceptive sites have been combined.
Experiment 2: Intensity Functions on Cold- and Heat-Sensitive Sites
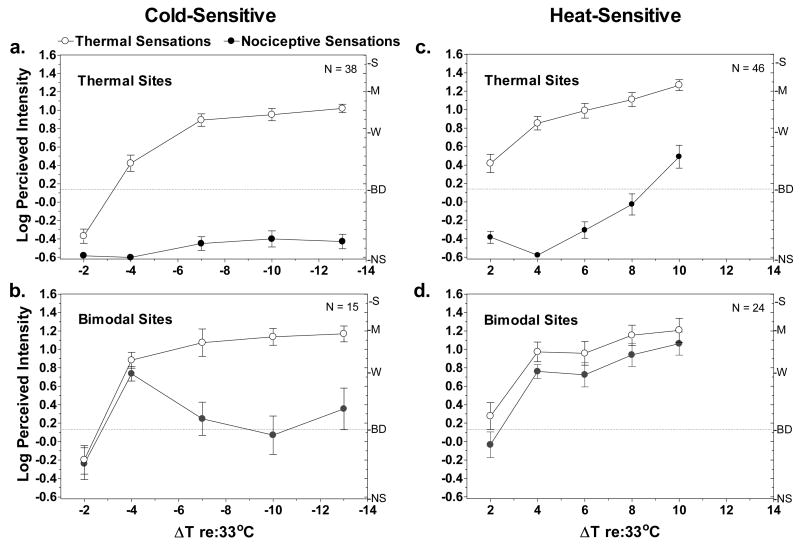
Looking first at the intensity functions for thermal sites (Fig. 7a,c), heat-sensitive sites were more sensitive to weak stimulation than were cold-sensitive sites. Heat-sensitive sites yielded perceptible thermal sensations when ΔT (re: 33°C) was just +2°, while a ΔT of −4° was required to produce an equally intense sensation of cold. Also, nociceptive sensations began to be reported on heat-sensitive thermal sites when ΔT= +10° (43°C), whereas nociceptive sensations remained negligible at ΔT= −13° (20°C) on cold-sensitive thermal sites.
Figure 7.
Log mean ratings of perceived intensity of thermal (empty circles) and nociceptive (filled circles) sensations are shown for cold- and heat-sensitive sites as a function of stimulus temperature. Vertical bars indicate standard errors of the means. Letters on the right y-axis of each graph represent descriptors on the psychophysical scale of perceived intensity (gLMS) that was used: NS = no sensation; BD = barely detectable; W = weak; M = moderate; S = Strong. The dashed line provides a reference to indicate stimuli that were rated above ‘barely detectable’.
On bimodal sites (Fig. 7b,d) the psychophysical functions for thermal sensations were very similar to those for thermal sites. Of greater interest was the difference in the functions for nociceptive sensations on cold- and heat-sensitive sites. On cold-sensitive sites, log-mean ratings of nociceptive sensations initially rose together with thermal ratings, then dropped to near ‘barely detectable’ at colder temperatures. On heat-sensitive sites nociceptive ratings closely paralleled thermal ratings throughout the stimulus range. Perusal of the raw data from cold-sensitive sites revealed that the drop in mean nociceptive ratings at colder temperatures was caused primarily by the complete disappearance of nociceptive sensations at some sites rather than by a decline in intensity ratings at all sites. Specifically, 5 sites from which nociceptive sensations had been reported at ΔT = −4° (29°C) yielded ratings of ‘no sensation’ at ΔT = −7° (26°C). These 5 sites remained insensitive together with an additional 3 sites that became insensitive at ΔT = −10° (23°C). Thus the general tendency found in experiment 1 for nociceptive sensations to be more labile than thermal sensations was also evident in experiment 2. However, nociceptive sensations evoked from heat-sensitive bimodal sites did not show the same tendency to “dropout” during the stimulation series. The latter result is consistent with the data from experiment 1, which showed both a greater stability of heat-sensitive compared to cold-sensitive nociceptive sites and a tendency for heat-sensitive bimodal sites to occasionally become nociceptive sites (Fig. 4e, f). In general, LTN was a more common and consistent characteristic of heating than of cooling.
