Abstract
Ambulatory arterial stiffness index (AASI) is a novel estimate of arterial stiffness which independently predicts cardiovascular mortality, even in normotensive subjects. Additionally, other markers derived from ambulatory blood pressure (BP) monitoring, including variability (BPV), pulse pressure (PP), nocturnal dipping, and morning BP surge have all been shown to be predictive of end-organ damage and cardiovascular disease. Exaggerated cardiovascular reactivity to sympathoexcitatory stimuli may also predict future incidence of hypertension. The purpose of this investigation was to test the hypothesis that AASI and other derivations of ambulatory BP, including PP, 24-hr BPV, dipping, and morning surge, would be correlated with the pressor response to common physiological stress maneuvers. We measured continuous HR and arterial BP during head-up tilt, mental stress, cold pressor test, and isometric handgrip to fatigue in 67 healthy, normotensive, non-obese individuals (43 women, 24 men, mean age ± SD: 28 ± 6 yr). Then 24-hr ambulatory BP was obtained, and AASI was defined as 1 minus the slope of diastolic on systolic BP in individual 24-h ambulatory BP recordings. Although all measures were widely variable among subjects, there was no relationship between AASI, PP, BPV, dipping, and morning surge with the pressor responses. We conclude that in the absence of aging, cardiovascular, or autonomic disease, the novel stiffness index (AASI) or other ambulatory BP indices are either poorly correlated with, or mechanistically unrelated to the complex pressor response to common provocations of sympathoexcitation.
Keywords: ambulatory blood pressure monitoring, arterial stiffness, head-up tilt, mental stress, cold pressor test, exercise pressor reflex
INTRODUCTION
Ambulatory arterial stiffness index (AASI), a novel index which is derived from ambulatory blood pressure (BP) recordings, has been suggested recently as an index of arterial stiffness. AASI has been shown to strongly correlate with classic measures of arterial stiffness, such as central and peripheral augmentation indexes, central pulse pressure (PP), carotid-femoral pulse wave velocity (PWV), and carotid intima-media thickness [1]. Li and colleagues also demonstrated a close correlation between AASI and PWV in normotensive volunteers; the correlation was stronger in women vs. men, and the correlation was significant when analyzing adults under age 40 [1, 2]. Moreover, in subjects under age 40, AASI but not PP correlated with systolic augmentation index, suggesting AASI might be an indicator of arterial dysfunction at a much younger age [1, 2]. Finally, AASI has also been postulated to be a predictor of cardiovascular mortality, even in normotensive subjects [3, 4].
In addition to AASI, other markers derived from ambulatory blood pressure monitoring may have diagnostic, prognostic, and therapeutic relevance. Exaggerated ambulatory BP variability (BPV) has been proposed to be a risk factor for cardiovascular disease in hypertensives [5]. Ambulatory BPV, PP, nocturnal dipping, and morning BP surge have all been shown to be predictive of end-organ damage and cardiovascular disease [6-8].
Growing evidence suggests that the degree of cardiovascular reactivity to laboratory stressors may be predictive of future development of cardiovascular disease. Four of the most common laboratory provocations of sympathoexcitatory stress target different integrative pathways: orthostatic stress, emotional stress, cold stimuli, and the exercise pressor reflex. Normotensive individuals who show a robust pressor response to sympathoexcitatory stimuli like mental stress (MS) and cold pressor test (CPT) are at increased risk for developing hypertension [9]. Therefore, it seems reasonable to predict that healthy individuals with markers of increased arterial stiffness might also generate greater pressor responses to sympathoexcitation. These “pre-clinical” characteristics are important because socioeconomic status, job strain, and chronic emotional stress have powerful widespread implications on cardiovascular health and wellness in general [10].
The purpose of this study was to evaluate the relationship between AASI and the pressor responses to common laboratory stressors in healthy normotensive young adults. A secondary aim was to compare the BPV, PP, degree of nocturnal dipping, and morning BP surge with the pressor responses. Though these ideas in general have been explored in humans with risk factors for cardiovascular disease, less is known how these intermediate physiologic traits compare in healthy individuals. We hypothesized that determinants of arterial stiffness from 24-hr ambulatory blood pressure monitoring, including AASI, ambulatory BPV, PP, nocturnal dipping, and morning surge would be correlated with the pressor response to four common laboratory sympathoexcitatory maneuvers.