Discussion
The present study has demonstrated that small, temperature-sensitive sites can be found that contain one or more spots from which burning, stinging or pricking can be evoked by mild heating or cooling. This result implies the nociceptive pathway can be stimulated via low-threshold primary afferent fibers, and thus conflicts with the classical theory that temperature and pain are served entirely by separate sensory receptors.
The evidence that LTN sites are strongly suppressed by normal contact supports the hypothesis that LTN spots escaped prior detection primarily because of their susceptibility to inhibition by touch (Fig. 5). The instability of LTN spots relative to warm spots and cold spots may also have been a factor. Without the expectation that nociceptive sensations can be perceived at mild temperatures, occasional reports of stinging or pricking during punctate searches may have been ignored. The alternative possibility that the present results are artifacts of the methods of thermal stimulation can be ruled out by the precise control of stimulus temperature afforded by the Peltier system together with use of a ‘classical’ metal thermode to locate and stimulate sensitive spots.
The neurophysiological basis of LTN spots is unclear. Although the spots were detected at temperatures served by cold fibers and warm fibers, it has been assumed that sensations of burning, stinging or pricking arise from stimulation of nociceptors, which by definition have high thresholds and respond to noxious stimulation (Bessou and Perl 1969;Iggo and Ogawa 1971). The test temperatures of 28° and 36°C were specifically chosen to avoid stimulation of C-polymodal nociceptors (CPNs), which have average cold thresholds below 20°C (Simone and Kajander 1996;Campero et al. 1996) and average heat thresholds above 40°C (Bessou and Perl 1969;Van Hees and Gybels 1981;Yarnitsky et al. 1992). However, Georgopoulos (1976) reported a few Aδ- and C-mechano-thermal nociceptors in primates that were unusually sensitive to cold, with some having thresholds as high as 30°C. More recently, Campero et al. (2001) discovered cold-sensitive C-fibers in humans that discharge statically to temperatures up to 30°C yet respond maximally below 20°C. These C-fibers and the sensitive C-mechano-thermal nociceptors described by Georgopoulos (1976) could potentially mediate cold LTN ≤30°C, although Campero et al. (2001) questioned whether the C-fibers contribute to thermal perception after a vigorous discharge in one of the fibers failed to evoke a sensation.
Recent evidence that some dorsal root ganglion (DRG) neurons in mice express two transient receptor potential (TRP) channels that have different thermal thresholds raises the possibility that LTN may arise from what might be described as ‘sensitive nociceptors’. The heat-sensitive channel TRPV3, a potential warm receptor with a threshold of 31°–39°C (Smith et al. 2002;Xu et al. 2002;Peier et al. 2002b), has been reported to be co-expressed in DRG neurons (Smith et al. 2002) with the capsaicin and heat-sensitive channel TRPV1 (Caterina et al. 1999;Szolcsanyi 2004). In addition, TRPV3 continues to respond to painfully hot temperatures (Peier et al. 2002b) knockout mice that lack TRPV3 show deficits in response to both noxious and innocuous heat (Moqrich et al. 2005). Similarly, the menthol and cold receptor TRPM8 (McKemy et al. 2002;Peier et al. 2002a), which was recently shown in mice to be the principal receptor for cold below 30°C (Bautista et al. 2007;Colburn et al. 2007;Dhaka et al. 2007;see however Munns et al. 2007), has been reported in some DRG and trigeminal ganglion neurons that are capsaicin-sensitive and/or were shown to express TRPV1 (McKemy et al. 2002;Okazawa et al. 2004;Abe et al. 2005;Xing et al. 2006;Hjerling-Leffler et al. 2007). (See, however, Kobayashi et al. 2005.) Studies have also shown that menthol can evoke nociceptive sensations (Green 1992;Cliff and Green 1994;Namer et al. 2005) via stimulation of C-fibers (Wasner et al. 2004). LTN sites and spots may therefore be attributable to primary afferent fibers that express TRPV1 together with TRPV3 or TRPM8..
However, the possibility that LTN arises from classically defined cold fibers and warm fibers cannot be ruled out, as the thresholds of fibers that express TRPM8 and TRPV3 fall within the range of putative cold and warm fibers. Support for involvement of cold fibers comes from their ‘paradoxical’ response to high temperatures (Dodt and Zotterman 1952;Long 1973;1977;Campero et al. 2001) and from the phenomenon of the heat grill illusion, or ‘synthetic heat’ (Green 1977;Green 2002;Fruhstorfer et al. 2003). Whereas the high threshold [>50°C; (Long 1977)] and temporal irregularity of the paradoxical discharge rule out a role for cold fibers in encoding heat pain, evidence that repeated heating sensitizes cold fibers to heat and lowered the threshold of the paradoxical discharge (Dubner et al. 1975;Long 1977) led to speculation that cold fibers may contribute to heat hyperalgesia (Dubner et al. 1975;Price and Dubner 1977). This possibility may seem to conflict with the phenomenon of ‘paradoxical cold’, in which heating cold spots to high temperatures sometimes evokes a sensation of cold (Boring 1942). However, under natural conditions cold fibers discharge paradoxically only when warm fibers and heat-sensitive nociceptors are also stimulated, and mimicking these conditions by heating and cooling adjacent areas of skin produces the heat grill illusion (Green 1977;Craig and Bushnell 1994;Green 2002;Fruhstorfer et al. 2003). Evidence that the heat grill illusion can also occur at very mild temperatures [e.g., 31° and 36°C; (Green 2002)] indicates either that simultaneous stimulation of warm fibers and cold fibers is sufficient to induce heat (Alrutz 1898), or that warm fiber stimulation inhibits the cold pathway and thereby disinhibits low-threshold stimulation in the nociceptive pathway. The latter hypothesis is a modification of Craig and Bushnell’s disinhibition interpretation of the heat grill illusion (Craig and Bushnell 1994;Craig et al. 1996) which allows for the likelihood that nociceptive stimulation can arise from low-threshold afferent fibers.
If cold fibers and warm fibers are responsible for LTN, they must project directly or via interneurons to nociceptive STT neurons. Evidence of wide-dynamic range (WDR) and polymodal spinal thalamic tract (STT) neurons in the dorsal horn, trigeminal nucleus caudalis and thalamus that respond to both innocuous and noxious thermal stimulation (Christensen and Perl 1970;Bushnell et al. 1993;Zhang et al. 2006;Zanotto et al. 2007) confirms that low-threshold thermal stimulation reaches the nociceptive pathway. However, because some ‘sensitive nociceptors’ apparently express TRPM8 or TRPV3, it is impossible to deduce from the available data which class of primary afferent fibers drive the low-threshold thermal response of spinal and trigeminal WDR neurons.
The ability of dynamic contact to inhibit LTN sites together with evidence that both nonpainful cold and tactile stimulation can inhibit pain (Pertovaara 1979;Bini et al. 1984;Craig and Bushnell 1994), suggests that low-threshold stimulation of the nociceptive pathway can be readily inhibited. Convincing evidence that stimulation from cold fibers inhibits cold-induced nociceptive stimulation comes from the aforementioned studies of ischemic or pressure block of A-δ cold fibers, which causes innocuous cooling to be felt as stinging, burning or hot (Fruhstorfer 1984;Kojo and Pertovaara 1986;Wahren et al. 1989;Yarnitsky and Ochoa 1990). Evidence that nociceptive stimulation might also be inhibited by stimulation of warm fibers (Kanui 1987) comes from the present finding that 33% of all spots within heat-sensitive thermal sites were nociceptive spots (Fig. 3d). Readily inhibited nociceptive stimulation might also explain the instability of nociceptive sites and spots and individual differences in reports of LTN. Fluctuations in inhibition of low-threshold nociceptive projections could cause nociceptive spots to fall silent or to become thermal spots. That bimodal and nociceptive sites sometimes changed to thermal sites supports the involvement of thermoreceptors in LTN, and implies that nociceptive projections are more susceptible to inhibition than are thermal projections. The latter possibility is consistent with the much weaker suppression of thermal sensations compared to nociceptive sensations (Fig. 5). Parallel discharge in the thermal pathways, or a tonic descending inhibition (Dickhaus et al. 1985), may be sufficient in some individuals to block relatively weak stimulation of the nociceptive pathway from low-threshold fibers.