METHODS
Subjects
A total of 67 subjects (43 females, 24 males) volunteered for this study, which was part of a large ongoing phenotyping protocol of adrenergic receptor gene variation and BP regulation as detailed elsewhere [11]. Institutional Review Board approval was obtained and each subject gave informed consent prior to participation. Subjects underwent a standard health screening to ensure they were healthy, non-obese, normotensive nonsmokers, and were not taking any medications (except for oral contraceptives in women). All women were studied during the early follicular phase of the menstrual cycle or in the placebo phase of oral contraceptives. All subjects were instrumented with a brachial arterial catheter for BP measurement, and HR was obtained by 3-lead ECG.
Head-up tilt
Following instrumentation and a 20 min period of quiet rest on a tilt table, participants were tilted to 60° for 5 min, then returned to level for 10 min. There were 6 participants who described symptoms of pre-syncope (i.e., light-headedness, nausea) before 5 min elapsed, and for safety were immediately returned to the supine position. Their data was included in the analysis. No subject described a history of recurrent syncope. Data were averaged during the 5 min tilt and compared to averages in the 2 min pre-tilt period. Baroreflex control of heart period was measured with the modified Oxford technique as described elsewhere and not included in the present analysis [11].
Stroop colored word test
Following transfer to a recumbent chair and 25 min of quiet rest, a computerized version of the Stroop colored word test lasting 3 min was administered as described previously [12]. Data were averaged during the 3 min stress test and compared to averages in the 2 min pre-stress control period.
Cold pressor test
After 10 min of quiet rest, a 2 min baseline period was recorded, followed by a cold pressor test as previously described [12]. Each subject’s dominant hand was immersed up to the wrist in a bucket of ice water (0-4°C) for 3 min. HR and MAP were averaged over the 3 min immersion, and then compared to the average values during the pre-stress period.
Isometric handgrip
After 25 min rest, subjects performed submaximal isometric handgrip (40% of max) as previously described [12]. Upon fatigue, subjects rested quietly for a 2 min recovery period. To account for individual subject differences in time to exhaustion, HR and BP data were averaged during the final 20% of endurance time, then compared to the averages obtained from the preceding rest period.
24-hr ambulatory BP monitoring
Following these measurements, a 24-hr ambulatory BP monitor (Spacelabs model 90207, Spacelabs Inc., Issaquah, WA, USA) was placed as detailed previously [11]. Participants with a daytime average BP > 135/85 mmHg were excluded from further analysis.
Data analysis
Laboratory stress data were digitized at 200 Hz, stored on computer, and analyzed off-line with signal processing software (Windaq; Dataq Instruments, Akron, OH for HUT; PowerLab, AD Instruments for MS, CPT, HG). From 24-hr ambulatory BP monitoring, AASI was defined as 1 minus the slope of diastolic on systolic BP in individual 24-h ambulatory BP recordings as previously described [1, 13]. Pulse pressure was defined as the average values for systolic minus diastolic BP over the 24-hr, daytime, and nighttime periods. Ambulatory BPV and HR variability (HRV) were calculated as the 24-hr, daytime, and nighttime standard deviation and coefficient of variation. Nocturnal dipping was expressed as the nocturnal fall in BP calculated as the difference between daytime and nighttime BP adjusted for the daytime BP level and expressed in percentages. Morning surge was defined as the average SBP, and DBP in the first 2 hr of awakening minus the respective averaged values in the 2-hr prior to awakening.
Statistics
Values are expressed as mean ± standard deviation. Pre-stress values to stress response values were compared by paired t-test. The pairwise association between the quantitative variables was evaluated using linear regression and Pearson’s correlation coefficient. A p-value < 0.05 was considered significant.
RESULTS
24-hour BP and heart rate
The mean age ± SD was 28.3 ± 6.4 years, and the mean BMI was 24.0 ± 2.1 kg/m2. The values for BP and heart rate from the ambulatory measures are given in Table 1. The nocturnal fall in SBP, DBP and MAP averaged 14 ± 6 mmHg, 15 ± 5 mmHg and 14 ± 5 mmHg, with the corresponding dipping percentages listed in table 1. The mean HR decreased by an average of 21 ± 7% at night.
Table 1.
BP and heart rate values during ambulatory BP monitoring in 67 normotensives
| 24-hr | daytime | nighttime | ||||
|---|---|---|---|---|---|---|
| mean | SD | mean | SD | mean | SD | |
| SBP (mmHg) | 116.2 | 7.5 | 120.6 | 7.8 | 106.3 † | 8.6 |
| DBP (mmHg) | 69.6 | 6.0 | 74.1 | 6.3 | 59.3 † | 6.9 |
| MAP (mmHg) | 84.9 | 5.2 | 89.2 | 5.5 | 74.9 † | 6.4 |
| PP (mmHg) | 46.6 | 7.2 | 46.5 | 7.6 | 47.1 | 7.2 |
| HR (bpm) | 72.7 | 8.7 | 77.5 | 10.0 | 61.2 † | 7.6 |
| MAP variability — SD(mmHg) | 10.3 | 2.0 | 7.9 | 1.8 | 6.9* | 2.4 |
| HR variability — SD(mmHg) | 13.3 | 3.5 | 11.5 | 3.6 | 7.4 † | 3.5 |
| Morning surge in SBP (mmHg) |
- | - | 11.4 | 8.1 | - | - |
| Morning surge in DBP (mmHg) |
- | - | 12.6 | 7.6 | - | - |
| MAP variability — CV (%) | 12.1 | 2.4 | 8.9 | 2.1 | 9.3 | 3.3 |
| HR variability — CV (%) | 18.3 | 4.1 | 14.8 | 4.2 | 12.1* | 5.5 |
| Nocturnal dipping in SBP (%) | - | - | - | - | 11.8 | 4.9 |
| Nocturnal dipping in DBP (%) | - | - | - | - | 19.9 | 7.1 |
| Nocturnal dipping in MAP (%) | - | - | - | - | 16.0 | 5.7 |
BP, blood pressure. SBP, systolic BP. DBP, diastolic BP. PP, pulse pressure. MAP, mean arterial pressure. HR, heart rate. SD, standard deviation. CV, coefficient of variation. -, not applied.
p < 0.05
p < 0.001 for comparison daytime versus nighttime
Pressor response to HUT, MS, CPT, and isometric HG
For HUT, the pre-stress SBP, DBP, and MAP averages were 124 ± 10, 70 ± 6, 89 ± 7 mmHg, respectively. During HUT, SBP was unchanged (123 ± 12 mmHg), while the DBP was 76 ± 8 mmHg, representing an increase of 9 ± 8% (p < 0.05), and MAP was 91 ± 9 mmHg, an increase of 3 ± 7% (p < 0.05). Pre-stress HR was 63 ± 9 bpm, which increased to 78 ± 11 bpm, an increase of 24 ± 12% (p < 0.01).
Subsequent pre-stress BP and HR values for MS, CPT, and HG were essentially identical to the pre-stress values from HUT. During MS, mean SBP increased to 138 ± 13 mmHg (12 ± 6%, p < 0.01), mean DBP increased to 72 ± 7 mmHg (19 ± 8%, p < 0.01), mean MAP increased to 97 ± 9 mmHg (18 ± 7%, p < 0.01) and mean HR increased to 87 ± 12 bpm (37 ± 20%, p < 0.01). During CPT, mean SBP increased to 147 ± 13 mmHg (17 ± 9%, p < 0.01), mean DBP increased to 80 ± 12 mmHg (26 ± 12%, p < 0.01), mean MAP increased to 105 ± 11 mmHg (24 ± 11% (p < 0.01) and mean HR increased to 72 ± 12 bpm (14 ± 12%, p < 0.01). During HG, mean SBP increased to 162 ± 17 mmHg (30 ± 9%, p < 0.01), mean DBP increased to 91 ± 12 mmHg (47 ± 14%, p < 0.01), mean MAP increased to 119 ± 15 mmHg (44 ± 13% (p < 0.01) and mean HR increased to 96 ± 15 bpm (46 ± 21%, p < 0.01).
AASI and Pressor response to HUT, MS, CPT, and isometric HG
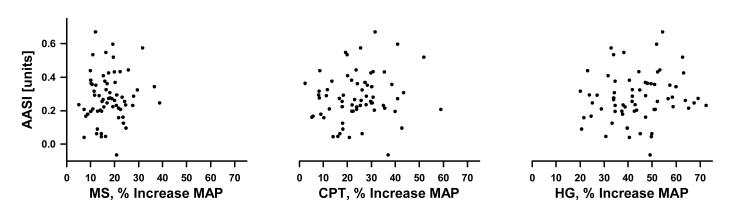
The AASI was widely variable among this healthy population. The mean AASI was 0.27 ± 0.16, with values ranging from -0.25 to 0.67. Interestingly, 2 individuals had an AASI less than zero. There was no relationship between AASI and age (r = -0.212, p = 0.084) or between AASI and BMI (r = 0). Figure 1 depicts the linear regression analyses for 24-hr AASI versus the increase in MAP during each stressor, expressed as a percentage increase in MAP from pre-stress baseline. The AASI versus HUT data was excluded from the figure because of the modest rise in MAP during HUT. The AASI was not correlated to absolute values, absolute change, or percent change of hemodynamic variables in response to HUT, MS, CPT, and HG.