Previous psychophysical studies have also provided evidence of a close relationship between temperature sensitivity and thermal pain. Green and Cruz (1998) found that small patches of healthy skin in which warmth sensitivity was absent had significantly higher heat pain thresholds than adjacent skin with normal warmth sensitivity. Similarly, Defrin et al. (2001;2002) reported that in spinal cord injury patients, regions of the body where warmth sensitivity had been lost had abnormally high heat pain thresholds. These earlier findings imply that stimulation in the warmth pathway convergences with stimulation in the nociceptive pathway. The present results imply that the nociceptive pathway can also be stimulated directly by primary afferent fibers that have low thresholds to heating or cooling. Both lines of evidence point to a functional overlap between temperature and pain that may prove to be important for understanding the source of painful neuropathies that can be triggered by mild temperatures.
Acknowledgments
This research was supported in part by a grant from the National Institutes of Health, RO1 NS038463. The authors thank Dr. Juyun Lim for her comments on an earlier version of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abe J, Hosokawa H, Okazawa M, Kandachi M, Sawada Y, Yamanaka K, Matsumura K, Kobayashi S. TRPM8 protein localization in trigeminal ganglion and taste papillae. Brain Res Mol Brain Res. 2005;136:91–98. doi: 10.1016/j.molbrainres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Alrutz S. On the temperature senses: II. The sensation ‘hot’. Mind. 1898;7:140–144. [Google Scholar]
- Bartoshuk LM, Duffy VB, Fast K, Green BG, Prutkin J, Snyder DJ. Labeled scales (e.g. category, Likert, VAS) and invalid across-group comparisons: what we have learned from genetic variation in taste. Food Quality and Preference. 2003;14:125–138. [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969;32:1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- Bini G, Cruccu G, Hagbarth K-E, Shady W, Torebjork E. Analgesic effect of vibration and cooling on pain induced by intraneural electrical stimulation. Pain. 1984;18:239–248. doi: 10.1016/0304-3959(84)90819-4. [DOI] [PubMed] [Google Scholar]
- Boring EG. Sensation and perception in the history of experimental psychology. NY: Appleton-Century-Crofts, Inc.; 1942. Tactual sensibility; pp. 463–522. [Google Scholar]
- Bushnell MC, Duncan GH, Tremblay N. Thalamic VPM nucleus in the behaving monkey. I. Multimodal and discriminative properties of thermosensitive neurons. J Neurophysiol. 1993;69:739–752. doi: 10.1152/jn.1993.69.3.739. [DOI] [PubMed] [Google Scholar]
- Campero M, Serra J, Bostock H, Ochoa JL. Slowly conducting afferents activated by innocuous low temperature in human skin. J Physiol. 2001;535:855–865. doi: 10.1111/j.1469-7793.2001.t01-1-00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campero M, Serra J, Ochoa JL. C-polymodal nociceptors activated by noxious low temperature in human skin. J Physiol. 1996;497 ( Pt 2):565–572. doi: 10.1113/jphysiol.1996.sp021789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Christensen BN, Perl ER. Spinal neurons specifically excited by noxious or thermal stimuli: marginal zone of the dorsal horn. J Neurophysiol. 1970;33:293–307. doi: 10.1152/jn.1970.33.2.293. [DOI] [PubMed] [Google Scholar]
- Cliff MA, Green BG. Sensory irritation and coolness produced by menthol: Evidence for selective desensitization of irritation. Physiol Behav. 1994;56:1021–1029. doi: 10.1016/0031-9384(94)90338-7. [DOI] [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D’Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Craig AD, Bushnell MC. The thermal grill illusion: Unmasking the burn of cold pain. Science. 1994;265:252–255. doi: 10.1126/science.8023144. [DOI] [PubMed] [Google Scholar]
- Craig AD, Reiman EM, Evans A, Bushnell MC. Functional imaging of an illusion of pain. Nature. 1996;384:258–260. doi: 10.1038/384258a0. [DOI] [PubMed] [Google Scholar]
- Dallenbach KM. The temperature spots and end organs. Amer J Psychol. 1927;39:402–427. [Google Scholar]
- Darian-Smith I, Johnson KO, LaMotte C, Shigenaga Y, Kenins P, Champness P. Warm fibers innervating palmar and digital skin of the monkey: responses to thermal stimuli. J Neurophysiol. 1979;42:1297–1315. doi: 10.1152/jn.1979.42.5.1297. [DOI] [PubMed] [Google Scholar]
- Defrin R, Ohry A, Blumen N, Urca G. Sensory determinants of thermal pain. Brain. 2002;125:501–510. doi: 10.1093/brain/awf055. [DOI] [PubMed] [Google Scholar]
- Defrin R, Ohry A, Blumen N, Urca G. Characterization of chronic pain and somatosensory function in spinal cord injury subjects. Pain. 2001;89:253–263. doi: 10.1016/s0304-3959(00)00369-9. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Dickhaus H, Pauser G, Zimmermann M. Tonic descending inhibition affects intensity coding of nociceptive responses of spinal dorsal horn neurones in the cat. Pain. 1985;23:145–158. doi: 10.1016/0304-3959(85)90056-9. [DOI] [PubMed] [Google Scholar]
- Dodt E, Zotterman Y. The discharge of specific cold fibers at high temperatures (the paradoxical cold) Acta Physiol Scand. 1952;26:358–365. doi: 10.1111/j.1748-1716.1952.tb00917.x. [DOI] [PubMed] [Google Scholar]
- Dubner R, Sumino R, Wood WI. A peripheral “cold” fiber population responsive to innocuous and noxious thermal stimuli applied to monkey’s face. J Neurophysiol. 1975;38:1373–1389. doi: 10.1152/jn.1975.38.6.1373. [DOI] [PubMed] [Google Scholar]
- Fruhstorfer H. Thermal sensibility changes during ischemic nerve block. Pain. 1984;20:355–361. doi: 10.1016/0304-3959(84)90112-X. [DOI] [PubMed] [Google Scholar]
- Fruhstorfer H, Harju EL, Lindblom UF. The significance of A-delta and C fibres for the perception of synthetic heat. Eur J Pain. 2003;7:63–71. doi: 10.1016/s1090-3801(02)00056-3. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP. Functional properties of primary afferent units probably related to pain mechanisms in primate glabrous skin. J Neurophysiol. 1976;39:71–83. doi: 10.1152/jn.1976.39.1.71. [DOI] [PubMed] [Google Scholar]
- Green BG. Synthetic heat at mild temperatures. Somatosens Mot Res. 2002;19:130–138. doi: 10.1080/08990220220220131524. [DOI] [PubMed] [Google Scholar]
- Green BG. The sensory effects of l-menthol on human skin. Somatosens Mot Res. 1992;9:235–244. doi: 10.3109/08990229209144774. [DOI] [PubMed] [Google Scholar]
- Green BG. Localization of thermal sensation: An illusion and synthetic heat. Percept Psychophys. 1977;22:331–337. [Google Scholar]
- Green BG, Cruz A. “Warmth-insensitive fields”: evidence of sparse and irregular innervation of human skin by the warmth sense. Somatosensory & Motor Research. 1998;15:269–275. doi: 10.1080/08990229870682. [DOI] [PubMed] [Google Scholar]