Figure 1.
Regression analyses between the 24-hr ambulatory arterial stiffness index (AASI) and the mean arterial pressure (MAP) response to mental stress (MS), cold pressor test (CPT), and isometric handgrip (HG), expressed as a percent change from baseline MAP prior to each stress maneuver in 67 healthy young adults. The AASI vs. HUT is not shown due to a modest rise in MAP. There was no relationship between the AASI and the pressor responses.
AASI and 24-hr BP
As shown in Table 2, there were positive correlations when comparing AASI to 24-hr SBP, daytime SBP, and nighttime SBP, with r-values of 0.427, 0.336 and 0.520, respectively. Similar to associations between systolic pressure and AASI, AASI was correlated with 24-hr PP, daytime PP, and nighttime PP with r-values of 0.578, 0.603 and 0.455, respectively. Morning surge in DBP, daytime DBP, and 24-hr MAP variability (when expressed as both SD and CV) demonstrated an inverse correlation with AASI. Nocturnal dipping in SBP, DBP and MAP had inverse correlations to AASI with r-values of 0.368, 0.540 and 0.511, respectively.
Table 2.
Correlations between ambulatory arterial stiffness index and 24-hr, daytime, and nighttime ambulatory variables monitoring in 67 normotensives
| 24-hr | daytime | nighttime | ||||
|---|---|---|---|---|---|---|
| r | p-value | r | p-value | r | p-value | |
| SBP (mmHg) | 0.43 | <0.001 | 0.34 | 0.005 | 0.52 | <0.001 |
| DBP (mmHg) | -0.16 | NS | -0.31 | 0.010 | 0.18 | NS |
| MAP (mmHg) | 0.10 | NS | -0.06 | NS | 0.36 | 0.003 |
| PP (mmHg) | 0.58 | <0.001 | 0.60 | <0.001 | 0.46 | <0.001 |
| HR (bpm) | -0.09 | NS | -0.07 | NS | -0.13 | NS |
| MAP variability — SD (mmHg) | -0.45 | <0.001 | -0.15 | NS | -0.09 | NS |
| HR variability — SD (mmHg) | -0.05 | NS | -0.01 | NS | -0.21 | NS |
| Morning surge SBP (mmHg) | -0.17 | NS | ||||
| Morning surge DBP (mmHg) | -0.31 | 0.010 | ||||
| MAP variability — CV (%) | -0.47 | <0.001 | -0.13 | NS | -0.17 | NS |
| HR variability — CV (%) | -0.02 | NS | 0.01 | NS | -0.18 | NS |
| Nocturnal dipping in SBP (%) | -0.37 | 0.002 | ||||
| Nocturnal dipping in DBP (%) | -0.54 | <0.001 | ||||
| Nocturnal dipping in MAP (%) | -0.51 | <0.001 | ||||
BP, blood pressure. SBP, systolic BP. DBP, diastolic BP. PP, pulse pressure. MAP, mean arterial pressure. HR, heart rate. SD, standard deviation. CV, coefficient of variation. r, correlation coefficient.
BPV and Pressor response to HUT, MS, CPT, and isometric HG
No parameter from 24h ambulatory variability indices, when expressed as either SD or CV, nocturnal dipping in SBP, DBP, and MAP, or morning surge in SBP and DBP, had any relationship to absolute values, absolute change, or percent change of hemodynamic variables in response to HUT, MS, CPT, and HG.
DISCUSSION
To our knowledge, this is the first study to compare the AASI with four common laboratory sympathoexcitatory maneuvers in healthy normotensive young adults. The major finding is that AASI was not related to the pressor response to HUT, MS, CPT, and isometric HG. Although AASI is not a diagnostic criterion for systolic hypertension, AASI positively correlated with daytime, nighttime, and 24-hr average SBP in these normotensive individuals. AASI positively correlated with pulse pressure, inversely correlated with the degree of systolic, diastolic, and mean BP nocturnal dipping, and inversely correlated with 24-hr MAP variability when expressed as both standard deviation and coefficient of variation. The morning surge in DBP was also inversely related to AASI, such that individuals with little or no rise in DBP had a higher AASI. Finally, none of the additional ambulatory BP measures correlated with the pressor response to the sympathoexcitatory maneuvers.
Recently Li et al. found that AASI derived from 24-hr ambulatory BP monitoring showed high correlations with several measures of arterial stiffness, including aortic PWV, central and peripheral pulse pressure, and systolic augmentation index, and was suggested as an easily obtainable marker of arterial stiffness [1]. In the Dublin Outcome Study, both AASI and 24-hr PP were predictors of cardiovascular mortality [13]. Furthermore, compared with the 24-hr PP, AASI was a strong predictor of fatal stroke, especially in normotensive subjects, whereas the opposite was true for 24-hr PP in relation to cardiac mortality [13].
In this context, we based our hypotheses on previous studies that have linked hypertension with cardiovascular reactivity to emotional stress [9, 10], cold pressor [14-16], handgrip [17], and orthostatic stress [18, 19], although the predictability of incident hypertension using these maneuvers is not uniform [20]. We reasoned that even in healthy normotensive individuals, AASI would be predictive of cardiovascular reactivity. In healthy aging, Lipman and colleagues compared common carotid artery mechanical stiffness and the pressor response to mental stress, and found that the HR and MAP responses to mental stress were correlated with arterial stiffness [21]. We extended this general idea to a young adult population based on reports linking stiffness parameters to blood pressure at rest or during sympathoexcitation [22-26]. In contrast to our hypothesis, there was no correlation between AASI and the pressor responses to all four maneuvers in the present study. There may be multiple potential reasons to explain this finding. For cardiovascular responsiveness to emotional stress, integrative physiological phenomena that might account for variation include perceived stress, brain stem regulation of autonomic and endocrine output, and peripheral vascular function including stiffness or receptor sensitivity [27].
The lack of association between AASI and the pressor responses may also be attributed to the controversial diagnostic utility of AASI, as other studies have criticized AASI as a predictor of arterial stiffness [28-30]. The AASI depends not only on the total arterial compliance or its inverse, arterial stiffness, but also on systemic vascular resistance, heart period, and pressure [31]. This would explain why AASI was positively correlated with 24-hr average SBP in the present study, consistent with recent findings by Dolan in normotensives and hypertensives that showed AASI was directly related to 24-hr SBP across quintiles in which the AASI ranged from < 0.28 to ≥ 0.55 [3]. We also found that AASI inversely correlated with nocturnal systolic, diastolic, and mean BP reduction in normotensives, which is consistent with a study by Schillaci et al. that showed AASI is strongly dependent on the degree of nocturnal BP fall in hypertensive patients [32]. That AASI positively correlated with 24-hr pulse pressure in our normotensive population is consistent with the idea that AASI correlates with other indicators of arterial stiffness, but by itself may be marker of ventriculo-arterial coupling and not a direct measure of stiffness [31].
AASI may also depend on the collection method of ambulatory BP. A recent summation of 5 studies examining AASI in normotensive and hypertensive individuals described the range of average AASI from 0.31 to 0.56, with the lowest AASI found in the population where every individual had hypertension [32, 33]. This apparent paradox was proposed to be a consequence of a greater number of nighttime BP measurements, with more readings causing a lower AASI, a greater degree of dipping, and a wider level of BP variability [33]. This may be consistent with our findings, where the group average AASI (0.27) was lower than any previous investigation, and 3 BP measurements were obtained per hr during the night, as recommended by the European hypertension guidelines [34]. However, our low AASI was also likely due to a combination of young age, no hypertension, and no risk factors of cardiovascular disease including smoking. Future physiologic stress studies are needed to determine if AASI is predictive of cardiovascular reactivity in disease states where pathologic arterial stiffness would be prevalent, such as hypertension, obesity or metabolic syndrome [35, 36].