- Green BG, Pope JV. Innocuous cooling can produce nociceptive sensations that are inhibited during dynamic mechanical contact. Exp Brain Res. 2003;148:290–299. doi: 10.1007/s00221-002-1280-9. [DOI] [PubMed] [Google Scholar]
- Green BG, Schoen KL. Evidence that tactile stimulation inhibits nociceptive sensations produced by innocuous contact cooling. Behav Brain Res. 2005;162:90–98. doi: 10.1016/j.bbr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Green BG, Shaffer GS, Gilmore MM. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem Senses. 1993;18:683–702. [Google Scholar]
- Hensel H. Thermal sensations and thermoreceptors in man. Springfield, Ill. USA: Charles C. Thomas; 1982. [Google Scholar]
- Hensel H, Boman K. Afferent impulses in cutaneous sensory nerves in human subjects. J Neurophysiol. 1960;23:564–578. doi: 10.1152/jn.1960.23.5.564. [DOI] [PubMed] [Google Scholar]
- Hensel H, Iggo A. Analysis of cutaneous warm and cold fibres in primates. Pflugers Arch. 1971;329:1–10. doi: 10.1007/BF00586896. [DOI] [PubMed] [Google Scholar]
- Hensel H, Zotterman Y. Action potentials of cold fibres and intracutaneous temperature gradient. J Neurophysiol. 1951;14:377–385. doi: 10.1152/jn.1951.14.5.377. [DOI] [PubMed] [Google Scholar]
- Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci. 2007;27:2435–2443. doi: 10.1523/JNEUROSCI.5614-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A, Ogawa H. Primate cutaneous thermal nociceptors. J Physiol. 1971;216:77P–78P. [PubMed] [Google Scholar]
- Jarvilehto T. Neural coding in the temperature sense (Sarja - Ser B 184) Helsinki, Suomalainen Tiedeakatemia; Annals Academie Scientiarium Fennice: 1973. Ref Type: Serial (Book, Monograph) [Google Scholar]
- Jenkins WL, Karr AC. Paradoxical warmth: A sufficient condition for its arousal. Am J Psychol. 1957;70:640–641. [PubMed] [Google Scholar]
- Kanui TI. Thermal inhibition of nociceptor-driven spinal cord neurones in the cat: a possible neuronal basis for thermal analgesia. Brain Res. 1987;402:160–163. doi: 10.1016/0006-8993(87)91060-2. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, Gallegos ES. Multiple temperature-sensitive spots innervated by single nerve fibers. Science. 1967;158:1064. doi: 10.1126/science.158.3804.1064. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- Kojo I, Pertovaara A. Effect of tourniquet-induced ischemia on cutaneous thermal thresholds. Acta Neurol Scand. 1986;74:383–386. doi: 10.1111/j.1600-0404.1986.tb03530.x. [DOI] [PubMed] [Google Scholar]
- Long RR. Cold fiber heat sensitivity: dependency of “paradoxical” discharge on body temperature. Brain Res. 1973;63:389–392. doi: 10.1016/0006-8993(73)90110-8. [DOI] [PubMed] [Google Scholar]
- Long RR. Sensitivity of cutaneous cold fibers to noxious heat: Paradoxical cold discharge. J Neurophysiol. 1977;40:489–502. doi: 10.1152/jn.1977.40.3.489. [DOI] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- Munns C, Alqatari M, Koltzenburg M. Many cold sensitive peripheral neurons of the mouse do not express TRPM8 or TRPA1. Cell Calcium. 2007;41:331–342. doi: 10.1016/j.ceca.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Namer B, Seifert F, Handwerker HO, Maihofner C. TRPA1 and TRPM8 activation in humans: effects of cinnamaldehyde and menthol. Neuroreport. 2005;16:955–959. doi: 10.1097/00001756-200506210-00015. [DOI] [PubMed] [Google Scholar]