We also postulated that individuals with greater degrees of ambulatory BPV, as measured by 24-hr coefficient of variation or standard deviation, nocturnal dipping, or morning BP surge, would evoke a greater pressor response to the stress maneuvers. This idea stemmed from studies in which exaggerated BPV in hypertensives is associated with hypertension-associated end organ damage or a higher rate of cardiovascular events [37, 38]. We were unable to demonstrate any correlations with these ambulatory BP derivations. This is most likely due to the widely variable and complex mechanism of the cardiovascular response to all four maneuvers in healthy normotensives, and it challenges the notion that ambulatory BP measures might predict cardiovascular reactivity in healthy subjects, in part consistent with a previous study showing the absence of such a relationship in young, mildly hypertensive subjects [39].
Several limitations of this investigation deserve mention. Arterial stiffness in our young, non-obese, predominantly female population may have been insufficient to detect a significant relationship with AASI, and modest initial changes in arterial properties may require a larger sample size. Moreover, PWV has been proposed as the “gold-standard” surrogate for arterial stiffness [40]. Taken together, the negative finding in the present study should be regarded with caution until additional investigations evaluate more direct indices of arterial properties in additional populations.
In summary, this study suggests that neither AASI nor other stiffness parameters derived from 24-hr ABPM have correlations with the pressor response to four common sympathoexcitatory maneuvers in lean, normotensive young adults, especially in female population. The widely variable and complex nature of cardiovascular reactivity in healthy subjects may not be related to markers of arterial stiffness, but implies that more focus is needed in target-organ response mechanisms.
ACKNOWLEDGEMENTS
We thank study coordination by Pamela A. Engrav, Shelly K. Roberts, Karen P. Krucker, and Ruth A. Kraft, the technical assistance from Lakshimi Somaraju and Maile Ceridon, and the statistical assistance by Darrell R. Schroeder. We also thank the study volunteers for their enthusiastic participation.
Sources of support: Science and Technology Department of Zhejiang Province, China (2006C30049; ZL); R01HL089331 (JE), NS 90530056/5J4205 (MJJ), DFG grant He 4605/1-1 (CH), NIH/NCRR and NIH Roadmap for Medical Research 1 KL2 RR024151-01.
Footnotes
Disclaimers/conflict of interest: NONE
REFERENCES
- 1.Li Y, Wang JG, Dolan E, Gao PJ, Guo HF, Nawrot T, et al. Ambulatory arterial stiffness index derived from 24-hour ambulatory blood pressure monitoring. Hypertension. 2006;47:359–364. doi: 10.1161/01.HYP.0000200695.34024.4c. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Dolan E, Wang JG, Thijs L, Zhu DL, Staessen JA, et al. Ambulatory arterial stiffness index: determinants and outcome. Blood Press Monit. 2006;11:107–110. doi: 10.1097/01.mbp.0000189791.90488.a1. [DOI] [PubMed] [Google Scholar]
- 3.Dolan E, Thijs L, Li Y, Atkins N, McCormack P, McClory S, et al. Ambulatory arterial stiffness index as a predictor of cardiovascular mortality in the Dublin Outcome Study. Hypertension. 2006;47:365–370. doi: 10.1161/01.HYP.0000200699.74641.c5. [DOI] [PubMed] [Google Scholar]
- 4.Kikuya M, Staessen JA, Ohkubo T, Thijs L, Metoki H, Asayama K, et al. Ambulatory arterial stiffness index and 24-hour ambulatory pulse pressure as predictors of mortality in Ohasama, Japan. Stroke. 2007;38:1161–1166. doi: 10.1161/01.STR.0000259604.67283.69. [DOI] [PubMed] [Google Scholar]
- 5.Kario K. Blood pressure variability in hypertension: a possible cardiovascular risk factor. Am J Hypertens. 2004;17:1075–1076. doi: 10.1016/j.amjhyper.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Birkenhager AM, van den Meiracker AH. Causes and consequences of a non-dipping blood pressure profile. Neth J Med. 2007;65:127–131. [PubMed] [Google Scholar]