- Norrsell U, Finger S, Lajonchere C. Cutaneous sensory spots and the “law of specific nerve energies”: history and development of ideas. Brain Res Bull. 1999;48:457–465. doi: 10.1016/s0361-9230(98)00067-7. [DOI] [PubMed] [Google Scholar]
- Okazawa M, Inoue W, Hori A, Hosokawa H, Matsumura K, Kobayashi S. Noxious heat receptors present in cold-sensory cells in rats. Neurosci Lett. 2004;359:33–36. doi: 10.1016/j.neulet.2004.01.074. [DOI] [PubMed] [Google Scholar]
- Pavlicek G, Jenkins JG. Paradoxical warmth. Amer J Psychol. 1933;45:350–353. [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP Channel that Senses Cold Stimuli and Menthol. Cell. 2002a;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002b;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- Pertovaara A. Modification of human pain threshold by specific tactile receptors. Acta Physiol Scand. 1979;107:339–341. doi: 10.1111/j.1748-1716.1979.tb06485.x. [DOI] [PubMed] [Google Scholar]
- Price DD, Dubner R. Neurons that subserve the sensory-discriminative aspects of pain. Pain. 1977;3:307–338. doi: 10.1016/0304-3959(77)90063-X. [DOI] [PubMed] [Google Scholar]
- Simone DA, Kajander KC. Excitation of rat cutaneous nociceptors by noxious cold. Neurosci Lett. 1996;213:53–56. doi: 10.1016/0304-3940(96)12838-x. [DOI] [PubMed] [Google Scholar]
- Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- Szolcsanyi J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides. 2004;38:377–384. doi: 10.1016/j.npep.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Van Hees J, Gybels J. C nociceptor activity in human nerve during painful and non painful skin stimulation. J Neurol Neurosurg Psychiatry. 1981;44:600–607. doi: 10.1136/jnnp.44.7.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren LK, Torebjork E, Jorum E. Central suppression of cold-induced C fibre pain by myelinated fibre input. Pain. 1989;38:313–319. doi: 10.1016/0304-3959(89)90218-2. [DOI] [PubMed] [Google Scholar]
- Wasner G, Schattschneider J, Binder A, Baron R. Topical menthol--a human model for cold pain by activation and sensitization of C nociceptors. b. 2004;127:1159–1161. doi: 10.1093/brain/awh134. [DOI] [PubMed] [Google Scholar]
- Xing H, Ling J, Chen M, Gu JG. Chemical and cold sensitivity of two distinct populations of TRPM8-expressing somatosensory neurons. J Neurophysiol. 2006;95:1221–1230. doi: 10.1152/jn.01035.2005. [DOI] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D, Ochoa JL. Release of cold-induced burning pain by block of cold-specific afferent input. Brain. 1990;113:893–902. doi: 10.1093/brain/113.4.893. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D, Simone DA, Dotson RM, Cline MA, Ochoa JL. Single C nociceptor responses and psychophysical parameters of evoked pain: effect of rate of rise of heat stimuli in humans. J Physiol. 1992;450:581–592. doi: 10.1113/jphysiol.1992.sp019144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanotto KL, Merrill AW, Carstens MI, Carstens E. Neurons in superficial trigeminal subnucleus caudalis responsive to oral cooling, menthol, and other irritant stimuli. J Neurophysiol. 2007;97:966–978. doi: 10.1152/jn.00996.2006. [DOI] [PubMed] [Google Scholar]
- Zhang X, Davidson S, Giesler GJ., Jr Thermally identified subgroups of marginal zone neurons project to distinct regions of the ventral posterior lateral nucleus in rats. J Neurosci. 2006;26:5215–5223. doi: 10.1523/JNEUROSCI.0701-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]