- 7.Mancia G, Parati G. Ambulatory blood pressure monitoring and organ damage. Hypertension. 2000;36:894–900. doi: 10.1161/01.hyp.36.5.894. [DOI] [PubMed] [Google Scholar]
- 8.Parati G, Valentini M. Prognostic relevance of blood pressure variability. Hypertension. 2006;47:137–138. doi: 10.1161/01.HYP.0000198542.51471.c4. [DOI] [PubMed] [Google Scholar]
- 9.Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110:74–78. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- 10.Markovitz JH, Matthews KA, Whooley M, Lewis CE, Greenlund KJ. Increases in job strain are associated with incident hypertension in the CARDIA Study. Ann Behav Med. 2004;28:4–9. doi: 10.1207/s15324796abm2801_2. [DOI] [PubMed] [Google Scholar]
- 11.Hesse C, Charkoudian N, Liu Z, Joyner MJ, Eisenach JH. Baroreflex sensitivity inversely correlates with ambulatory blood pressure in healthy normotensive humans. Hypertension. 2007;50:41–46. doi: 10.1161/HYPERTENSIONAHA.107.090308. [DOI] [PubMed] [Google Scholar]
- 12.Eisenach JH, McGuire AM, Schwingler RM, Turner ST, Joyner MJ. The Arg16/Gly beta2-adrenergic receptor polymorphism is associated with altered cardiovascular responses to isometric exercise. Physiol Genomics. 2004;16:323–328. doi: 10.1152/physiolgenomics.00152.2003. [DOI] [PubMed] [Google Scholar]
- 13.Dolan E, Li Y, Thijs L, McCormack P, Staessen JA, O’Brien E, Stanton A. Ambulatory arterial stiffness index: rationale and methodology. Blood Press Monit. 2006;11:103–105. doi: 10.1097/01.mbp.0000200478.19046.dd. [DOI] [PubMed] [Google Scholar]
- 14.Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Kasagi F, Akahoshi M, Shimaoka K. Relation between cold pressor test and development of hypertension based on 28-year follow-up. Hypertension. 1995;25:71–76. doi: 10.1161/01.hyp.25.1.71. [DOI] [PubMed] [Google Scholar]
- 16.Menkes MS, Matthews KA, Krantz DS, Lundberg U, Mead LA, Qaqish B, et al. Cardiovascular reactivity to the cold pressor test as a predictor of hypertension. Hypertension. 1989;14:524–530. doi: 10.1161/01.hyp.14.5.524. [DOI] [PubMed] [Google Scholar]
- 17.Brorson L, Wasir H, Sannerstedt R. Haemodynamic effects of static and dynamic exercise in males with arterial hypertension of varying severity. Cardiovasc Res. 1978;12:269–275. doi: 10.1093/cvr/12.5.269. [DOI] [PubMed] [Google Scholar]
- 18.Drummond PD. Cardiovascular reactivity in mild hypertension. J Psychosom Res. 1983;27:291–297. doi: 10.1016/0022-3999(83)90051-x. [DOI] [PubMed] [Google Scholar]
- 19.London GM, Weiss YA, Pannier BP, Laurent SL, Safar ME. Tilt test in essential hypertension. Differential responses in heart rate and vascular resistance. Hypertension. 1987;10:29–34. doi: 10.1161/01.hyp.10.1.29. [DOI] [PubMed] [Google Scholar]
- 20.Majahalme S, Turjanmaa V, Tuomisto M, Lu H, Uusitalo A. Blood pressure responses to exercise as predictors of blood pressure level after 5 years. Am J Hypertens. 1997;10:106–116. doi: 10.1016/s0895-7061(96)00298-1. [DOI] [PubMed] [Google Scholar]
- 21.Lipman RD, Grossman P, Bridges SE, Hamner JW, Taylor JA. Mental stress response, arterial stiffness, and baroreflex sensitivity in healthy aging. J Gerontol A Biol Sci Med Sci. 2002;57:B279–284. doi: 10.1093/gerona/57.7.b279. [DOI] [PubMed] [Google Scholar]
- 22.Lydakis C, Momen A, Blaha C, Gugoff S, Gray K, Herr M, et al. Changes of central haemodynamic parameters during mental stress and acute bouts of static and dynamic exercise. J Hum Hypertens. 2008;22:320–328. doi: 10.1038/jhh.2008.4. [DOI] [PubMed] [Google Scholar]
- 23.Nurnberger J, Dammer S, Opazo Sae, z A, Philipp T, Schafers RF. Diastolic blood pressure is an important determinant of augmentation index and pulse wave velocity in young, healthy males. J Hum Hypertens. 2003;17:153–158. doi: 10.1038/sj.jhh.1001526. [DOI] [PubMed] [Google Scholar]
- 24.Rajzer MW, Klocek M, Kawecka-Jaszcz K, Czarnecka D, Baran W, Dudek K, Petriczek T. Aortic pulse wave velocity in young normotensives with a family history of hypertension. J Hypertens. 1999;17:1821–1824. doi: 10.1097/00004872-199917121-00006. [DOI] [PubMed] [Google Scholar]
- 25.Reid KF, Conway MA. Haemodynamic determinants of elevated pulse wave velocity during acute isometric handgrip exercise. Ir J Med Sci. 2006;175:13–19. doi: 10.1007/BF03169166. [DOI] [PubMed] [Google Scholar]
- 26.Vlachopoulos C, Kosmopoulou F, Alexopoulos N, Ioakeimidis N, Siasos G, Stefanadis C. Acute mental stress has a prolonged unfavorable effect on arterial stiffness and wave reflections. Psychosom Med. 2006;68:231–237. doi: 10.1097/01.psy.0000203171.33348.72. [DOI] [PubMed] [Google Scholar]
- 27.Lovallo WR, Gerin W. Psychophysiological reactivity: mechanisms and pathways to cardiovascular disease. Psychosom Med. 2003;65:36–45. doi: 10.1097/01.psy.0000033128.44101.c1. [DOI] [PubMed] [Google Scholar]
- 28.Laurent S. Surrogate measures of arterial stiffness: do they have additive predictive value or are they only surrogates of a surrogate? [Letter] Hypertension. 2006;47:325–326. doi: 10.1161/01.HYP.0000200701.43172.9a. [DOI] [PubMed] [Google Scholar]
- 29.Benetos A, Lacolley P. From 24-hour blood pressure measurements to arterial stiffness: a valid short cut? [Letter] Hypertension. 2006;47:327–328. doi: 10.1161/01.HYP.0000200705.61571.95. [DOI] [PubMed] [Google Scholar]
- 30.Gavish B. Correlating ambulatory blood pressure measurements with arterial stiffness: a conceptual inconsistency? [Letter] Hypertension. 2006;48:e108. doi: 10.1161/01.HYP.0000248120.73770.26. [DOI] [PubMed] [Google Scholar]
- 31.Westerhof N, Lankhaar JW, Westerhof BE. Ambulatory arterial stiffness index is not a stiffness parameter but a ventriculo-arterial coupling factor. [Letter] Hypertension. 2007;49:e7. doi: 10.1161/01.HYP.0000254947.07458.90. [DOI] [PubMed] [Google Scholar]
- 32.Schillaci G, Parati G, Pirro M, Pucci G, Mannarino MR, Sperandini L, Mannarino E. Ambulatory arterial stiffness index is not a specific marker of reduced arterial compliance. Hypertension. 2007;49:986–991. doi: 10.1161/HYPERTENSIONAHA.106.082248. [DOI] [PubMed] [Google Scholar]
- 33.Dechering DG, Adiyaman A, van der Steen M, Thien T. Interstudy variability in the ambulatory arterial stiffness index. Hypertension. 2007;50:e65. doi: 10.1161/HYPERTENSIONAHA.107.096065. [DOI] [PubMed] [Google Scholar]
- 34.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. 2007. [DOI] [PubMed] [Google Scholar]
- 35.Leoncini G, Ratto E, Viazzi F, Vaccaro V, Parodi A, Falqui V, et al. Increased ambulatory arterial stiffness index is associated with target organ damage in primary hypertension. Hypertension. 2006;48:397–403. doi: 10.1161/01.HYP.0000236599.91051.1e. [DOI] [PubMed] [Google Scholar]
- 36.Leoncini G, Ratto E, Viazzi F, Vaccaro V, Parodi A, Falqui V, et al. Metabolic syndrome and ambulatory arterial stiffness index in non-diabetic patients with primary hypertension. J Hum Hypertens. 2007;21:802–807. doi: 10.1038/sj.jhh.1002240. [DOI] [PubMed] [Google Scholar]
- 37.Floras JS, Hassan MO, Jones JV, Sleight P. Pressor responses to laboratory stresses and daytime blood pressure variability. J Hypertens. 1987;5:715–719. doi: 10.1097/00004872-198712000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens. 1987;5:93–98. doi: 10.1097/00004872-198702000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Olga V, Lucio M, Giuseppe G, Stefano M, Paolo P. Blood pressure response to stress tests does not reflect blood pressure variability and degree of cardiovascular involvement in young hypertensives. Int J Cardiol. 1995;48:303–310. doi: 10.1016/0167-5273(94)02237-d. [DOI] [PubMed] [Google Scholar]
- 40.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]